Abstract
Reperfusion therapy after cerebral ischemia often leads to reperfusion injury which may cause brain edema and blood-brain barrier (BBB) dysfunction. As a natural bioflavonoid, quercetin may exert protective effects on BBB dysfunction. This study aimed to investigate effects of quercetin in a rat model of global cerebral ischemia reperfusion (I/R) injury and explore the potential mechanism. Male rats were randomly divided into 4 groups: sham group, I/R group, quercetin-treated group (25 μmol/kg twice daily for 3 consecutive days before I/R), and quercetin/DKK-1-treated group. Global cerebral I/R was induced by bilateral common carotid artery occlusion combined with hypotension for 20 min and reperfusion for 24 h. Neurological function was scored, and then rats were sacrificed. The brain was harvested for HE staining, NeuN staining, and detection of brain water content. The BBB structure and permeability were examined by transmission electron microscopy and Evans blue extravasation, respectively. The protein expression of MMP-9, ZO-1, Claudin-5, β-catenin, and GSK-3β, and the mRNA expression of Axin and LEF1 were detected in either the absence or presence of Wnt/β-catenin inhibitor DKK-1. Results showed that quercetin reduced brain edema and BBB leakage, and improved BBB dysfunction. Quercetin could increase the expression of ZO-1, Claudin-5, β-catenin, and LEF1, and decrease the expression of MMP-9, GSK-3β and Axin. And all these protective effects of quercetin could be reversed by DKK-1. Thus, quercetin can alleviate BBB dysfunction after global cerebral I/R in rats and the mechanism may be related to the activation of canonical Wnt/β-catenin signaling pathway.
Keywords: Quercetin, blood-brain barrier dysfunction, cerebral ischemia reperfusion, MMP-9, Wnt/β-catenin signaling pathway
Introduction
The blood-brain barrier (BBB), as a diffusion barrier, consists mainly of capillary endothelial cells, a basal lamina, astrocytic foot processes, and sealed tight junctions (TJs) (such as claudins, zonula occludens [ZO] and occludin) [1]. The integrity and function of BBB are closely related to the neuronal survival and neurological function [2]. There is evidence showing that BBB breakdown is a major event in the cerebral ischemia/reperfusion (I/R) injury [3,4]. Studies on cerebral I/R animal models have revealed the expression of TJ-associated proteins is significantly reduced, which eventually results in BBB dysfunction [5]. Brain edema due to the increased permeability of damaged endothelium is an inevitable consequence of BBB disruption. The resulting fluid leakage may aggravate infarction, increase intracranial pressure and cause brain hernia [6]. Furthermore, BBB disruption also leads to long-term complications of cerebral I/R injury, such as cognitive impairments [7]. Therefore, deep understanding of the mechanisms underlying brain injury due to BBB breakdown may provide novel targets for prevention and treatment of cerebral I/R injury.
Quercetin (3,3’,4’,5,7-pentahydroxyflavone) is a common flavonol in a variety of fruits and vegetables [8]. It is characterized by two benzene rings linked through a heterocyclic pyrone ring in chemical structure [9]. It possesses strong antioxidant and anti-inflammatory activities, and may exert protective effects in several pathological conditions including cardiovascular diseases, metabolic disorders, neurodegenerative disorders, diabetes, cancer, and obesity [10-12]. Recent findings have indicated that quercetin can exert neuroprotective effects on ischemic damage [13] and maintain BBB integrity [14]. However, exact mechanisms involved in its neuroprotective effects remain unknown.
Wnt signaling pathway is a highly conserved intracellular communication system and closely related to various physiological and pathological processes [15], including proliferation, apoptosis, migration, polarization, stemness maintenance and differentiation [16]. There are two different Wnt pathways: non-canonical and canonical Wnt pathways. A recent study indicates that the canonical Wnt/β-catenin signaling pathway can not only induce but also maintain BBB characteristics throughout lifetime [17], and its dysfunction is associated with BBB breakdown in Alzheimer’s disease [18]. Matrix metalloproteinase-9 (MMP-9) is expressed in a variety of cell types, such as neurons, endothelial cells, activated astrocytes, microglia and infiltrating neutrophils [19]. Studies have confirmed that MMP-9 can degrade TJ in cerebral endothelial cells in vitro and in middle cerebral artery occlusion (MACO) rats, which is ascribed to the increased BBB permeability [20-22]. Moreover, Wnt signaling pathway is able to regulate MMP expression at transcriptional level [23]. Activation of Wnt signaling pathway may inhibit MMP-9 activation and BBB disruption. Thus, we hypothesized that quercetin could attenuate MMP-9 activation by activating canonical Wnt/β-catenin pathway, eventually protecting BBB dysfunction in I/R injury.
Materials and methods
Animals and reagent
All the procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the whole study was approved by the Animal Care and Use Ethics Committee of Wuhan University. Healthy adult male Sprague-Dawley (SD) rats, weighing 250-280 g, were purchased from the Experimental Animal Center of Wuhan University. Rats were housed under standard condition with 12:12 h light/dark cycle and given ad libitum access to food and water throughout the study. Quercetin was purchased from Sigma-Aldrich (India), Evans blue from Urchem Ltd. (Shanghai, P.R. China), and rat DKK-1 (4010-DK-010) recombinant protein (1341-WF-050) from R&D System (Minnesota, USA). All other chemicals used in this study were of analytical grade.
Experimental protocols
Rats were randomly divided into four groups (n = 12 per group): (1) sham-operated group (S), (2) global cerebral I/R group (I/R), (3) quercetin-treated group (Q), (4) quercetin/DKK-1-treated group (Q+DKK). Quercetin at 25 μmol/kg was administered twice daily for 3 consecutive days before I/R. DKK-1 (5 μg/kg) was injected intracerebroventricularly 30 min before the establishment of the model in Q+DKK group (Figure 1).
Figure 1.
