Abstract
Accumulating evidence has demonstrated the vital roles of long noncoding RNA (lncRNA) in prostate cancer (PCa). However, the function of small nucleolar RNA host gene 20 (SNHG20) in PCa is still unclear. This study aimed to investigate the expression and biological roles of SNHG20 in PCa. SNHG20 expression in PCa tissues and cell lines was measured by quantitative real-time PCR. Gain and loss-of-function experiments were conducted to examine the biological roles of SNHG20. Bioinformatic analysis and dual luciferase activity reporter assay were conducted to establish the SNHG20/secretoglobin family 2A member 1 (SCGB2A1)/microRNA-6516-5p (miR-6516-5p) axis. SHNG20 expression was found markedly elevated in PCa tissues and cell lines. Overexpression of SNHG20 increased PCa cell proliferation and invasion but decreased cell apoptosis. However, the knockdown of SNHG20 will cause the opposite effects on PCa cell behaviors. Mechanical investigation found that SNHG20 could relive the SCGB2A1 protein expression via sponging the miR-6516-5p, acting as miRNA sponge. In conclusion, this finding suggests the SNHG20/miR-6516-5p/SCGB2A1 axis in PCa tumorigenesis, providing the novel insight for the molecular mechanism of PCa.
Keywords: SNHG20, miR-6516-5p, SCGB2A1, prostate cancer, ceRNA
Introduction
In a worldwide range, newly diagnosed cases for prostate cancer (PCa) is 1,276,106 in 2018, while the death numbers caused by this cancer type is 358,989 [1]. The incidence rate of PCa is increasing in recent years due to the economy development and screening methods improvement [2]. The treatment outcome for patients at early stages is relatively good due to the advances in treatment strategies, however, the treatment for patients at advanced stages is inefficient [3,4]. Hence, further revealing the mechanisms behind PCa progression is essential to identify novel targets for PCa treatment.
Non-coding RNAs including long non-coding RNAs (lncRNAs), microRNAs (miRNAs), circular RNAs (circRNAs), and pseudogenes were used to be regarded as “junk genes” but their crucial roles in regulating human cancer development have been appreciated in recent years [5-7]. lncRNAs are a group of RNAs with the average length of around 200 nucleotides [8]. The competing endogenous RNA (ceRNA) theory raised by Salmena has provided novel insight into the action mechanism of lncRNA and open a new window to investigate the function of lncRNA in cancer [9].
Long noncoding RNA small nucleolar RNA host gene 20 (SNHG20) has been generally recognized as crucial regulators in cancer progression. In epithelial ovarian cancer, high SNHG20 was found correlated with shorter overall survival and could be used as indicator for prognosis prediction [10]. In non-small cell lung cancer, it was found the knockdown of SNHG20 was able to suppress cancer cell growth and metastasis but promote apoptosis via functioning as miR-154 sponge [11]. In gastric cancer, SNHG20 was shown to be higher expressed in cancer tissues compared with normal tissues [12]. The knockdown of SNHG20 decreases tumor growth in vitro and in vivo through regulating the miR-495-3p/zinc finger protein X-linked axis [12]. However, it was not determined whether SNHG20 has a role in PCa.
Our study was aimed to investigate the clinical significance of SNHG20 in PCa. Our results indicated that SNHG20 was upregulated in PCa tissues and cell lines. The knockdown of SNHG20 was able to inhibit cell growth and invasion but promote apoptosis by regulating the miR-6516-5p/secretoglobin family 2A member 1 (SCGB2A1), while the overexpression of SNHG20 caused the opposite effects. These findings suggested a potential target for the treatment of PCa.
Materials and methods
Human tissues
PCa tissues and adjacent nontumor samples were obtained from 68 patients who underwent treatment at Peking Union Medical College Hospital, Chinese Academy of Medical Sciences (Beijing, China). The patient who has ever received anti-cancer treatment was excluded from this study. These tissues were snap-frozen in liquid nitrogen and storage at -80°C for usage. The study protocol was approved by the ethic committee of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and written informed consent has been obtained from all participates.
