Abstract
The expression and function of microRNA (miR)-30a-3p in several types of human cancer have been explored. However, the biological function of miR-30a-3p in renal cell carcinoma (RCC) remains largely unknown. In this study, we demonstrate that expression of miR-30a-3p is down-regulated in RCC tissues compared to adjacent normal tissues. Furthermore, ectopic expression of miR-30a-3p significantly suppressed the proliferation, migration, and invasion of a human RCC cell line in vitro, while miR-30a-3p inhibited tumor growth in vivo as well. TargetScan software identified Wnt2 as a potential direct target of miR-30a-3p. To confirm this relationship, Wnt2 was ectopically expressed. The effects of miR-30a-3p on RCC cell proliferation and invasion were subsequently restored. Therefore, the results of this study support an anti-tumor role for miR-30a-3p in RCC progression which is potentially mediated via Wnt2.
Keywords: Renal cell carcinoma, MicroRNA-30a-3p, WNT2
Introduction
Renal cell carcinoma (RCC) is currently the most common urologic malignancy diagnosed. It accounts for 2-3% [1] of all adult malignancies and for approximately 5% [2] of epithelial cancers worldwide. Advances in RCC therapy over the past few decades have enhanced patient outcomes [3]. However, the 5-year survival rate for patients who are diagnosed at a metastatic stage of RCC remains less than 10% [4]. Therefore, the identification of factors involved in RCC tumorigenesis and progression is imperative in order to identify novel targets and improve the clinical outcome of patients with metastatic RCC.
MicroRNAs (miRNAs) are a class of highly conserved, noncoding RNAs which are approximately 22 nucleotides in length and endogenously expressed. MiRNAs have been shown to negatively regulate a large number of genes by binding to the 3’-untranslated region (UTR) of corresponding target mRNAs [5,6]. It has also been demonstrated that a variety of miRNAs are dysregulated in various types of human cancer, including RCC [6-8]. MiRNAs have been found to function as either tumor suppressors or oncogenes, depending on the role of their target genes. We hypothesize that a better understanding of the role of miRNAs in RCC will provide a new approach for management of this disease.
MiR-30a is dysregulated in many types of cancer, including glioma and lung cancer [9]. In a recent study, it was revealed that miR-30a suppresses RCC progression by targeting disintegrin and metalloproteinase domain-containing protein 9 (ADAM9) [10]. Similarly, miR-30a-3p, a member of the miR-30 family, is down-regulated in several cancers [11]. Moreover, down-regulation of miR-30a-3p has been observed in both high grade and advanced stage RCC [12]. However, the gene(s) which miR-30a-3p targets to affect RCC progression and metastasis remain unknown, and identification of these target(s) requires further investigation.
Here, we investigated the role of miR-30a-3p in an RCC cell line to explore its potential for mediating tumor growth and metastasis and for serving as a therapeutic target in RCC. We demonstrate that miR-30a-3p suppressed the proliferation, migration, and invasion of a human RCC cell line. In addition, miR-30a-3p inhibited tumor growth in vivo. We identify Wnt2 as a direct target of miR-30a-3p, and restoration of Wnt2 is shown to reverse the anti-tumor effects of miR-30a-3p in a RCC cell line. Collectively, these findings indicate that miR-30a-3p is a promising target and should be further investigated in RCC.
Materials and methods
Human tissue sample collection
This study was approved by the ethical board of Renmin Hospital of Wuhan University and it complies with the Declaration of Helsinki. Thirty pairs of RCC tissues and adjacent normal renal tissues were collected from patients undergoing radical nephrectomy at Renmin Hospital of Wuhan University (Hubei, China). Informed consent was obtained for each sample collection.
Cell lines, cell culture, and cell transfection
Human RCC cell lines, A498, Caki-1 and 786-O, were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). In addition, a normal renal cell line (HK-2) was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All four cell lines were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco Life Technologies, Grand Island, NY, USA), 1% glutamine, and 1% penicillin/streptomycin (Invitrogen). Cell cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. A miR-30a-3p mimic and a negative control were purchased from GenePharma (Shanghai, China) and transfected into cells with Lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol.
