Abstract
The effect of bone marrow mesenchymal stem cells (BMSCs) on RhoA/ROCK signal pathway expression in severe acute pancreatitis (SAP) was investigate in the present study. SAP model was established by retrograde injection of 5% sodium taurocholate into biliopancreatic duct. SD rats were then randomly divided into four groups: normal control, untreated SAP, BMSCs transplant + SAP and ROCK inhibitor + SAP groups (N = 30 each). All rats were sacrificed at 6, 12 and 24 h followed by analysis of serum amylase, TNF-α and IL-6 levels by ELISA, RhoA and ROCK I expression in pancreatic tissues by Western blot, morphological change by HE staining. CM-Dil labelled BMSC can be observed in transplant group. Compared to control group, untreated SAP group had significantly elevated serum amylase, ascites, and levels of TNF-α and IL-6 (P<0.05) in a time-dependent manner, with enhanced pancreatic RhoA and ROCK I protein expression (P<0.05). However, BMSCs transplant group showed decreased serum amylase, ascites, TNF-α and IL-6, plus lower RhoA or ROCK I protein expression (P<0.05). Meanwhile, Y-27632 intervention group also showed lower serum amylase or ascites, plus lower RhoA or ROCK I (P<0.05). HE staining showed improved pathological score in BMSCs transplant or Y-27632 intervention group (only at 6 h time point) compared to untreated SAP group (P<0.05). Pancreatic expression of RhoA and ROCK I is up-regulated in SAP, with severe pancreatic tissue damage. BMSCs can alleviate pancreatic injury possibly through decreasing serum inflammatory factor level and inhibiting RhoA/ROCK signal pathway.
Keywords: Bone marrow mesenchymal stem cells, severe acute pancreatitis, RhoA/Rho kinase signal pathway
Introduction
Severe acute pancreatitis (SAP) has sudden onset, rapid progression and difficulty in treatment, with frequent development of multi-organ failures (MODs) [1]. The incidence of SAP is relatively higher with unfavorable prognosis, with a mortality rate about 15-20% [2,3]. Major reason causing SAP patient death is inflammatory response as a result of abundantly released inflammatory mediators, leading to systemic inflammatory response syndrome (SIRS) and MODs [4]. In various diseases including SAP, inflammatory cytokines and angiotensin can activate RhoA/ROCK signal pathway to participate in several patho-physiological processes [5,6]. Therefore, regulation of RhoA/ROCK signal pathway activation may affect SAP prognosis.
In recent years, bone marrow mesenchymal stem cells (BMSCs) have multiple advantages including anti-inflammation, migration towards injury organ/tissues, regulating cell apoptosis, facilitating tissue repair, modulating immunity and low antigenicity, all of which make it become one hot topic for studying SAP. However, whether BMSCs play a role in prevention or treatment of SAP remains poorly understood. The aim of the present study was to investigate effect of BMSCs on SAP as well as on RhoA/ROCK signal pathway.
Materials and methods
Experimental animals
Healthy male SPF grade Sprague-Dwley (SD) rats (body weight 250-300 g), and SPF grade male juvenile SD rat (body weight 60-80 g) were purchased from SLC Laboratory Animal (Certificate No. SCXK-2014-0005, China).
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of our hospital.
Drugs and reagents
DMEM/F12, fetal bovine serum (FBS), streptomycin/penicillin, EDTA and trypsin were purchased from Gibco (US). Anti-mouse CD29, CD34, CD45 and CD90/PE antibody was purchased from Biolegend (US). CM-Dil was purchased from Invitrogen (US). Amylase test kit was purchased from Meikang Biotech (China). Rat interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) ELISA kits were purchased from eBioscience (US). RhoA and ROCK I primary antibody were purchased from Abcam (US). Horseradish peroxidase labelled IgG secondary antibody and DAB chromogenic substrate were purchased from Maixin Biotech (China). Other reagents were provided in-house.
