Abstract
Our aim was to study the osteogenic effect of cnidium lactone for bone marrow mesenchymal stem cells (BMSCs) and its potential mechanism. BMSCs were isolated and identified by flow cytometry and multidirectional differentiation capacity. Cell Counting Kit-8 (CCK-8) was used to identify the optimal concentration of cnidium lactone. Alkaline Phosphatase (ALP) and Alizarin Red S (ARS) was performed to identify whether cnidium lactone has effect on osteogenesis at early and late phase respectively. Moreover, we used estrogen receptor antagonist, ICI182780, to identify the receptor of cnidium lactone. The expression of Runt-related transcription factor 2 (RUNX2), Osterix (OSX), osteopontin (OPN), estrogen receptor (ER), Smad4, p-Smad1, Smad1 and bone morphogenetic protein 2 (BMP-2) protein were measured by PCR and western blot. Cnidium lactone (2 μM) demonstrated increased osteogenesis after osteogenic inducible medium (OIM) induction, as evidenced by more ALP activity and mineralization. When blocked with ICI182780, osteogenesis capacity was decreased. Moreover, the polymerase chain reaction (PCR) and western blotting results indicated that cnidium lactone enhanced ER, BMP2, Smad1, Smad4, RUNX2, OSX, and OPN expression and Smad1 phosphorylation. Cnidium lactone can effectively stimulates osteogenic differentiation of BMSCs via BMP-2/Smad-Signaling cascades mediated by ER.
Keywords: Cnidium lactone, bone marrow mesenchymal stem cells, smad signaling, estrogen receptor
Introduction
Osteoporosis (OP) is a harmful disease with the high incidence that resulting in elevated fracture risk [1,2]. OP is mainly due to excessive bone resorption and decreased bone formation. Bone mesenchymal stem cells (BMSCs) are a class of cells with multipotency and can fully differentiate towards various cell types such as osteoblasts, chondrocytes and adipocytes [3,4]. Due to multi-directional differentiation properties, BMSCs are a potential cell source for bone regeneration. Recent studies shown that transplant with BMSCs and promotion of osteogenic differentiation is a hot topic.
Cnidium lactone is a fundamental component with pharmacological activity extracted from Chinese Medicine cnidium monnieri [5]. Cnidium lactone has been reported to inhibit osteoclastogenesis to prevent ovariectomy-induced bone loss in mice [6]. In vitro experiment found that cnidium lactone inhibits osteoclasts formation and bone resorption by regulating NF-κB signaling and NFATc1 activations stimulated by receptor activator of nuclear factor-κB ligand (RANKL) [7]. Estrogen combined with estrogen receptor (ER) and promote bone formation. BMP-2, a member of the transforming growth factor-beta (TGF-β) superfamily, plays a vital role in inducing osteogenic differentiation of BMSCs [8]. The mature BMP-2 protein is secreted from cells, and can then act in a paracrine and/or autocrine manner. When BMP-2 binds to corresponding receptors, then downstream genes were up-regulated and finally stimulate bone formation [9,10]. Hsieh et al. [11] transfection of BMP-2 to BMSCs could induce osteoblastic differentiation in a rat calvarial defect model. BMP-2/Smad signaling pathway is crucial for bone remodeling. Li et al. [12] found that Macrolactin F promotes osteoblastogenesis through a BMP-2/smad/Akt/Runx2 signaling pathway. Dong et al. [13] revealed that silicon stimulates collagen type 1 and osteocalcin synthesis in Human Osteoblast-Like Cells through the BMP-2/Smad/RUNX2 signaling pathway.
All in all, these studies revealed that cnidium lactone is a potential new drug for treating osteoporosis. However, whether cnidium lactone could stimulates osteogenic differentiation of BMSCs through ER/BMP-2/Smad signaling cascades was unknown.
