Abstract
Endometrial regenerative cells (ERCs) are easily isolated from menstrual blood, and can be cultured in large amounts. Although, ERCs can ameliorate DSS-induced colitis in mice, the molecular mechanism underlying ERCs-mediated immunosuppression is unclear. This study was aimed to assess the function of PD-L1 expressed on ERCs in colitis attenuation. ERCs with and without anti-PD-L1 mAb-pretreatment were administered to mice by injection at 2, 5 and 8 days after colitis induction by DSS treatment. Blood, spleen and colon samples were obtained 15 days post-DSS-induction. Then, clinicopathological alterations, cytokine levels, immune cell types and cell tracking were assessed. ERCs or ERCs preincubated with anti-PD-L1 antibody were co-cultured with splenocytes, whose phenotypes was analyzed by flow cytometry. We found that PD-L1 on ERCs was upregulated by IFN-γ stimulation. The transplanted PKH26-labeled ERCs were engrafted to the lung, liver, spleen and injured colon. Interestingly, ERC-based therapy markedly attenuated mouse colitis, but blockade of PD-L1 on ERCs with a specific monoclonal antibody conferred severe colitis to the animals. These effects of PD-L1 inhibition on colitis were associated with reduced amounts of pro-inflammatory cytokines and infiltrated immune cells, including CD3+CD4+ T lymphocytes, CD3+CD8+ T lymphocytes, CD11c+MHC-II+ Dendritic cells and F4/80+ macrophages, both in vivo and in vitro, as well as with elevated levels of anti-inflammatory cytokines and regulatory immune cells, including CD4+CD25+Foxp3+ Tregs and F4/80+CD206+ macrophages. These findings demonstrated that ERCs-based treatment promotes immune tolerance in mouse colitis, in association with PD-L1, thus indicating that PD-L1 modulates immunosuppression by ERCs.
Keywords: Colitis, endometrial regenerative cells, immunosuppression, mice, PD-L1
Introduction
Ulcerative colitis (UC) is a major type of inflammatory bowel disease (IBD) featuring chronic, relapsing, nonspecific inflammatory reactions in the colorectal area [1]. UC prevalence and incidence are currently elevated in developed nations, and have been quickly increasing in other countries, especially in China, in past decades [2]. Although a clear-cut etiology has not been proposed for UC, it is generally admitted that this ailment is associated with genetic susceptibility, environmental factors, changes in the gut microbiota and dysregulation of immune response [3]. UC is routinely treated with anti-inflammatory medicines and immunosuppressors, and even surgical removal of the colon [1]. However, satisfactory results are rarely obtained. In recent years, there has been increasing evidence that shows mesenchymal stromal cells (MSCs) therapy to be effective in the treatment of UC [4].
MSCs are multipotent stromal cells with self-renewal potential. They promote tissue repair and wound healing, and show immunomodulatory and anti-inflammatory features in UC [5]. Endometrial regenerative cells (ERCs), isolated from menstrual blood as a novel group of MSCs, also display tissue repair and immunomodulatory functions. Compared with MSCs from many conventional sources, ERCs present further advantages, including rich source, noninvasive method for harvesting, high abundance, easy purification, rapid proliferation, relatively unlimited expandability, and the potential to differentiate into more lineages [6]. Moreover, ERCs can produce matrix metalloprotease and a large number of growth factors to promote tissue repair [7]. In our previous study, ERCs were selected for the treatment of UC, and the results showed that systemic infusion of ERCs attenuates experimental colitis in mice with significantly reduced disease activity index (DAI). The intra-colon levels of proinflammatory cytokines were decreased while there was an increase in anti-inflammatory cytokines. It was also found that immune reactive cells in the spleen decreased, while regulatory T cells increased [8]. However, the molecular mechanism underlying ERC-mediated immunosuppression in the colitis model remains largely unclear.
MSCs modulate some immune cells, including DCs, macrophages and T and B cells via secretion of soluble factors [5,9,10]. In addition, MSCs exert immunosuppressive effects on immune cells by direct cell-to-cell interactions (e.g. PD-L1 interacts with PD-1) [11], and production of surface adhesion molecules [12] and human leukocyte antigen-G [13]. MSC-expressed PD-L1 plays a significant role in MSC-mediated immune suppression [14]. Our previous research indicated that MSCs require PD-L1 for the induction of immune tolerance in a cardiac allotransplantation model [15]. Moreover, Song et al found that PD-L1-Fc markedly alleviates DSS associated acute colitis as well as T-cell dependent chronic colitis, by inducing colonic CD4+ T cell and DC regulation [16]. We detected that ERCs as mesenchymal-like stem cells also express PD-L1. Thus, we speculate that PD-L1 also plays an important role in immunosuppressive effects during the ERCs treatment for colitis.
Materials and methods
Animals
BALB/c mice (Male, 8 weeks, 18-22 g; Aoyide Co., Tianjin, China) were maintained routinely under a cycle of 12 h-12 h light-dark in the vivarium of the Tianjin General Surgery Institute, with standard rodent chow and drinking water ad libitum. The study was approved by the Institutional Ethics Committee, following the Chinese Council on Animal Care guidelines.
Preparation of ERCs
The menstrual blood was collected from a 30-year-old healthy woman upon informed consent. ERCs were isolated following the isolation method as described by Y Lv et al previously [8]. In brief, menstrual blood was obtained by the Diva cup method in a solution supplemented with antibiotics. Approximately 10 ml of menstrual blood samples were used for ERCs isolation each time. Then, the Ficoll method was employed for mononuclear cell separation. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) high glucose containing 10% fetal bovine serum (FBS) overnight, with marginal adherence to tissue culture flasks. After 2 weeks of culture (with 2 medium changes weekly), adherent cells with a fibroblast-like morphology were obtained. A culture was started with about 1×107 adherent cells. Phenotypic analysis of ERCs was performed by flow cytometry (Becton Dickinson FACS Canto II, Franklin Lakes, NJ, USA) after staining with antibodies against CD34, CD45, CD90 and CD105 (Becton Dickinson and Company, http://www.BD.com/), respectively, as directed by the manufacturer. In addition, ERCs were positive for PD-L1 (BioLegend, http://www.biolegend.com/) as evaluated by FACS. In functional experiments, PD-L1 on ERCs was inhibited by anti-PD-L1 antibodies (BioLegend, http://www.biolegend.com/) as reported in previous studies [15,17,18]. ERCs at passages 3-4 were used for in vitro and in vivo.
Induction and assessment of DSS-associated colitis
The mouse DSS colitis model was established according to previous study [19], by administration of 3% Dextran sulfate sodium (DSS) (MW 5000 kDa; Sigma-Aldrich, St Louis, United States) in drinking water for 7 consecutive days. Weight, stool consistency and occult blood assessments were carried out daily.
In vivo treatment and experimental groups
Thirty-two animals were randomly divided into four groups (n=8), including sham (Group 1; drinking water for 14 days), colitis model (Group 2; 3% DSS for 7 days, and drinking water for 7 days), ERCs treatment (Group 3; DSS models intravenously administered ERCs in PBS at 1×106/0.2 mL/mouse at 2, 5 and 8 days, respectively), and anti-PD-L1-pretreated ERCs group (Group 4; DSS models intravenously administered anti-PD-L1 mAb-pretreated ERCs in PBS at 1×106/0.2 mL/mouse at 2, 5 and 8 days, respectively).
Gross inspection and DAI
Body weight, stool consistency and stool features were evaluated during the experiment. DAI was employed to assess intestinal inflammation [20]. The animals were euthanized at 15 days after DSS initiation, and blood, spleen and colon specimens were obtained from different groups. The colon length was measured; after rinsing with PBS, each colon was divided and employed for histology, mRNA purification, and protein extraction.
Histological examination
Colon specimens were fixed with 10% formaldehyde, paraffin embedded and sectioned at 5 μm for hematoxylin and eosin staining. The samples were histologically scored in a blinded manner based on inflammation severity (scores of 0 to 3), extent of injury (0 to 3), crypt damage (0 to 4), and percent involvement (1-4); the overall histopathology score was obtained as a sum of the above subscores [21].