BMSCs culture, identification and labelling
Male SD juvenile rats (body weight 60-80 g) were sacrificed via cervical dislocation and immersed in 75% ethanol. Under sterile conditions, bilateral femoral bones were separated. BMSCs were isolated from bilateral femoral bone marrow tissues. Allogenic cells were cultured using whole bone marrow attached growth method. When cell confluence reached about 90% of attachment, all cells were inoculated into new flask at 1:2 and 1:3 ratios until P3 generation. Cells were then digested by trypsin and collected for counting and further use. Flow cytometry was performed to measure the expression of MSC surface markers including CD29, CD34, CD45 and CD90. Cells were then labelled by CM-Dil, and P3 generation cells with good growth status were collected, digested, washed once in PBS. Cell concentration was adjusted to 106/ml and mixed with 5 μl CM-Dil labelling buffer (2 mg/ml) for gentle mixture. After incubation at 37°C for 5 min, cells were then incubated at 4°C for 15 min followed by being centrifuged at 1000 rpm for 5 min. After discarding supernatants and twice washing in PBS, labelled cells were removed to culture flask for continuous incubation. Cell morphology was observed and imaged under an inverted fluorescent microscope.
SAP animal model preparation
Rats were fasted 12 h before experiments but with water ad libitum. SAP model was prepared following previous literature [7]. In brief, SD rats were anesthetized and located for pancreatic duct under microscope. A 0.5 cm puncture was made on bile-pancreas duct from extra-intestinal site proximal of the junction between bile-pancreas duct and duodenum, plus clapping of distal arterioles. A micro-injection pump was connected for retro-infusion of 5% sodium deoxy-taurocholate solution (flow rate = 0.04 ml/min, dosage = 0.1 ml/100 g). Pressure was kept for 10 min after injection. Successful model generation was confirmed with visible hematoma and edema on pancreatic tissues. In control group, pancreas gland was gently removed and the abdominal cavity was closed. After surgery, rats were fasted with water ad libitum. 2 ml/100 g saline was replenished at multiple sites on the back with 6 h time interval. Experimental animal protocol followed guidelines with laboratory animals.
Animal grouping
A total of 120 male adult SD rats were used for establishment of SAP model, and randomly assigned into BMSCs + SAP group, ROCK inhibitor Y-27632 + SAP group, untreated SAP group and control group (N = 30). Within each group, 10 rats were treated allocated at 6 h, 12 h and 24 h time points. After model preparation, a micropump was used to infuse following drugs via tail veins: 5 ml/kg BMSCs (about 106/ml) in BMSCs-SAP group, 5 ml/kg ROCK inhibitor Y-27632 (5 mg/kg) in Y-27632-SAP group. Control-SAP and control groups received 5 ml/kg saline. Blood and pancreatic tissue samples were collected at all time points for investigating biochemical indexes and inflammatory factors, for observing pathological structures of pancreas, and for assay of pancreatic RhoA or Rock-1 expression.
In vivo MSCs tracing
SAP and control rats received BMSCs with CM-Dil labelling via tail veins. Pancreas tissues were collected at 6 h, 12 h and 24 h after sacrifice, and were kept in liquid nitrogen for preparing 7 μm slices. Observation was performed under a fluorescent microscope for cell tracing assay.
Rat serum index assay
Blood samples were collected from cardiac apex, and were kept at 10 min under room temperature. After centrifugation at 4°C for 10 min under 3000 g, serum was obtained and kept at -70°C for further assays. Serum amylase was measured by Olympus AV2700 automatic biochemical analyzer. Serum TNF-α and IL-6 were measured by ELISA following manual instruction of ELISA kits. A450 values were measured on a microplate reader, and concentrations of those factors were calculated based on standard curves.
Pancreatic tissue pathology and morphology assays
Samples were fixed for 24 h in formalin, dehydrated and embedded in paraffin. 4 μm thickness slices were collected form cross-sections along the long axis of left ventricles for HE staining. Pancreatic tissue morphology was observed under a microscope.
Rat pancreatic RhoA and Rock-1 immunohistochemistry (IHC) assay
Tissues were fixed in 10% formalin and were embedded in paraffin. After de-wax, rehydration and sectioning, tissue slices were processed in 0.01 mol/L citric acid buffer (pH 6.0) for heat antigen-retrieval, and were immersed in 3% formaldehyde-H2O2 to quench endogenous peroxidase. 5% bovine serum albumin (BSA) was used for blocking under room temperature for 20 min. Primary antibody (1:200) was added for 1 h room temperature incubation, followed by 20 min room temperature incubation with secondary antibody. After Tween rinsing, streptavidin-biotin-peroxidase complex (SABC) was added for 20 min room temperature incubation. After PBS rinsing, DAB substrate was added for development, followed by hematoxylin counter-staining, dehydration, resin mounting and microscopic observation.