Materials and methods
BMSC and cnidium lactone preparation
Rat BMSCs was isolated from Sprague Dawley (SD) rats according to previously reports [14]. Rats femurs and tibias were harvested from 21-days-old rats, and then the bone marrow stromal cells were flushed out from the bone marrow cavity with a syringe and culture in complete medium with 20% fetal bovine serum (FBS) at 37°C and 5% CO2. After 24 h period culture, unattached cells were discarded. Medium was replaced every 3 days. Passages 3 BMSCs were used for experiment. We used flow-cytometric analysis to characterize their immunophenotype, and mesenchymal and non-mesenchymal stem cell-associated surface markers were measured.
Antibodies combined with phcoerythrin (PE), fluorescein isothiocyanate (FITC) or APC against CD44, CD90, CD29, CD11b/c and CD 45 (TBD Science, Tianjin, China) were added into each tube loaded with nearly 5×105 BMMSCs/200 μl of PBS, and then the BMMSCs were incubated for 1 hour at 4°C for further analyses. Flow cytometry was conducted by a FACSCanto™ II Flow Cytometer (BD Biosciences, San Jose, CA, USA).
To investigate the osteogenic differentiation potential, BMSCs were exposed to the osteogenic induced medium (OIM), which is complete medium supplemented with 10 nM dexamethasone (Solarbio, Beijing, China), 10 mM β-glycerophosphate (Solarbio, Beijing, China), and 0.05 mM ascorbic acid-2-phosphate (Solarbio, Beijing, China). To investigate the adipogenic differentiation potential, rBMSCs were cultured in adipogenic differentiation medium, which is CM supplemented with 100 nM dexamethasone, 10 ng/ml insulin, and 0.5 μM isobutyl-methylxanthine (IBMX) (Solarbio, Beijing, China). To investigate chondrogenic differentiation, We used DMEM supplemented with 10% FBS, penicillin (100 U/mL)/streptomycin (100 μg/mL), 100 μg/mL sodium pyruvate, 300 μg/mL L-glutamine, 40 μg/mL L-proline, 50 μg/mL L-ascorbic acid-2-phosphate, 1.5 mg/mL bovine serum albumin (BSA; Roche, San Francisco, CA, USA), 1× insulin-transferrin-selenium, 100 nM dexamethasone (all from Sigma, St Louis, MO, USA) and 10 ng/mL TGF-β3 to induce chondrogenic differentiation for 28 days.
Cnidium lactone was purchased from Aladdin Company (Shanghai, China) and was dissolved in DMSO. We used medium to diluent experiment concentration. And final concentration in each experiment group was less than 0.1%.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8) (Solarbio, Beijing, China) was used to determine the cnidium lactone on cell viability and proliferation assays of BMSCs. BMSCs were seeded at 10000 cells in 96 well plates in triplicate and cultured in DMEM. BMSCs were cultured for 24 hours, then each well plates were treated with cnidium lactone at the different concentrations (0, 0.1, 1, 2, 4 and 8-μM) at 1, 3, 5 and 7 days. Each time point ends, 10 μL CCK-8 was added to every well, and plates were incubated at 37°C for 3 h. The absorbance was detected at 450 nm in a Spectra Max 190 Enzyme standard instrument (Molecular Devices).
Alkaline phosphatase (ALP) activity assay
After gelatine wrap the 6-well plates, BMSCs were seeded in 6-well plates and waited BMSCs reached 70% confluence. BMSCs were then divided into the following four groups: control group, OIM group, OIM+ cnidium lactone group (2 μM), E2 group. We further used estrogen receptor inhibitor ICI182780 to explore whether cnidium lactone could bind to the estrogen receptor to stimulate osteogenic differentiation. The concentration of ICI182780 was determined from previous report and identified as 1 μM [15]. ALP activity was quantified in cell lysates using an ALP assay kit (Solarbio, Beijing, China), according to the manufacturer’s instructions. ALP staining was performed with BCIP/NBT Alkaline Phosphatase Colour Development Kit (Beyotime) according to manufacturer’s instructions.