Cell culture
PCa cell lines PC3 and DU145 were purchased at American Type Culture Collection (ATCC, Manassas, VA, USA). Normal human prostate epithelial cells (NHPE) were purchased from Lonza, Inc. (Allendale, NJ, USA) and cultured in prostate epithelial cell growth medium containing growth medium and supplements (cat no. CC-3166; Lonza, Inc.). PCa cells were maintained in RPMI-1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen). All these cells were incubated in a 37°C humidified atmosphere containing 5% of CO2.
Cell transfection
Oligonucleotides targeting SNHG20 (sh-SNHG20, 5’-GCCACUCACAAGAGUGUAUTT-3’) and the negative control (sh-NC, 5’-GGATACGGAGTACTATAGC-3’) were synthesized by RiboBio Inc. (Guangzhou, China). Full-length of SNHG20 was PCR amplified and cloned into pcDNA3.1 to name it as pSNHG20. miR-6516-5p mimic (5’-UUUGCAGUAACAGGUGUGAGCA-3’) and NC-mimic (5’-AGGAUGUAUUACCAGUGAUCGG-3’) was purchased from RiboBio Inc. (Guangzhou, China). The pcDNA3.1 contains the open reading frame of SCGB2A1 (pSCGB2A1) was obtained from GenScript (Nanjing, China). Cell transfection was conducted with the help of Lipofectamine 3000 (Invitrogen).
RNA extraction and quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted using TRIzol Regent (Invitrogen) according to the manufacturer’s protocol. After quantified with NanoDrop 2000 (Thermo Fisher Scientifc, Inc.), equal amount of RNA sample was reverse transcribed into complementary DNA using M-MLV Reverse Transcriptase (Promega, Madison, WI, USA). miR-6516-5p expression level was detected using SYBR PrimeScript miRNA RT PCR kit (Takara, Dalian, China) at ABI 7500 system (Applied Biosystems, Foster City, CA, USA) with U6 snRNA as internal control. SNHG20 and SCGB2A1 expression levels were measured using SYBR Green Master Mixture (Takara) at ABI 7500 system (Applied Biosystems) with GAPDH as internal control. The primers used were as follows: SNHG20 forward 5’-AGCAACCACTATTTTCTTCC-3’, reverse 5’-CCTTGGCGTGTATCTATTTAT-3’; SCGB2A1 forward 5’-GCTATGCCAGTGGTTCTGGCT-3’, reverse 5’-CGTCATCACGCAACATTTTCAAC-3’; GAPDH forward 5’-TCGGAGTCAACGGATTTGGT-3’, reverse 5’-TTGGAGGGATCTCGCTCCT-3’; miR-6516-5p forward 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGCTCACA-3’, reverse 5’-ACACTCCAGCTGGGTTTGCAGTAACAGGTG-3’; U6 snRNA forward 5’-CTCGCTTCGGCAGCACA-3’, reverse 5’-AACGCTTCACGAATTTGCGT-3’. Relative expression levels were calculated with 2-ΔΔCt method. The thermocycling conditions were as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 12 s and 58°C for 40 s.
Western blot assay
Total protein was isolated using radio immunoprecipitation assay lysis buffer (Beyotime, Haimen, Jiangsu, China). After quantified with bicinchoninic acid kit (Beyotime), equal amount of protein sample was separated at 10% SDS-PAGE and then transferred to polyvinylidene fluoride membrane (Beyotime). Membranes were blocked with 5% fat-free milk and then incubated with antibodies against SCGB2A1 (ab251853) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, ab181602, Abcam, Cambridhe, MA, USA) at 4°C for overnight. Then, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (ab6721, Abcam). Protein signals were visualized with BeyoECL kit (Beyotime) and quantitated with Quantity One software (Bio-Rad, Hercules, CA, USA).
Cell proliferation assay
Cell counting kit-8 (CCK-8, Beyotime) assay was performed to measure cell proliferation rate. 5,000 cells were seeded into 96-well plates and incubated until 10 μl CCK-8 reagent was added to the well at various time points. Optical density was measured at 450 nm with microplate reader (Bio-Rad) after further incubation for another 4 h.
