Abstract
Breast cancer is one of the most common cancers and the second leading cause of cancer mortality in women worldwide. Novel therapies and chemo-therapeutic drugs are still in urgent need to be developed for the treatment of breast cancer. One of the most important metabolic hallmarks of breast cancer cells is enhanced lipogenesis. Increasing evidences suggest that fatty acid synthase (FAS) plays an important role in the development of human breast cancer, for the expression of FAS is significantly higher in breast cancer cells than in normal cells. In addition, FAS inhibitors, such as curcumin, ursolic acid, and resveratrol, have shown anti-cancer potential. In the present study, we discovered that vitisin B, a natural stilbene isolated from the seeds of Iris lactea Pall. var. chinensis (Fisch.), was a novel FAS inhibitor. We found that vitisin B could down-regulate FAS expression and inhibit intracellular FAS activity in MDA-MB-231 cells. Also, we reported for the first time that vitisin B exhibited apoptotic effect on human breast cancer cells. Given all of this, we proposed a hypothesis that vitisin B has an application potential in the chemoprevention and treatment of breast cancer.
Keywords: Fatty acid synthase, vitisin B, stilbene, inhibitor, apoptosis, breast cancer cell
Introduction
Breast cancer is one of the most common human cancers worldwide [1]. As in other cancers, elevated lipogenesis is one of the most important metabolic hallmarks of breast cancer cells [2]. Cancer cells acquire fatty acids mainly through de novo lipogenesis to support their growth and proliferation [3-5]. The upregulated fatty acid synthesis in cancer cells is reflected by significant increase in both expression and activity of fatty acid synthase (FAS, EC 2.1.3.85) [6]. FAS catalyzes the synthesis of long chain saturated fatty acids from acetyl-CoA and malonyl-CoA in the presence of the reducing substrate NADPH [7,8]. The activation of FAS is important for carcinogenesis and cancer cells survival, so FAS may constitute a rational therapeutic target for cancer treatment [8,9].
Several FAS inhibitors (such as cerulenin, C75, EGCG) have been recognized as potential therapeutic agents in cancer treatment [10-12]. However, the drawbacks of these already investigated FAS inhibitors should be taken into account. Cerulenin, the first reported FAS inhibitor, was chemically unstable because of the reactive epoxide group in its structure [10]. C75, the first reported synthetic FAS inhibitor, was found to have the side effect of causing weight loss and anorexia [11]. EGCG, the first FAS inhibitor isolated from plants, showed relatively weak inhibitory activity on FAS with the half inhibitory concentration (IC50) of 24 µg/ml [12]. Therefore, it is important to explore novel and highly active FAS inhibitors, both natural and synthetic, in order to test their anti-cancer potential.
Vitisin B is a natural stilbene isolated from the seeds of Iris lactea Pall. var. chinensis (Fisch.) Koidz [13]. As an oligomer of resveratrol, vitisin B has been reported to have several biological functions such as inhibition on the activities of HMG-CoA [14], and BACE-1 [15], and induction of mitochondrial swelling and cytochrome C release [16]. Wu et al., reported that vitisin B significantly inhibited leukemia cell proliferation through activation of JNK and Fas death-signal transduction [17]. Empl et al., reported that vitisin B showed cytotoxicity in prostate cancer LNCaP cells at fairly low concentrations [18]. The mechanisms exerted by vitisin B involve a cell cycle arrest and the activation of caspases (i.e. apoptosis).
By screening a variety of natural products, we found that vitisin B could inhibit FAS activity. The aim of the present study was to investigate the inhibitory effect of vitisin B on both extracellular and intracellular FAS activities, as well as its apoptotic effect on FAS over-expressed human breast cancer MDA-MB-231 cells.
Materials and methods
Reagents
Acetyl-CoA, malonyl-CoA, NADPH and DMSO were purchased from Sigma (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco (Beijing, China). Fetal bovine serum (FBS) was purchased from Sijiqing Biological Engineering Material Company (Beijing, China). Cell Counting Kit (CCK-8) was purchased from Dojindo Laboratories (Kumamoto, Japan). Antibodies of FAS, PARP, Bax, Bcl-2 and GAPDH were purchased from Cell Signaling Technology (Denvers, MA, USA). Vitisin B (purity ≥ 97%, HPLC) was isolated and purified from the ethanol extracts from seed kernel of I. lactea as previously described [13] and it was dissolved in DMSO before use.
Preparation of FAS and its substrates
The preparation of FAS from chicken liver was performed as described previously [19]. The concentrations of FAS and its substrates were determined by a UV-vis spectrophotometer (Amersham Pharmacia Ultrospec 4300, England, UK) with the following experimental parameters: FAS, 4.83 × 105 M-1 cm-1 at 279 nm; Ac-CoA, 1.54 × 104 M-1 cm-1 at 259 nm, pH 7.0; Mal-CoA, 1.46 × 104 M-1 cm-1 at 260 nm, pH 6.0; acetoacetyl-CoA, 1.59 × 104 M-1 cm-1 at 259 nm, pH 7.0; NADPH, 6.02 × 103 M-1 cm-1 at 340 nm, and 1.59 × 104 M-1 cm-1 at 259 nm, pH 9.0 [19].