Alizarin red S (ARS) staining
After 21 days of induction, calcium mineral deposits were measured by ARS. In briefly, BMSCs were washed with PBS for 3 times and then fixed in 4% paraformaldehyde for 10 minutes. Then extracellular matrix mineralized nodules were measured by ARS (0.2%, pH 4.2, Solarbio) for 30 min at room temperature. Then, wells were washed by water for 3 times and observed by microscope. The absorbance values were measured at 560 nm.
Quantitative real-time PCR (qPCR)
The total RNA was isolated using Trizol reagent (Invitrogen, USA) and reverse-transcribed to cDNA templates using the ReverTra Ace qPCR RT Kit (Toyobo Co. Ltd., Osaka, Japan), and amplified by quantitative the RT-PCR using SYBR® Green Real-Time PCR Master Mix (Toyobo Co. Ltd., Osaka, Japan) according to the manufacturer’s guidelines. All reactions were run in triplicate, and the mRNA levels were normalized to GADPH. Sequences of the primers of the target and housekeeping genes used for RT-PCR can be seen in Table 1.
Table 1.
Sequences of the primers of the target and housekeeping genes used for RT-PCR
| Gene | Sense primer | Antisense primer |
|---|---|---|
| GAPDH | 5-GGCCTTCCGTGTTCCTACC-3 | 5-TGCCTGCTTCACCACCTTC-3 |
| RUNX2 | 5-CCAAGTAGCCAGGTTCAACG-3 | 5-GGTGAAACTCTTGCCTCGTC-3 |
| OSX | 5-CTTTCGTCTGCAACTGGCTT-3 | 5-TAAAGCGCTTGGAACAGAGC-3 |
| OPN | 5-CAGCCATGAGTCAAGTCAGC-3 | 5-TGTGGCTGTGAAACTTGTGG-3 |
Western blot analysis
Total protein was extracted by RIPA combined with PMSF. The concentrations of proteins determined according to the method of BCA Protein Quantitation Kit (Thermo Scientific, USA). Each group of samples was loaded with 40 μg, separated by SDS-PAGE and transferred to a PVDF membrane (Millipore, USA). The membrane was blocked with 5% skim milk for 1 h, then primary antibody anti-RUNX2, OSX, OPN, ERα, Smad4, p-Smad1, Smad1 and BMP-2 antibody (1:1000, sc390518, Santa Cruz Biotechnology, USA) were added and incubated at 4°C overnight, TBST (TBS, 1 ml/L Tween-20) washed the membrane 3 times, 5 min/time; added HRP-labeled secondary goat anti-mouse IgG (1:5000, sc2005, Santa Cruz Biotechnology, USA), incubated for 2 h at room temperature. The membrane was washed with TBST three times, 10 min/time; ECL chemiluminescence was developed in darkroom. The protein expression levels were normalized to β-actin and grayscale scanning and quantitation were performed by Image J 1.46 (National Institutes of Health, USA) software.
Statistical analysis
The obtained data are expressed as means ± standard deviation (SD). Means of multiple groups were compared by one-way analysis of variance (ANOVA) followed by a Bonferroni multiple comparison test for pairwise comparison. Statistical analysis was conducted using SPSS 20.0 (IBM Corp., Armonk, NY, USA). P values less than 0.05 were considered significant.
Results
Identification of BMSCs
Flow cytometry analysis results shown that BMSCs were positive for CD44, CD90, CD29 but negative for CD11b/c and CD45. BMSCs were capable of differentiation into adipocytes and chondrocytes, and were calcified under the proper conditions (Figure 1). In adipogenic medium, colonies stained well with oil red O. In calcification medium, colonies produced abundant calcium. In chondrogenic medium, much glycosaminoglycan was stained diffusely with toluidine blue (Figure 1).
Figure 1.