Transwell invasion assay
Cell invasion was measured using 24-well chamber with 8-μm pore size polycarbonate membrane (Corning, NY, USA) and pre-coated with Matrigel (BD Biosciences, San Jose, CA, USA). 5 × 104 cells in serum-free medium were seeded into the upper chamber, while the medium containing 10% FBS was added to the lower chamber. After transfection for 48 h, the invasive cells were fixed with 4% paraformaldehyde, stained with 0.5% crystal violet, and counted in 5 randomly fields using Olympus fluorescence microscope (Tokyo, Japan).
Cell apoptosis assay
Cell apoptosis was determined using the fluorescein isothiocyanate (FITC) Annexin V-propidium iodide (PI) Kit purchased from Beyotime (Haime Jiangsu, China). Cell apoptosis rate was analyzed at FACSCalibur flow cytometer (BD Biosciences) according to the manufacturer’s protocol and the cells with FITC Annexin V stained were regarded to undergoing apoptosis.
Luciferase reporter assay
Targets of SNHG20 were predicted by LncBase Predicted v2 and found miR-6516-5p contains binding site for SNHG20. Target of miR-6526-5p was analyzed at TargetScan (http://www.targetscan.org/vert_72/) and found SCGB2A1 was a potential target. Sequences contain wild-type or mutant SNHG20 and SCGB2A1 were synthesized by GenScript and inserted into pmirGLO vector (Promega, Madison, WI, USA) to generate wt SNHG20/SCGB2A1 or mt SNHG20/SCGB2A1. Cells were co-transfected with miRNAs and these luciferase vectors. Following transfection for 48 h, relative luciferase activity was measured using dual luciferase reporter assay system (Promega) using Renilla luciferase activity as internal control.
Statistical analysis
Data were presented as the mean ± standard deviation (SD) from at least three independent experiments with similar results. Two-tailed Student’s t-test and one-way analysis of variance followed by Tukey post hoc test were conducted to determine differences in groups. Pearson’s correlation analysis was performed to analyze the correlation of miR-6516-5p and SNHG20 or SCGB2A1. P < 0.05 was regarded as statistically significant difference.
Results
SNHG20 was upregulated in PCa tissues and cell lines
To examine the expression of SNHG20, we examined SNHG20 expression in PCa tissues and cell lines. We found SNHG20 expression level was significantly higher in PCa tumor tissues compared with normal tissues (Figure 1A). Moreover, SNHG20 was statistically significant increased in PCa cell lines compared with the NHPE cell line (Figure 1B). These results indicated SNHG20 plays a pivotal role in PCa.
Figure 1.
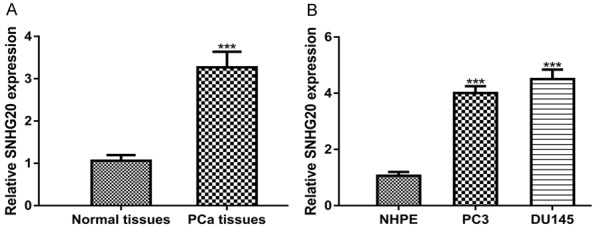
SNHG20 expression was elevated in PCa (A) tissues and (B) cell lines as examined by qRT-PCR. SNHG20: small nucleolar RNA host gene 20; PCa: prostate cancer; qRT-PCR: quantitative real-time PCR.
Overexpression of SNHG20 promotes cell proliferation and invasion but inhibits apoptosis
To further investigated the role of SNHG20 in PCa, pSNHG20 was transfected into DU145 cell line whose expression was relatively high in PCa cells investigated. qRT-PCR analysis result showed that SNHG20 was increased by pSNHG20 (Figure 2A). CCK-8 assay showed that cell growth rate was increased by pSNHG20 (Figure 2B). Transwell invasion assay revealed that cell invasion was also stimulated by pSNHG20 (Figure 2C). Cell apoptosis assay demonstrated that cell apoptosis rate was significantly inhibited by pSNHG20 (Figure 2D).
Figure 2.