Quantitative reverse-transcriptase PCR (qRT-PCR)
Total RNA from tissues and cells was harvested with TRIzol reagent (Invitrogen) and purified with a RNeasy Mini kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. A SYBR-Green PCR master mix (Applied Biosystems, Foster City, CA, USA) and 7500 Real-time PCR system (Applied Biosystems) were used for qRT-PCR assays. All of the reactions were setup in triplicate. The PCR primers used included: miR-30a-3p: 5’-CGCTTTCAGTCGGATGTTTG-3’ and 5’-GTGCAGGGTCCGAGGT-3’; U6: 5’-CTCGCTTCGGCAGCACA-3’ and 5’-AACGCTTCACGAATTTGCGT-3’; 18S: 5’-CATTCGTATTGCGCCGCT-3’ and 5’-CGACGGTATCTGATCGTC-3’; and Wnt2: 5’-CATAGCCCCCCACCACTGT-3’ and 5’-AGTTCCTTCGCTATGTGATGTTTCT-3’. A relative quantification value for each gene was calculated according to the 2-ΔΔCt method with U6 used as an internal control.
MTT, colony formation, and cell cycle assays
Cell proliferation was determined in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays which were performed according to the manufacturer’s protocol (Sigma-Aldrich, St. Louis, MO, USA). Optical density (OD) was measured at 490 nm with an enzyme immunoassay instrument (BioRad, Hercules, CA, USA). For colony formation assays, cells were transfected with the indicated constructs and then seeded into 6-well plates in growth medium to grow. After 7 d, the colonies present were fixed with 4% formaldehyde and then stained with 0.5% crystal violet. Stained colonies with a diameter greater than 1 mm were counted. To analyze cell cycle distribution, cells were harvested, washed, and fixed with 70% ethanol overnight at 4°C. The cells were subsequently resuspended in fluorescence-activated cell sorting (FACS) solution supplemented with RNase and propidium iodide (PI). Cell cycle distribution was analyzed with a FACScan flow cytometer (BD Biosciences).
Cell migration assay
Twenty-four well Transwell plates (8-μm pore size, BD Biosciences, Franklin Lakes, NJ, USA) were used to perform cell migration assays. Briefly, 48 h after 786-O cells were transfected with the indicated constructs, aliquots of 100,000 cells from each transfection were resuspended in DMEM without FBS and loaded into the collagen IV-coated upper chambers. The lower chambers were filled with 0.4 ml DMEM containing 10% FBS. After 24 h, the cells were fixed with 10% formalin and stained with 0.1% crystal violet solution. The cells that migrated were imaged and counted in five randomly selected fields under a light microscope at × 200 magnification. All of the assays were performed in triplicate.
Wound healing assay
Cell migration was also evaluated in wound healing assays. Briefly, cells were seeded into 6-well plates (25,000 cells/well). After 24 h, the cells were transfected with the indicated constructs. After an additional 24 h, each confluent cell monolayer was wounded with a sterile 200 ul pipette tip. The medium from each well was subsequently removed and the cultures were replenished with fresh medium. After 24 h, cellular migration was examined under phase contrast objectives (× 10 magnification) of a CK2 inverted microscope (DM 16000, Leica, Germany).
Tumor growth assay
Male, 4-week-old Balb/c nude mice were housed in an animal facility maintained at 23 ± 2°C and 50 ± 5% humidity. 786-O cells (2 × 106) stably expressing miR-30a-3p or a negative control, were injected subcutaneously into the right dorsal flank of these mice. Tumor volumes were measured once a week. After five weeks, the mice were sacrificed and their tumors were removed and weighed. The experimental procedures were performed in accordance with the animal experimental guidelines of Wuhan University.