Immunohistochemistry staining
To assess the amounts of neutrophils, T cells and M2 macrophages (total or activated), the colonic sections were stained with antibodies raised against mouse Ly6g, CD3 and CD206 (Abcam, Cambridge, MA), separately. Briefly, 5 μm sections of paraffin embedded samples were deparaffinized and rehydrated, followed by antigen retrieval (microwave) and treatment with 3% H2O2. After blocking with 5% bovine serum albumin (BSA), the samples were exposed to antibodies raised against mouse Ly6g, CD3 and CD206 overnight at 4°C, respectively. In negative controls, the primary antibodies were omitted. A Streptavidin-Biotin Complex (SABC) kit was employed for detection; imaging was photographed (Olympus Imaging America, Center Valley, PA).
Real-time quantitative PCR
TNF-α, IFN-γ, IL-10 and TGF-β mRNA amounts in colon specimens were assessed by real-time quantitative PCR (MJ Research Inc., USA), with the following PCR primers: TNF-α, forward 5’-CATCTTCTCAAAATTCGAGTGACAA-3’ and reverse 5’-TGGGAGTAGACAAGGTACAACCC-3’; IFN-γ, forward 5’-GCCGCGTCTTGGTTTTGCAG-3’ and reverse 5’-TACCGTCCTTTTGCCAGTTCCTCCA-3’; IL-10, forward 5’-AGAAGCATGGCCCAGAAATCA-3’ and reverse 5’-GGCCTTGTAGACACCTTGGT-3’; TGF-β, forward 5’-CAGCAACAATTCCTGGCGATAC-3’ and reverse 5’-GCTAAGGCGAAAGCCCTCAAT-3’.
ELISA
The protein levels of TNF-α, IFN-γ, IL-10 and TGF-β in colon tissue and serum specimens were evaluated with specific ELISA kits (eBioscience, http://www.ebioscience.com/) as instructed by the manufacturer.
Flow-cytometry
To quantitate CD3+CD4+ T, CD3+CD8+ T and CD4+CD25+Foxp3+ T lymphocytes, as well as CD11c+MHC-II+ DCs, macrophages (F4/80+) and the sub-population of macrophages positive for CD206+ in spleen specimens, the cell concentration of suspensions was adjusted to 1×107 cells/mL before incubation with fluorescent antibodies targeting CD3, CD4, CD8 (BioLegend, http://www.biolegend.com/), CD25, Foxp3, CD11c, MHC-II, F4/80 and CD206 (eBioscience, http://www.ebioscience.com/), respectively. Flowjo was used for data analysis.
Co-cultures of ERCs with splenocytes
In order to assess PD-L1’s role in ERCs immunoregulation in vitro, splenocytes were co-cultured with ERCs blocked with anti-PD-L1 mAb or not, via transwell plates. ERCs (104 cells/well) were pre-incubated with anti-PD-L1 antibody or isotype-control antibody (20 μg/mL) for 1 h, and co-cultured with splenocytes obtained from BALB/c mice. In non-contact assays, 3 μm pore-Transwell was employed for ERCs separation from splenocytes. To assess whether Tregs differentiation is modulated by ERCs, splenocytes (2×105/well) were co-cultured in different groups, and administered a mixture of anti-CD3 antibody (100 ng/mL) and anti-CD28 antibody (200 ng/mL) for 72 h [15]. For CD4+ T and CD8+ T cell quantitation, splenocytes were co-cultured with ERCs by using the same grouping as above, and administered lipopolysaccharide (LPS) (20 μg/mL) [15]. DCs were stimulated with ConA (10 μg/mL) for assessment [22], while macrophages were stimulated with IL-4 (20 ng/mL) [23]. Analysis was performed by flow-cytometry (Becton Dickinson FACS Canto II, United States).
ERCs labeling and in vivo tracking
To track ERCs in vivo after treatment, cells labeling with PKH26 Fluorescent Cell Linker Kit (Sigma-aldrich, St Louis, USA) were performed as directed by the manufacturer. Then, PKH26-labeled ERCs (6×106 cells/0.2 mL) were injected via tail vein into normal and colitis mice on day 5, respectively. After sacrifice 48 h following treatment, mouse liver, lung, kidney, spleen and colon specimens were obtained and snap-frozen in liquid nitrogen. Cryosections (4 µm) were assessed by fluorescence microscopy for ERCs detection.
Statistical analysis
SPSS 20.0 software (SPSS Inc., USA) was used for all analyses. Data are mean ± SD, and were assessed by one-way analysis of variance (ANOVA) followed by post hoc least significant difference (LSD) test. P<0.05 indicated statistical significance.
Results
Phenotypic identification of ERCs and PD-L1 expression on ERCs
ERCs are plastic adherent cells. Flow cytometric analysis revealed the expression of CD105 and CD90 while lacking of CD34 and CD45 (Figure 1A). These results were consistent with previous reports on ERCs [7]. For assessing PD-L1 levels and its response to IFN-γ in ERCs, these cells were treated with IFN-γ (PeproTech, Rocky Hill, USA) or medium control. Basal PD-L1 levels were detectable on ERCs and were increased with the IFN-γ concentration (0.5-5 ng/mL) in a dose-dependent manner (Figure 1B).
Figure 1.
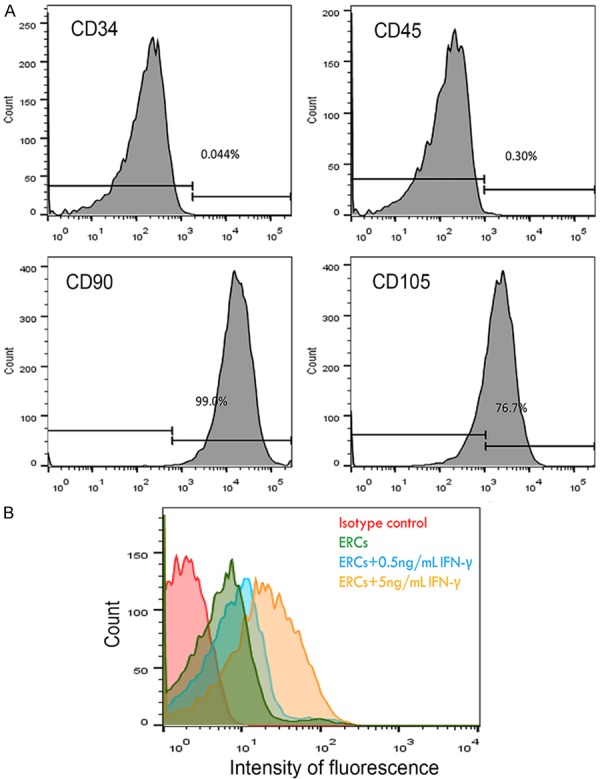
A. Representative flow cytometric analysis of surface expression of phenotypic markers in ERCs. ERCs express CD105, CD90, while lacking of CD34, CD45. B. PD-L1 expression of ERCs was analyzed by using the FACS. Basal PD-L1 levels were found on ERCs and dose-dependently increased with the IFN-γ concentration (0.5-5 ng/mL). The result was representative in three separate experiments.
PD-L1 is required for ERCs-mediated attenuation of colitis
It was demonstrated that ERCs as a novel cell therapy significantly alleviate DSS-induced colitis in our previous report [8]. To investigate the PD-L1’s role in ERC-based therapy, ERC-expressed PD-L1 was neutralized with anti-PD-L1 mAb. As shown in Figure 2, untreated mice with colitis progressively showed the following symptoms: body weight loss, lethargy, and loose and even bloody stool (Figure 2A), with the highest DAI (Figure 2C) and histopathological scores (Figure 2D). This was accompanied by severe inflammatory reactions featured by epithelial and crypt structural damage, disarranged glandular, less goblet cell renewal and pronounced inflammatory cell infiltration into the mucosal layers (Figure 2B). Compared with the colitis model group, ERCs therapy strikingly relieved the above clinical signs (Figure 2A), including the DAI (P<0.05 vs. model group; Figure 2C) and histopathological scores (P<0.05 vs. model group; Figure 2D). Overt bowel dilation was absent. Colon lengths in the ERC-treated group were starkly greater in comparison with those of the model group (Figure 2A). In addition, mucosal hyperemia and edema were attenuated, with blunted ulceration. Histologically, the colon showed improved epithelial and crypt structures, with remarkably reduced infiltration of inflammatory cells and larger goblet cell amounts (Figure 2B). However, functional blockage of ERCs by pretreatment with anti-PD-L1 mAb markedly reduced the therapeutic effects of ERCs in clinical signs (Figure 2A). Our results also showed that DAI and histopathological score alterations in mice administered anti-PD-L1 mAb-pretreated ERCs (*ERCs) were more pronounced than in the ERC-treatment group (ERCs) (both DAI and histopathological scores, P<0.05 for ERCs vs. *ERCs; Figure 2C and 2D). These findings indicated that functional PD-L1 blockage via anti-PD-L1 mAb markedly reduced the efficacy of ERCs-based treatment of colitis.