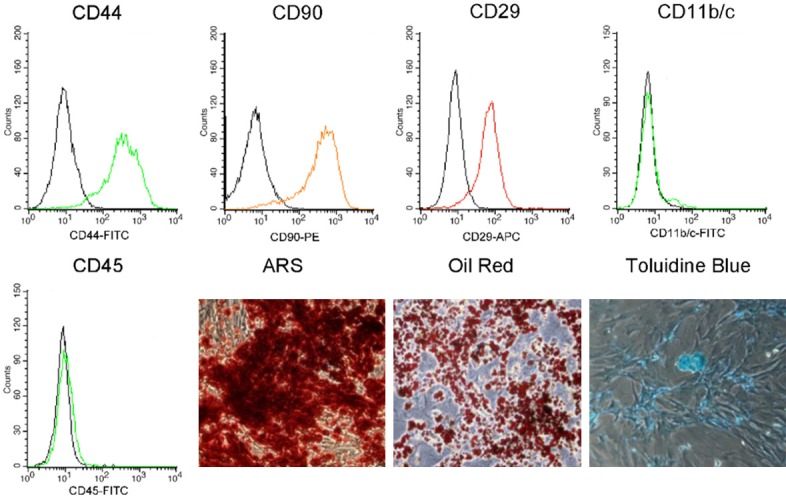
Flow cytometry analysis of BMSCs surface antigens CD11b, CD29, CD44, CD45, CD90 and MHC II Expression. Multi-directional differentiation into osteoblast, adipocytes and chondrocytes that identified by ARS, Oil-Red and Toluidine Blue respectively.
Cnidium lactone enhanced cell proliferation
The effect of cnidium lactone on BMSCs viability was performed by CCK-8 assay. The results revealed that, as time extended, the number of BMSCs in all different treating-groups increased gradually. However, following 1, 3 and 5 days treatment, the average number of viable cells in cnidium lactone group showed increase than control group (Figure 2). Among the five doses of cnidium lactone, 2 μM cnidium lactone possess the most obvious viable cells than other doses. At 5 days, only cnidium lactone with 2 μM has statistically significant than control group (P<0.05). There were no statistically significant different between the number of BMSCs in cnidium lactone and control group following 7 days treatment.
Figure 2.
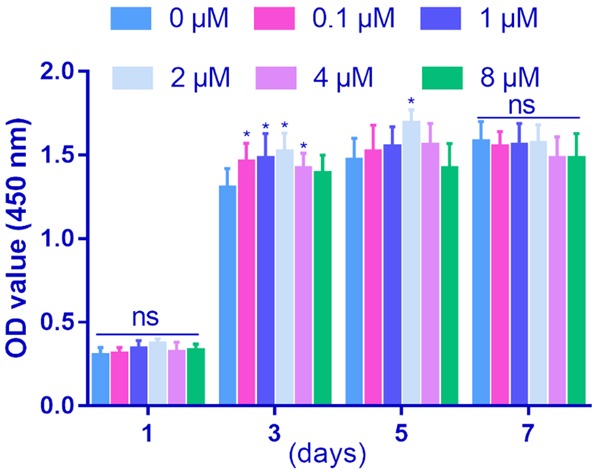
Effect of cnidium lactone on cell proliferation in BMSCs from day 1, 3, 5 and 7. *P<0.05, compared with control group.
Cnidium lactone enhanced osteoblastic differentiation and extracellular matrix production and mineralization
Compared with the control group, BMSCs treatment with cnidium lactone (2 μM) demonstrated increased osteogenesis after OIM induction, as evidenced by more ALP activity and mineralization. E2 group has associated with no superior than cnidium lactone in terms of the ALP activity and mineralization (Figure 3).
Figure 3.
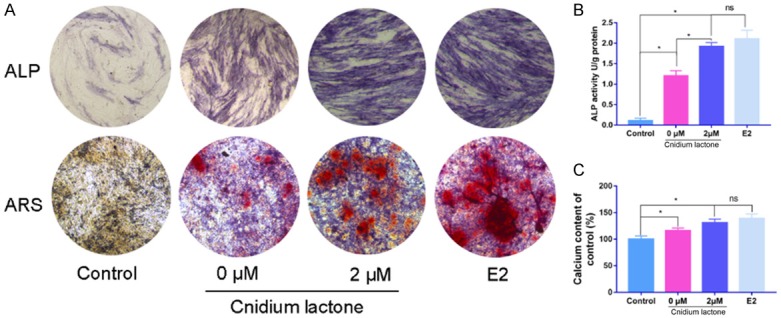
Effects of cnidium lactone on ALP staining and matrix mineralization. (A) General observation of ALP staining and ARS. Quantitative analysis of ALP activity (B) and calcium content (C) in each group. *P<0.05, vs control group.