Dual luciferase assay
The 3’-UTR of Wnt2 which contains a putative miR-30a-3p binding site was cloned into the psi-CHECK vector (Promega, Madison, WI, USA). After confirming the sequence of the assembled plasmid, it was transfected into 786-0 cells seeded in 12-well plates along with a corresponding reporter construct, a control vector, and indicated constructs. Thirty hours later, luciferase activity was measured with a Dual-Luciferase Reporter assay kit (Promega).
Western blotting
Western blotting was performed as previously described [13]. Briefly, cells were lysed in a modified radioimmunoprecipitation (RIPA) buffer, proteins were separated by electrophoresis, and then the proteins were transferred to polyvinylidene membranes. The membranes were subsequently probed with anti-β-actin and anti-Wnt2 primary antibodies overnight at 4°C. Bound antibodies were visualized with horseradish peroxidase (HRP)-linked secondary antibodies for 1 h at room temperature. Western blots were developed with an enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Piscataway, NJ, USA).
Statistical analysis
The results of multiple experiments are presented as the mean ± standard deviation (SD) from three separate experiments and analyzed using SPSS16.0 statistical software. Student’s t-test was used to evaluate the statistical significance of differences between groups. P-values less than 0.05 were considered statistically significant.
Results
MiR-30a-3p is down-regulated in RCC specimens and cell lines
Expression of miR-30a-3p was detected in 30 RCC specimens and matched adjacent normal tissues by qRT-PCR. The levels of miR-30a-3p were significantly lower in the clinical RCC specimens compared to the corresponding non-tumor tissues (Figure 1A). The expression levels of miR-30a-3p detected in three RCC cells lines (A498, Caki-1, and 786-O) were also markedly reduced compared with the normal human renal cell line, HK-2 (Figure 1B).
Figure 1.
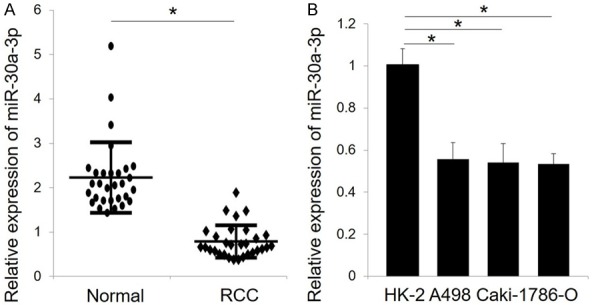
MiR-30a-3p is down-regulated in RCC specimens and cell lines. A. Relative expression of miR-30a-3p was detected in 30 paired clinical RCC specimens and adjacent normal tissues by qRT-PCR. B. The expression level of miR-30a-3p in 3 RCC cell lines (786-O, A498, Caki-1) and one normal renal cell line (HK-2) was also analyzed by qRT-PCR. U6 served as an internal reference. All data are expressed as the mean ± SD of three independent experiments. *P < 0.05.
MiR-30a-3p inhibits RCC cell proliferation and disrupts cell cycle progression
To examine the role of miR-30a-3p in RCC cell growth, 786-O cells were transfected with a miR-30a-3p mimic or negative control. After 72 h, cell proliferation was evaluated in MTT assays. The effect of miR-30a-3p was also validated by qRT-PCR (Figure 2A). Transfection of miR-30a-3p was found to significantly inhibit the growth of 786-O cells (Figure 2B) and it suppressed their ability to form colonies (Figure 2C and 2D). When the effect of miR-30a-3p on cell cycle progression was investigated, flow cytometry analyses revealed a greater number of cells in the S phase of the cell cycle, while the G0/G1 and G2/M populations decreased (Figure 2E).
Figure 2.