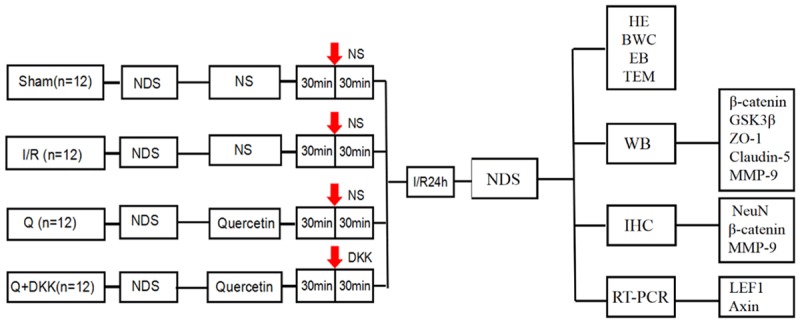
Experimental protocol diagram. Forty-eight male SD rats were randomly divided into 4 groups (n = 12 each): Sham, I/R, Q and Q+DKK group. At 30 min before ischemia/reperfusion (red arrow), intracerebroventricular injections were performed. DKK-1 and NS were given respectively. At 24 h after reperfusion, neurological assessment of the rats was performed using NDS. Then the rats were sacrificed and the brains were harvested for HE staining, NeuN staining and detection of brain water content (BWC). BBB ultrastructure and permeability were examined by transmission electron microscopy (TEM) and Evans blue (EB) extravasation, respectively. Western blot, RT-PCR and immunohistochemical analysis were performed for the relevant key factors of Wnt signaling pathway.
Induction of global cerebral I/R injury in rats
Induction of global cerebral I/R was performed according to previously described with slight modifications [24,25]. In brief, rats were intraperitoneally anesthetized with pentobarbital at 50 mg/kg. A midline ventral incision was made in the neck. Both right and left common carotid arteries were separated and a cotton thread was passed below each carotid artery. The global ischemia was induced by occluding bilateral common carotid arteries (BCCA) with a surgical knot. During ischemia, arterial blood pressure was monitored continuously and the mean arterial pressure (MAP) was lowered to 35-45 mmHg after blood collection (2.1-2.4 ml/100 g body weight) through the jugular vein. After 20-min global cerebral ischemia, reperfusion was induced by removing surgical knots from both arteries and the collected blood was reinfused at 1.2 ml/min for 24 h. During the surgical procedure, the body temperature was maintained around 37 ± 0.5°C with a heating surgical platform. Rats in sham group received same surgical procedure, except for BCCA occlusion. All surgical procedures were carried out under sterile conditions.
Neurological scoring
Neurological function was scored using the Neurological Deficit Score (NDS) system by the same investigator, who was blind to the grouping at 24 h after reperfusion [26]. The NDS ranges from 100 (best) to 0 (brain dead) and it evaluates the consciousness, breathing, smell, vision and hearing, reflexes, motor function, overall activity, orientation, and seizures. All randomized rats were evaluated at baseline to ensure normal neurological function.
Histopathological examination
After 24-h reperfusion, rats were sacrificed and the brains were collected immediately. The brain was fixed in 10% neutral buffered formalin (NBF), embedded in paraffin and cut into 3-mm slices. Coronal sections (6 μm in thickness) of the dorsal hippocampus were prepared and stained with hematoxylin and eosin (HE).
Detection of brain water content
The brain water content (BWC) was used to evaluate cerebral edema after global cerebral I/R injury. Rats were sacrificed and brains were quickly removed. Then, the brains were weighed as the wet weight. The brains were heated in an oven at 105°C for 24 h, and weighed again as the dry weight. The brain water content is calculated as follow:
Brain water content (%) = (wet weight - dry weight)/wet weight × 100
Evaluation of BBB function by Evans blue extravasation
Evans blue (EB) (2%, 3 ml/kg, Sigma) in normal saline was intravenously injected at 3 h before brain collection. After perfusion with saline, each hemisphere was weighed and homogenized in methylformamide (1 mL/100 mg brain tissue), incubated for 24 h at 60°C, and centrifuged for 5 min at 1,000 rpm. The supernatant was harvested and the absorbance (A) of supernatant was detected at 620 nm by spectrophotometry. The amount of EB (μg/g) was calculated from a standard curve. The extravasation of EB was also observed under a fluorescence microscope.
Transmission electron microscopy
After 24-h reperfusion, rats were sacrificed and the brains were collected and fixed in 2% paraformaldehyde/2% glutaraldehyde buffered at 4°C overnight. After washing thoroughly, samples were post-fixed with 1% OsO4 in the same buffer, containing 7.5% sucrose, at 4°C for 2 h and then stained with 2% aqueous solution of uranyl acetate for 1 h. Subsequently, the brains were dehydrated with a graded series of ethanol and embedded in epon. Serial ultrathin sections were obtained with the ultramicrotome, stained with lead citrate and uranyl acetate, and observed under a transmission electron microscope.
Immunohistochemistry
Paraffin sections of brain tissue from each group were baked overnight at 37°C. Then the sections (5 μm thick) were deparaffinized and incubated with 3% hydrogen peroxide for 15 min. The slices were treated with microwave for thermal repair of tissue antigen and then left to cool for 30 min at room temperature. Then the brain tissues were incubated with mouse anti-neuronal nuclei monoclonal antibody, rabbit anti-MMP-9 and rabbit anti-β-catenin polyclonal antibodies. Finally, the brain tissues were incubated with appropriate fluorescent or biotin-labeled secondary antibodies to observe protein expression.
Western blotting
The proteins were extracted from the brain tissue after 24-h reperfusion according to the manufacturer’s instructions, then separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% non-fat dry milk in phosphate buffer saline (PBS) and then incubated with primary antibodies against claudin-5, ZO-1, MMP-9, β-catenin or GSK-3β overnight at 4°C. After incubation with secondary antibodies for 2 h, membranes were washed twice with PBS (10 min for each), and a densitometric analysis was performed using the Western blotting detection system.
Quantitative RT-PCR
Total RNA was extracted from the brain tissue after 24-h reperfuion with the RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. Equal amounts of RNA were used to synthesize cDNA using the QuantiTect Reverse Transcription Kit (Qiagen, Germany). Quantitative RT-PCR was carried out using the QuantiTect SYBR® Green RT-PCR Kit (Qiagen, Germany). The primers used in the RT-PCR were as follows: Axin, 5’-ACCTCACATTCCTCGCAC-3’ (forward) and 5’-AGAAGGCATTTCCCCATC-3’ (reverse); LEF1, 5’-AGCCTG- TTTATCCCATCACG-3’ (forward) and 5’-TGAGGCTTCACGTGCATTAG-3’ (reverse); β-actin, 5’-CACCCGCGAGTACAACCTTC-3’ (forward) and 5’-CCCAT-ACCCACCATCACACC-3’ (reverse). β-actin was used as an internal reference.