Cnidium lactone upregulates osteoblast-specific marker gene expression
As shown in Figure 4, compared with control group, administration with cnidium lactone was associated with an increase of the expression of RUNX2, OSX, and OPN with statistically significant (P<0.05).
Figure 4.
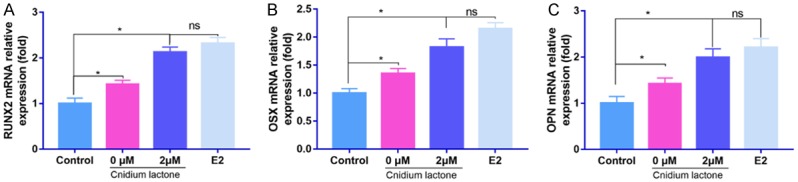
Effects of cnidium lactone on RUNX2, OSX and OPN mRNA expression in each group.
Treatment with cnidium lactone combined with OIM upregulated RUNX2, OSX, and OPN expression compared with treatment with the cnidium lactone (0 μM) combined with OIM group. There was no statistically significant difference between cnidium lactone (2 μM) and E2 groups in terms of the RUNX2, OSX, and OPN expression (P>0.05).
Cnidium lactone regulates osteoblast-specific gene expression via the ER
The addition of 1 μM ICI182780 in cnidium lactone (2 μM) inhibited ALP activity and mineralization than cnidium lactone (2 μM) group. While, cnidium lactone can rescue the inhibition of osteogenesis effect caused by ICI182780 (Figure 5).
Figure 5.
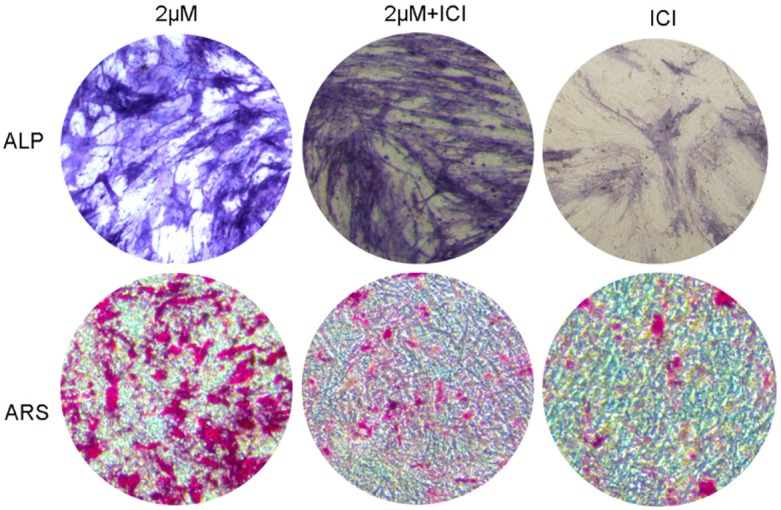
ALP activity and matrix mineralization was decreased by ICI182780.
Cnidium lactone regulates osteoblast marker protein expression
As shown in Figure 6, ERα, OPN, OSX and RUNX2 expression levels in the 2 μM cnidium lactone group were upregulated compared with control group. Compared with control group, cnidium lactone group was associated with a reduction of the ERα, OPN, OSX and RUNX2 protein expression. In ICI group, when added 2 μM cnidium lactone, the ERα, OPN, OSX and RUNX2 protein expression could recover in some extent.
Figure 6.