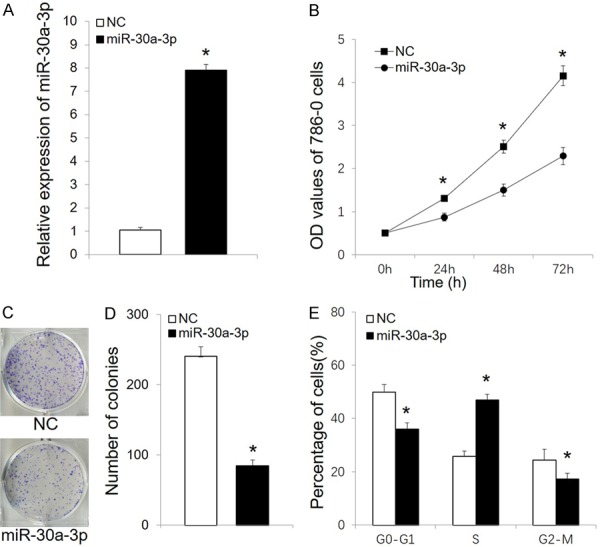
MiR-30a-3p suppresses RCC cell proliferation. A. Relative expression of miR-30a-3p was detected by qRT-PCR in 786-O cells after transfections with miR-30a-3p and a negative control (NC). B. MTT assays were performed to measure 786-O cell proliferation following transfection with miR-30a-3p or NC. C. The colonies formed after transfection with miR-30a-3p or NC are shown. D. Stained colonies with a diameter greater than 1 mm were counted. E. Cell cycle distribution was analyzed in 786-O cells transfected with miR-30a-3p or NC. All data are expressed as the mean ± SD for three independent experiments. *P < 0.05. NC, negative control.
MiR-30a-3p inhibits tumor growth in vivo
To clarify the potential tumor suppressor effects of miR-30a-3p in vivo, 786-O cells were transfected with a miR-30a-3p mimic or negative control and then were injected subcutaneously into the right dorsal flank of Balb/c mice. The miR-30a-3p mice exhibited a significant reduction in tumor size and weight compared with the negative control mice (Figure 3A and 3B).
Figure 3.
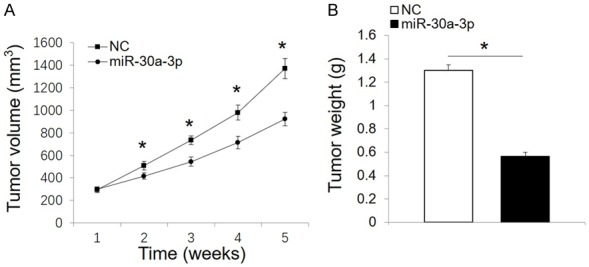
MiR-30a-3p inhibits tumor growth in vivo. A. Tumor growth curves were generated based on measurements of tumor volume that were recorded weekly following the injection of tumor cells. B. Tumor weights were recorded five weeks after the initial injections of tumor cells. All data are expressed as the mean ± SD. *P < 0.05. NC, negative control.
MiR-30a-3p inhibits RCC cell motility
To study the effect of miR-30a-3p on RCC cell motility, wound healing and Transwell migration assays were performed. Following the transfection of miR-30a-3p, both the migration and invasion phenotypes of 786-O cells were significantly suppressed (Figure 4A and 4B).
Figure 4.
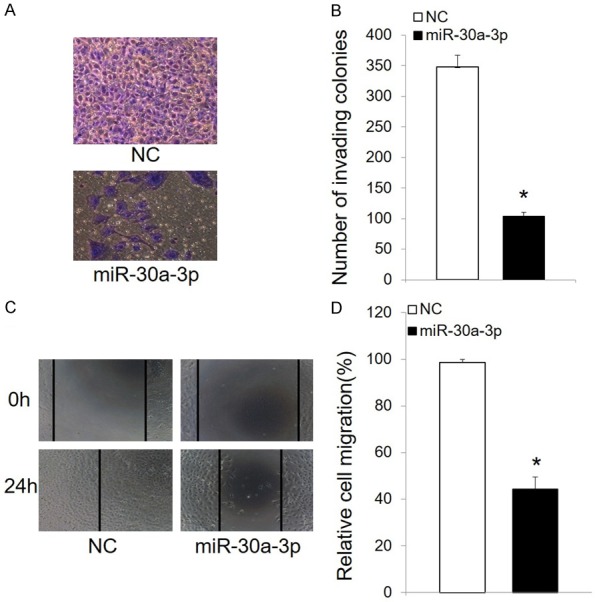
MiR-30a-3p inhibits RCC cell migration. A. Representative images (200 × magnification) of 786-O cells stained with crystal violet after transfection with miR-30a-3p or NC. B. Quantification of the number of cells that migrated in Transwell assays. Cells were counted in five randomly selected fields. C. Wound healing assays were performed to assess cell migration. Overexpression of miR-30a-3p decreased the numbers of migrating 786-O cells after 24 h. D. Quantification of the cell migration data. The data presented are the mean ± SD. *P < 0.05. NC, negative control.