Figure 2.
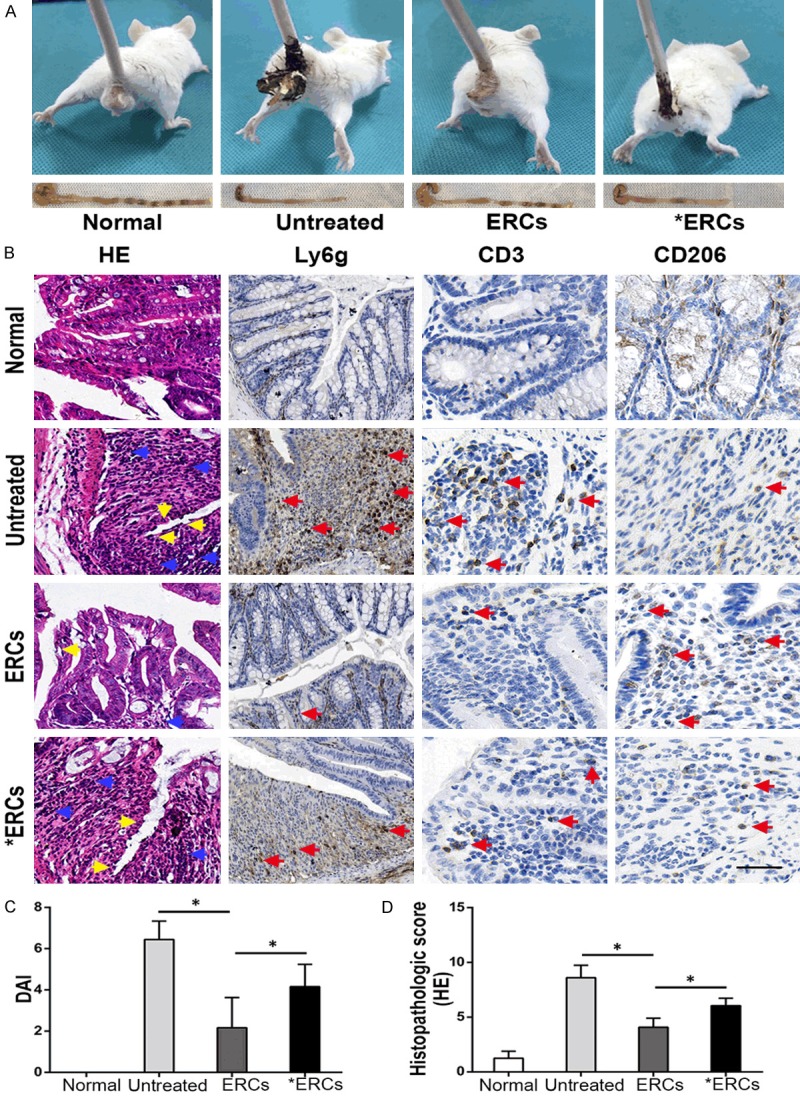
A. In vivo, the representative photos of mice with general performance were shown from different groups. B. Microscopic observation of the colon from different groups. In the untreated group, it was characterized by damaged epithelium (yellow arrow) and massive inflammatory cells infiltration (blue arrow) into the mucosa and submucosa. In ERCs-treated group, the colon showed improved epithelial and crypt structures (yellow arrow) and remarkably reduced infiltration of inflammatory cells (blue arrow). ERCs suppress intra-colon Ly6g+ cells and CD3+ T cells (pro-inflammatory) infiltration (red arrow) and promote CD206+ cells (anti-inflammatory) infiltration (red arrow) in the colitis mice. However, the blockage of ERCs with anti-PD-L1 mAb significantly impaired the therapeutic efficacy. C. The comparison of DAI was analyzed in different groups on day 15 (n=8 per group). Bar=100 μm. D. The comparison of the histopathological scores was performed in different groups on day 15 (n=8 per group). ERCs indicated ERCs treatment. *ERCs indicated anti-PD-L1 antibody pretreated ERCs treatment. *P<0.05.
ERCs reduce the amounts of neutrophil, CD3+ T cell and enhance M2 macrophage levels in the colon via PD-L1
To assess whether ERCs treatment via PD-L1 could influence inflammatory cell infiltration in the mouse colitis model, intra-colon neutrophils (Ly6g-positive), intra-colon CD3+ T cells and CD206+ (a biomarker for M2 macrophages) cells infiltrated were evaluated among different groups. ERCs administration markedly decreased the amounts of neutrophils and CD3+ T cells, while enhancing CD206+ cells in colon samples in comparison with control colitis mice (Figure 2B). However, after pretreatment of ERCs with anti-PD-L1 mAb, neutrophil and CD3+ T cell amounts were significantly increased, and those of CD206+ cells significantly decreased (Figure 2B). These results suggested that ERCs significantly suppressed colonic inflammation via PD-L1.
PD-L1 is required for ERCs-induced changes of pro-inflammatory and anti-inflammatory cytokines in mouse colitis
In a previous report, we demonstrated that ERCs concomitantly downregulate pro-inflammatory cytokines and upregulate anti-inflammatory cytokines [8]. In this study, pro-inflammatory cytokine (TNF-α, IFN-γ) amounts in serum (protein) as well as the colon (mRNA and protein) were overtly elevated in control mice with colitis (Figure 3); therefore, administration of ERCs resulted in markedly decreased pro-inflammatory cytokine levels (both TNF-α and IFN-γ, P<0.05, Untreated vs. ERCs; Figure 3A-C). Compared with the amounts in ERCs-treated animals, the levels of intra-colonic pro-inflammatory cytokines were enhanced in ERCs pre-conditioned with anti-PD-L1 mAb (both TNF-α and IFN-γ, P<0.05, ERCs vs. *ERCs; Figure 3A-C). Meanwhile, administration of ERCs resulted in enhanced levels of anti-inflammatory cytokines (IL-10, TGF-β) in serum and colon samples (both IL-10 and TGF-β, P<0.05, Untreated vs. ERCs; Figure 3A-C). However, functional blockade of ERCs-expressing PD-L1 with anti-PD-L1 mAb resulted in reduced levels of anti-inflammatory cytokines compared with the ERCs-treatment group (both IL-10 and TGF-β, P<0.05, ERCs vs. *ERCs; Figure 3A-C). These findings indicated PD-L1 in ERCs-based therapy has a critical function in regulating cytokine profiles.
Figure 3.
