Abstract
Prostate cancer (PCa) is a worldwide malignant tumor which seriously threats the reproductive health of middle-aged and senior male. Sperm-associated antigen 9 (SPAG9), which belongs to the cancer testis (CT) antigen, overexpressed in multiple human malignant tumors and promoted tumor proliferation, invasion and metastasis. However, little attention has been focused on the relationship between SPAG9 and PCa. SPAG9 protein level was measured by immunohistochemical staining in the PCa tissues. SPAG9 mRNA and protein expression were investigated in various PCa cells by qRT-PCR and Western blot. Depletion and overexpression of SPAG9 were proceeded in PCa cells to evaluate their effects by various malignant approaches in vitro and in vivo. SPAG9 was significantly upregulated in the PCa tissues, mainly expressed in the cytoplasm and occasionally in the nucleus of some cells, while SPAG9 was not detected in normal prostate tissue. SPAG9 protein was detected in three PCa cells. Furthermore, these results revealed that upregulation of SPAG9 could promote cell proliferation, migration, motility and cycle of PC-3 cell line, vice versa, downregulation of SPAG9 resulted in the opposite effect. In vivo, knockout of SPAG9 expression induced suppression of tumor growth in athymic nude mice. In summary, the present study indicated that SPAG9 was closely related to the Gleason scores of PCa. SPAG9 could promote cell proliferation, migration, motility and cell cycle via MAPK signaling pathway, suggesting that SPAG9 may be a potential therapeutic target for PCa.
Keywords: Sperm-associated antigen 9, prostate neoplasm, cell migration, cell cycle, cell proliferation
Introduction
Prostate cancer (PCa) is one of the most common malignant tumors worldwide which seriously threats the reproductive health of middle-aged and senior male reproductive health [1]. However, most PCa patients have suffered distant metastasis at the initial diagnosis and lost the optimal opportunity for surgical treatment. For patients who own the chance of operation, early distant metastasis would still leads to poor postoperative recovery. Although serum prostate specific antigen (PSA) detection can be used for large-scale screening of PCa patients, it cannot predict patients; prognosis and the tumor malignancy. Newly approved urinary prostate cancer antigen 3 (PCA3) detection has been applied for clinical assessment of the risk of PCa in western countries [2]. However, due to ethnic differences, this new approach was not prevalent in China [3]. In order to achieve an early diagnosis and optimal treatment, it is necessary for us to investigate the potential molecular mechanism of PCa.
It has been reported that SPAG9 gene, which belongs to the cancer test is (CT) antigen, was specifically expressed in testicular tissue [4]. In addition, SPAG9 gene rarely expressed in the normal tissues, except for testicles and embryo. More importantly, several researches have also confirmed that SPAG9 can induce autoimmune response and presented strong immunogenicity in cancer patients [5]. Considering the important role of SPAG9 gene in testicular tissues, Garg M. et al. have explored the role of SPAG9 in promoting tumor development via c-Jun N-terminal Kinase (JNK) signaling pathway besides its immunogenicity [6]. Engstrom W. et al. have confirmed that SPAG9 can bind with JNK, suggesting that SPAG9 may promote signaling pathway activation through JNK-binding domain [7]. All these studies have shown that SPAG9 gene participates in multiple pathophysiological process through activating JNK signaling pathway as a cofactor. SPAG9 was abnormally overexpressed in multiple human malignant tumors as an oncogene, such as renal cancer, lung cancer, cervical cancer, breast cancer, thyroid cancer and chronic myeloid leukemia [8-13]. For instance, SPAG9 expression has been found in 90% of the ovarian cancer tissue, while all early stage cases (grade I and II) have SPAG9 enrichment. Enzyme-linked immunosorbent assay (ELISA) has revealed that SPAG9 could be detected in 67% of the ovarian cancer patients’ serum, whereas it could not be detected in normal cohorts [14]. Similar phenomenon was observed in chronic myelogenous leukemia [13].