Statistical analysis
All the data are expressed as mean ± standard deviation. Comparisons among various groups were done with one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls test. A value of P<0.05 was considered statistically significant. Statistical Program for Social Sciences (SPSS) 17.0 was used for statistical analysis in the present study.
Results
Quercetin improved neurologic function and neuronal damage after cerebral I/R injury
Neurological function was evaluated after 24-h reperfusion with NDS. No neurological deficit was observed in sham group, while severe neurological deficits were found in I/R group (P<0.05 vs sham group). Quercetin pretreatment significantly improved the neurological deficit as compared to I/R group (P<0.05). While with the application of DKK-1, the neurocognitive impairment were severe compared to Q group (P<0.05; Figure 2A).
Figure 2.
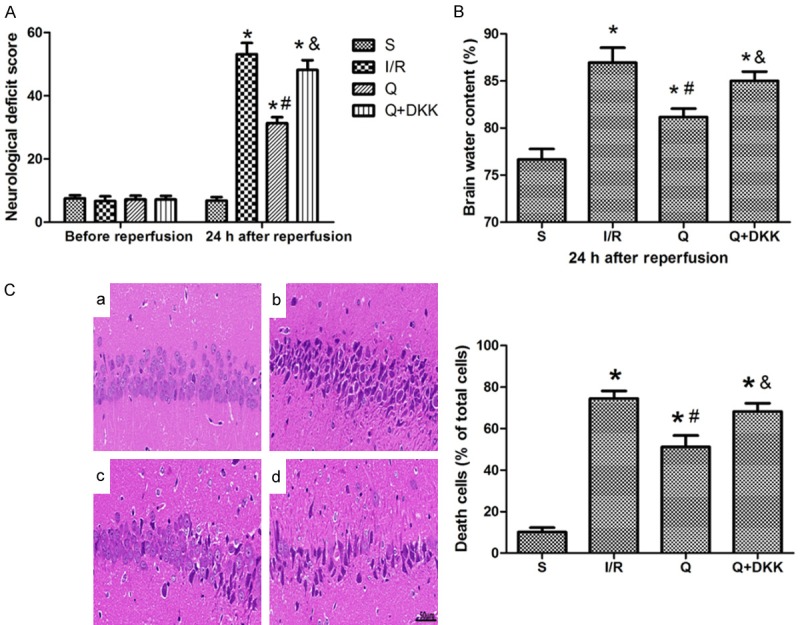
Quercetin reduced cerebral I/R-induced neuronal damage at 24 h after reperfusion. A. Pretreatment with quercetin reduced neurological deficit scores (NDS) in rats at 24 h after reperfusion. B. The value of brain water content (BWC) decreased in the Q group as compared to the I/R group. C. HE-staining (× 400 magnification) showed that quercetin could alleviate the pathological changes in the hippocampal CA1 region. a Group S, b Group I/R, c Group Q, d Group Q+DKK. Data are presented as the mean ± SD and analyzed by one-way analysis of variance. *P<0.05 versus S group, #P<0.05 versus I/R group, &P<0.05 versus Q group.
Brain water content (BWC) was used to assess brain edema after 24-h reperfusion. Compared with sham group, the BWC significantly increased in I/R group (P<0.05 vs sham group). After quercetin pretreatment, the BWC decreased as compared to I/R group (P<0.05), while in Q+DKK group the BWC increased as compared to Q group (P<0.05; Figure 2B).
HE staining of hippocampal CA1 region showed there were three or four layers of pyramidal cells in the hippocampal CA1 area in sham group, with complete cell structure and big and regular nuclei. After cerebral I/R, pyramidal cells in the CA1 region were extensively damaged, which was characterized by neuronal shrinkage and nuclear chromatin condensation. However, these pathological changes in the hippocampal CA1 region were significantly reduced in the presence of quercetin pretreatment characterized by normal shaped pyramidal cells in most area. In Q+DKK group, a large number of pyramidal cells were reduced in size, nuclear pyknosis and hyperchromatic (P<0.05; Figure 2C).
Neuron survival was assessed at 24 h after reperfusion by NeuN staining. In the sham group, there were a large number of surviving pyramidal neurons in hippocampal CA1 region that could be observed. After cerebral I/R injury, the amount of NeuN staining-positive cells decreased in the I/R group, while there was a marked increase in the amount of NeuN staining-positive cells in the Q group compared with the I/R group (P<0.05; Figure 3). In the Q+DKK group, the addition of antagonist DKK-1 could not increase the survival of hippocampal neurons.
Figure 3.
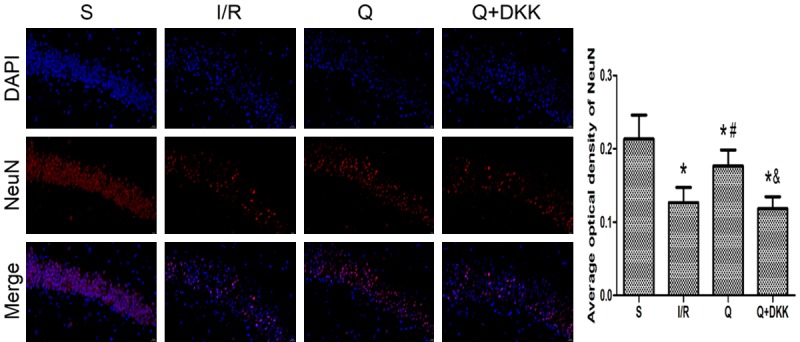
Effect of quercetin on neuron survival at 24 h after reperfusion. The expression of NeuN in the hippocampus of different groups was evaluated by immunofluorometric assay. The results showed that the amount of NeuN staining-positive cells increased in the Q group compared with the I/R group. Data are presented as the mean ± SD and analyzed by one-way analysis of variance. *P<0.05 versus S group, #P<0.05 versus I/R group, &P<0.05 versus Q group.