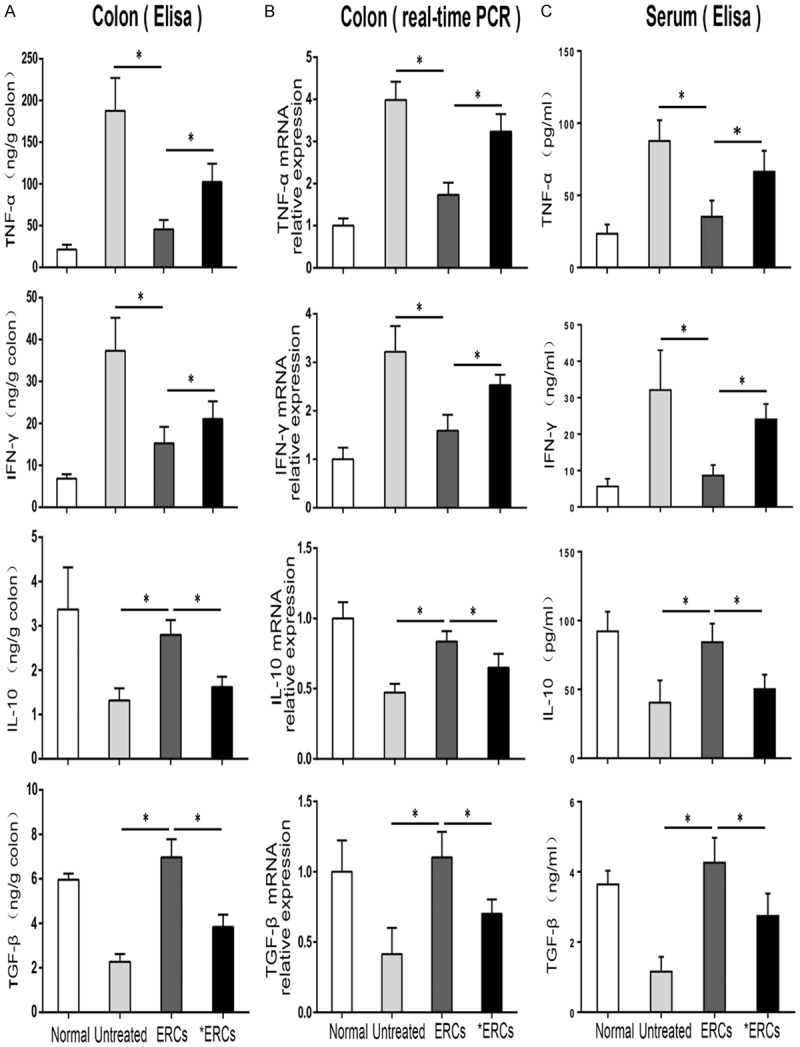
Comparison of the colonic cytokine protein levels (A), colonic mRNA transcriptional levels (B) and serum cytokine protein levels (C) were performed in different groups on day 15 (n=8 per group). ERCs indicated ERCs treatment. *ERCs indicated anti-PD-L1 pretreated ERCs treatment. *P<0.05.
ERCs via PD-L1 reduce the amounts of CD4+ T and CD8+ T cell in cell culture and animal model
To determine PD-L1’s effects on T cell populations in ERCs-treated mice with colitis, we used flow-cytometric analysis to assess CD4+ T and CD8+ T cell changes in the spleen in vivo or in co-cultures of ERCs with splenocytes in vitro. As depicted in Figure 4, the population of splenic CD4+ T and CD8+ T cells showed markedly reduced in ERCs-treated animals compared with untreated mice with colitis (both CD4+ T and CD8+ T, P<0.05, Untreated vs. ERCs; Figure 4B). However, the numbers of splenic CD4+ T and CD8+ T cells were significantly increased by pretreatment of ERCs with anti-PD-L1 mAb (both CD4+ T and CD8+ T, P<0.05, ERCs vs. *ERCs; Figure 4B). In vitro, addition of ERCs resulted in lower CD4+ T and CD8+ T cell numbers in co-cultures stimulated with LPS (both CD4+ T and CD8+ T, P<0.05, Splenocytes Vs. Splenocytes + ERCs; Figure 4B). These effects, however, were specifically blunted by neutralization with anti-PD-L1 mAb (both CD4+ T and CD8+ T, P<0.05, Splenocytes + ERCs vs. Splenocytes + *ERCs; Figure 4B) or separation of ERCs from splenocytes in a transwell plate (both CD4+ T and CD8+ T, P<0.05, Splenocytes + ERCs vs. Splenocytes + ERCs transwell; Figure 4B).
Figure 4.
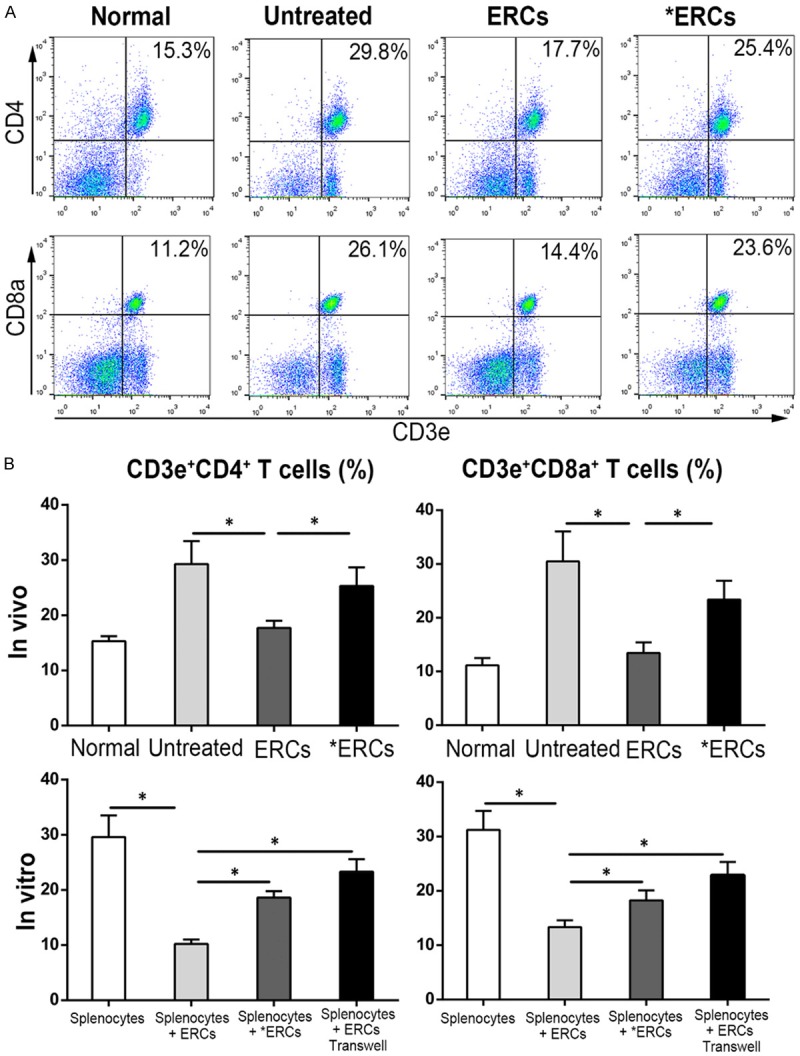
ERCs can suppress CD4+ T and CD8+ T cell populations through PD-L1. The proportion of CD4+ T and CD8+ T cells was detected by using FACS in different groups. A. Dot plots of CD4+ T and CD8+ T cells in vivo. B. Percentage of CD4+ T and CD8+ T cells in vivo (n=8 per group) and in vitro (n=6 per group). ERCs indicated ERCs-treated group. *ERCs indicated anti-PD-L1 antibody pretreated ERCs treatment. *P<0.05.
ERCs via PD-L1 increase Treg amounts
Tregs significantly contribute to immune suppression [24]. As depicted in Figure 5, ERCs-treated colitis mice had starkly elevated numbers of splenic CD4+CD25+Foxp3+ cells (Tregs) (P<0.05, Untreated vs. ERCs; Figure 5B). In contrast, Tregs were significantly decreased after pre-treatment with anti-PD-L1 of ERCs (*ERCs) compared with the ERCs-treatment group (P<0.05, ERCs vs. *ERCs; Figure 5B). Similarly, Treg numbers were higher in co-culture in presence of ERCs (P<0.05, Splenocytes Vs. Splenocytes + ERCs; Figure 5B). Meanwhile, when the co-cultured ERCs were pre-treated with anti-PD-L1 antibody, CD4+CD25+Foxp3+ Tregs were reduced (P<0.05, Splenocytes + ERCs vs. Splenocytes + *ERCs; Figure 5B). In addition, Treg levels were also decreased by employing a transwell plate (P<0.05, Splenocytes + ERCs vs. Splenocytes + ERCs transwell; Figure 5B).
Figure 5.