Western blot assay
Total tissue proteins were extracted from pancreas. 80 μg total protein samples were extracted from all groups, separated in 10% SDS-PAGE, transferred to PVDF membrane and blocked with 5% defatted milk powder. Polyclonal antibody against mouse RhoA and ROCK I (1:2000 dilution) was added for 4°C overnight incubation. Horseradish peroxidase labelled goat anti-rabbit IgG antibody (1:4000) was added for 2 h room temperature incubation, followed by addition of ECL. After exposure, Quantity One imaging analysis software was used to reveal absorbance (A) values of each band. Protein expression was presented as the ratio relative to endogenous actin.
Statistical methods
Measurement data were presented as mean ± standard deviation (SD). SPSS13.0 statistical software was used for analysis. Student t-test was performed for comparison between two independent groups. One-way analysis of variance (ANOVA) was used for multi-group comparison. A statistical significance was defined when P<0.05.
Results
Separation, culture and identification of MSC
On average, cells showed attached growth after 24~72 h, with tendency of colony growth. After several passages, cell component was gradually purified. Under inverted microscope, P3 generation cells showed fibroblast-like morphology with spiral-like distribution and membrane pavement in single morphology. Cells were labelled with fluorescent dye, and showed about 90% staining on membrane under fluorescent microscope. Flow cytometry analysis of the surface markers showed 96.70% CD29 positive rate, 99.80% CD90 positive rate, 1.90% DC34 positive rate and 0.80% CD45 positive rate, indicating MSCs (Figures 1 and 2).
Figure 1.
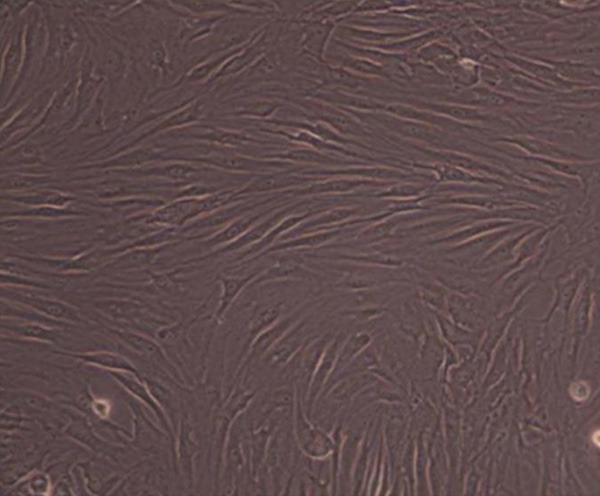
Attached BMSCs morphology 3 d after passage. BMSCs were cultured and collected on day 3 after passage for analysis of its morphology under a microscope.
Figure 2.
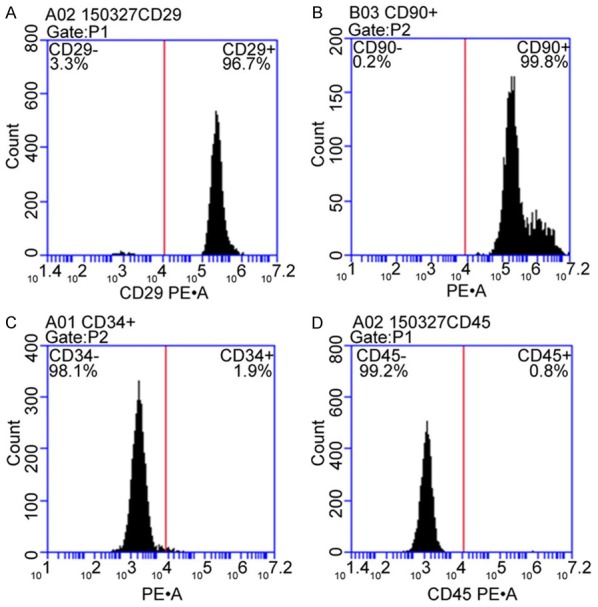
Surface antigen expression in BMSCs. Cultured BMSCs in P3 generation were collected for analysis of the expression of CD29 (A), CD90 (B), CD34 (C) and CD45 (D) by flow cytometry.