Quercetin improved BBB permeability after cerebral I/R injury
EB is red under a fluorescence microscope and EB content can reflect the BBB permeability. After 24-h reperfusion, a large amount of EB was observed in I/R group (P<0.05 vs sham group), while quercetin pretreatment significantly reduced the EB content (P<0.05; Figure 4B, 4C). Moreover, the EB content in Q+DKK group was significantly higher than in Q group (P<0.05; Figure 4B, 4C).
Figure 4.
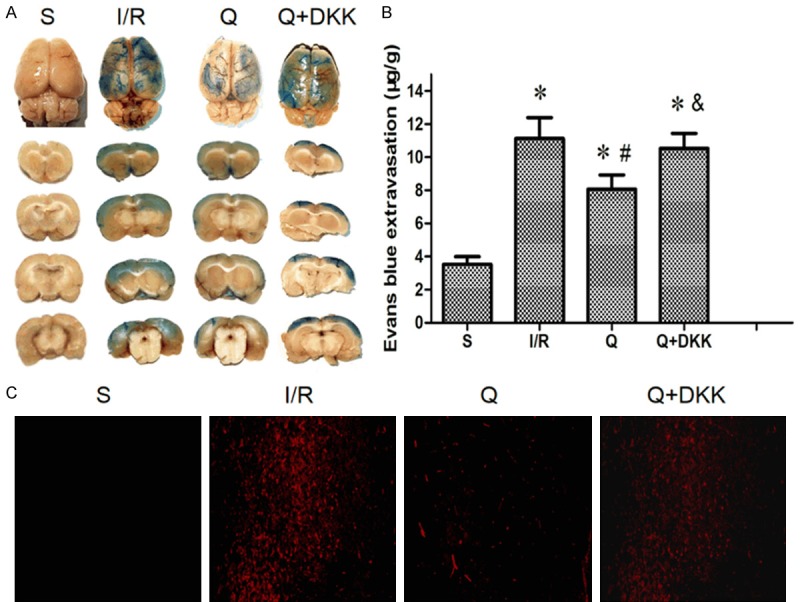
Effect of quercetin on blood-brain barrier permeability to Evans blue (EB) at 24 h after reperfusion. A. Representative images of brain sections showing extravasation of Evans blue dye. With the pretreatment of quercetin, the extravasation of Evans blue dye to cerebral cortex reduced. B, C. The leakage of EB in brain tissue observed by a fluorescence microscope. The results showed that the EB content reduced in the Q group compared with the I/R group. Data are presented as the mean ± SD and analyzed by one-way analysis of variance. *P<0.05 versus S group, #P<0.05 versus I/R group, &P<0.05 versus Q group.
Quercetin ameliorated ultrastructure of BBB after cerebral I/R injury
In sham group, capillary endothelial cells were regular without swelling. TJs in the plasma membrane of adjacent endothelial cells appeared intact and no gaps were observed. After 24-h reperfusion, the capillary endothelial cells showed swelling and irregular thickening, and the TJs between endothelial cells were widened in I/R group. Moreover, the irregular endothelial cells in both thin and thick cytoplasmic regions were filled with numerous vesicles and vacuoles. In quercetin-treated group, the endothelial cells turned to be regularly flattened with less vesicles and vacuoles and attenuation of swelling. In Q+DKK group, the basement membrane of endothelial cells was irregularly thickened, and extensive TJs with points and lines of fusion between plasma membrane external leaflets were observed (Figure 5).
Figure 5.
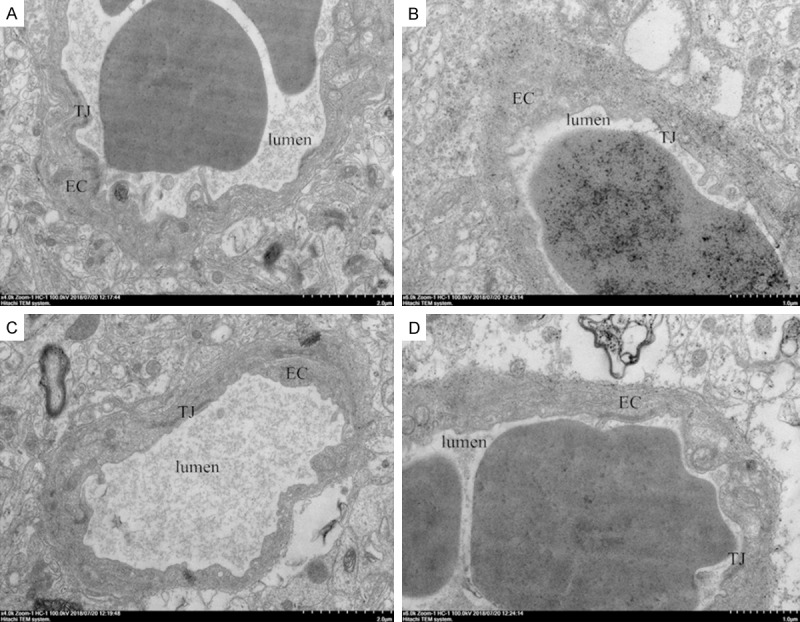
Effect of quercetin on blood-brain barrier ultrastructure alterations in different groups at 24 h after reperfusion. Quercetin could ameliorate the ultrastructure of BBB which manifested as regularly flattened endothelial cells with less vesicles and attenuation of swelling. A Group S, B Group I/R, C Group Q, D Group Q+DKK. EC, endothelial cells; TJ, tight junctions; Scale bar: 0.5 μm.
Quercetin regulated protein expression of claudin-5, ZO-1 and MMP-9 after cerebral I/R injury
Western blotting revealed that the expression of claudin-5 and ZO-1 decreased, and MMP-9 expression increased in I/R group as compared to sham group (P<0.05). Meanwhile, quercetin pre-treatment significantly increased the expression of claudin-5 and ZO-1 and decreased MMP-9 expression (P<0.05 vs I/R group). In Q+DKK group, the Wnt antagonist DKK-1 used together with quercetin reversed the effects of quercetin, manifesting as the expression of claudin-5 and ZO-1 decreased, and MMP-9 expression increased as compared to Q group (P<0.05; Figure 6).
Figure 6.