Assays of FAS activity
The FAS activity was measured at 37°C by the spectrophotometer at 340 nm (NADPH absorption). The overall reaction system contained 100 mM KH2PO4-K2HPO4 buffer, 1 mM EDTA, 1 mM dithiothreitol, 3 μM Ac-CoA, 10 μM Mal-CoA, 35 μM NADPH, and 10 μg FAS in a total volume of 2 ml as previously described [19,20].
Assays of FAS inhibition
The inhibition assays followed the same procedure as the activity assay but with the inhibitor (vitisin B). Vitisin B was dissolved in DMSO and added to the reaction mixture described above. The final concentration of DMSO was under 0.5% (v/v), to avoid the interference with FAS activity. The activities of FAS with and without vitisin B were represented as Ai and A0. The value of Ai/A0 × 100% was the residual activity (R.A.) of FAS. The IC50 was calculated from the plot of R.A. versus vitisin B concentration with Origin v. 7.5 (OriginLab, MA, USA).
Cell line and cultures
Human breast cancer MDA-MB-231 cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Cells were cultured in DMEM containing 10% FBS at 37°C in a humidified atmosphere containing 5% CO2.
Cell viability assay
Cell viability was assessed with the CCK-8 assay as previously described [21]. Briefly, MDA-MB-231 cells were seeded into 96-well plates at a concentration of 1 × 104 cells per 200 μl per well, and allowed an overnight period for attachment. The medium was removed, and fresh medium along with various concentrations (0, 2, 4, 6, 8, 10, 12, 14 µg/ml) of vitisin B were added to cultures in parallel. In following treatment, a drug-free medium (100 µl per well) and 10 µl CCK-8 solution were added to the cells, which were then incubated for 1-4 h at 37°C. The optical density (OD) value (absorbance) was measured at 450 nm by a microplate spectrophotometer (Multiskan MK3, Thermo Scientific, Shanghai, China). All experiments were performed in sextuplicate on three separate occasions.
Analysis of apoptosis
Cell apoptosis detection was performed with an Annexin-V-FITC Apoptosis Detection Kit (Becton Dickinson, Franklin Lakes, NJ, USA) according to the manufacturer’s protocol [22]. Briefly, cells were collected after 24 h treatment with vitisin B. The cells were washed thrice with cold phosphate buffered solution (PBS) and resuspended in 1× binding buffer at a concentration of 1 × 106 cells/ml. Then 500 µl cell suspension was incubated with 5 µl Annexin-V-FITC and 5 µl PI for 15 min in the dark and analyzed by an FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) within 1 h. Three replicates of the apoptosis assay were performed.
Intracellular FAS activity assay
Cells were treated with vitisin B for 24 h and were harvested by trypsinization, pelleted by centrifugation, washed twice, and resuspended in cold PBS. Cells were sonicated at 4°C and centrifuged at 13,000 rpm for 15 min at 4°C to obtain particle-free supernatants. The FAS activity was determined spectrophotometrically by measuring the decrease of absorbance at 340 nm due to oxidation of NADPH as others previously described [23]. 50 µl Particle-free supernatant, 25 mM KH2PO4-K2HPO4 buffer, 0.25 mM EDTA, 0.25 mM dithiothreitol, 30 µM acetyl-CoA, 350 µM NADPH (pH 7.0) in a total volume of 500 µl were monitored at 340 nm for 60 s to measure background NADPH oxidation. After the addition of 100 mM malonyl-CoA, the reaction was assayed for an additional 60 s to determine the FAS dependent oxidation of NADPH.
Western-blot assay
Western blotting was carried out as previously described [24]. Briefly, after 24 h treatment of vitisin B, cells were washed twice with cold PBS and harvested in RIPA lysis buffer with 1 mM PMSF and then lysed for 10 min on ice. Then a particle free supernatant solution was obtained by centrifugation at 13,000 rpm for 15 min at 4°C. Protein concentrations of cell lysates were measured by the Pierce BCA protein assay kit using bovine serum albumin (BSA) as a standard control. Comparable amounts of protein (50 µg FAS, 30 µg PARP, 25 µg Bcl-2, 25 µg Bax were put into each lane during SDS-PAGE) were heated in sodium dodecylsulphate (SDS) sample buffer (Laemmli) for 15 min at 95°C, separated using a 10%-12% SDS polyacrylamide gel and transferred to PVDF membranes. Then blocked with 5% skimmed milk for 1-2 h at room temperature to prevent nonspecific antibody binding, and probed with various primary antibodies at dilutions recommended by the suppliers overnight at 4°C. Then washed thrice with TBST (10 mM Tris, 10 mM NaCl, 0.1% Tween 20), and incubated 1 h with corresponding peroxidase conjugated secondary antibody and developed with a commercial kit (West Pico chemiluminescent substrate). Blots were reprobed with an antibody against β-actin as the control of protein loading and transfer.