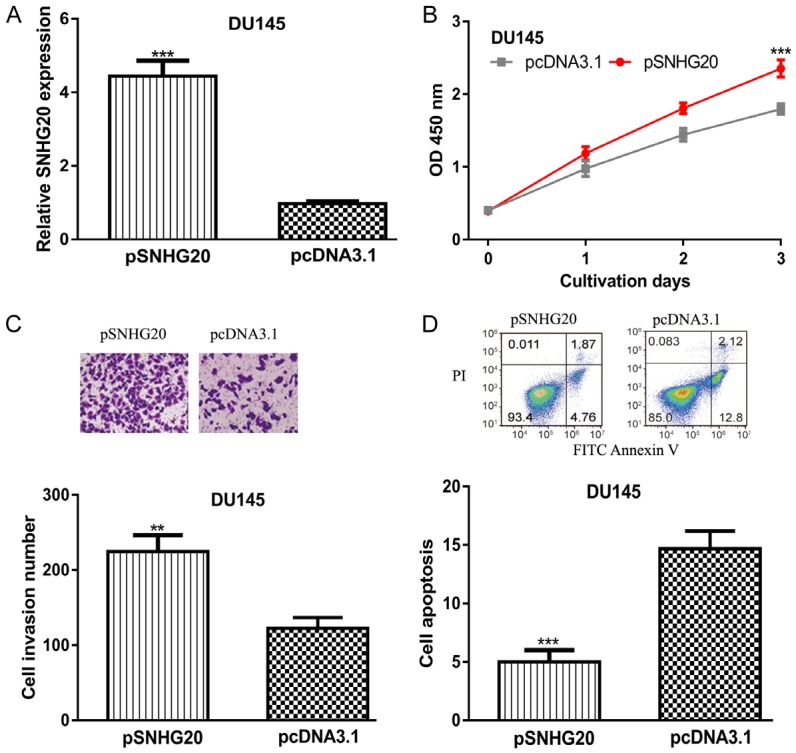
Overexpression of SNHG20 promotes PCa cell proliferation and invasion but inhibits apoptosis. (A) SNHG20 expression, (B) Cell proliferation, (C) Cell invasion, and (D) Cell apoptosis in PCa cells transfected with pSNHG20 or pcDNA3.1 transfection. SNHG20: small nucleolar RNA host gene 20; PCa: prostate cancer.
Knockdown of SNHG20 inhibits cell proliferation and invasion but promotes apoptosis
Next, sh-SNHG20 was transfected into PC3 cell line whose expression was relatively low in PCa cells investigated. Not surprisingly, SNHG20 expression level was decreased by sh-SNHG20 in comparison with sh-NC (Figure 3A). In vitro experiments demonstrated that the knockdown of SNHG20 inhibits PC3 cell proliferation and invasion but promotes apoptosis (Figure 3B-D).
Figure 3.
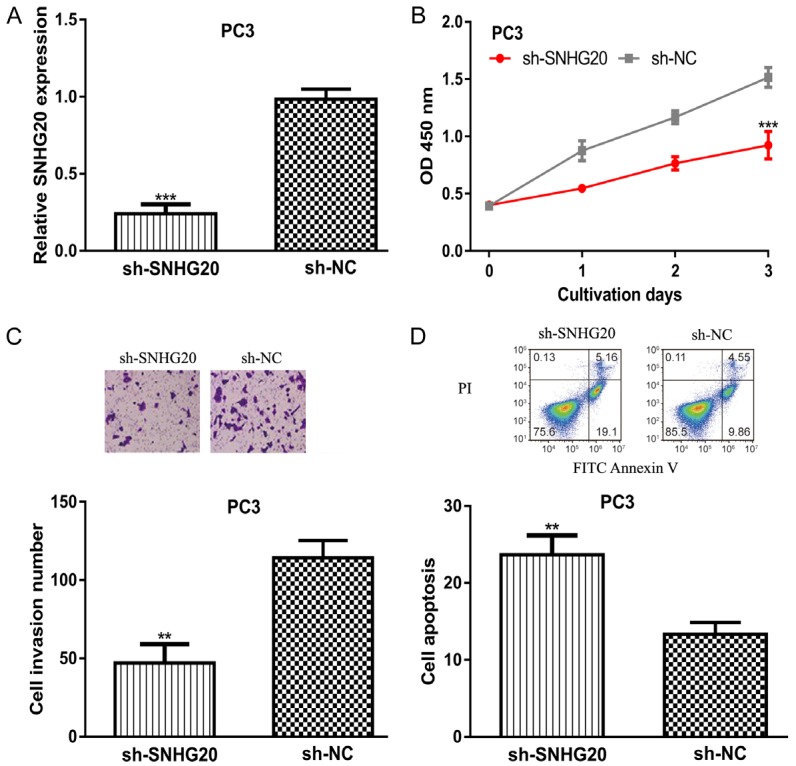
Knockdown of SNHG20 inhibits PCa cell proliferation and invasion but promotes apoptosis. (A) SNHG20 expression, (B) Cell proliferation, (C) Cell invasion, and (D) Cell apoptosis in PCa cells transfected with sh-SNHG20 or sh-NC transfection. SNHG20: small nucleolar RNA host gene 20; PCa: prostate cancer; sh-SHNG20: small hairpin RNA targeting SNHG20; sh-NC: negative control small hairpin RNA.