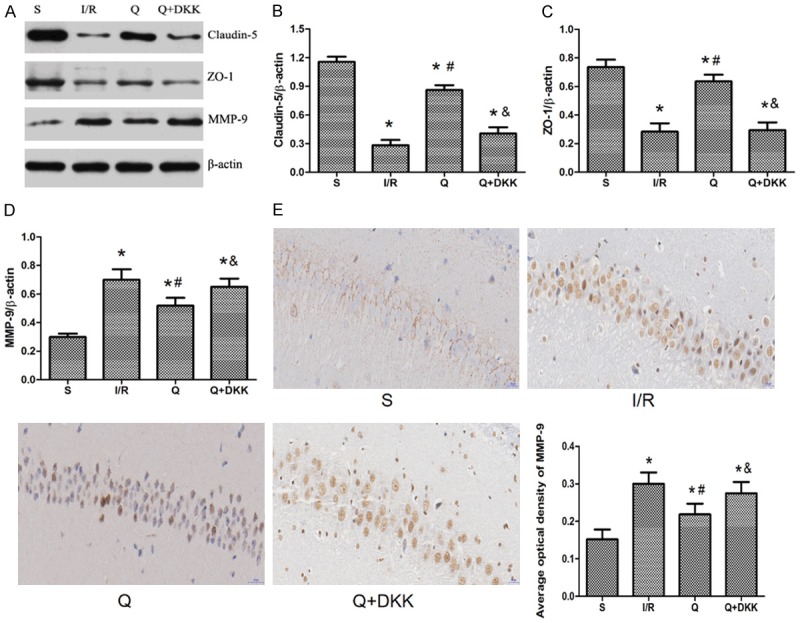
Effect of quercetin on the expression of MMP-9, Claudin-5 and ZO-1 at 24 h after reperfusion. A-D. Western blotting revealed that the expression of claudin-5 and ZO-1 increased, and the expression of MMP-9 decreased with the pretreatment of quercetin in the Q group compared with the I/R group. E. Immunohistochemical analysis showed that quercetin could downregulate the expression of MMP-9. Data are presented as the mean ± SD and analyzed by one-way analysis of variance. *P<0.05 versus S group, #P<0.05 versus I/R group, &P<0.05 versus Q group.
Immunohistochemical staining of brain section showed that MMP-9 was mainly expressed in the cytoplasm and the positive cells were brown (Figure 6). After cerebral I/R injury, the number of positive cells increased in the I/R group while with the pretreatment of quercetin there was a marked decrease in the number of positive cells in the Q group compared with the I/R group (P<0.05; Figure 6). In the Q+DKK group, the number of positive cells decreased compared with the Q group (P<0.05; Figure 6).
Quercetin influenced protein expression of β-catenin and GSK-3β after cerebral I/R injury
Western blotting showed the β-catenin expression was up-regulated, whereas GSK-3β expression was down-regulated after cerebral I/R injury as compared to sham group (P<0.05). Quercetin pre-treatment significantly augmented β-catenin expression and reduced GSK-3β expression as compared to I/R group (P<0.05). In the presence of DKK-1 treatment, the expression of β-catenin decreased and the expression of GSK-3β increased as compared to Q group (P<0.05; Figure 7). In addition, immunohistochemical results showed that at 24 h after reperfusion, β-catenin expression increased in the I/R group with the same findings in the Q group. All these results were consistent with the western blot results.
Figure 7.
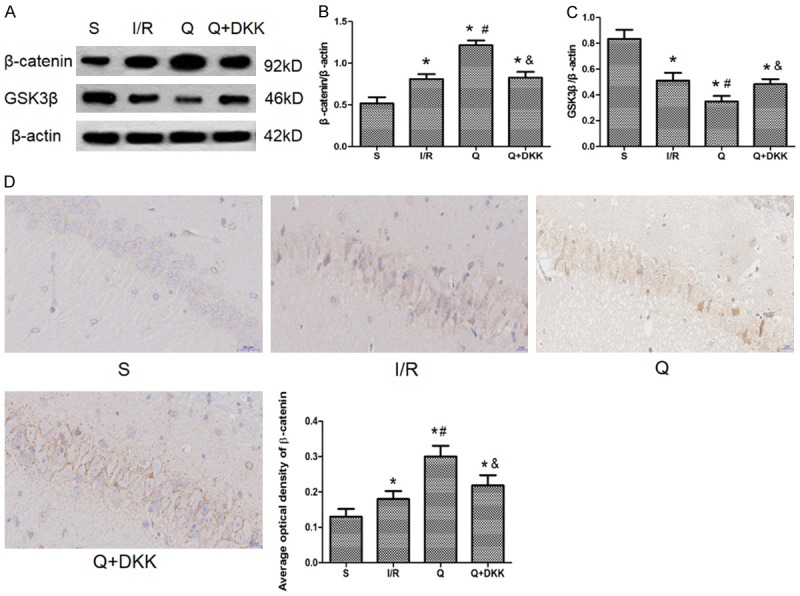
Effect of quercetin on the protein expression of β-catenin and GSK-3β. A-C. Western blot was performed to detect the protein expression of β-catenin and GSK-3β and the results indicated that quercetin could upregulate the β-catenin expression and downregulate the GSK-3β expression. D. Immunohistochemistry was used to show the expression of β-catenin and with the pretreatment of quercetin the expression of β-catenin increased. Data are presented as the mean ± SD and analyzed by one-way analysis of variance. *P<0.05 versus S group, #P<0.05 versus I/R group, &P<0.05 versus Q group.
Quercetin influenced mRNA expression of Axin and LEF1 after cerebral I/R injury
Real-time RT-PCR for Axin and LEF1, two major components of Wnt/β-catenin signaling pathway in the central nervous system, showed the Axin expression was down-regulated, whereas LEF1 expression up-regulated after cerebral I/R injury as compared to sham group (P<0.05). Quercetin pre-treatment significantly augmented Axin expression and increased LEF1 expression as compared to I/R group (P<0.05). In the presence of DKK-1 treatment, the expression of Axin increased and the expression of LEF1 decreased as compared to Q group (P<0.05; Figure 8A, 8B).
Figure 8.