RT-PCR analysis
Total RNA was extracted and purified from MDA-MB-231 cells using RNAsimple Total RNA Kit (TianGen Biotech, Beijing, China). A 1 μg amount of total RNA of each sample was reverse-transcribed to cDNA with a cDNA Synthesis Kit (TianGen Biotech, Beijing, China). The gene expression levels of FAS were analyzed by quantitative real-time PCR (Mx 3000P, USA). The conditions for PCR were as follows: initial denaturation at 95°C for 5 min and followed by 45 cycles (95°C, 15 s, 55°C, 15 s, 72°C, 20 s). The primer sequences used for qPCR were as follows. β-Actin: Forward 5’-GTGGGCCGCTCTAGGCACCAA-3’ and Reverse 5’-CTCTTTGATGTCACGACGATTTC-3’; FAS: Forward 5’-TTCGTACCTCCTTGGCAAAC-3’ and Reverse: 5’-GGCTGCAGTGAATGAATTTG-3’ [25].
Statistical analysis
Data represent the mean ± standard deviation (SD) from at least three independent experiments. The unpaired Student’s t test was used to compare the means of two groups. The statistical differences among three or more groups were determined by one-way ANOVA with Tukey’s post-test using Prism 5 software (GraphPad, San Diego, CA, USA). Statistical significance was determined at the level of P < 0.05.
Results
Inhibitory effect of vitisin B on FAS activity
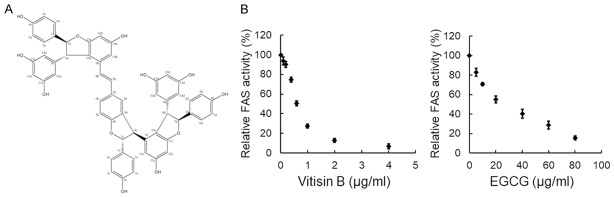
The inhibitory activity of vitisin B on the FAS catalyzed reaction was assayed in vitro. Vitisin B exhibited dose-dependent inhibition on chicken FAS. The data obtained from Figure 1B showed that 0.617 μg/ml (0.681 μM) vitisin B inhibited 50% of the overall reaction activity.
Figure 1.
The inhibitory effect of vitisin B on FAS activity. A. Chemical structure of vitisin B. B. The activity for the FAS overall reaction was assayed to determine the inhibitory capability of vitisin B (0, 0.1, 0.2, 0.4, 0.6, 1.0, 2.0, 4.0 µg/ml). EGCG was chosen as a positive control to compare the inhibitory effect of vitisin B on FAS.
Vitisin B reduced the viability of MDA-MB-231 cells
To evaluate the effect of vitisin B on cell viability, MDA-MB-231 cells were incubated with various concentrations of vitisin B (0, 2, 4, 6, 8, 10, 12, 14 µg/ml) for 24 h, followed by a CCK-8 assay. As shown in Figure 2, vitisin B exhibited a dose-dependent inhibitory effect on MDA-MB-231 cells with an IC50 value of 8.45 µg/ml (9.32 µM).
Figure 2.
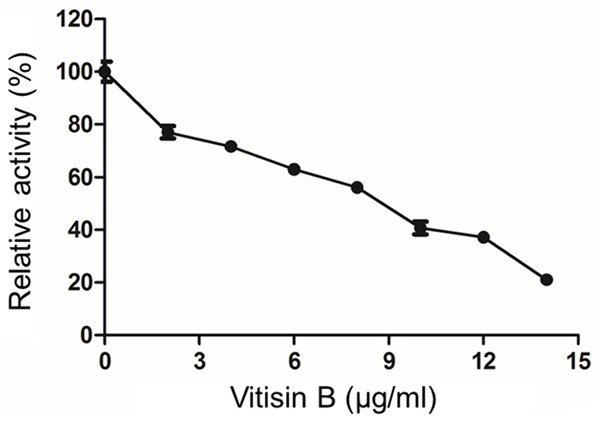
Vitisin B reduced the viability of MDA-MB-231 cells. MDA-MB-231 cells were treated with 0, 2, 4, 6, 8, 10, 12, 14 µg/ml vitisin B for 24 h. Cell viability was then determined by the CCK-8 assay. The percentage of cell viability was calculated as the ratio of treated cells to control cells (0.1% DMSO). Data were expressed as the mean ± SD of three independent experiments.