Compared with the important role of SPAG9 gene in testicular tissues, there are only few researches about the role of SPAG9 gene in PCa. Li H et al. have reported that SPAG9 gene was overexpressed in PCa and was closely related to prostate cancer proliferation, invasion and metastasis [15]. However, as a protein in JIP family, the underlying mechanism of SPAG9 protein in regulating JNK signaling pathway is still unclear. Thus, the present study aimed to investigate the role of SPAG9 protein on the biological behavior of PCa using technology of immunohistochemistry (IHC), quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blot.
Materials and methods
Patients and specimens
From January 2012 to December 2016, tissue samples from 62 cases of PCa were obtained from patients who had underwent radical prostatectomy in our institution. No patients have received hormonal therapy or radiotherapy or chemotherapy prior to surgery. Histological evaluation have followed the World Health Organization guidelines [16]. Clinical and histopathological data including histopathological diagnosis and tumor grade were extracted from the medical records systems. This retrospective study was conducted with the approval of the ethics committee of The Third Affiliated Hospital of Sun Yat-sen University, and all patients signed the informed consents for using their surgically-resected tissues and related information.
Immunohistochemistry (IHC)
IHC staining was performed with the streptavidin-biotin peroxidase (SP) conjugated two-step method and a standard SP kit (Zhongshan Biotech Inc., Beijing, China). After deparaffinization and antigen retrieval, tissue sections were proceeded with methanol containing 0.3% hydrogenperoxide (H2O2) to block endogenous peroxidase. Subsequently, tissue sections were incubated overnight at 4°C with mouse monoclonal antibody to SPAG9 (1:100, 5519S, Cell Signaling Technology Inc., MA, USA), followed by incubation with secondary antibody for 30 minutes at 37°C. With DAKO En-Vision System (Dako Diagnostics Inc., Zug, Switzerland), five random visions were examined per slide, and 500 cells were observed per view at ×400 magnification. All tumor slides were examined by two independent investigators randomly. Immunostaining intensity of SPAG9 was evaluated with semiquantitative immunoreactivity score (IRS) analysis, which was defined as the representative tumor areas, the intensity, and percentage of cells. Tumor cells with cytoplasmic staining was defined as positive immunostaining. SPAG9 protein in cancer cells were stained at showing more than 10% of specimen, and were considered the positive.
Cell lines and cell culture
Human prostate carcinoma cell lines PC-3, DU145 and C4-2 were purchased from The American Type Culture Collection (ATCC, VA, USA). These cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen Inc, CA, USA) containing 10% fetal bovine serum (FBS) (Gibco Inc., MD, USA) and 1% streptomycin/penicillin (Gibco Inc., MD, USA). Cells were grown on sterilized culture dishes and were passaged every 2 days with 0.25% trypsin (HyClone Laboratories Inc., UT, USA) at 37°C in a humidified incubator supplemented with 5% CO2.
Transfection of plasmids and expression vectors and gene transfections
The plasmids creating transient tumor cells overexpressing SPAG9 were obtained from the biotechnology company (GeneCopoeia, MD, USA) and transfected into subconfluent cells with Lipofectamine® LTX & Plus Reagent (Invitrogen Inc., CA, USA) following manufacturer’s instruction. The commercially constructed shRNA specifically targeting SPAG9 and scrambled shRNA (GenePharma Inc., Shanghai, China) were transfected into the cells with X-tremeGENE HP DNA transfection reagent (Roche Applied Science Inc., IN, USA) according to the operating protocol. PC-3 and DU145 cells were passaged and G418 (300 μg/ml) was added to select positive cells 48 hours post-transfection, we used RT-PCR and Western blot to detect the effect of transfection.