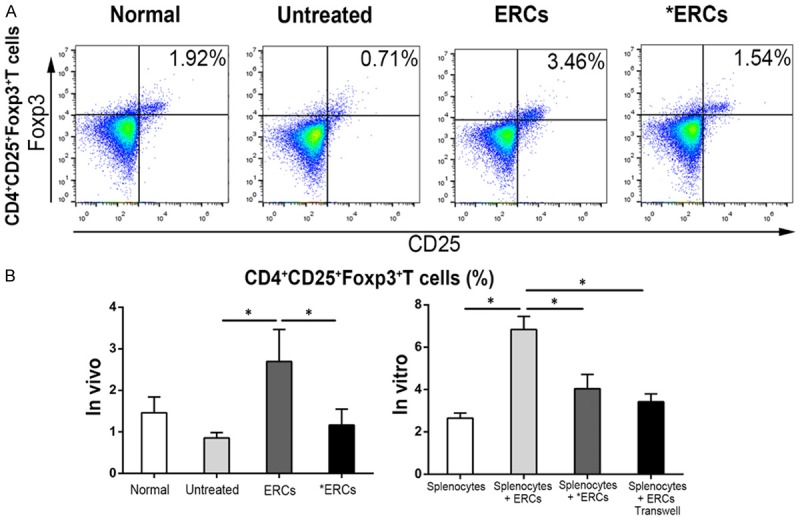
ERCs enhance the percentage of CD4+CD25+Foxp3+ Tregs via PD-L1 and induce the protection against colitis. Flow cytometric analysis of CD4+CD25+Foxp3+ Tregs was performed in different groups. A. Dot plot of CD4+CD25+Foxp3+ Tregs in vivo. B. Percentage of CD4+CD25+Foxp3+ Tregs in vivo (n=8 per group) and in vitro (n=6 per group). ERCs indicated ERCs-treatment. *ERCs indicated anti-PD-L1 pretreated ERCs treatment. *P<0.05.
ERCs via PD-L1 decrease the percentage of CD11c+MHC-II+ DCs in vivo and in vitro
DCs are critical for regulating immune responses in the antigen-rich gastrointestinal environment [25]. As shown in Figure 6, the CD11c+MHC-II+ DCs population in the spleen was markedly decreased after administration of ERCs in comparison with control animals with colitis (P<0.05, Untreated vs. ERCs; Figure 6B). Meanwhile, the number of these cells in the spleen induced by anti-PD-L1 mAb pretreated ERCs was increased, but not significantly, in comparison with the ERCs-treatment group (P>0.05, ERCs vs. *ERCs; Figure 6B). Addition of ERCs in vitro resulted in decreased numbers of CD11c+MHC-II+ DCs in co-cultures (P<0.05, Splenocytes + ERCs vs. Splenocytes; Figure 6B); these cells were more abundant when anti-PD-L1 antibody pretreated ERCs were co-cultured with splenocytes (P<0.05, Splenocytes + ERCs vs. Splenocytes + *ERCs; Figure 6B). Meanwhile, the number of CD11c+MHC-II+ DCs was increased in ERCs and splenocytes co-culture performed in a transwell plate (P<0.05, Splenocytes + ERCs vs. Splenocytes + ERCs transwell; Figure 6B). Collectively, these data suggested that ERCs treatment decreases DCs maturation via PD-L1.
Figure 6.
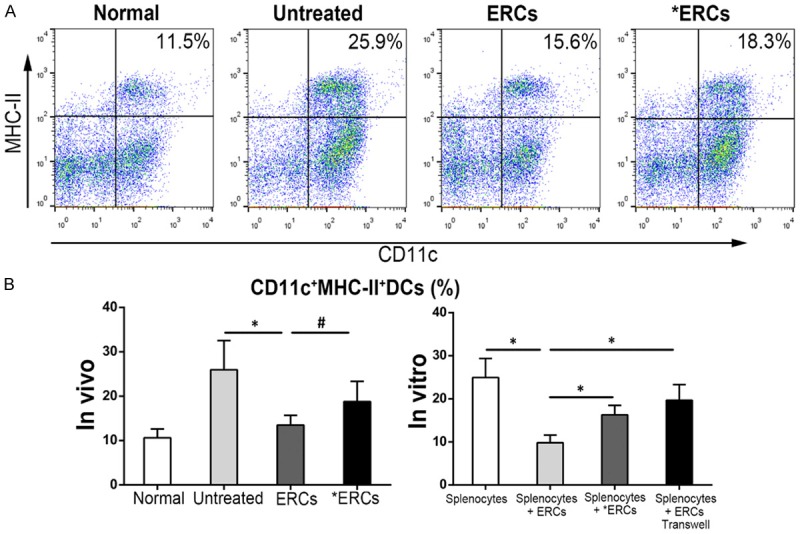
ERCs regulate the CD11c+MHC-II+ DCs percentage via PD-L1. The analysis of CD11c+MHC-II+ DCs was performed by using FACS in different groups. A. Dot plot of CD11c+MHC-II+ DCs in vivo. B. Percentage of CD11c+MHC-II+ DCs in vivo (n=8 per group) and in vitro (n=6 per group). ERCs indicated ERCs-treatment. *ERCs indicated anti-PD-L1 mAb-pretreated ERCs-treatment. *P<0.05. #P>0.05.
ERCs via PD-L1 reduce total macrophage amounts, but relatively increase the number of M2 macrophages in vivo and in vitro
Macrophages have critical functions in body protection from the luminal content at an extremely large interface [26]. To assess whether ERCs-associated colitis alleviation is related to macrophage profile, and the potential effect of PD-L1 on macrophage amounts, total macrophages positive for F4/80 and the M2 macrophage subtype with double F4/80 and CD206 staining were evaluated in vitro and in vivo by the flow-cytometric analysis (Figure 7). Interestingly, ERCs efficiently reduced total macrophage numbers compared with control values (P<0.05, Untreated vs. ERCs; Figure 7B). However, M2 macrophages were relatively more abundant in the ERCs-treatment group than control mice with colitis (P<0.05, Untreated vs. ERCs; Figure 7B). Compared with the ERCs-treatment group, the percentage of splenic total macrophages in mice administered ERCs-pretreated with anti-PD-L1 mAb was enhanced but showed no significant difference (P>0.05, ERCs vs. *ERCs; Figure 7B); meanwhile, the M2 sub-population was relatively reduced, with a significant difference (P<0.05, ERCs vs. *ERCs; Figure 7B). Similarly, ERCs reduced the number of total macrophages after co-culture of splenocytes with ERCs (P<0.05, Splenocytes vs. Splenocytes + ERCs; Figure 7B), but relatively increased the M2 macrophage population (P<0.05, Splenocytes vs. Splenocytes + ERCs; Figure 7B). However, the percentage of total macrophages in the co-culture was correspondingly enhanced by using anti-PD-L1 antibody-pretreated ERCs (P<0.05, Splenocytes + ERCs vs. Splenocytes + *ERCs; Figure 7B) or separating ERCs from splenocytes in transwell plates (P<0.05, Splenocytes + ERCs vs. Splenocytes + ERCs transwell; Figure 7B). The M2 macrophage sub-population in co-culture was reduced after neutralization with anti-PD-L1 mAb (P<0.05, Splenocytes + ERCs vs. Splenocytes + *ERCs, Figure 7B) or separating ERCs from splenocytes in transwell plates (P<0.05, Splenocytes + ERCs vs. Splenocytes + ERCs transwell; Figure 7B).
Figure 7.