In vivo cell tracing
Control rat pancreatic tissues had no CM-Dil labelled BMSCs but were found in SAP injury pancreas. With time elapsed, BMSCs number was gradually increased (Figure 3).
Figure 3.
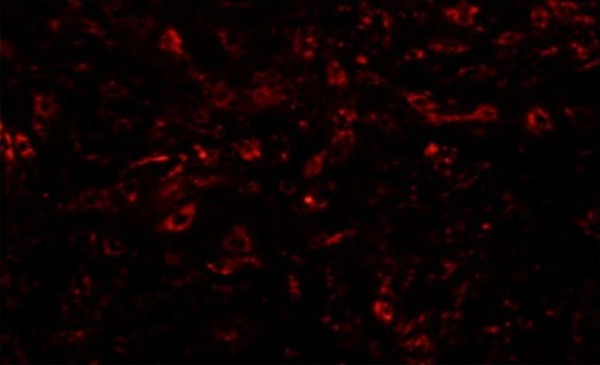
CM-Dil labelling for BMSCs tracing at 24 h. P3 generation cells were collected and mixed with 5 μl CM-Dil labelling buffer for gentle mixture followed by analysis of the cell morphology under an inverted fluorescent microscope.
Rat survival
At 6 h, 12 h and 24 h, control group rats all survived whilst SAP group rats had 1, 3 and 4 deaths, and Y-27632-SAP group showed 0, 3 and 5 deaths, plus 0, 3 and 4 deaths in BMSCs-SAP group. No significant difference existed in mortality rate among SAP, Y-27632-SA and BMSCs-SAP groups (P>0.05). With time elapsed, mortality rate was higher than control group (P<0.05).
Rat serum index assay
Compared to control group, SAP group showed significantly elevated serum amylase, ascites volume, TNF-α and IL-6 concentration (P<0.05) in a time dependent manner. However, compared to SAP group, BMSCs-SAP group had significantly lower serum amylase, ascites volume, TNF-α or IL-6 concentration (P<0.05). Compared to SAP group, Y-27632-YAP group only showed lower serum amylase and ascites volume at 6 h (P<0.05) but not at 12 h or 24 h (P>0.05), without significant differences of TNF-α or IL-6 concentration at all time points (P>0.05, Table 1).
Table 1.
Serum amylase, IL-6, TNF-α and ascites volume (mean ± sd, n = 10)
| Group | Index | Time point | ||
|---|---|---|---|---|
|
| ||||
| 6 h | 12 h | 24 h | ||
| BMSCs-SAP | Amylase (IU/L) | 4369.10±962.87* | 5391.20±557.40* | 7764.30±752.98* |
| Ascites volume (ml) | 5.95±1.99* | 10.63±2.36* | 17.50±4.52* | |
| IL-6 (pg/mL) | 18.77±1.08* | 27.03±4.23* | 26.17±3.84* | |
| TNF-α (pg/mL) | 105.53±7.95* | 144.36±12.80* | 175.26±13.13* | |
| Y-27632-SAP | Amylase (IU/L) | 4662.50±637.35▲ | 6939.21±953.00 | 9489.60±1661.24 |
| Ascites volume (ml) | 6.13±4.45▲ | 13.20±3.20 | 20.56±4.21 | |
| IL-6 (pg/mL) | 20.37±6.32 | 30.62±3.23 | 34.98±5.32 | |
| TNF-α (pg/mL) | 127.31±15.2 | 187.76±7.45 | 231.86±9.32 | |
| SAP | Amylase (IU/L) | 6772.70±3057.13# | 7716.30±4037.44# | 10376.3±3186.00# |
| Ascites volume (ml) | 9.96±2.41# | 14.96±4.41# | 22.70±7.10# | |
| IL-6 (pg/mL) | 24.17±4.61# | 33.11±3.27# | 38.21±8.63# | |
| TNF-α (pg/mL) | 138.02±14.87# | 190.22±8.57# | 254.72±30.40# | |
| Control | Amylase (IU/L) | 1078.7±190.81 | 955.30±112.50 | 858.50±99.33 |
| Ascites volume (ml) | 0.59±0.26 | 0.44±0.23 | 0.73±0.28 | |
| IL-6 (pg/mL) | 15.47±3.32 | 16.75±4.25 | 17.98±2.32 | |
| TNF-α (pg/mL) | 73.28±6.73 | 75.63±8.66 | 86.77±7.54 | |
P<0.05 comparing between BMSCs-SAP and SAP group at the same time point;
P<0.05 comparing between Y-27632-SAP and SAP group;
P<0.05 comparing between SAP and control group.