Western blot analysis
For total protein extraction, cells were homogenizd in a lysis buffer (Cell Signaling Technology Inc., MA. USA) containing protease and phosphatase inhibitors (Roche Applied Science Inc., IN, USA), and quantified with Bradford method using BCA Protein Assay Kit (Thermo Fisher Scientific Inc., CA, USA). Subsequently, an equal amount of protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride membrane (PVDF) membranes (EMD Millipore Inc., MA, USA). After blocked by 5% non-fat milk for 1 hour, the PVDF membranes were incubated with primary antibodies at 4°C overnight and secondary antibody for 1 hours at room temperature. Primary antibodies used in the present study are as follows: rabbit monoclonal antibody to SPAG9 (5519), rabbit monoclonal antibody to Cyclin D1 (2978), mouse monoclonal antibody to p-JNK (9255), rabbit antibody to JNK (9252), rabbit monoclonal antibody to p-ERK (4370), rabbit monoclonal antibody to ERK (4695), rabbit monoclonal antibody to p-p38 (4511), rabbit monoclonal antibody to p38 (8690) (1:1000, Cell Signaling Technology Inc., MA, USA), CDK2 (1:1000, sc-136191, Santa Cruz Biotech Inc., CA, USA), GAPDH (1:1000, AF0006, Beyotime Biotechnology Inc., Shanghai, China). Anti-mouse (1:5000) and anti-rabbit (1:5000) secondary antibodies conjugated to horseradish peroxidase (Zhongshan Biotech Inc., Beijing, China) were apllied to detect the primary antibodies. Proteins bends were visualized with enhanced chemiluminescence (ECL, EMD Millipore Inc., MA, USA) and detected by the FluorChem M system (ProteinSimple Inc., CA, USA).
Cell proliferation test
The effects of SPAG9 overexpression and inhibition on cell viability of PC-3 and DU145 cells were examined by Cell Counting Kit-8 (CCK-8, Dojindo Laboratories Inc., Kumamoto, Japan). The cells were seeded into 96-well plates at a density of 4000 cells/well and cultured for 24 h. Cells were transfected with SPAG9 overexpression or sh-SPAG9 plasmids according to standard protocols after reaching approximately 70% confluence. At the indicated time points, each well was treated with 10 ul CCK-8 at 37°C for 2 hours, then the numbers of cells per well were measured by absorbance read at 450 nm with an automatic microplate reader (Infinite M200, TECAN, Männedorf, Switzerland).
Cell cycle analysis
PC-3 and DU145 cells were acquired after the transfection of SPAG9 overexpression and control plasmids and negative control and SPAG9 shRNA at the logarithmic growth phase, respectively. Cells were cultured in 6-well plates, until reaching 70% confluence at 37°C in a humidified incubator with 5% CO2. Cells were harvested after treated with 0.25% trypsin (HyClone Laboratories Inc., UT, USA), fixed with 70% ethanol overnight at 4°C. Then cells were resuspended in phosphate buffer saline (PBS, Gibco Inc., MD, USA) containing 40 μg/mL PI and 0.1 mg/mL RNase (Sigma-Aldrich Inc., MO, USA), and cell nuclei were stained for 30 minutes at 37°C subsequently. Cell cycle analysis was performed using a flow-cytometer (Becton-Dickinson, CA, USA). Each experiment was performed at least thrice in triplicates.
Monolayer wound healing assay
Wound-healing assay was employed as previously described [17]. PC-3 cells transfected with SPAG9 and control plasmids were seeded at a density of 1 × 106 on a 24-well plate. Cells were grown for 24 hours to reach 100% confluence, subsequently scratched to create a scratch wound by 10 μL plastic pipet tip. Cells were washed with PBS solution (Gibco Inc., MD, USA) 3 times, and cultured in the incubator with RPMI 1640 medium. Cells were photographed at 0 hours and at a time interval of 12, 24, 48 and 72 hours. All experiment was performed in triplicates and repeated 3 times.