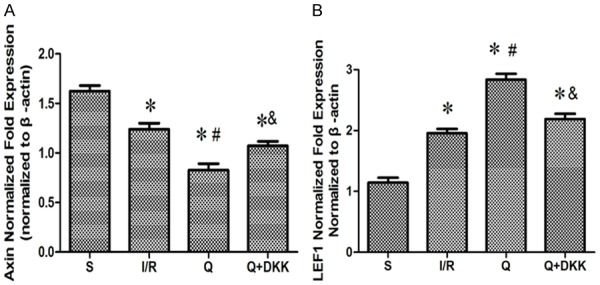
Effect of quercetin on the mRNA expression of Axin and LEF1. RT-PCR was performed to show the mRNA expression of Axin and LEF1 and the results indicated that quercetin could downregulate the Axin expression (A) and upregulate the LEF1 expression (B). Data are presented as the mean ± SD and analyzed by one-way analysis of variance. *P<0.05 versus S group, #P<0.05 versus I/R group, &P<0.05 versus Q group.
Discussion
Our study indicated that quercetin could alleviate morphological damage and improve neurological function in rats with global cerebral I/R injury. Furthermore, the permeability of BBB and the expression of MMP-9 decreased, and the expression of ZO-1 and claudin-5 increased in the presence of quercetin pre-treatment, indicating that quercetin may improve BBB dysfunction following cerebral I/R injury. In addition, quercetin could up-regulate the expression of β-catenin and down-regulate the expression of GSK-3β. While after the application of DKK-1, all these morphological, neurological and biochemical changes were reversed. Thus, these findings suggest that quercetin can alleviate BBB dysfunction after global cerebral I/R injury in rats via activating canonical Wnt/β-catenin signaling pathway.
The maintenance of BBB integrity is helpful to protect the neuronal microenvironment from circulating harmful substances [27]. BBB dysfunction has been found as a key event in the I/R-induced cerebral impairment [28,29]. BBB disturbance allows the infiltration of neutrophils. This, on one hand, initiates inflammation, and on the other hand, causes the leakage of plasma proteins leading to brain edema, both of which contribute to the neurological damage [30]. TJs between adjacent endothelial cells are the major components of BBB and crucial for the permeability of BBB [31]. Cerebral I/R injury can induce the degradation of these components, resulting in the increased BBB permeability [32]. Consistently, in this study, our results showed bilateral common carotid artery occlusion significantly enhanced cerebral vascular leakage, as indicated by EB extravasation assay. In the presence of quercetin pretreatment, the BBB permeability to macromolecules was significantly improved, as indicated by EB extravasation assay. In accordance with these results, transmission electron microscopy revealed quercetin inhibited the perivascular space widening, TJs opening and ultrastructural swelling of capillary endothelial cells after cerebral I/R injury.
To further investigate the mechanism underlying the protective effects of quercetin on BBB dysfunction, the MMP-9 expression was detected in rat brains. Studies have confirmed a close relationship between MMP-9 activation and BBB permeability after cerebral I/R injury [33]. MMP-9 can degrade TJ proteins (claudin-5 and ZO-1) [22]. In the ischemic brain, MMP-9 was activated to degrade TJ proteins and increase BBB permeability [34]. Our study showed the MMP-9 expression increased significantly after cerebral I/R injury, in accordance with previous studies [35]. Furthermore, in the presence of quercetin pretreatment, the MMP-9 expression decreased markedly as compared to I/R group. Moreover, the expression of claudin-5 and ZO-1 decreased in I/R group as compared to sham group, but quercetin pretreatment markedly up-regulated the expression of both TJ proteins. These results suggest that the neuroprotection of quercetin against BBB dysfunction may be related to the inhibition of MMP-9 and regulation of TJ protein expression.
Recent studies indicate that the canonical Wnt/β-catenin signaling pathway is involved in the regulation of MMP expression [36,37]. Some types of MMPs, such as MMP-2 and MMP-9, are the direct transcription targets of Wnt/β-catenin signaling pathway [23]. Meanwhile, there is evidence showing that Wnt β-catenin signaling pathway plays a vital role in the cerebrovascular development and BBB formation [2,38,39]. Dickkopf-1, a negative regulator of Wnt signaling pathway, participates in the development of ischemic neuronal death [15]. Activation of Wnt signaling pathway protects the BBB against dysfunction in various cerebral diseases [40]. Consistently, in the present study, the MMP-9 expression decreased in the presence of quercetin pretreatment, but concomitant DKK-1 treatment failed to affect the MMP-9 expression in Q+DKK group as compared to I/R group. These results indicate that the effect of quercetin on MMP-9 expression has involvement of Wnt/β-catenin signaling pathway.
To further confirm the role of Wnt signaling pathway in the neuroprotective effects of quercetin, the expression of key components in Wnt/β-catenin pathway was detected after 24-h reperfusion. The protein expression of β-catenin, a key component in the canonical Wnt/β-catenin pathway activation [41], was up-regulated in Q group as compared to I/R group. GSK-3β and Axin are inhibitors of Wnt signaling pathway [2]. Quercetin pretreatment downregulated the expression of GSK-3β and Axin as compared to I/R group. Moreover, the mRNA expression of LEF1, a downstream target of β-catenin, was up-regulated in Q group as compared to I/R group. These findings were reversed in the presence of DKK-1, a negative regulator of Wnt pathway. In accordance with these results, the morphological damage and NDS showed no differences between I/R group and Q+DKK group, while quercetin pre-treatment exclusively alleviated morphological damage and improved neurological function. These results indicated that the neuroprotection of quercetin may be related to the early activation of Wnt/β-catenin signaling pathway.
However, there is evidence showing that Wnt signaling pathway has crosstalk with other signaling pathways [42]. Wnt signaling pathway can interact with Notch signaling pathway, leading to the inhibition of differentiation of neural stem cells into glial cells [43]. GSK-3β can regulate a variety of pathways besides Wnt signaling pathway, and plays an important role in the PI3-kinase signaling pathway. Evidence shows that cerebral ischemic preconditioning can activate PI3K/Akt/GSK-3β signaling pathway, enhance p-PDK1 expression and inhibit PP2A expression. It promotes Akt activation and increase p-GSK-3β expression, eventually alleviating cerebral I/R injury [44]. All these findings indicate that Wnt signaling pathway exerts specific biological effects through synergistic interaction with different signaling pathways. At present, the specific cellular and molecular mechanisms in these processes are still unclear, and further research is needed.