Wnt2 is the downstream target of miR-30a-3p in RCC cells
To identify genes targeted by miR-30a-3p, TargetScan software was used. Among the possibilities, Wnt2 was identified as a potential target (Figure 5A). To confirm this target, luciferase activity assays were performed. Transfection of miR-30a-3p resulted in a significant reduction in luciferase activity in the presence of the WT 3’-UTR of Wnt2 (Figure 5B). In contrast, no reduction in luciferase activity was observed in the presence of a mutated Wnt2 3’-UTR (Mut) in 786-O cells (Figure 5B). Furthermore, overexpression of miR-30a-3p was found to significantly suppress both mRNA and protein levels of WNT2 (Figure 5C and 5D). In addition, miR-30a-3p markedly inhibited the expression of β-catenin (Figure 5D).
Figure 5.
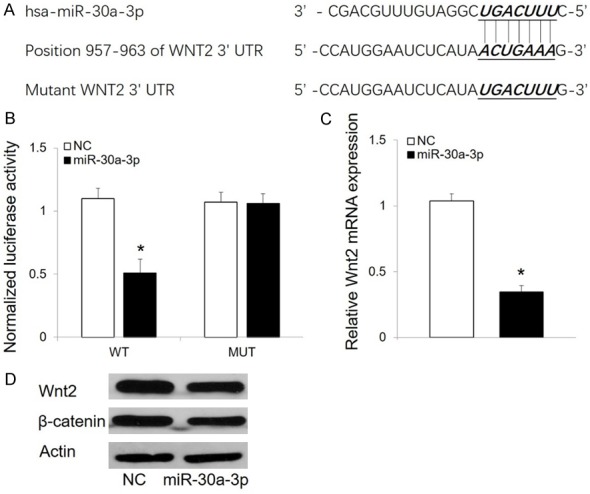
Wnt2 is a direct target of miR-30a-3p. A. TargetScan identified a highly conserved putative miR-30a-3p binding site in the 3’-UTR of Wnt2. The corresponding sequences of a miR-30a-3p mimic and a NC mutant were designed and synthesized. B. In luciferase reporter assays, overexpression of miR-30a-3p reduced luciferase activity in the presence of a WT Wnt2 reporter gene, but not in the presence of a mutated reporter gene, in 786-O cells. C. Overexpression of miR-30a-3p inhibited transcription of Wnt2 mRNA in 786-O cells. D. Western blot analysis of Wnt2 and β-catenin expression in 786-O cells transfected with miR-30a-3p and NC. Detection of actin served as a loading control. The data presented are the mean ± SD. *P < 0.05. NC, negative control.
Restoration of Wnt2 reverses the anti-tumor effects of miR-30a-3p in 786-O cells
To further verify a functional relationship between miR-30a-3p and Wnt2, we co-transfected 786-O cells with a miR-30a-3p mimic and a vector to provide overexpression of Wnt2 (Figure 6A). Cell proliferation, colony formation, cell cycle, and migration assays were subsequently performed. Overexpression of Wnt2 significantly attenuated the inhibitory effect of miR-30a-3p on 786-O cell growth (Figure 6B and 6C). Cell cycle arrest induced by miR-30a-3p was also reversed with overexpression of Wnt2 (Figure 6D). Furthermore, restoration of Wnt2 significantly promoted the invasion and migration of 786-O cells (Figure 6E and 6F).
Figure 6.