SNHG20 regulate miR-6516-5p expression in PCa
Bioinformatic analysis showed SNHG20 processes a possible binding site for miR-6516-5p (Figure 4A). Luciferase activity reporter assay showed that luciferase activity was significantly inhibited by miR-6516-5p mimic in cells transfected with wt SNHG20 (Figure 4B). qRT-PCR analysis result showed miR-6516-5p expression was decreased in PCa cell lines compared with the normal cell line (Figure 4C). Further analysis on PCa tissues revealed that SNHG20 and miR-6516-5p expression was inversely correlated (Figure 4D).
Figure 4.
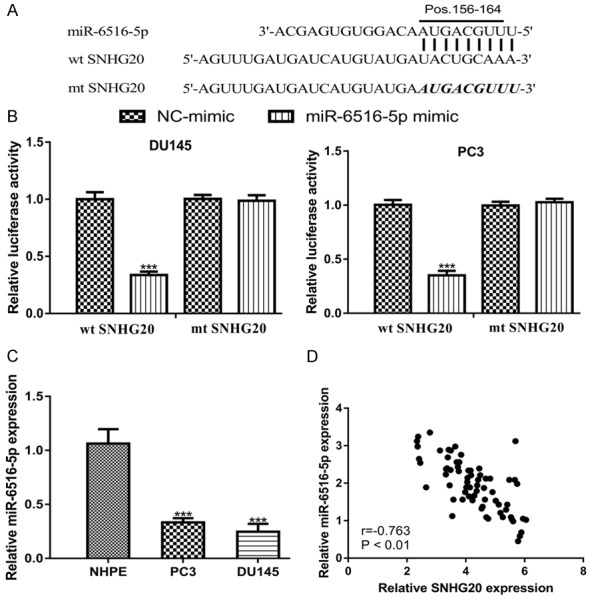
SNHG20 directly target miR-6516-5p in PCa. A. A putative binding site of SNHG20 was located in miR-6516-5p. B. Transfection miR-6516-5p mimic decreased the luciferase activity of wt SNHG20 but not mt SNHG20. C. miR-6516-5p expression in PCa cell lines and normal cell line. D. Correlation of SNHG20 and miR-6516-5p in PCa tissues. SNHG20: small nucleolar RNA host gene 20; PCa: prostate cancer; miR-6516-5p: microRNA-6516-5p; wt: wild-type; mt: mutant; NC-mimic: negative control miR-6516-5p mimic.
miR-6516-5p regulates SCGB2A1 expression in PCa
Next, we searched for the targets of miR-6516-5p and found SCGB2A1 was a potential target (Figure 5A). Luciferase activity reporter assay showed that overexpression of miR-6516-5p inhibited the luciferase activity in cells transfected with wt SCGB2A1 (Figure 5B). Western blot showed that SCGB2A1 expression was increased in PCa cells compared with normal cell line (Figure 5C). Pearson’s correlation showed miR-6516-5p and SCGB2A1 expression was also negatively correlated (Figure 5D).
Figure 5.