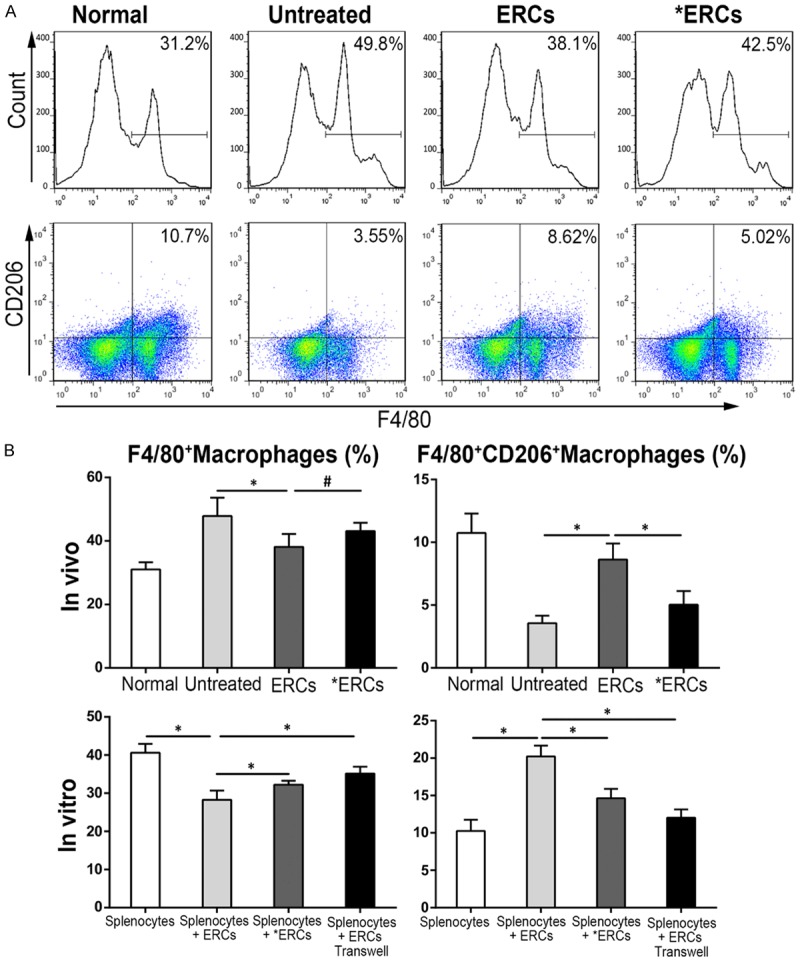
ERCs regulate the number and type of macrophage via PD-L1. Total macrophages positive for F4/80 and the M2 macrophage subtype with double F4/80 and CD206 staining were evaluated in vitro and in vivo flow-cytometrically. A. Flow cytometry of total macrophages positive for F4/80 and M2 macrophage subtype with double F4/80 and CD206 in vivo. B. Percentage of total macrophages and M2 macrophages in vivo (n=8 per group) and in vitro (n=6 per group). ERCs indicated ERC-treatment. *ERCs indicated anti-PD-L1 mAb-pretreated ERCs-treatment. *P<0.05. #P>0.05.
In vivo ERCs tracking after engraftment
To assess whether PHK26-ERCs could be engrafted in the colon tissue of colitis mice, ERCs were administered by tail-vein injection into normal control and colitis mice, which were sacrificed after 48 hours. Interestingly, PKH26-positive ERCs were found by fluorescence microscopy in colon (injured tissue) and spleen (lymphoid organ) samples from ERCs-treated mice (Figure 8). Moreover, fluorescence intensity of labeled ERCs after 48 hours had the following order: lung, liver, spleen and colon. There was no fluorescence observed in the kidneys. Fluorescence intensities in colon samples from mice with DSS-induced colitis were greater than those of normal control animals.
Figure 8.
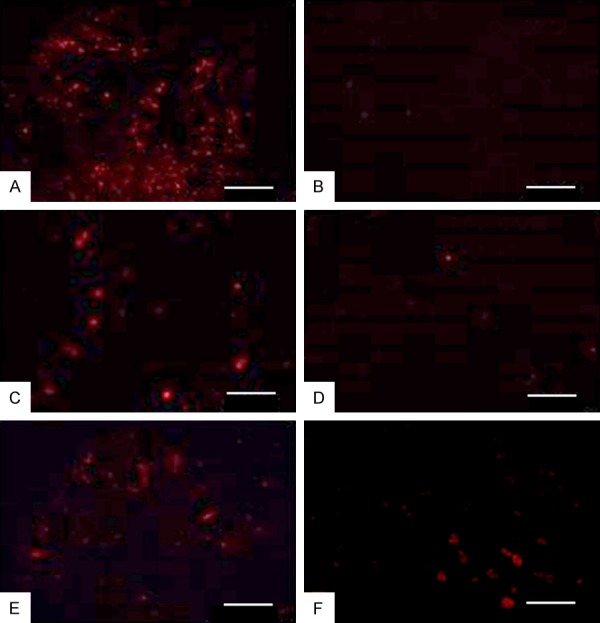
The figure shows the distribution of ERCs in experimental animals under fluorescence microscopy. ERCs were injected into both of the normal control mice and colitis mice via the tail vein, and the internal organs were sacrificed after 48 hours. The fluorescence intensity of the colitis mice was showed in the following order: lung (A), liver (C), spleen (E), colon (F). No fluorescence was observed in the kidney (B). Fluorescence intensity of colon in the colitis group (F) was stonger than the normal group (D). Bar=100 μm.
Discussion
MSC-based therapy constitutes an efficient method in treating IBD [4]. ERCs, as an attractive stem cell type obtained from menstrual blood, show several advantages over MSCs of other origins. ERCs are harvested from female donors in a non-invasive manner, representing an ethically compliant substitute of autologous stem cells. Meanwhile, ERCs can be rapidly and massively expanded while maintaining a normal karyotype until the 68th doubling [7]. In our previous studies, ERCs were shown to regulate the immune system and induce immune tolerance [27-30]. In the present study, intravenously injected ERCs ameliorate clinical signs and histological alterations in the colon of DSS-colitis mice. Intact epithelium and crypts in the colon of normal mice were observed by microscopy, which showed intestinal layers orderly arranged, large amounts of goblet cells, and limited inflammatory cells. Untreated mice displayed significant inflammatory alterations, including epithelial and crypt damage, glandular disarrangement, reduced goblet cell amounts, and pronounced inflammatory cells (including neutrophils, T lymphocytes and macrophages) infiltration into the mucosal layers. In mice administered ERCs, the colon showed markedly improved symptoms. However, the molecular mechanism of ERCs-mediated immunosuppression remains unclear.
MSCs modulate the functions of certain immune cells by means of soluble factor secretion or direct cell-to-cell interactions [12]. MSCs derived from the bone marrow (BM) or umbilical cord (UC) constitutively express PD-L1, which plays a significant role in MSC-associated immune suppression [14,31]. Our previous research has shown that PD-L1 expression is a requirement in BM-MSCs for immune tolerance to heart transplants together with rapamycin administration [15]. Furthermore, the negative effects of MSCs-expressing PD-L1 neutralization on graft survival are related to decreased amounts of regulatory immune cells and increased levels of alloantibodies (IgG and IgM), both in the graft and blood stream. In cell cultures, blockade of MSC-associated antibody synthesis and B cell proliferation is associated with PD-L1 as well as interactions of CD19+ B cells with MSCs [15]. Therefore, PD-L1 plays an important role in MSC-mediated immunosuppression.
Our research showed that PD-L1 was also expressed on the cell surface of ERCs. PD-L1 levels in ERCs increased with the stimulating factor concentration. Meanwhile, PD-1 as a PD-L1 receptor is found at high levels in activated T lymphocytes, B lymphocytes, DCs and macrophages [32-36]. PD-1 and PD-L1 interactions modulate immune cells and induce immune tolerance. Indeed, ligand binding to PD-1 results in suppressed T cell receptor (TCR) signaling via reduction of CD3z chain phosphorylation and Zap-70 binding [37]. PD-1/PD-L1 signaling can downregulate both Ras and Bcl-xL, which regulate cell growth and survival, respectively [38]. In addition, PD-1 suppresses phosphatidylinositol 3-kinase (PI3K)/Akt signaling by reducing PI3K activation [39]. This further downregulates mechanistic target of rapamycin (mTOR) and increases the half-life of FoxO1 [40]. In this setting, inflammatory cells show reduced proliferation and effector function, resulting in a dysfunctional phenotype.
PD-1/PD-L1 signaling is important in inducing immune suppression and establishing a therapeutic strategy for autoimmune diseases [41-43]. For example, mice not expressing PD-1 develop systemic autoimmune diseases spontaneously [44]. In addition, PD-1-associated inhibitory signals are important in alleviating colon inflammation, and PD-L1-Fc constitutes a very potential therapeutic tool for treating IBD [16]. Thus, we evaluated the role of PD-L1 in ERCs-mediated treatment of colitis. ERCs could reduce Ly6g+ cells and CD3+ T cells infiltration and enhance CD206+ cells infiltration in the colon. ERCs reduced levels of pro-inflammatory cytokines and enhanced levels of anti-inflammatory cytokines in the colon and serum. However, blockade of ERCs with anti-PD-L1 mAb disrupted their immunosuppressive properties. In addition, the distribution of ERCs in colitis mice remains unknown; therefore, xenogeneic PHK26 labeled ERCs were grafted via tail vein, and found to migrate to the lung, liver, spleen, colon tissue in the mouse colitis model. The colon tissue in the colitis group showed significantly higher fluorescence intensity compared with that of the normal group. Most ERCs were distributed in the lung since they first pass through the lung after intravenous injection. Since the distribution of early transplanted cells is associated with the degree of capillary richness in different organs, elevated fluorescence was found in the liver. Meanwhile, large amounts of fluorescently labeled cells were detected in the spleen; therefore, splenic immune cell populations were assessed to examine the association of ERCs with systemic immune reactions. ERCs circulation in the body and the chemotactic effects of inflammatory factors would cause ERCs to gradually migrate to the diseased colon tissue. Therefore, fluorescence intensity in colon samples from the colitis group was much stronger than that of the normal group.