Pancreatic morphology change
Pancreatic pathology damage was scored based on previous literatures [7,8]. Control group showed no significant edema in pancreas. BMSCs-SAP group showed lower pancreatic pathology damage score than SAP group (P<0.05). Y-27632-SAP group showed alleviated pancreatic pathology damage at 6 h compared to control-SAP group (P<0.05), but with similar damage condition at 12 h or 24 h (P>0.05, Figure 4; Table 2).
Figure 4.
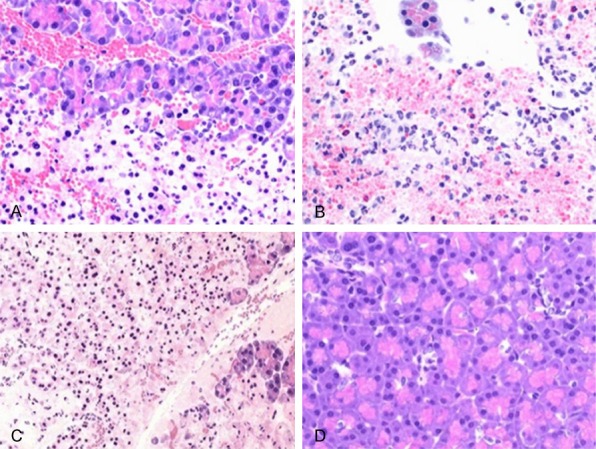
Pathology slices of pancreatic tissues from different groups at 24 h (×200, bright-field microscope). Pancreatic tissue were collected, fixed, dehydrated and embedded in paraffin. 4 μm thickness slices were collected form cross-sections along the long axis of left ventricles for HE staining analysis of the pathology changes of pancreatic tissue. A. 24 h of BMSCs-SAP group; B. 24 h of Y-27362-SAP group; C. 24 h of SAP group; D. Control group.
Table 2.
Pathological grade of pancreatic tissues of all four groups of mice (mean ± sd)
| Group | 6 h | 12 h | 24 h |
|---|---|---|---|
| BMSCs + SAP | 6.51±0.54# | 9.38±0.35# | 12.42±0.69# |
| Y-27632 + SAP | 5.8±0.2★ | 12.92±0.50 | 16.36±0.76 |
| SAP | 8.56±0.63* | 13.40±0.79* | 16.88±0.87* |
| Control | 0.13±0.10 | 0.15±0.11 | 0.16±0.14 |
P<0.05 comparing between SAP and control group;
P<0.05 comparing between BMSCs + SAP group and SAP group;
P<0.05 comparing between Y-27632 + SAP group and SAP group.
Rat pancreatic RhoA and ROCK I IHC assay
IHC staining showed normal expression of RhoA and Rock-1 in pancreatic glandular vesicle and endothelial cell cytoplasm. At 24 h after SAP model preparation, expression of RhoA and Rock-1 was significantly potentiated in pancreatic glandular vesicle and endothelial cells. MSCs intervention showed weakened expressional strength by pancreatic staining. However, at 24 h after Y-27632 intervention, RhoA and ROCK I expression showed similar profile as untreated SAP group (Figures 5 and 6).
Figure 5.
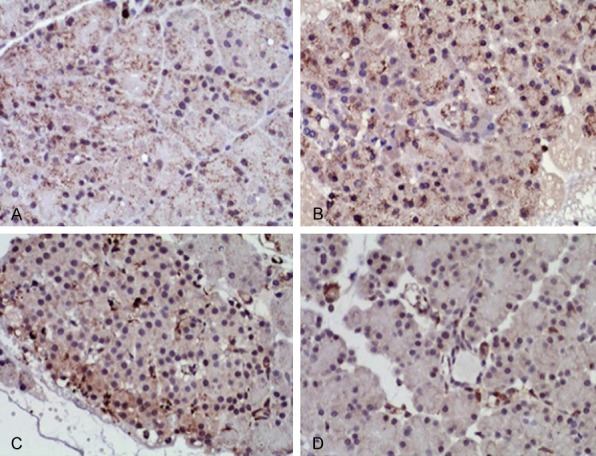
RhoA IHC staining in pancreatic tissues at 24 h (DAB staining, ×200). Pancreatic tissues were collected, fixed and embedded in paraffin followed by antigen-retrieval and blocking followed by measuring the expression of RhoA by IHC staining. A. 24 h of BMSCs-SAP group; B. 24 h of Y-27362-SAP group; C. 24 h of SAP group; D. 24 h of control group.