Vitisin B induced MDA-MB-231 cells apoptosis
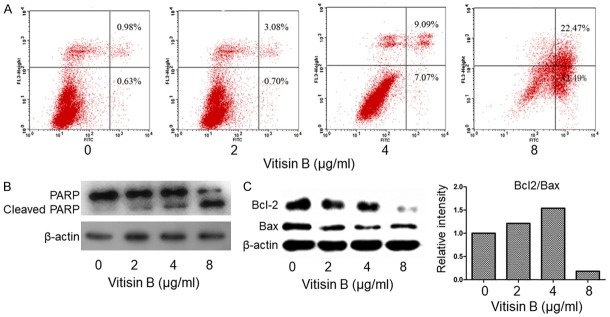
The apoptotic effect of vitisin B on MDA-MB-231 cells was analyzed and quantified by flow cytometry using the annexin V-FITC Apoptosis Detection Kit. As shown in Figure 3A, vitisin B induced MDA-MB-231 cell apoptosis in a dose-dependent manner, reaching 54.96% at 8 µg/ml (32.49% early apoptosis plus 22.47% late apoptosis). Corresponding to that, only 1.61% cell apoptosis was found in control group (0.63% early apoptosis plus 0.98% late apoptosis). The apoptotic activity of vitisin B on MDA-MB-231 cells was also confirmed by Western blotting, which showed cleavage of PARP. MDA-MB-231 cells treated with vitisin B for 24 h expressed a marked increase in the levels of the PARP cleavage product (89 kDa band) in a dose-dependent manner (Figure 3B). Western blotting analysis showed that 8 μg/ml vitisin B down-regulated Bcl-2 expression level, thereby causing a significant decrease in the Bcl-2/Bax ratio that favored cell apoptosis (Figure 3C). The expression levels of Bax were also decreased after treating with 2, 4, 8 μg/ml vitisin B (Figure 3C).
Figure 3.
Apoptotic effect of vitisin B on MDA-MB-231 cells. A. Apoptosis was evaluated using an annexin V-FITC apoptosis detection kit and flow cytometry. The X- and Y-axes represent annexin V-FITC staining and PI staining, respectively. The representative pictures were from MDA-MB-231 cells incubated with different concentrations of vitisin B (0, 2, 4 and 8 µg/ml). The gate setting distinguished between living (bottom left), necrotic (top left), early apoptotic (bottom right), and late apoptotic (top right) cells. The experiment was repeated three times. B. Vitisin B induced apoptosis in MDA-MB-231 cells as assessed by PARP cleavage (note intact PARP at 116 kDa and its cleavage product at 89 kDa). Shown gels were representative of those obtained from at least three independent experiments. C. The expression levels of Bcl-2 and Bax were down-regulated after treatment with vitisin B. Shown gels were representative of those obtained from at least three independent experiments.
Vitisin B inhibited FAS expression and activity in MDA-MB-231 cells
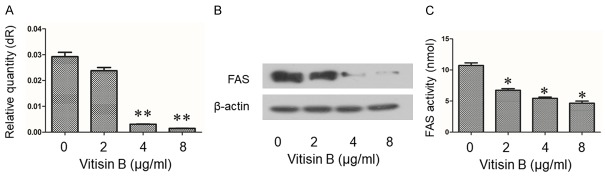
In order to examine the impact of vitisin B on FAS mRNA, we measured the changes of FAS on mRNA level. The results showed that compared to the control group, the mRNA level of FAS significantly decreased after vitisin B administration (Figure 4A). Moreover, MDA-MB-231 cells incubated with vitisin B resulted in a dose-dependent reduction of FAS expression (Figure 4B). The intracellular FAS activity in vitisin B treated MDA-MB-231 cells was inhibited in a dose-dependent manner (Figure 4C). These results showed that vitisin B down-regulated both mRNA and protein level of FAS, as well as inhibited intracellular FAS activity in MDA-MB-231 cells.
Figure 4.
Inhibitory effect of vitisin B on FAS expression and intracellular FAS activity in MDA-MB-231 cells. A. Effect of vitisin B on FAS mRNA. MDA-MB-231 cells were treated with 0, 2, 4, 8 μg/ml vitisin B. After 24 h, mRNA was extracted and quantified via RT-PCR, and normalized to β-actin mRNA. Data were normalized to control cells without vitisin B (0 μg/ml). Data was analyzed using unpaired Student’s t test, **P < 0.01 compared to the control (0 μg/ml). B. Representative pictures for FAS protein expression by western blot analysis. Cells were treated with 0, 2, 4, 8 μg/ml vitisin B. Vitisin B down-regulated FAS expression in MDA-MB-231 cells in a dose-dependent manner. Shown gels were representative of those obtained from at least three independent experiments. C. Intracellular FAS activity measured in MDA-MB-231 cells using NADPH by spectrophotometry at 340 nm. Relative FAS activities were represented as the means ± SD from three independent experiments with similar results. *P < 0.05 compared to the control (0 μg/ml).