CD4+ T and CD8+ T lymphocytes as well as Tregs induced by ERCs-based therapy were markedly alleviated by pre-treatment of ERCs with mAb targeting PD-L1 in vitro and in vivo. ERCs downregulated pro-inflammatory cytokines while upregulating anti-inflammatory cytokines. Moreover, compared with the ERCs-treatment group, colonic pro-inflammatory cytokine amounts were enhanced while anti-inflammatory immune mediators were reduced when ERCs were pre-conditioned with mAb against PD-L1. Most studies suggest that PD-L1 exerts negative regulation of T cells and inhibits inflammation via PD-1 [45-47], and our study is consistent with these studies. Tregs comprise 5 to 10% of all CD4+ T cells in healthy human and rodents, as important players in peripheral tolerance which suppress effector T (Teff) cells and reduce tissue damage associated with immune reactions [48]. Binding of PD-1 to PD-L1 enhances the suppressive ability of Tregs as well as their interactions with Teffs [43]. In presence of TGF-β, PD-L1-Ig induces an important production of iTregs from naive CD4+ T cells. Meanwhile, PD-L1 blockade decreases Treg amounts and reduces CD4+Foxp3- cell differentiation into IL-17 positive T cells [49]. Furthermore, PD-L1-Ig administration upregulates Foxp3 and increases suppressive function in iTregs [50].
DCs are critical for immune regulation in the gut. DCs activation is a prerequisite for DSS-associated colitis [51]. In agreement, DCs accumulate at sites of inflammation in patients with IBD [25]. As shown above, addition of ERCs markedly reduced the number of CD11c+MHC-II+ DCs in co-culture, which, however, was reversed by neutralization with anti-PD-L1 mAb in vitro. Splenic CD11c+MHC-II+ DCs were enhanced by pretreatment of ERCs with anti-PD-L1 mAb although no statistical significance was found in vivo; this result may be related to sample size, indicating the need to further enlarge the animal number. Previous evidence reveals PD-1 on DCs negatively modulates their functions since mice not expressing PD-1 show a greater ability in innate protection of the animals from Listeria infection and elevated IL-12 and TNF-γ synthesis [36]. Moreover, Song et al. demonstrated that in DSS colitis, PD-L1-Fc activates PD-1/PD-L1 signaling pathway primarily in DCs, causing DC dysfunction and therefore reducing the severity of colon inflammation [16].
Macrophages play critical roles in tissue homeostasis and remodeling [52], with various functions and phenotypes in distinct metabolic and immune microenvironments [53]. Two macrophage subsets are broadly acknowledged, including the classically (M1) and alternatively (M2) activated subgroups [54]. Macrophages have anti-inflammatory phenotype and function in the normal intestinal lamina propria [55]. However, in individuals with active IBD, macrophages are more abundant in the intestine, expressing pro-inflammatory phenotypic and functional characteristics at the sites of mucosal inflammation [56]. As shown above, administration of ERCs resulted in increased amounts of M2 cells, which likely have a critical function in the alleviation of DSS-induced colitis. This corroborates previous findings that inflammation suppression in UC might be associated with induced M2 polarization [57]. Besides, a previous study also showed that MSCs regulate the M1/M2 balance and may contribute to immune-tolerance [58]. However, the molecular mechanism of ERC-mediated M2 polarization remains unclear. It has been demonstrated that PD-L1 plays a significant role in MSC-associated immune suppression. Binding of PD-L1 and PD-1 acts in a negative feedback mechanism to inhibit immune reactions. Since PD-1 is found on the macrophage surface, alleviation of DSS-associated colitis by PD-L1-Fc administration could be modulated by interaction with macrophages, controlling downstream macrophage effects [16]. Therefore, we also evaluated the regulating effects of ERC-expressing PD-L1 on macrophages. The results showed M2 cells were relatively reduced after pretreatment of ERCs with anti-PD-L1 mAb. Indeed, PD-L1 interaction with PD-1 leads M2 polarization and thus repair of the intestinal mucosa.
Conclusion
ERCs can provide an immuno-privileged environment for the regulation of immune responses. The present study showed that PD-L1 plays a critical role in mediating ERCs therapy of colitis. However, the in-depth molecular mechanism of ERCs in immune regulation is complex and deserves further investigation.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China [grant numbers 81273257, 81471584]; Tianjin Application Basis and Cutting-Edge Technology Research Grant [grant number 14JCZDJC35700]; Natural Science Foundation of Tianjin [grant number 18JCZDJC35800]; Li Jieshou Intestinal Barrier Research Special Fund [grant number LJS_201412]; Tianjin Medical University Talent Fund; and Research Fund of the Second Hospital of Tianjin Medical University [grant number 2017ydey08].
Disclosure of conflict of interest
None.
Abbreviations
- CD
Crohn’s disease
- DAI
Disease activity index
- DCs
Dendritic cells
- DSS
Dextran sulfate sodium
- ERCs
Endometrial regenerative cells
- HLA
Human leukocyte antigen
- IBD
Inflammatory bowel disease
- IL
Interleukin
- LP
Lamina propria
- MHC
Major histocompatibility complex
- MSCs
Mesenchymal stromal cells
- M
Macrophages
- PBS
Phosphate buffered saline
- SPF
Specific pathogen free
- TGF-β
Transforming growth factor-β
- Th
Helper T cell
- TNF
Tumor necrosis factor
- Tregs
Regulatory T cells
- UC
Ulcerative colitis
References
- 1.Sonnenberg E, Siegmund B. Ulcerative colitis. Digestion. 2016;94:181–185. doi: 10.1159/000452621. [DOI] [PubMed] [Google Scholar]
- 2.Ye L, Cao Q, Cheng J. Review of inflammatory bowel disease in China. ScientificWorldJournal. 2013;2013:296470. doi: 10.1155/2013/296470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 4.Singh UP, Singh NP, Singh B, Mishra MK, Nagarkatti M, Nagarkatti PS, Singh SR. Stem cells as potential therapeutic targets for inflammatory bowel disease. Front Biosci (Schol Ed) 2010;2:993–1008. doi: 10.2741/s115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He XW, He XS, Lian L, Wu XJ, Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci. 2012;57:3136–3144. doi: 10.1007/s10620-012-2290-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Z, Patel AN, Ichim TE, Riordan NH, Wang H, Min WP, Woods EJ, Reid M, Mansilla E, Marin GH, Drago H, Murphy MP, Minev B. Feasibility investigation of allogeneic endometrial regenerative cells. J Transl Med. 2009;7:15. doi: 10.1186/1479-5876-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, Thebaud B, Riordan NH. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv Y, Xu X, Zhang B, Zhou G, Li H, Du C, Han H, Wang H. Endometrial regenerative cells as a novel cell therapy attenuate experimental colitis in mice. J Transl Med. 2014;12:344. doi: 10.1186/s12967-014-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 10.Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012;67:1–8. doi: 10.1111/j.1600-0897.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- 11.Luz-Crawford P, Noel D, Fernandez X, Khoury M, Figueroa F, Carrion F, Jorgensen C, Djouad F. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One. 2012;7:e45272. doi: 10.1371/journal.pone.0045272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren G, Zhao X, Zhang L, Zhang J, L’Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasef A, Mathieu N, Chapel A, Frick J, Francois S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D, Fouillard L. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84:231–237. doi: 10.1097/01.tp.0000267918.07906.08. [DOI] [PubMed] [Google Scholar]