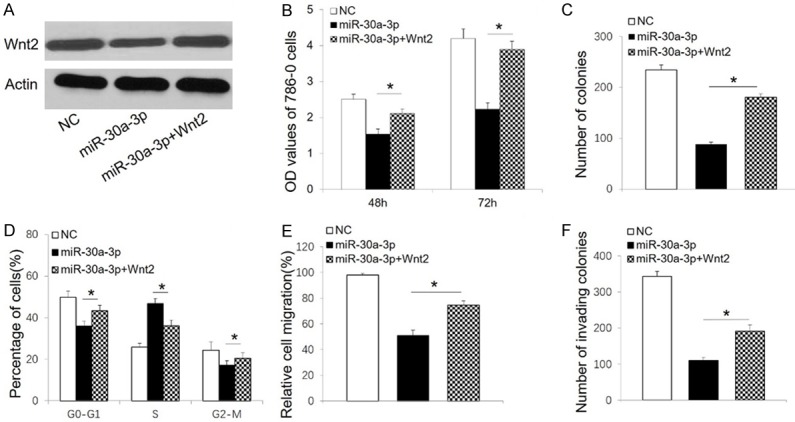
Ectopic overexpression of Wnt2 reverses the anti-tumor effects induced by miR-30a-3p in 786-O cells. A. Western blot analysis of Wnt2 in 786-O cells transfected with the indicated constructs. Actin was detected as a loading control. B. MTT proliferation assay results for 786-O cells transfected with the indicated constructs. C. Number of 786-O colonies formed after transfections with the indicated constructs. D. Cell cycle distribution of 786-O cells after transfections with the indicated constructs. E. Wound healing assay results for 786-O cells after transfections with the indicated constructs. F. Cell migration data from Transwell chamber assays after transfection of 786-O cells with the indicated constructs. All data are presented as the mean ± SD. *P < 0.05. NC, negative control.
Discussion
It has been reported that miR-30a is a critical regulator in carcinogenesis and tumor progression by functioning as an oncogene or tumor suppressor gene [14,15]. MiR-30a-3p is a member of the miR-30a family and a number of studies have indicated that miR-30a-3p plays an essential role in tumorigenesis [11,16,17]. MiR-30a-3p is down-regulated in hepatocellular carcinoma and can suppress tumor proliferation, metastasis, and invasiveness [11]. MiR-30a-3p has also been shown to be down-regulated in RCC [12]. To the best of our knowledge, the experiments described here provide the first evidence that miRNA-30a-3p can inhibit RCC progression by regulating Wnt2 expression. In both RCC cell lines and tissues, the expression level of miRNA-30a-3p was significantly down-regulated. Conversely, both in vitro and in vivo studies demonstrated that overexpression of miR-30a-3p inhibits RCC proliferation and growth. The migration potential of miR-30a-3p RCC cells was also decreased compared to control cells. Following the identification of Wnt2 as a direct target of miRNA-30a-3p, restoration of Wnt2 was shown to reverse miRNA-30a-3p-induced inhibition of cell proliferation. Thus, it appears that miRNA-30a-3p has a tumor suppressor role to negatively affect RCC progression.
The Wnt signaling pathway is frequently dysregulated in various types of tumors. Correspondingly, Wnt signaling has been found to have important roles in tumor development and progression, including specific roles in regulating cell proliferation, invasion, and migration [18-20]. In several cancers, the Wnt2/β-catenin pathway plays a crucial role in disease pathogenesis [17,21,22]. For example, Wnt2 has been shown to promote cancer progression by activating the Wnt/β-catenin pathway in both non-small cell lung cancer [23] and in colorectal cancer [24]. Moreover, Wnt2 induces pancreatic cancer metastasis through activation of the Wnt/β-catenin pathway [25,26]. The present results demonstrate that Wnt2 is a direct target of miR-30a-3p in RCC cells. Moreover, miR-30a-3p overexpression correlates with Wnt2 down-regulation and subsequent inhibition of cell proliferation and migration. These findings were confirmed when restoration of Wnt2 reversed the miR-30a-3p-induced inhibition of cell proliferation. Taken together, these data suggest that the tumor suppressor role of miR-30a-3p in RCC may be mediated via regulation of Wnt2 expression.