Discussion
Due to the differential expression levels between cancer and normal cells, FAS has been suggested as a potential molecular target for anticancer drug development [26,27]. Fatty acids contribute to cancer progression because they are the building blocks for newly-synthesized membrane phospholipids [28]. It is now widely recognized that cancer cells frequently enhanced ability of intracellular lipids synthesis [6,29,30], a process which is tightly related to FAS activity.
In the present work, we found that vitisin B was an FAS inhibitor with an IC50 value of 0.617 µg/ml, which was significantly lower than those of classical FAS inhibitors like cerulenin (IC50 = 20 µg/ml) [10] and EGCG (IC50 = 24 µg/ml) [12], as well as some newly reported natural FAS inhibitors like resveratrol (11.1 µg/ml), curcumin (10.5 µg/ml), α-mangostin (2.27 µg/ml) [31-33]. Considering that the activities of these compounds were measured within the same assay, vitisin B is one of the strongest natural FAS inhibitors so far discovered.
The amino acid sequences of human and other animal FASs are closely similar [34], however, the human FAS preparations showed lower activity than FASs of other animals [35]. So the chicken and duck FAS were used commonly for the study of FAS inhibitors [36]. In this study, chicken FAS was applied to measure the inhibitory activity of vitisin B. The amino acid sequence of chicken FAS has 63% identity with that of human FAS [35].
We found that vitisin B down-regulated not only protein expression level, but also the mRNA level of FAS. In cancer cells, newly synthesized lipids catalyzed by FAS preferentially transfer to phospholipids which could be involved in cell signaling and biosynthesis of cell membrane [3]. Inhibition of FAS has been proven to be an obstacle of cancer cell growth and an induction to cell death [9,37].
As one of the widely researched stilbenes, resveratrol was reported to be an antitumor component that lead to breast cancer cell apoptosis [38-41]. However, not many studies had referred to the anti-cancer activity of vitisin B [17]. In the present study, we found that vitisin B, a stilbene tetramer, exhibited dose-dependent anti-proliferative activity in human breast cancer cells. In our previous study, we found that the inhibitory effect of catechin polymer was stronger than catechin, and condensed tannin was stronger than tannin monomer [42,43]. These results indicated that polyphenol oligomers may be more effective than monomers in FAS inhibition. Compared with our previous studies on stilbene monomers and oligomers, such as resveratrol, pallidol, rhaponticin, and desoxyrhaponticin, vitisin B still has stronger inhibitory activity on FAS [24,44]. The possible reason may be that vitisin B could affect more functional domains of FAS because of its big molecular structure, although the mechanism involved need to be detailed investigated. Willenberg et al., have reported that the intestinal absorption of stilbene oligomers like hopeaphenol, whose structure is similar to vitisin B, was much lower than that of resveratrol [45]. The bioavailability of vitisin B may not be good and the clinical application of vitisin B should be evaluated with bioavailability. From this point of view, vitisin B may not be a promising anti-cancer drug candidate. FAS is an enzyme which catalyzes fatty acid synthesis reaction in the cytoplasm. In general, if a compound could not enter the cell, it seemed unable to affect intracellular FAS activity. However, we could not rule out the possibility that vitisin B might indirectly inhibit FAS activity by sticking on the cell surface, since it has been reported that cell surface receptors such as growth factor receptors and hormone receptors can play essential roles in tumor-related FAS over-expression [46].
Apoptosis signaling pathway is strictly regulated by a fine balance between pro- and anti-apoptotic Bcl-2 family proteins [47,48]. Overexpression of anti-apoptotic Bcl-2 family proteins, such as Bcl-2, has been demonstrated as a major contributing factor for apoptosis resistance in breast cancer and many other cancers [47,48]. In this study, we investigated the effect of vitisin B on two Bcl-2 family proteins in MDA-MB-231 cells. The expression levels of Bcl-2 and Bax were all reduced by vitisin B. Compared with the apoptotic effect of α-mangostin, a known FAS inhibitor, vitisin B showed the similar effects [23]. It is interesting that up to 4 µg/ml vitisin B seems to favor an anti-apoptotic Bcl-2/Bax ratio. However, vitisin B treatment at concentration of 8 µg/ml showed major reduction of Bcl-2/Bax ratio. So Bcl-2 family proteins may be involved in vitisin B induced apoptosis and the specific mechanism may be complicated.
Many natural stilbenes with high anticancer efficacy and acceptable levels of toxicity to normal tissues have been suggested as candidates for cancer treatment [49]. Ong et al., has reported that vitisin B showed no toxic effect on vascular smooth muscle cells [50]. In the present work, we found that vitisin B induced both early and late apoptosis, which was also similar to α-mangostin, as shown in Figure 3A. However, we could not confirm whether vitisin B induced apoptosis was due to its toxic effect.