- 14.Gu YZ, Xue Q, Chen YJ, Yu GH, Qing MD, Shen Y, Wang MY, Shi Q, Zhang XG. Different roles of PD-L1 and FasL in immunomodulation mediated by human placenta-derived mesenchymal stem cells. Hum Immunol. 2013;74:267–276. doi: 10.1016/j.humimm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Qi F, Dai X, Tian W, Liu T, Han H, Zhang B, Li H, Zhang Z, Du C. Requirement of B7-H1 in mesenchymal stem cells for immune tolerance to cardiac allografts in combination therapy with rapamycin. Transpl Immunol. 2014;31:65–74. doi: 10.1016/j.trim.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Song MY, Hong CP, Park SJ, Kim JH, Yang BG, Park Y, Kim SW, Kim KS, Lee JY, Lee SW, Jang MH, Sung YC. Protective effects of Fc-fused PD-L1 on two different animal models of colitis. Gut. 2015;64:260–271. doi: 10.1136/gutjnl-2014-307311. [DOI] [PubMed] [Google Scholar]
- 17.Saudemont A, Jouy N, Hetuin D, Quesnel B. NK cells that are activated by CXCL10 can kill dormant tumor cells that resist CTL-mediated lysis and can express B7-H1 that stimulates T cells. Blood. 2005;105:2428–2435. doi: 10.1182/blood-2004-09-3458. [DOI] [PubMed] [Google Scholar]
- 18.Ye K, Lan X, Wang G, Zhang B, Xu X, Li X, Zhao Y, Wang H. B7-H1 expression is required for human endometrial regenerative cells in the prevention of transplant vasculopathy in mice. Stem Cells Int. 2018;2018:2405698. doi: 10.1155/2018/2405698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kihara N, de la Fuente SG, Fujino K, Takahashi T, Pappas TN, Mantyh CR. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut. 2003;52:713–719. doi: 10.1136/gut.52.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevceva L, Pavli P, Husband A, Ramsay A, Doe WF. Dextran sulphate sodium-induced colitis is ameliorated in interleukin 4 deficient mice. Genes Immun. 2001;2:309–316. doi: 10.1038/sj.gene.6363782. [DOI] [PubMed] [Google Scholar]
- 21.Chidlow JH Jr, Langston W, Greer JJ, Ostanin D, Abdelbaqi M, Houghton J, Senthilkumar A, Shukla D, Mazar AP, Grisham MB, Kevil CG. Differential angiogenic regulation of experimental colitis. Am J Pathol. 2006;169:2014–2030. doi: 10.2353/ajpath.2006.051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abomaray FM, Al Jumah MA, Kalionis B, AlAskar AS, Al Harthy S, Jawdat D, Al Khaldi A, Alkushi A, Knawy BA, Abumaree MH. Human chorionic villous mesenchymal stem cells modify the functions of human dendritic cells, and induce an anti-inflammatory phenotype in CD1+ dendritic cells. Stem Cell Rev. 2015;11:423–441. doi: 10.1007/s12015-014-9562-8. [DOI] [PubMed] [Google Scholar]
- 23.Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, Abomaray FM, Fatani AS, Chamley LW, Knawy BA. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev. 2013;9:620–641. doi: 10.1007/s12015-013-9455-2. [DOI] [PubMed] [Google Scholar]
- 24.Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008;125:145–153. doi: 10.1111/j.1365-2567.2008.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niess JH. Role of mucosal dendritic cells in inflammatory bowel disease. World J Gastroenterol. 2008;14:5138–5148. doi: 10.3748/wjg.14.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhl AA, Erben U, Kredel LI, Siegmund B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol. 2015;6:613. doi: 10.3389/fimmu.2015.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Li X, Gu X, Zhang B, Tian W, Han H, Sun P, Du C, Wang H. Prolongation of cardiac allograft survival by endometrial regenerative cells: focusing on B-Cell responses. Stem Cells Transl Med. 2017;6:778–787. doi: 10.5966/sctm.2016-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun P, Liu J, Li W, Xu X, Gu X, Li H, Han H, Du C, Wang H. Human endometrial regenerative cells attenuate renal ischemia reperfusion injury in mice. J Transl Med. 2016;14:28. doi: 10.1186/s12967-016-0782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu S, Shi G, Xu X, Wang G, Lan X, Sun P, Li X, Zhang B, Gu X, Ichim TE, Wang H. Human endometrial regenerative cells alleviate carbon tetrachloride-induced acute liver injury in mice. J Transl Med. 2016;14:300. doi: 10.1186/s12967-016-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan X, Wang G, Xu X, Lu S, Li X, Zhang B, Shi G, Zhao Y, Du C, Wang H. Stromal cell-derived Factor-1 mediates cardiac allograft tolerance induced by human endometrial regenerative cell-based therapy. Stem Cells Transl Med. 2017;6:1997–2008. doi: 10.1002/sctm.17-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tipnis S, Viswanathan C, Majumdar AS. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol. 2010;88:795–806. doi: 10.1038/icb.2010.47. [DOI] [PubMed] [Google Scholar]
- 32.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao A, Liu F, Chen K, Tang L, Liu L, Zhang K, Yu C, Bian G, Guo H, Zheng J, Cheng P, Ju G, Wang J. Programmed death 1 deficiency induces the polarization of macrophages/microglia to the M1 phenotype after spinal cord injury in mice. Neurotherapeutics. 2014;11:636–650. doi: 10.1007/s13311-013-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boussiotis VA, Chatterjee P, Li L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J. 2014;20:265–271. doi: 10.1097/PPO.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, Xu H, Ruff W, Broadwater M, Choi IH, Tamada K, Chen L. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG, Comin-Anduix B, Ribas A. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20:3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng X, Veverka V, Radhakrishnan A, Waters LC, Muskett FW, Morgan SH, Huo J, Yu C, Evans EJ, Leslie AJ, Griffiths M, Stubberfield C, Griffin R, Henry AJ, Jansson A, Ladbury JE, Ikemizu S, Carr MD, Davis SJ. Structure and interactions of the human programmed cell death 1 receptor. J Biol Chem. 2013;288:11771–11785. doi: 10.1074/jbc.M112.448126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saresella M, Rainone V, Al-Daghri NM, Clerici M, Trabattoni D. The PD-1/PD-L1 pathway in human pathology. Curr Mol Med. 2012;12:259–267. doi: 10.2174/156652412799218903. [DOI] [PubMed] [Google Scholar]
- 42.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12:2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 45.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 46.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li T, Ma R, Zhu JY, Wang FS, Huang L, Leng XS. PD-1/PD-L1 costimulatory pathway-induced mouse islet transplantation immune tolerance. Transplant Proc. 2015;47:165–170. doi: 10.1016/j.transproceed.2014.10.043. [DOI] [PubMed] [Google Scholar]
- 48.Himmel ME, Yao Y, Orban PC, Steiner TS, Levings MK. Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology. 2012;136:115–122. doi: 10.1111/j.1365-2567.2012.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, Levine BL, June CH, Medin JA, Fowler DH. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3:111ra120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berndt BE, Zhang M, Chen GH, Huffnagle GB, Kao JY. The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J Immunol. 2007;179:6255–6262. doi: 10.4049/jimmunol.179.9.6255. [DOI] [PubMed] [Google Scholar]
- 52.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 54.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 55.Weber B, Saurer L, Mueller C. Intestinal macrophages: differentiation and involvement in intestinal immunopathologies. Semin Immunopathol. 2009;31:171–184. doi: 10.1007/s00281-009-0156-5. [DOI] [PubMed] [Google Scholar]
- 56.Rugtveit J, Bakka A, Brandtzaeg P. Differential distribution of B7.1 (CD80) and B7.2 (CD86) costimulatory molecules on mucosal macrophage subsets in human inflammatory bowel disease (IBD) Clin Exp Immunol. 1997;110:104–113. doi: 10.1046/j.1365-2249.1997.5071404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campos N, Magro F, Castro AR, Cabral J, Rodrigues P, Silva R, Appelberg R, Rodrigues S, Lopes S, Macedo G, Sarmento A. Macrophages from IBD patients exhibit defective tumour necrosis factor-alpha secretion but otherwise normal or augmented pro-inflammatory responses to infection. Immunobiology. 2011;216:961–970. doi: 10.1016/j.imbio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]