Conclusion
In summary, the results of this study indicate that miR-30a-3p is down-regulated in RCC specimens and cell lines and it plays an important role in the malignant progression of RCC cells by directly regulating Wnt2 expression. These findings suggest that miR-30a-3p may represent a potential therapeutic target for treatment of RCC and further studies are warranted.
Acknowledgements
This work was supported by Fundamental Research Funds for Central Universities (No. 2042014kf0624).
Disclosure of conflict of interest
None.
References
- 1.Guo J, Ma J, Sun Y, Qin S, Ye D, Zhou F, He Z, Sheng X, Bi F, Cao D, Chen Y, Huang Y, Liang H, Liang J, Liu J, Liu W, Pan Y, Shu Y, Song X, Wang W, Wang X, Wu X, Xie X, Yao X, Yu S, Zhang Y, Zhou A. Chinese guidelines on the management of renal cell carcinoma (2015 edition) Ann Transl Med. 2015;3:279. doi: 10.3978/j.issn.2305-5839.2015.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslam MZ, Matthews PN. Cytoreductive nephrectomy for metastatic renal cell carcinoma: a review of the historical literature and its role in the era of targeted molecular therapy. ISRN Urol. 2014;2014:717295. doi: 10.1155/2014/717295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banumathy G, Cairns P. Signaling pathways in renal cell carcinoma. Cancer Biol Ther. 2010;10:658–664. doi: 10.4161/cbt.10.7.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387:894–906. doi: 10.1016/S0140-6736(15)00046-X. [DOI] [PubMed] [Google Scholar]
- 5.Oom AL, Humphries BA, Yang C. MicroRNAs: novel players in cancer diagnosis and therapies. Biomed Res Int. 2014;2014:959461. doi: 10.1155/2014/959461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Li Y, Liu S, Duan Q, Chen L, Wu T, Qian H, Yang S, Xin D. Downregulation of miR-193a-3p inhibits cell growth and migration in renal cell carcinoma by targeting PTEN. Tumour Biol. 2017;39:1010428317711951. doi: 10.1177/1010428317711951. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Liu S, Duan Q, Chen L, Wu T, Qian H, Yang S, Xin D, He Z, Guo Y. MicroRNA-142-5p promotes cell growth and migration in renal cell carcinoma by targeting BTG3. Am J Transl Res. 2017;9:2394–2402. [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng B, Zhu H, Gu D, Pan X, Qian L, Xue B, Yang D, Zhou J, Shan Y. MiRNA-30a-mediated autophagy inhibition sensitizes renal cell carcinoma cells to sorafenib. Biochem Biophys Res Commun. 2015;459:234–239. doi: 10.1016/j.bbrc.2015.02.084. [DOI] [PubMed] [Google Scholar]
- 10.Jiang L, Liu Y, Ma C, Li B. MicroRNA-30a suppresses the proliferation, migration and invasion of human renal cell carcinoma cells by directly targeting ADAM9. Oncol Lett. 2018;16:3038–3044. doi: 10.3892/ol.2018.8999. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Wang W, Lin H, Zhou L, Zhu Q, Gao S, Xie H, Liu Z, Xu Z, Wei J, Huang X, Zheng S. MicroRNA-30a-3p inhibits tumor proliferation, invasiveness and metastasis and is downregulated in hepatocellular carcinoma. Eur J Surg Oncol. 2014;40:1586–1594. doi: 10.1016/j.ejso.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Gowrishankar B, Ibragimova I, Zhou Y, Slifker MJ, Devarajan K, Al-Saleem T, Uzzo RG, Cairns P. MicroRNA expression signatures of stage, grade, and progression in clear cell RCC. Cancer Biol Ther. 2014;15:329–341. doi: 10.4161/cbt.27314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Ai J, Xiao W, Liu J, Wang Y, Xin D, He Z, Guo Y, Wang Z. ELL is an HIF-1alpha partner that regulates and responds to hypoxia response in PC3 cells. Prostate. 