Figure 6.
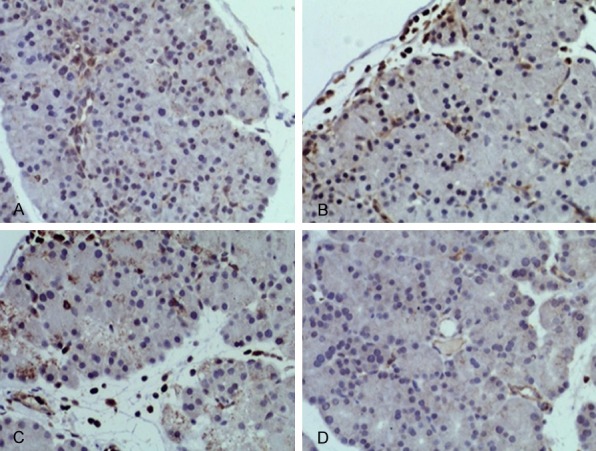
ROCK I IHC staining in pancreatic tissues at 24 h (DAB staining, ×200). Pancreatic tissues were collected, fixed and embedded in paraffin followed by antigen-retrieval and blocking followed by measuring the expression of ROCK I by IHC staining. A. 24 h of BMSCs-SAP group; B. 24 h of Y-27362-SAP group; C. 24 h of SAP group; D. 24 h of control group.
Western blot for pancreatic RhoA and ROCK I protein expression
At different time points, control group showed no significant difference of RhoA or Rock-1 protein expression. SAP group had significantly higher RhoA and Rock-1 expression than control group at the same time point (P<0.05) in a time-dependent manner. BMSCs-SAP group showed decreased RhoA or Rock-1 expression compared to SAP group at the same time point (P<0.05). Y-27632-SAP group showed decreased RhoA or Rock-1 expression at 6 h compared to SAP group (P<0.05) but not at 12 h or 24 h time points (P>0.05, Figure 7; Table 3).
Figure 7.

RhoA and Rock-I protein expression in pancreas across different groups. Total proteins were isolated from different groups for measuring the expression of RhoA and Rock-1 by western blot. From left to right, 6 h, 12 h and 24 h of BMSCs-SAP group; 6 h, 12 h and 24 h of Y-27632-SAP group; 6 h, 12 h and 24 h of SAP group; and control group.
Table 3.
RhoA and Rock-1 protein expression level in pancreatic tissues (mean ± sd, n = 10)
| Index | Group | Time point | ||
|---|---|---|---|---|
|
| ||||
| 6 h | 12 h | 24 h | ||
| RhoA/β-actin | BMSCs + SAP | 0.19±0.01★ | 0.37±0.04★ | 0.62±0.08★ |
| Y-27632 + SAP | 0.18±0.03# | 0.41±0.5 | 0.70±0.06 | |
| SAP | 0.30±0.06▲ | 0.50±0.06▲ | 0.80±0.07▲ | |
| Control | 0.14±0.03 | 0.14±0.04 | 0.15±0.03 | |
| Rock-1/β-actin | BMSCs + SAP | 0.19±0.02★ | 0.26±0.03★ | 0.44±0.04★ |
| Y-27632 + SAP | 0.16±0.03# | 0.26±0.11 | 0.48±0.05 | |
| SAP | 0.27±0.03▲ | 0.35±0.04▲ | 0.57±0.06▲ | |
| Control | 0.16±0.04 | 0.18±0.05 | 0.17±0.06 | |
P<0.05 comparing between BMSCs-SAP group and control-SAP group at the same time point (6 h, 12 h and 24 h).
P<0.05 comparing between Y-27632-SAP group and control-SAP group;
P<0.05 comparing between SAP and control group.