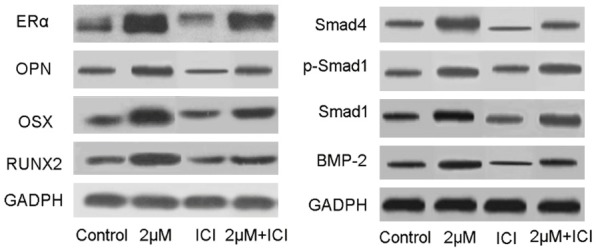
Western-blot analysis of RUNX2, OSX, OPN, ERα, Smad4, p-Smad1, Smad1 and BMP-2.
Meanwhile, BMP-2, Smad1, p-Smad and Smad 4 expression levels in the group treated with 2 μM cnidium lactone was higher than those in the control group. Compared with control group, ICI group was associated with a reduction of the Smad 4, p-Smad 1, Smad 1 and BMP-2 protein expression. In ICI group, when added 2 μM cnidium lactone, the Smad 4, p-Smad 1, Smad 1 and BMP-2 protein expression could recover in some extent.
Discussion
BMSCs own multipotential in differentiation, and BMSCs has a prospective alternative autologous cell-based therapy for bone defect. BMSCs could differentiated into three types of cells including osteoblast, adipocyte and chondrocyte. In this study, we used surface marker and multi-directional differentiation capacity to identify the BMSCs. Results shown that BMSCs were positive for CD44, CD90 and CD29, while were negative for CD11b/c and CD45. ARS results shown that a lot of calcium nodule deposited on the extracellular. Oil red shown that after induced for 10 days, many fat droplet could be seen. Alcian blue staining revealed that many chondrocyte-like cells could be seen.
First, we used CCK-8 to identify the optimal dose of cnidium lactone for next treatment. Results found that 2 μM cnidium lactone was the most obvious dose that could stimulate BMSCs proliferation. Increasing in ALP activity and calcium nodule formation in cnidium lactone-treated BMSCs suggested that it promoted osteogenic differentiation, which was consistent with previous findings. Zhang et al. [16] revealed that cnidium lactone enhances osteogenesis in osteoblasts by elevating transcription factor Osterix (OSX) via cAMP/CREB signaling. Jia et al. [17] concluded that cnidium lactone may represent new pharmacological tools for the treatment of osteoporosis. Ming et al. [18] revealed that cnidium lactone could stimulate the osteoblastic differentiation of rat calvarial osteoblast cultures by the BMP-2/p38MAPK/Runx-2/osterix pathway.
BMP-2 pathway has been well-defined as a vital positive modulator of bone homeostasis [19,20]. Activated type I BMP receptors propagate BMP signals by phosphorylating Smad1/5/8 [21]. We found that cnidium lactone could stimulate the BMP-2 expression and subsequent Smad signaling. When blocked the ER by ICI, the expression of BMP-2 and Smad proteins were down-regulated. ALP and ARS staining has similar results. When blocked with ICI, ALP activity and calcium nodule formation were downregulated with statistically significant. When added ICI in cnidium lactone, the ALP activity and calcium nodule formation could rescue to some degree. This result indicated that cnidium lactone partially combined with ERα and activate BMP-2/smad signaling pathway to induce osteogenic differentiation of BMSCs.
OPN, OSX and RUNX2 protein expression level were used for assess the osteogenic capacity. Compared with control group, cnidium lactone could significantly enhance the relative expression of OPN, OSX and RUNX2. When added ICI, the relative expression of OPN, OSX and RUNX2 was decreased than control group and cnidium lactone group. Thus, we speculated that cnidium lactone could bind with ER and subsequent stimulate BMP-2/Smad signaling pathway.
Previous studies BMP have a close relationship between estrogen receptor [22,23]. Lee et al. [24] reported that dehydrodiconiferyl alcohol promotes BMP-2-induced osteoblastogenesis through its agonistic effects on estrogen receptor. When estrogen receptor activated, BMP-2 and subsequent Smad signaling was upregulated. Finally, BMSCs were differentiated and mineralization. Thus, we concluded cnidium lactone combined with ERα and other receptors, then activated BMP-2/Smad signaling pathway.