In most normal cells, de novo fatty acid synthesis is suppressed. Only a few normal tissues and cells such as adipocytes, hepatocytes, hormone sensitive cells, the cycling endometrium, and fetal lung tissue may keep a very active fatty acid synthesis pathway [51]. And even those with comparatively high proliferation rates of normal cells preferentially use dietary/exogenous lipids for synthesis of new structural lipids [2,46] whereas most cancer cells display increased endogenous fatty acid biosynthesis regardless of extracellular lipid availability. Therefore, FAS is a promising target for obstacle the growth of cancer cells without adverse effect on the survival of normal cells. Hence vitisin B may induce cancer cells apoptosis without affecting the lipids metabolism in normal cells.
Conclusions
Targeting intracellular FAS activity may represent a new approach to prevent or treat human breast cancer. Therefore, more safe and effective FAS inhibitors along this line should be developed for cancer treatment. In the present study, we demonstrate that vitisin B is a novel FAS inhibitor. In addition, we found that vitisin B decreased FAS expression and inhibited intracellular FAS activity in MBA-MD-231 cells. Vitisin B also induced MDA-MB-231 cell apoptosis, as observed by flow cytometry (with 54.96% apoptosis at 8 µg/ml compared to the control, 1.61%) and evidenced by increasing expression level of cleaved PARP. Since FAS has been found to be a potent anticancer drug target, the results of our present work may furnish some useful ideas and new clues in developing drugs in treatment of human breast cancer.
Acknowledgements
This work was supported by the Beijing Municipal Education Commission (KM201910029001), the Fusion of Science and Education Special Fund, College of Life Sciences, University of Chinese Academy of Sciences (KJRH2015-012); Application Basic Research Project of Qinghai Province (2015-ZJ-728); Youth Innovation Promotion Association, CAS (2012315); 2014 Youth National Natural Science Foundation of China (No. 31300292); The Key Program of “The Dawn of West China” Talent Foundation of CAS (2012); 2014 Youth National Natural Science Foundation of China (No. 31300292).
Disclosure of conflict of interest
None.
References
- 1.Ginsburg OM, Love RR. Breast cancer: a neglected disease for the majority of affected women worldwide. Breast J. 2011;17:289–295. doi: 10.1111/j.1524-4741.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandey PR, Xing F, Sharma S, Watabe M, Pai SK, Iiizumi-Gairani M, Fukuda K, Hirota S, Mo YY, Watabe K. Elevated lipogenesis in epithelial stem-like cell confers survival advantage in ductal carcinoma in situ of breast cancer. Oncogene. 2013;32:5111–5122. doi: 10.1038/onc.2012.519. [DOI] [PubMed] [Google Scholar]
- 3.Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA, Pettus JR, Froehlich HM, Memoli VA, Morganelli PM, Swinnen JV, Timmerman LA, Chaychi L, Fricano CJ, Eisenberg BL, Coleman WB, Kinlaw WB. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10:427–436. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniëls VW, Machiels J, Vanderhoydonc F, Smans K, Waelkens E, Verhoeven G, Swinnen JV. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 6.Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91:47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 7.Wakil SJ. Fatty-acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28:4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 8.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 9.Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vance D, Goldberg I, Mitsuhashi O, Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972;48:649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- 11.Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Moran TH, Ronnett GV. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem. 2004;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Tian WX. Green tea epigallocatechin gallate: a natural inhibitor of fatty-acid synthase. Biochem Biophys Res Commun. 2001;288:1200–1206. doi: 10.1006/bbrc.2001.5923. [DOI] [PubMed] [Google Scholar]