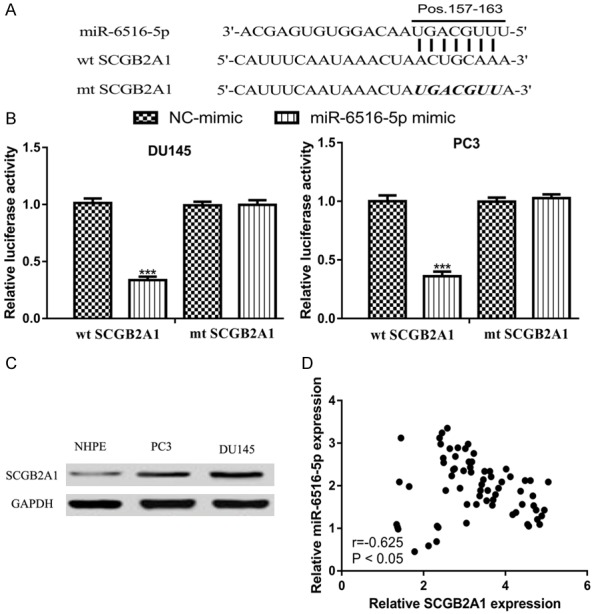
miR-6516-5p directly target SCGB2A1 in PCa. A. A putative binding site of SCGB2A1 was located in miR-6516-5p. B. Transfection miR-6516-5p mimic decreased the luciferase activity of wt SCGB2A1 but not mt SCGB2A1. C. SCGB2A1 expression in PCa cell lines and normal cell line. D. Correlation of SCGB2A1 and miR-6516-5p in PCa tissues. SCGB2A1: secretoglobin family 2A member 1; PCa: prostate cancer; miR-6516-5p: microRNA-6516-5p; wt: wild-type; mt: mutant; NC-mimic: negative control miR-6516-5p mimic.
SCGB2A1, a downstream target of SNHG20/miR-6516-5p, regulates PCa cell behaviors
Furthermore, we are interested to validate the connection among SNHG20/miR-6516-5p/SCGB2A1. Western blot showed the co-expression of pSNHG20 or pSCGB2A1 and miR-6516-5p mimic could reverse the effect of miR-6516-5p mimic on SCGB2A1 expression (Figure 6A). We found miR-6516-5p mimic transfection decreased PCa cell proliferation, invasion but promotes apoptosis (Figure 6B-D). Importantly, we found the effects of miR-6516-5p could be partially reversed by pSNHG20 or pSCGB2A1 (Figure 6B-D). These results indicated that SNHG20/miR-6516-5p/SCGB2A1 axis functions as crucial roles in regulating PCa cell behaviors.
Figure 6.
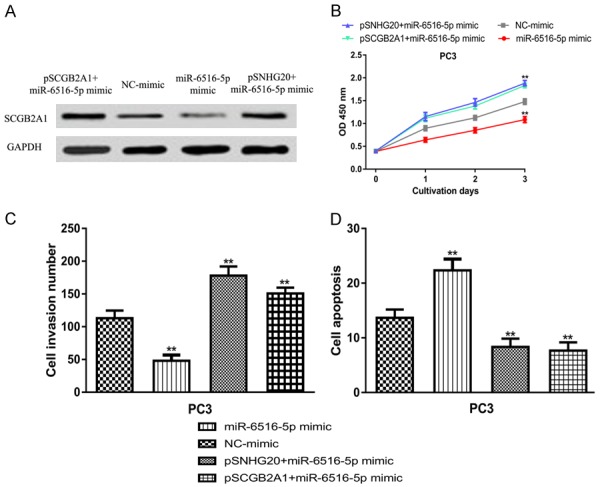
Role of SNHG20/miR-6516-5p/SCGB2A1 axis in regulating PCa cell behaviors. (A) SCGB2A1 expression, (B) Cell proliferation, (C) Cell invasion, and (D) Cell apoptosis in PCa cell lines transfected with miR-6516-5p mimic, pSNHG20+ miR-6516-5p mimic, or pSCGB2A1+ miR-6516-5p mimic transfection. SNHG20: small nucleolar RNA host gene 20; SCGB2A1: secretoglobin family 2A member 1; PCa: prostate cancer; miR-6516-5p: microRNA-6516-5p.