The mechanism of quercetin action in a rat model of global cerebral I/R injury is illustrated in Figure 9.
Figure 9.
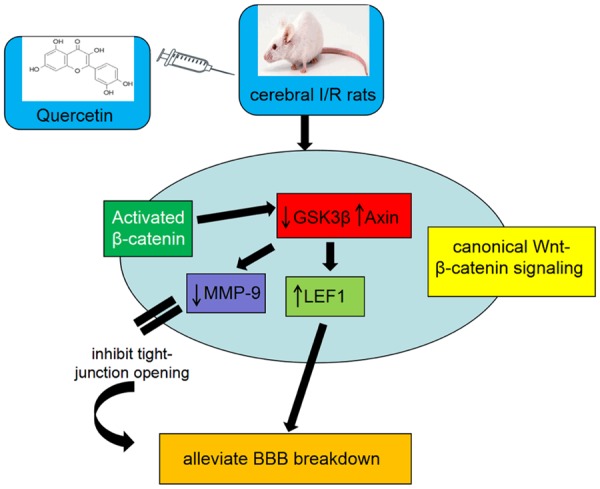
Proposed model for the roles of Wnt/β-catenin signaling regulated by quercetin in protection of BBB breakdown. In ischemic stroke, quercetin preteatment activates the canonical Wnt/β-catenin signaling. Quercetin increased the expression of β-catenin. Improved interaction of nuclear β-catenin then leads to the downregulation of GSK-3β and upregulation of Axin. Then LEF1, which is a downstream target gene of β-catenin, was upregulated resulting in alleviation of BBB breakdown and, concomitantly, MMP-9 was downregulated resulting in tight-junction opening inhibition, which also leads to the alleviation of BBB dysfunction.
Conclusions
In summary, the global cerebral I/R injury induces BBB dysfunction and brain edema. Quercetin, a flavonoid found in various plants and foods, can protect the BBB against dysfunction and attenuate early I/R injury. The neuroprotective effects of quercetin may be related to the early activation of Wnt/β-catenin signaling pathway. However, we cannot exclude the involvement of other signaling pathways in the neuroprotection of quercetin due to the complex microenvironments and the interaction of Wnt pathways with other signaling pathways. The present study provides experimental evidence on the neuroprotection of quercetin on cerebral I/R injury, but the specific mechanism is needed to be further confirmed.
Acknowledgements
This work was supported by the Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (No. znpy2018020) and the National Natural Science Foundation of China (No. 81871553).
Disclosure of conflict of interest
None.
References
- 1.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Gothert JR, Malik AB, Valyi-Nagy T, Zhao YY. Endothelial beta-catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation. 2016;133:177–186. doi: 10.1161/CIRCULATIONAHA.115.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu H, Yang X, Huang C, Gao Z, Tang Y, Dong Q. Apelin-13 protects against ischemic blood-brain barrier damage through the effects of aquaporin-4. Cerebrovasc Dis. 2017;44:10–25. doi: 10.1159/000460261. [DOI] [PubMed] [Google Scholar]
- 4.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 5.Brouns R, Wauters A, De Surgeloose D, Marien P, De Deyn PP. Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur Neurol. 2011;65:23–31. doi: 10.1159/000321965. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Wang D, Wang H, Qu Y, Xiao X, Zhu Y. The protective effect of HET0016 on brain edema and blood-brain barrier dysfunction after cerebral ischemia/reperfusion. Brain Res. 2014;1544:45–53. doi: 10.1016/j.brainres.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Li Y, Tang Y, Tang G, Yang GY, Wang Y. CXCR4 antagonist AMD3100 protects blood-brain barrier integrity and reduces inflammatory response after focal ischemia in mice. Stroke. 2013;44:190–197. doi: 10.1161/STROKEAHA.112.670299. [DOI] [PubMed] [Google Scholar]
- 8.Lee JC, Kim J, Park JK, Chung GH, Jang YS. The antioxidant, rather than prooxidant, activities of quercetin on normal cells: quercetin protects mouse thymocytes from glucose oxidase-mediated apoptosis. Exp Cell Res. 2003;291:386–397. doi: 10.1016/s0014-4827(03)00410-5. [DOI] [PubMed] [Google Scholar]
- 9.Suganthy N, Devi KP, Nabavi SF, Braidy N, Nabavi SM. Bioactive effects of quercetin in the central nervous system: focusing on the mechanisms of actions. Biomed Pharmacother. 2016;84:892–908. doi: 10.1016/j.biopha.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Cho JY, Kim IS, Jang YH, Kim AR, Lee SR. Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global cerebral ischemia. Neurosci Lett. 2006;404:330–335. doi: 10.1016/j.neulet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Galvez J, Zarzuelo A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 2005;35:584–592. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- 12.Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song YS, Lee YH, Jin C, Lee YS, Cho J. Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res. 2003;965:130–136. doi: 10.1016/s0006-8993(02)04150-1. [DOI] [PubMed] [Google Scholar]
- 13.Hwang IK, Lee CH, Yoo KY, Choi JH, Park OK, Lim SS, Kang IJ, Kwon DY, Park J, Yi JS, Bae YS, Won MH. Neuroprotective effects of onion extract and quercetin against ischemic neuronal damage in the gerbil hippocampus. J Med Food. 2009;12:990–995. doi: 10.1089/jmf.2008.1400. [DOI] [PubMed] [Google Scholar]
- 14.Selvakumar K, Prabha RL, Saranya K, Bavithra S, Krishnamoorthy G, Arunakaran J. Polychlorinated biphenyls impair blood-brain barrier integrity via disruption of tight junction proteins in cerebrum, cerebellum and hippocampus of female wistar rats: neuropotential role of quercetin. Hum Exp Toxicol. 2013;32:706–720. doi: 10.1177/0960327112464798. [DOI] [PubMed] [Google Scholar]
- 15.Reis M, Liebner S. Wnt signaling in the vasculature. Exp Cell Res. 2013;319:1317–1323. doi: 10.1016/j.yexcr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 17.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Wan W, Xia S, Kalionis B, Li Y. Dysfunctional Wnt/beta-catenin signaling contributes to blood-brain barrier breakdown in Alzheimer’s disease. Neurochem Int. 2014;75:19–25. doi: 10.1016/j.neuint.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100. doi: 10.1016/s0006-8993(99)01843-0. [DOI] [PubMed] [Google Scholar]