In conclusion, this research revealed that cnidium lactone binds to the receptor of ER and stimulated BMP/Smad signaling, then osteogenic-related genes were up-regulated and ultimately stimulated BMSCs differentiation and mineralization.
Disclosure of conflict of interest
None.
References
- 1.Cauley JA. Osteoporosis: fracture epidemiology update 2016. Curr Opin Rheumatol. 2017;29:150–156. doi: 10.1097/BOR.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 2.Fuggle NR, Curtis EM, Ward KA, Harvey NC, Dennison EM, Cooper C. Fracture prediction, imaging and screening in osteoporosis. Nat Rev Endocrinol. 2019;15:535–547. doi: 10.1038/s41574-019-0220-8. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Wang CL, Xiao F, Wang CD, Zhu JF, Shen C, Zuo B, Wang H, Li , Wang XY, Feng WJ, Li ZK, Hu GL, Zhang X, Chen XD. Gremlin2 suppression increases the BMP-2-induced osteogenesis of human bone marrow-derived mesenchymal stem cells via the BMP-2/Smad/Runx2 signaling pathway. J Cell Biochem. 2017;118:286–297. doi: 10.1002/jcb.25635. [DOI] [PubMed] [Google Scholar]
- 5.You L, Feng S, An R, Wang X. Osthole: a promising lead compound for drug discovery from a traditional Chinese medicine (TCM) Nat Prod Commun. 2009;4:297–302. [PubMed] [Google Scholar]
- 6.Zhao D, Wang Q, Zhao Y, Zhang H, Sha N, Tang D, Liu S, Lu S, Shi Q, Zhang Y, Dong Y, Wang Y, Shu B. The naturally derived small compound Osthole inhibits osteoclastogenesis to prevent ovariectomy-induced bone loss in mice. Menopause. 2018;25:1459–1469. doi: 10.1097/GME.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Wang L, Zheng S, Xu J, Pan Y, Tu P, Sun J, Guo Y. Osthole inhibits osteoclasts formation and bone resorption by regulating NF-kappaB signaling and NFATc1 activations stimulated by RANKL. J Cell Biochem. 2019;120:16052–16061. doi: 10.1002/jcb.28886. [DOI] [PubMed] [Google Scholar]
- 8.Wang CG, Liao Z, Xiao H, Liu H, Hu YH, Liao QD, Zhong D. LncRNA KCNQ1OT1 promoted BMP2 expression to regulate osteogenic differentiation by sponging miRNA-214. Exp Mol Pathol. 2019;107:77–84. doi: 10.1016/j.yexmp.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Kim HY, Park SY, Choung SY. Enhancing effects of myricetin on the osteogenic differentiation of human periodontal ligament stem cells via BMP-2/Smad and ERK/JNK/p38 mitogen-activated protein kinase signaling pathway. Eur J Pharmacol. 2018;834:84–91. doi: 10.1016/j.ejphar.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Xia P, Wang S, Qi Z, Zhang W, Sun Y. BMP-2-releasing gelatin microspheres/PLGA scaffolds for bone repairment of X-ray-radiated rabbit radius defects. Artif Cells Nanomed Biotechnol. 2019;47:1662–1673. doi: 10.1080/21691401.2019.1594852. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh MK, Wu CJ, Chen CC, Tsai TT, Niu CC, Wu SC, Lai PL. BMP-2 gene transfection of bone marrow stromal cells to induce osteoblastic differentiation in a rat calvarial defect model. Mater Sci Eng C Mater Biol Appl. 2018;91:806–816. doi: 10.1016/j.msec.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Sapkota M, Gao M, Choi H, Soh Y. Macrolactin F inhibits RANKL-mediated osteoclastogenesis by suppressing Akt, MAPK and NFATc1 pathways and promotes osteoblastogenesis through a BMP-2/smad/Akt/Runx2 signaling pathway. Eur J Pharmacol. 2017;815:202–209. doi: 10.1016/j.ejphar.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Dong M, Jiao G, Liu H, Wu W, Li S, Wang Q, Xu D, Li X, Liu H, Chen Y. Biological silicon stimulates collagen type 1 and osteocalcin synthesis in human osteoblast-like cells through the BMP-2/Smad/RUNX2 signaling pathway. Biol Trace Elem Res. 2016;173:306–315. doi: 10.1007/s12011-016-0686-3. [DOI] [PubMed] [Google Scholar]