Discussion
In recent years, the importance of lncRNAs in regulating cancer progression has attracted increasingly attentions since they can regulate almost all the hallmarks of cancers [13]. lncRNA SNHG20 has been identified to be an oncogenic molecular in several human cancers including epithelial ovarian cancer, non-small cell lung cancer, and gastric cancer [10-12]. However, the biological roles of SNHG20 in PCa remain unclear.
In this present study, we enrolled PCa patients into this study and examined the expression of SNHG20 using qRT-PCR. The results showed that SNHG20 expression was upregulated in PCa tissues. In addition, SNHG20 expression was also higher in PCa cell lines than in the normal cell line. These results indicated that SNHG20 may have a crucial role in PCa. The vital role of lncRNA in cancers are increasingly investigated in decades [14,15]. For example, knockdown lncRNA HCP5 exerted anti-tumor effect via sponging miR-128-3p in anaplastic thyroid cancer [14]. Besides, increased metastasis associated lung adenocarcinoma transcript 1 was identified in colorectal cancer, and functioned as ceRNA via targeting miR-15/RUNX family transcription factor 2 to promote cancer metastasis [15].
To examine the biological roles of SNHG20 in PCa, gain-of and loss-of function experiments were conducted in PCa cell lines. As expected, the pSNHG20 transfection increased, while sh-SNHG20 transfection decreased SNHG20 expression in PCa cell lines. Functional assays revealed that overexpression of SNHG20 was able to increase PCa cell proliferation, invasion but decrease cell apoptosis. Not surprisingly, the knockdown of SNHG20 caused exactly the opposite effects on PCa cell behaviors. Therefore, we confirmed that lncRNA SNHG20 could promote the PCa progression.
To data, it has been appreciated that lncRNA mainly exert its role through a ceRNA theory [9]. In our study, we found miR-6516-5p acted as the downstream of SNHG20, meanwhile, the miR-6516-5p could also bind with the 3’-UTR of SCGB2A1 through bioinformatic analyses, dual-luciferase activity reporter assay, and correlation analysis on PCa tissues. SCGB2A1 expression was found elevated in colorectal cancer, and force the expression of SCGB2A1 promoted cancer cell proliferation and decreased the chemosensitivity to 5-fluorouracil and oxaliplatin [16]. Besides that, SCGB2A1 was revealed to be abnormally expressed in ovarian cancer [17]. Functional assays showed that SCGB2A1 functions a downstream target of SNHG20/miR-6516-5p to regulate PCa cell behaviors. Further experiments using in vivo animal model should be performed to validate the association among SNHG20/miR-6516-5p/SCGB2A1, which we believe will provide more evidence to regard SNHG20 as a treatment target for PCa in clinical.
Conclusions
In conclusion, our study revealed that SNHG20 functions as oncogenic role to promote PCa progression via targeting miR-6516-5p/SCGB2A1. This work highlighted the importance role of SNHG20/miR-6516-5p/SCGB2A1 in PCa. These results suggested that SNHG20 might be a target for PCa treatment.
Acknowledgements
This work was supported by the Natural Science Foundation of Beijing, China (No. 7192159).