2010;70:797–805. doi: 10.1002/pros.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Chen G, Ma X, Yang Y, Chen Y, Peng Y, Bai Z, Zhang Z, Pei H, Guo W. MiR-30a regulates cancer cell response to chemotherapy through SNAI1/IRS1/AKT pathway. Cell Death Dis. 2019;10:153. doi: 10.1038/s41419-019-1326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Ji Q, Zhang C, Liu Y, Liu N, Sui H, Zhou L, Wang S, Li Q. miR-30a acts as a tumor suppressor by double-targeting COX-2 and BCL9 in H. pylori gastric cancer models. Sci Rep. 2017;7:7113. doi: 10.1038/s41598-017-07193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park D, Kim H, Kim Y, Jeoung D. miR-30a regulates the expression of CAGE and p53 and regulates the response to anti-cancer drugs. Mol Cells. 2016;39:299–309. doi: 10.14348/molcells.2016.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi B, Wang Y, Chen ZJ, Li XN, Qi Y, Yang Y, Cui GH, Guo HZ, Li WH, Zhao S. Down-regulation of miR-30a-3p/5p promotes esophageal squamous cell carcinoma cell proliferation by activating the Wnt signaling pathway. World J Gastroenterol. 2017;23:7965–7977. doi: 10.3748/wjg.v23.i45.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Lu X, He H, Cui R, Wang X, Wu X. A novel culture system robustly maintained pluripotency of embryonic stem cells and accelerated somatic reprogramming by activating Wnt signaling. Am J Transl Res. 2017;9:4534–4544. [PMC free article] [PubMed] [Google Scholar]
- 20.Li K, Pan J, Wang J, Liu F, Wang L. MiR-665 regulates VSMCs proliferation via targeting FGF9 and MEF2D and modulating activities of Wnt/beta-catenin signaling. Am J Transl Res. 2017;9:4402–4414. [PMC free article] [PubMed] [Google Scholar]
- 21.Taciak B, Pruszynska I, Kiraga L, Bialasek M, Krol M. Wnt signaling pathway in development and cancer. J Physiol Pharmacol. 2018;69 doi: 10.26402/jpp.2018.2.07. [DOI] [PubMed] [Google Scholar]
- 22.Ai G, Meng M, Wang L, Shao X, Li Y, Cheng J, Tong X, Cheng Z. microRNA-196a promotes osteogenic differentiation and inhibit adipogenic differentiation of adipose stem cells via regulating beta-catenin pathway. Am J Transl Res. 2019;11:3081–3091. [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Ma R, Xu Y, Li N, Li Z, Yue J, Li H, Guo Y, Qi D. Wnt2 promotes non-small cell lung cancer progression by activating WNT/beta-catenin pathway. Am J Cancer Res. 2015;5:1032–1046. [PMC free article] [PubMed] [Google Scholar]
- 24.Jung YS, Jun S, Lee SH, Sharma A, Park JI. Wnt2 complements Wnt/beta-catenin signaling in colorectal cancer. Oncotarget. 2015;6:37257–37268. doi: 10.18632/oncotarget.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Li H, Huang C, Zhao T, Zhang H, Zheng C, Ren H, Hao J. Wnt2 protein plays a role in the progression of pancreatic cancer promoted by pancreatic stellate cells. Med Oncol. 2015;32:97. doi: 10.1007/s12032-015-0513-2. [DOI] [PubMed] [Google Scholar]
- 26.Ling J, Dong X, Wang L, Xue Y, Jia X, Song W, Li Q. MiR-27a-regulated FOXO1 promotes pancreatic ductal adenocarcinoma cell progression by enhancing Wnt/beta-catenin signaling activity. Am J Transl Res. 2019;11:3069–3080. [PMC free article] [PubMed] [Google Scholar]