Matrigel invasion assay
For cell matrigel invasion assays, cells were determined using a 24-well Transwell chamber of 8 μm pore (EMD Millipore Inc., MA, USA). Matrigel (BD Bioscience, USA) was diluted by serum-free medium to the total concentration of 2 mg/mL. After the transfection of 24 hours, Cells transfected with plasmid were seeded into transwell upper chamber with serum-free medium at a density of 1 × 105 cells, and 0.5 mL culture medium supplemented with 10% FBS was added into the lower chamber. After incubation for 24 hours, the invaded cells were washed with PBS, fixed with 4%paraformaldehyde (Sigma-Aldrich Inc., MO, USA) for 20 minutes, and stained with 0.1% crystal violet solution (Sigma-Aldrich Inc., MO, USA) for 15 minutes. Three randomly selected fields were counted per chamber with a light microscope (Olympus Optical, Tokyo, Japan), and the results were repeated at least thrice in triplicate.
Prostate cancer cells xenograft assays
To carry out in vivo studies, human cancer xenograft model were established in 4-week-old athymic nude mice (National Institute of Immunology [NII], National Institutes of Health, nu/nu). DU145 cells stably expressing SPAG9 shRNA and control shRNA were injected subcutaneously into the right backside of the nude mice at a density of 1 × 106 cells and mice were divided into experimental group and control group. The tumors size was monitored for 4 weeks and measured regularly twice a week. After 4 weeks, the tumor was harvested from the back of nude for weighting and measurement. Tumors were then excised, fixed, embedded in paraffin and sectioned for SPAG9 and Ki-67 expression detection with IHC staining. Statistical analysis all experimental data were presented as the Mean ± S.D. of three separate experiments performed in triplicate and analyzed with GraphPad Prism 6 software (GraphPad, San Diego, CA, USA). The data obtained for different groups were compared by unpaired two-tailed Student’s t-test or one-way ANOVA (P<0.05 was considered as statistically significant).
Results
Expression of SPAG9 with clinicopathological characteristics of PCa
Expression level of SPAG9 protein in paraffin-embedded formalin fixed tissue slides from 62 cases of PCa was measured by IHC staining and results were shown in Table 1. Firstly, SPAG9 was mainly present in the cytoplasm and occasionally in the nucleus of some cells. SPAG9 significantly increased in the prostate cancer tissues (Figure 1C, 1D), while negative expression of SPAG9 in normal prostate tissue (Figure 1A). Next, we evaluated the relationship between SPAG9 expression and clinical pathological characteristics of prostate cancer and results are shown in Table 1. An increased SPAG9 expression was related to Gleason scores (P=0.019), tumor staging (P=0.002). But, there was no significant correlation between the SPAG9 expression and age (P=0.544), nodal status (P=0.582), PSA levels (P=0.306).
Table 1.
Patient characteristics and SPAG9 expression
| Characteristics | Number | Negative/weak (%) | Positive (%) | p |
|---|---|---|---|---|
| Age | ||||
| <60 | 14 | 8 (57.14%) | 6 (48.26%) | 0.544 |
| ≥60 | 48 | 23 (47.91%) | 25 (52.09%) | |
| Gleason Score | ||||
| <8 | 24 | 17 (70.83%) | 7 (29.17%) | 0.019 |
| ≥8 | 38 | 14 (36.84%) | 24 (63.16%) | |
| Tumor stage | ||||
| pT2a-c | 32 | 22 (68.75%) | 10 (31.25%) | 0.002 |
| pT3a-4 | 30 | 9 (30.00%) | 21 (70.00%) | |
| Nodal status | ||||
| pN0 | 43 | 23 (53.48%) | 20 (46.52%) | 0.582 |
| pN1 | 29 | 8 (42.10%) | 11 (57.90%) | |
| PSA levels | ||||
| <10 ng/mL | 27 | 16 (59.26%) | 11 (40.74%) | 0.306 |
| ≥10 ng/mL | 35 | 15 (42.86%) | 20 (57.14%) |
P-values are obtained from Chi-square test.
Figure 1.