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, Thorat MA, Andriole G, Brawley OW, Brown PH, Culig Z, Eeles RA, Ford LG, Hamdy FC, Holmberg L, Ilic D, Key TJ, La Vecchia C, Lilja H, Marberger M, Meyskens FL, Minasian LM, Parker C, Parnes HL, Perner S, Rittenhouse H, Schalken J, Schmid HP, Schmitz-Dräger BJ, Schröder FH, Stenzl A, Tombal B, Wilt TJ, Wolk A. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484–e492. doi: 10.1016/S1470-2045(14)70211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane JA, Donovan JL, Davis M, Walsh E, Dedman D, Down L, Turner EL, Mason MD, Metcalfe C, Peters TJ, Martin RM, Neal DE, Hamdy FC ProtecT study group. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol. 2014;15:1109–1118. doi: 10.1016/S1470-2045(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 4.Teply BA, Wang H, Luber B, Sullivan R, Rifkind I, Bruns A, Spitz A, DeCarli M, Sinibaldi V, Pratz CF, Lu C, Silberstein JL, Luo J, Schweizer MT, Drake CG, Carducci MA, Paller CJ, Antonarakis ES, Eisenberger MA, Denmeade SR. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study. Lancet Oncol. 2018;19:76–86. doi: 10.1016/S1470-2045(17)30906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adelman K, Egan E. Non-coding RNA: more uses for genomic junk. Nature. 2017;543:183–185. doi: 10.1038/543183a. [DOI] [PubMed] [Google Scholar]
- 6.Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18:5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira HJ, Esteller M. Non-coding RNAs, epigenetics, and cancer: tying it all together. Cancer Metastasis Rev. 2018;37:55–73. doi: 10.1007/s10555-017-9715-8. [DOI] [PubMed] [Google Scholar]
- 8.McFadden EJ, Hargrove AE. Biochemical methods to investigate lncRNA and the influence of lncRNA: protein complexes on chromatin. Biochemistry. 2016;55:1615–1630. doi: 10.1021/acs.biochem.5b01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Dai J, Hou S, Qian Y. LncRNA SNHG20 predicts a poor prognosis and promotes cell progression in epithelial ovarian cancer. Biosci Rep. 2019;39 doi: 10.1042/BSR20182186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingling J, Xiangao J, Guiqing H, Jichan S, Feifei S, Haiyan Z. SNHG20 knockdown suppresses proliferation, migration and invasion, and promotes apoptosis in non-small cell lung cancer through acting as a miR-154 sponge. Biomed Pharmacother. 2019;112:108648. doi: 10.1016/j.biopha.2019.108648. [DOI] [PubMed] [Google Scholar]
- 12.Cui N, Liu J, Xia H, Xu D. LncRNA SNHG20 contributes to cell proliferation and invasion by upregulating ZFX expression sponging miR-495-3p in gastric cancer. J Cell Biochem. 2019;120:3114–3123. doi: 10.1002/jcb.27539. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira JC, Oliveira LC, Mathias C, Pedroso GA, Lemos DS, Salviano-Silva A, Jucoski TS, Lobo-Alves SC, Zambalde EP, Cipolla GA, Gradia DF. Long non-coding RNAs in cancer: another layer of complexity. J Gene Med. 2019;21:e3065. doi: 10.1002/jgm.3065. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Zhao D, Meng Q. Knockdown of HCP5 exerts tumor-suppressive functions by up-regulating tumor suppressor miR-128-3p in anaplastic thyroid cancer. Biomed Pharmacother. 2019;116:108966. doi: 10.1016/j.biopha.2019.108966. [DOI] [PubMed] [Google Scholar]
- 15.Ji Q, Cai G, Liu X, Zhang Y, Wang Y, Zhou L, Sui H, Li Q. MALAT1 regulates the transcriptional and translational levels of proto-oncogene RUNX2 in colorectal cancer metastasis. Cell Death Dis. 2019;10:378. doi: 10.1038/s41419-019-1598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munakata K, Uemura M, Takemasa I, Ozaki M, Konno M, Nishimura J, Hata T, Mizushima T, Haraguchi N, Noura S, Ikenaga M, Okamura S, Fukunaga M, Murata K, Yamamoto H, Doki Y, Mori M. SCGB2A1 is a novel prognostic marker for colorectal cancer associated with chemoresistance and radioresistance. Int J Oncol. 2014;44:1521–1528. doi: 10.3892/ijo.2014.2316. [DOI] [PubMed] [Google Scholar]
- 17.Bellone S, Tassi R, Betti M, English D, Cocco E, Gasparrini S, Bortolomai I, Black JD, Todeschini P, Romani C, Ravaggi A, Bignotti E, Bandiera E, Zanotti L, Pecorelli S, Ardighieri L, Falchetti M, Donzelli C, Siegel ER, Azodi M, Silasi DA, Ratner E, Schwartz PE, Rutherford TJ, Santin AD. Mammaglobin B (SCGB2A1) is a novel tumour antigen highly differentially expressed in all major histological types of ovarian cancer: implications for ovarian cancer immunotherapy. Br J Cancer. 2013;109:462–471. doi: 10.1038/bjc.2013.315. [DOI] [PMC free article] [PubMed] [Google Scholar]