- 13.Lv H, Wang H, He Y, Ding C, Wang X, Suo Y. Separation and purification of four oligostilbenes from Iris lactea Pall. var. chinensis (Fisch.) Koidz by high-speed counter-current chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;988:127–134. doi: 10.1016/j.jchromb.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 14.Koo M, Kim SH, Lee N, Yoo MY, Ryu SY, Kwon DY, Kim YS. 3-Hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitory effect of Vitis vinifera. Fitoterapia. 2008;79:204–206. doi: 10.1016/j.fitote.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Choi YH, Yoo MY, Choi CW, Cha MR, Yon GH, Kwon DY, Kim YS, Park WK, Ryu SY. A new specific BACE-1 inhibitor from the stembark extract of Vitis vinifera. Planta Med. 2009;75:537–540. doi: 10.1055/s-0029-1185311. [DOI] [PubMed] [Google Scholar]
- 16.Seya K, Kanemaru K, Sugimoto C, Suzuki M, Takeo T, Motomura S, Kitahara H, Niwa M, Oshima Y, Furukawa K. Opposite effects of two resveratrol (trans-3,5,4’-trihydroxystilbene) tetramers, vitisin A and hopeaphenol, on apoptosis of myocytes isolated from adult rat heart. J Pharmacol Exp Ther. 2009;328:90–98. doi: 10.1124/jpet.108.143172. [DOI] [PubMed] [Google Scholar]
- 17.Wu SS, Chen LG, Lin RJ, Lin SY, Lo YE, Liang YC. Cytotoxicity of (-)-vitisin B in human leukemia cells. Drug Chem Toxicol. 2013;36:313–319. doi: 10.3109/01480545.2012.720990. [DOI] [PubMed] [Google Scholar]
- 18.Empl MT, Albers M, Wang S, Steinberg P. The resveratrol tetramer r-viniferin induces a cell cycle arrest followed by apoptosis in the prostate cancer cell line LNCaP. Phytother Res. 2015;29:1640–1645. doi: 10.1002/ptr.5443. [DOI] [PubMed] [Google Scholar]
- 19.Tian WX, Hsu RY, Wang YS. Studies on the reactivity of the essential sulfhydryl groups as a conformational probe for the fatty acid synthetase of chicken liver. Inactivation by 5,5’-dithiobis-(2-nitrobenzoic acid) and intersubunit cross-linking of the inactivated enzyme. J Biol Chem. 1985;260:11375–11387. [PubMed] [Google Scholar]
- 20.Soulié JM, Sheplock GJ, Tian WX, Hsu RY. Transient kinetic studies of fatty acid synthetase. A kinetic self-editing mechanism for the loading of acetyl and malonyl residues and the role of coenzyme A. J Biol Chem. 1984;259:134–140. [PubMed] [Google Scholar]
- 21.Lu YY, Chen TS, Qu JL, Pan WL, Sun L, Wei XB. Dihydroartemisinin (DHA) induces caspase-3-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. J Biomed Sci. 2009;16:16. doi: 10.1186/1423-0127-16-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menendez JA, Vellon L, Colomer R, Lupu R. Pharmacological and small interference RNA-mediated inhibition of breast cancer-associated fatty acid synthase (oncogenic antigen-519) synergistically enhances Taxol (paclitaxel)-induced cytotoxicity. Int J Cancer. 2005;115:19–35. doi: 10.1002/ijc.20754. [DOI] [PubMed] [Google Scholar]
- 23.Li P, Tian W, Ma X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol Cancer. 2014;13:138. doi: 10.1186/1476-4598-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P, Tian W, Wang X, Ma X. Inhibitory effect of desoxyrhaponticin and rhaponticin, two natural stilbene glycosides from the Tibetan nutritional food Rheum tanguticum Maxim. ex Balf., on fatty acid synthase and human breast cancer cells. Food Funct. 2014;5:251–256. doi: 10.1039/c3fo60484e. [DOI] [PubMed] [Google Scholar]
- 25.Nie F, Liang Y, Xun H, Sun J, He F, Ma X. Inhibitory effects of tannic acid in the early stage of 3T3-L1 preadipocytes differentiation by down-regulating PPARγ expression. Food Funct. 2015;6:894–901. doi: 10.1039/c4fo00871e. [DOI] [PubMed] [Google Scholar]
- 26.Ogino S, Nosho K, Meyerhardt JA, Kirkner GJ, Chan AT, Kawasaki T, Giovannucci EL, Loda M, Fuchs CS. Cohort study of fatty acid synthase expression and patient survival in colon cancer. Clin Oncol. 2008;26:5713–5720. doi: 10.1200/JCO.2008.18.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi S, Graner E, Febbo P, Weinstein L, Bhattacharya N, Onody T, Bubley G, Balk S, Loda M. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res. 2003;1:707–715. [PubMed] [Google Scholar]
- 28.Zaidi N, Lupien L, Kuemmerle NB, Kinlaw WB, Swinnen JV, Smans K. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52:585–589. doi: 10.1016/j.plipres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 30.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang HZ, Quan XF, Tian WX, Hu JM, Wang PC, Huang SZ, Cheng ZQ, Liang WJ, Zhou J, Ma XF, Zhao YX. Fatty acid synthase inhibitors of phenolic constituents isolated from Garcinia mangostana. Bioorg Med Chem Lett. 2010;20:6045–6047. doi: 10.1016/j.bmcl.2010.08.061. [DOI] [PubMed] [Google Scholar]
- 32.Zhao J, Sun XB, Ye F, Tian WX. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Mol Cell Biochem. 2011;351:19–28. doi: 10.1007/s11010-010-0707-z. [DOI] [PubMed] [Google Scholar]