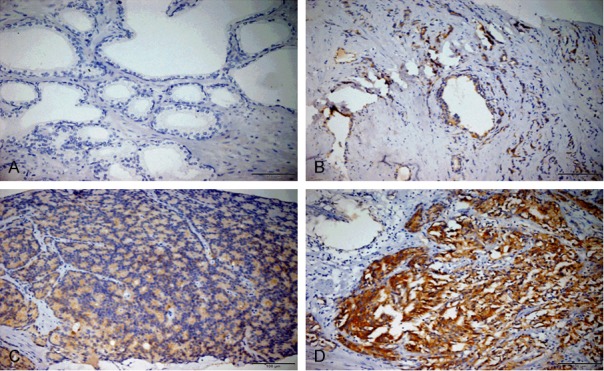
Protein expression of SPAG9 determined by IHC in different tissues. A. Negative SPAG9 expression in normal prostate tissues. B. Weak SPAG9 expression in tumor-adjacent PCa tissues. C. Weak SPAG9 expression in prostate cancer with low Gleason score. D. Strong SPAG9 expression in prostate cancer with high Gleason score (magnification: 200×).
SPAG9 depletion inhibits prostate cancer cell migration and its over-expression promotes cell migration
The pioneer work confirmed that SPAG9 was most associated with the prostate cancer, especially with tumor staging. Aim to test these influence, western blot assays analyzed the expression of SPAG9 in three prostate cancer cell lines. We found high expression of SPAG9 in DU145 cell, while PC3 cell line showed low expression. (Figure 2A). In order to investigate the biological roles of SPAG9 in prostate cancer cells, we employed SPAG9-targeting sh-RNAs in DU145 cell line and SPAG9 over-expression plasmid in PC-3 cell line to confirm knockdown and transfection efficiency (Figure 2B). First, our data revealed that there was a significant tendency of invasive ability in the PC3 cell migration experiment (Control vs SPAG9 plasmid: 315±10 vs 442±8, P<0.05). In accordance with result in PC3, after transfection sh-SPAG9in DU145, the ability of cell migration was obviously reduced (NC vs sh-SPAG9 plasmid: 340±4 vs 231±3, P<0.05, Figure 2C). Consequently, in order to test SPAG9 influence on cellular motility by wound healing assay, PC3 cells wound closure was followed at 12 h, 24 h, 48 h and 72 h. After 48 h, we found that treatment of SPAG9 over expression plasmid resulted in a significant accelerated compared to negative control (Figure 2D).
Figure 2.