Discussion
Rho is a member of Ras super-family, with RhoA, RhoB and RhoC isomers, in which RhoA is the major isomer with GTPase activity. The most potent downstream effector of Rho is Rho kinase (ROCK) [9] consisting of Rock-1 and Rock-2 isomers. Rock-1 is mainly expressed in skeletal muscle, cardiomyocyte, pancreas and kidney tissues, whilst Rock-2 is mainly expressed in nervous system. In various diseases, RhoA/ROCK signal pathway can be activated by inflammatory cytokines and angiotensin to induce various biological stress response to participate in body inflammatory response, cell apoptosis and reconstruction of endothelial cell skeleton [5,6]. A recent study showed that RhoA/ROCK was a major factor governing vascular endothelial permeability as demonstrated by that activation of Rho/ROCK signal pathway led to elevated permeability of pulmonary microvessels and pulmonary edema [10]. Leakage of pulmonary vascular endothelium in SAP rats is also related with RhoA/ROCK signal pathway activation [11]. This study utilized retrograde injection of 5% sodium taurocholate into biliopancreatic duct to establish SAP model. Through comparing between untreated SAP group and control group, we found significantly elevated serum amylase, ascites volume, TNF-α and IL-6 levels in untreated SAP group, which also had higher expression of RhoA and ROCK I expression in pancreatic tissues, with aggravated pathology damage, suggesting the involvement of RhoA/ROCK signal pathway in SAP pathogenesis, probably via inflammatory factor to activate RhoA/ROCK signal pathway for organ damage.
BMSCs is a group of mesoderm derived pluripotent stem cells that can be self-renewal and induced to be differentiated into specific tissue cells, with advantages including unique immune modulation and low antigenicity [12]. In this study, cultured P3 generation cells showed high levels of CD29 and CD90 plus lower CD34 and CD45 levels, all of which fitted into the characteristics of BMSCs. Recently various animal and clinical studies have demonstrated that injected BMSCs could migrate towards injury tissues [13,14] to repair tissues and inhibit inflammatory response [15,16]. Previous studies showed that BMSCs could ameliorate SAP disease via suppressing over-response of inflammation and repairing damaged pancreatic tissues [17-20]. Chen et al showed that BMSCs could reduce blood creatine, urea nitrogen and amylase concentration, and alleviate SPA-induced kidney damage, indicating important roles of BMSCs in managing inflammatory disease [21]. However, whether BMSCs can improve SAP disease condition via mediating RhoA/ROCK signal pathway remains poorly understood. In the present study, we demonstrated that BMSCs could migrate and implant in SAP-injury kidneys. Further study found significantly lower serum amylase, ascites volume, IL-6 and TNF-α levels at 6 h, 12 h and 24 h time points in BMSCs treated SAP animals compared to SAP group, plus alleviated pancreas damage, and lower RhoA or Rock-1 protein expression. These results suggested that BMSCs could relieve pancreas damage possibly through decreasing inflammatory factor release, and inhibiting RhoA/ROCK signal pathway expression. ROCK inhibitor Y-27632 treated-SAP rats showed significantly lower serum amylase or ascites volume at 6 h compared to untreated SAP rats, plus lower expression of pancreatic RhoA and Rock-1, and alleviated pancreas damage. At 12 h and 24 h time points, RhoA and Rock-1 expression showed no significant difference with untreated SAP group. These two groups showed no significant differences of inflammatory factors including IL-6 and TNF-α at all three time points, indicating that Y-27632 might have no inhibitory effects on the release of inflammatory factors, but directly inhibit RhoA/ROCK signal pathway expression to alleviate pancreatic damage. Probably due to half-life, Y-27632 can only exert inhibitory role on RhoA/ROCK signal pathway within short time span, whilst BMSCs have continuous self-renewal after migrating towards injury organs for mediating RhoA/ROCK signal pathway.
Conclusion
BMSCs can alleviate pancreas injury via migrating towards injury site for implantation, leading to continuous inhibition of the release of inflammatory factor as well as inhibition of activation of RhoA/ROCK signal pathway for alleviating pancreas damage and improving SAP disease, thus providing evidences for novel treatment of SAP.
Disclosure of conflict of interest
None.