- 33.Quan X, Wang Y, Ma X, Liang Y, Tian W, Ma Q, Jiang H, Zhao Y. α-Mangostin induces apoptosis and suppresses differentiation of 3T3-L1 cells via inhibiting fatty acid synthase. PLoS One. 2012;7:e33376. doi: 10.1371/journal.pone.0033376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chirala SS, Huang WY, Jayakumar A, Sakai K, Wakil SJ. Animal fatty acid synthase: functional mapping and cloning and expression of the domain I constituent activities. Proc Natl Acad Sci U S A. 1997;94:5588–5593. doi: 10.1073/pnas.94.11.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayakumar A, Tai MH, Huang WY, al-Feel W, Hsu M, Abu-Elheiga L, Chirala SS, Wakil SJ. Human fatty acid synthase: properties and molecular cloning. Proc Natl Acad Sci U S A. 1995;92:8695–8699. doi: 10.1073/pnas.92.19.8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li LC, Tian WX. New evolution: Inhibitors of fatty acid synthase and fat-reducing study. Chin Sci Bull. 2002;47:89–91. [Google Scholar]
- 37.De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799–3804. [PubMed] [Google Scholar]
- 38.Filomeni G, Graziani I, Rotilio G, Ciriolo MR. Trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr. 2007;2:295–305. doi: 10.1007/s12263-007-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang HY, Shih A, Cao HJ, Davis FB, Davis PJ, Lin HY. Resveratrol-induced cyclooxygenase-2 facilitates p53-dependent apoptosis in human breast cancer cells. Mol Cancer Ther. 2006;5:2034–2042. doi: 10.1158/1535-7163.MCT-06-0216. [DOI] [PubMed] [Google Scholar]
- 40.Alkhalaf M, El-Mowafy A, Renno W, Rachid O, Ali A, Al-Attyiah R. Resveratrol-induced apoptosis in human breast cancer cells is mediated primarily through the caspase-3-dependent pathway. Arch Med Res. 2008;39:162–168. doi: 10.1016/j.arcmed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Liu J, Liu X, Xing K, Wang Y, Li F, Yao L. Resveratrol-induced cell inhibition of growth and apoptosis in MCF7 human breast cancer cells are associated with modulation of phosphorylated Akt and caspase-9. Appl Biochem Biotechnol. 2006;135:181–192. doi: 10.1385/abab:135:3:181. [DOI] [PubMed] [Google Scholar]
- 42.Du YT, Wang X, Wu XD, Tian WX. Keemun black tea extract contains potent fatty acid synthase inhibitors and reduces food intake and body weight of rats via oral administration. J Enzyme Inhib Med Chem. 2005;20:349–356. doi: 10.1080/14756360500148841. [DOI] [PubMed] [Google Scholar]
- 43.Zhang SY, Zheng CG, Yan XY, Tian WX. Low concentration of condensed tannins from catechu significantly inhibits fatty acid synthase and growth of MCF-7 cells. Biochem Biophys Res Commun. 2008;371:654–658. doi: 10.1016/j.bbrc.2008.04.062. [DOI] [PubMed] [Google Scholar]
- 44.Bao L, Ma X, Song X, Wang M, Liu H. Two new resveratrol tetramers isolated from Cayratia japonica (Thunb. ) Gagn. with strong inhibitory activity on fatty acid synthase and antioxidant activity. Chem Biodivers. 2010;7:2931–2940. doi: 10.1002/cbdv.200900394. [DOI] [PubMed] [Google Scholar]
- 45.Willenberg I, Michael M, Wonik J, Bartel LC, Empl MT, Schebb NH. Investigation of the absorption of resveratrol oligomers in the Caco-2 cellular model of intestinal absorption. Food Chem. 2015;167:245–250. doi: 10.1016/j.foodchem.2014.06.103. [DOI] [PubMed] [Google Scholar]
- 46.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100:1369–1372. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kvansakul M, Hinds MG. The Bcl-2 family: structures, interactions and targets for drug discovery. Apoptosis. 2015;20:136–150. doi: 10.1007/s10495-014-1051-7. [DOI] [PubMed] [Google Scholar]
- 48.Kvansakul M, Hinds MG. Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis. 2013;4:e909. doi: 10.1038/cddis.2013.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sirerol JA, Rodríguez ML, Mena S, Asensi MA, Estrela JM, Ortega AL. Role of natural stilbenes in the prevention of cancer. Oxid Med Cell Longev. 2016;2016:3128951. doi: 10.1155/2016/3128951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ong ET, Hwang TL, Huang YL, Lin CF, Wu WB. Vitisin B, a resveratrol tetramer, inhibits migration through inhibition of PDGF signaling and enhancement of cell adhesiveness in cultured vascular smooth muscle cells. Toxicol Appl Pharmacol. 2011;256:198–208. doi: 10.1016/j.taap.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Wagle S, Bui A, Ballard PL, Shuman H, Gonzales J, Gonzales LW. Hormonal regulation and cellular localization of fatty acid synthase in human fetal lung. Am J Physiol. 1999;277:L381–390. doi: 10.1152/ajplung.1999.277.2.L381. [DOI] [PubMed] [Google Scholar]