- 14.Wu SP, Yang Z, Li FR, Liu XD, Chen HT, Su DN. Smad7-overexpressing rat BMSCs inhibit the fibrosis of hepatic stellate cells by regulating the TGF-beta1/Smad signaling pathway. Exp Ther Med. 2017;14:2568–2576. doi: 10.3892/etm.2017.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang XG, Cong Y, Bao NR. Quercetin stimulates bone marrow mesenchymal stem cell differentiation through an estrogen receptor-mediated pathway. Biomed Res Int. 2018;2018:4178021–4178021. doi: 10.1155/2018/4178021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang ZR, Leung WN, Li G, Kong SK, Lu X, Wong YM, Chan CW. Osthole enhances osteogenesis in osteoblasts by elevating transcription factor osterix via cAMP/CREB signaling in vitro and in vivo. Nutrients. 2017;9:588–588. doi: 10.3390/nu9060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia M, Li Y, Xin HL, Hou TT, Zhang ND, Xu HT, Zhang QY, Qin LP. Estrogenic activity of osthole and imperatorin in MCF-7 cells and their osteoblastic effects in Saos-2 cells. Chin J Nat Med. 2016;14:413–420. doi: 10.1016/S1875-5364(16)30037-1. [DOI] [PubMed] [Google Scholar]
- 18.Ming LG, Zhou J, Cheng GZ, Ma HP, Chen KM. Osthol, a coumarin isolated from common cnidium fruit, enhances the differentiation and maturation of osteoblasts in vitro. Pharmacology. 2011;88:33–43. doi: 10.1159/000328776. [DOI] [PubMed] [Google Scholar]
- 19.Moon SH, Kim I, Kim SH. Mollugin enhances the osteogenic action of BMP-2 via the p38-Smad signaling pathway. Arch Pharm Res. 2017;40:1328–1335. doi: 10.1007/s12272-017-0964-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim EC, Yoon SJ, Noh K, Lee DW. Dual effect of curcumin/BMP-2 loaded in HA/PLL hydrogels on osteogenesis in vitro and in vivo. J Nanosci Nanotechnol. 2017;17:143–152. doi: 10.1166/jnn.2017.12380. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Nie B, Du Z, Zhang S, Long T, Yue B. Bacitracin promotes osteogenic differentiation of human bone marrow mesenchymal stem cells by stimulating the bone morphogenetic protein-2/Smad axis. Biomed Pharmacother. 2018;103:588–597. doi: 10.1016/j.biopha.2018.04.084. [DOI] [PubMed] [Google Scholar]
- 22.Guo AJ, Choi RC, Zheng KY, Chen VP, Dong TT, Wang ZT, Vollmer G, Lau DT, Tsim KW. Kaempferol as a flavonoid induces osteoblastic differentiation via estrogen receptor signaling. Chin Med. 2012;7:10. doi: 10.1186/1749-8546-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foroughinia G, Fazileh A, Eghbalsaied S. Expression of genes involved in BMP and estrogen signaling and AMPK production can be important factors affecting total number of antral follicles in ewes. Theriogenology. 2017;91:36–43. doi: 10.1016/j.theriogenology.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Lee W, Ko KR, Kim HK, Lim S, Kim S. Dehydrodiconiferyl alcohol promotes BMP-2-induced osteoblastogenesis through its agonistic effects on estrogen receptor. Biochem Biophys Res Commun. 2018;495:2242–2248. doi: 10.1016/j.bbrc.2017.12.079. [DOI] [PubMed] [Google Scholar]