- 21.Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Fu B, Zhang X, Chen L, Zhang L, Zhao X, Bai X, Zhu C, Cui L, Wang L. Neuroprotective effect of bicyclol in rat ischemic stroke: down-regulates TLR4, TLR9, TRAF6, NF-kappaB, MMP-9 and up-regulates claudin-5 expression. Brain Res. 2013;1528:80–88. doi: 10.1016/j.brainres.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farbiszewski R, Bielawski K, Bielawska A, Sobaniec W. Spermine protects in vivo the antioxidant enzymes in transiently hypoperfused rat brain. Acta Neurobiol Exp (Wars) 1995;55:253–258. doi: 10.55782/ane-1995-1084. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Tang C, Tai LW, Yao W, Guo P, Hong J, Yang X, Li X, Jin Z, Ke J, Wang Y. Flurbiprofen axetil attenuates cerebral ischemia/reperfusion injury by reducing inflammation in a rat model of transient global cerebral ischemia/reperfusion. Biosci Rep. 2018;38 doi: 10.1042/BSR20171562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geocadin RG, Ghodadra R, Kimura T, Lei H, Sherman DL, Hanley DF, Thakor NV. A novel quantitative EEG injury measure of global cerebral ischemia. Clin Neurophysiol. 2000;111:1779–1787. doi: 10.1016/s1388-2457(00)00379-5. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Zhong W, Jiang Z, Tang X. New progress in the approaches for blood-brain barrier protection in acute ischemic stroke. Brain Res Bull. 2019;144:46–57. doi: 10.1016/j.brainresbull.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Lorberboym M, Lampl Y, Sadeh M. Correlation of 99mTc-DTPA SPECT of the blood-brain barrier with neurologic outcome after acute stroke. J Nucl Med. 2003;44:1898–1904. [PubMed] [Google Scholar]
- 29.Chen K, Wu Q, Hu K, Yang C, Wu X, Cheung P, Williams KJ. Suppression of hepatic FLOT1 (flotillin-1) by type 2 diabetes mellitus impairs the disposal of remnant lipoproteins via syndecan-1. Arterioscler Thromb Vasc Biol. 2018;38:102–113. doi: 10.1161/ATVBAHA.117.310358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sifat AE, Vaidya B, Abbruscato TJ. Blood-brain barrier protection as a therapeutic strategy for acute ischemic stroke. AAPS J. 2017;19:957–972. doi: 10.1208/s12248-017-0091-7. [DOI] [PubMed] [Google Scholar]
- 31.Ma Q, Dasgupta C, Li Y, Huang L, Zhang L. MicroRNA-210 suppresses junction proteins and disrupts blood-brain barrier integrity in neonatal rat hypoxic-ischemic brain injury. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang L, Cao W, Deng Y, Zhu G, Han Y, Zeng H. Hypertonic saline alleviates experimentally induced cerebral oedema through suppression of vascular endothelial growth factor and its receptor VEGFR2 expression in astrocytes. BMC Neurosci. 2016;17:64. doi: 10.1186/s12868-016-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke. 2003;34:2165–2170. doi: 10.1161/01.STR.0000088062.86084.F2. [DOI] [PubMed] [Google Scholar]
- 34.Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86:324–333. [PubMed] [Google Scholar]
- 35.Yamashita T, Abe K. Therapeutic approaches to vascular protection in ischemic stroke. Acta Med Okayama. 2011;65:219–223. doi: 10.18926/AMO/46846. [DOI] [PubMed] [Google Scholar]
- 36.Xu K, Ma C, Xu L, Ran J, Jiang L, He Y, Adel Abdo Moqbel S, Wang Z, Wu L. Polygalacic acid inhibits MMPs expression and osteoarthritis via Wnt/beta-catenin and MAPK signal pathways suppression. Int Immunopharmacol. 2018;63:246–252. doi: 10.1016/j.intimp.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z, Li K, Liang Q, Zheng G, Zhang S, Lao X, Liang Y, Liao G. Elevated hydrostatic pressure promotes ameloblastoma cell invasion through upregulation of MMP-2 and MMP-9 expression via Wnt/beta-catenin signalling. J Oral Pathol Med. 2018;47:836–846. doi: 10.1111/jop.12761. [DOI] [PubMed] [Google Scholar]
- 38.Artus C, Glacial F, Ganeshamoorthy K, Ziegler N, Godet M, Guilbert T, Liebner S, Couraud PO. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metab. 2014;34:433–440. doi: 10.1038/jcbfm.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziegler N, Awwad K, Fisslthaler B, Reis M, Devraj K, Corada M, Minardi SP, Dejana E, Plate KH, Fleming I, Liebner S. β-catenin is required for endothelial Cyp1b1 regulation influencing metabolic barrier function. J Neurosci. 2016;36:8921–8935. doi: 10.1523/JNEUROSCI.0148-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polakis P. Formation of the blood-brain barrier: wnt signaling seals the deal. J Cell Biol. 2008;183:371–373. doi: 10.1083/jcb.200810040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji XK, Xie YK, Zhong JQ, Xu QG, Zeng QQ, Wang Y, Zhang QY, Shan YF. GSK-3beta suppresses the proliferation of rat hepatic oval cells through modulating wnt/beta-catenin signaling pathway. Acta Pharmacol Sin. 2015;36:334–342. doi: 10.1038/aps.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zechner D, Muller T, Wende H, Walther I, Taketo MM, Crenshaw EB 3rd, Treier M, Birchmeier W, Birchmeier C. Bmp and Wnt/beta-catenin signals control expression of the transcription factor Olig3 and the specification of spinal cord neurons. Dev Biol. 2007;303:181–190. doi: 10.1016/j.ydbio.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 43.Wu MV, Hen R. The young and the restless: regulation of adult neurogenesis by wnt signaling. Cell Stem Cell. 2013;12:139–140. doi: 10.1016/j.stem.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valvezan AJ, Klein PS. GSK-3 and wnt signaling in neurogenesis and bipolar disorder. Front Mol Neurosci. 2012;5:1. doi: 10.3389/fnmol.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]