References
- 1.Bogdan J, Elsaftawy A, Kaczmarzyk J, Jablecki J. Epidemiological characteristic of acute pancreatitis in Trzebnica district. Pol Przegl Chir. 2012;84:70–75. doi: 10.2478/v10035-012-0011-6. [DOI] [PubMed] [Google Scholar]
- 2.Brignier AC, Gewirtz AM. Embryonic and adult stem cell therapy. J Allergy Clin Immunol. 2010;125(Suppl 2):S336–344. doi: 10.1016/j.jaci.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Hyun I. The bioethics of stem cell research and therapy. J Clin Invest. 2010;120:71–75. doi: 10.1172/JCI40435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerlach H. Risk management in patients with severe acute pancreatitis. Crit Care. 2004;8:430–432. doi: 10.1186/cc3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes KP, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases: what is the link? Cell Mol Life Sci. 2010;67:3823–3836. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grewal HP, Mohey el Din A, Gaber L, Kotb M, Gaber AO. Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-alpha polyclonal antibody. Am J Surg. 1994;167:214–218. doi: 10.1016/0002-9610(94)90076-0. discussion 218-219. [DOI] [PubMed] [Google Scholar]
- 9.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carles M, Lafargue M, Goolaerts A, Roux J, Song Y, Howard M, Weston D, Swindle JT, Hedgpeth J, Burel-Vandenbos F, Pittet JF. Critical role of the small GTPase RhoA in the development of pulmonary edema induced by Pseudomonas aeruginosa in mice. Anesthesiology. 2010;113:1134–1143. doi: 10.1097/ALN.0b013e3181f4171b. [DOI] [PubMed] [Google Scholar]
- 11.Yu QH, Guo JF, Chen Y, Guo XR, Du YQ, Li ZS. Captopril pretreatment protects the lung against severe acute pancreatitis induced injury via inhibiting angiotensin II production and suppressing Rho/ROCK pathway. Kaohsiung J Med Sci. 2016;32:439–445. doi: 10.1016/j.kjms.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Sensebe L, Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87(Suppl):S49–53. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- 13.Lee HK, Finniss S, Cazacu S, Bucris E, Ziv-Av A, Xiang C, Bobbitt K, Rempel SA, Hasselbach L, Mikkelsen T, Slavin S, Brodie C. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget. 2013;4:346–361. doi: 10.18632/oncotarget.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vertelov G, Kharazi L, Muralidhar MG, Sanati G, Tankovich T, Kharazi A. High targeted migration of human mesenchymal stem cells grown in hypoxia is associated with enhanced activation of RhoA. Stem Cell Res Ther. 2013;4:5. doi: 10.1186/scrt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SH, Jang AS, Kim YE, Cha JY, Kim TH, Jung S, Park SK, Lee YK, Won JH, Kim YH, Park CS. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res. 2010;11:16. doi: 10.1186/1465-9921-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semedo P, Palasio CG, Oliveira CD, Feitoza CQ, Goncalves GM, Cenedeze MA, Wang PM, Teixeira VP, Reis MA, Pacheco-Silva A, Camara NO. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol. 2009;9:677–682. doi: 10.1016/j.intimp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011;140:998–1008. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q, Wang F, Hou Y, Chen S, Wang B, Lu F, Huang H, Chen Y. The effect of allogenetic bone marrow-derived mesenchymal stem cell transplantation on lung aquaporin-1 and -5 in a rat model of severe acute pancreatitis. Hepatogastroenterology. 2012;59:965–976. doi: 10.5754/hge12094. [DOI] [PubMed] [Google Scholar]
- 19.Jung KH, Yi T, Son MK, Song SU, Hong SS. Therapeutic effect of human clonal bone marrow-derived mesenchymal stem cells in severe acute pancreatitis. Arch Pharm Res. 2015;38:742–751. doi: 10.1007/s12272-014-0465-7. [DOI] [PubMed] [Google Scholar]
- 20.Hua J, He ZG, Qian DH, Lin SP, Gong J, Meng HB, Yang TS, Sun W, Xu B, Zhou B, Song ZS. Angiopoietin-1 gene-modified human mesenchymal stem cells promote angiogenesis and reduce acute pancreatitis in rats. Int J Clin Exp Pathol. 2014;7:3580–3595. [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z, Lu F, Fang H, Huang H. Effect of mesenchymal stem cells on renal injury in rats with severe acute pancreatitis. Exp Biol Med (Maywood) 2013;238:687–695. doi: 10.1177/1535370213490629. [DOI] [PubMed] [Google Scholar]