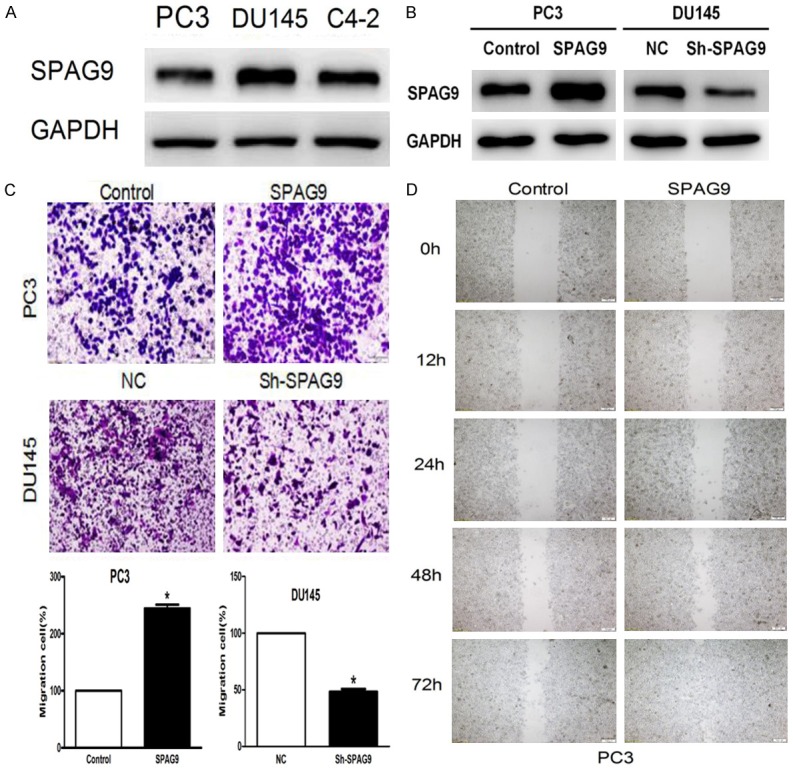
SPAG9 depletion inhibits prostate cancer cell migration and its over-expression promotes cell migration. A. The protein expression of SPAG9 in three prostate cell lines PC-3, DU145, C4-2; B. Western blot analysis showed that SPAG9-targeting sh-RNAs in DU145 cell line and SPAG9 over-expression plasmid in PC-3 cell line to confirm knockdown and transfection efficiency; C. Effects of SPAG9 over-expression on PC-3 cell migration in vitro. **P<0.001 compared with control; The affection of SPAG9 Sh-RNA on DU145 cell migration in vitro. ***P<0.0001 compared with Negative control Sh-RNA. D. PC-3 cell transfected with SPAG9 over-expression showed significantly increased cellular motility even compared with the control group after 48 h. Observations based on three experimental triplicates.
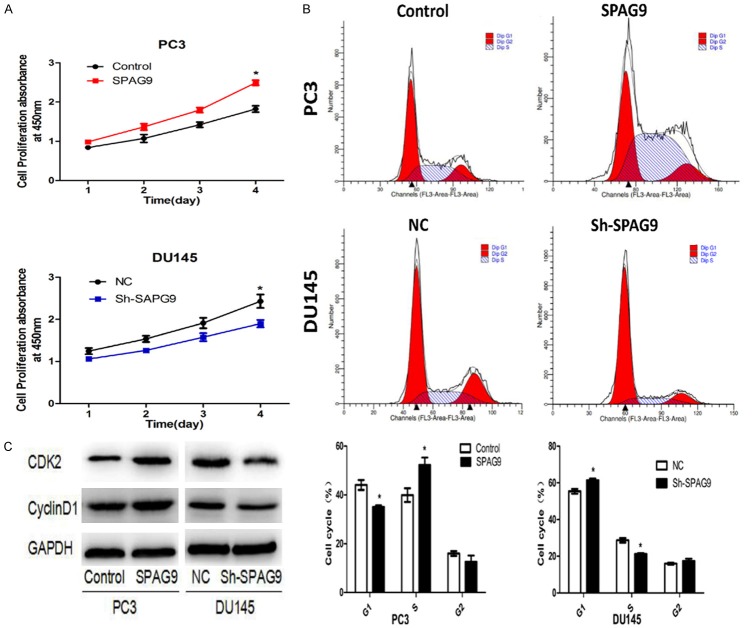
SPAG9 enhances the PCa cells proliferation
To validate the role of SPAG9 during cell proliferation in PCa cells, we altered its expression by the transfection eukaryotic overexpression plasmid and specific sh-RNA in PC-3 and DU145 respectively. Overexpression and knockdown of SPAG9 were confirmed by Western Blot as mentioned above (Figure 2B), CCK-8 assay revelaed that SPAG9 overexpression in PC-3 cell promoted cell proliferation, while the knockdown exhibited a contrary effect in DU145 cell (P<0.05, Figure 3A). To illuminate the specific regulatory mechanisms of SPAG9 on PCa proliferation, we detected the change of cell cycle-related protein after SPAG9 transfection/knockdown. SPAG9 overexpression in the PC-3 cell accelerated the cell cycle (S phase 37.24±2.2% vs 54.32±3%), and SPAG9 knockdown in DU145 cell blocked the cell cycle (S phase 28.67±1.2% vs 21.26±0.4%) (P<0.05) (Figure 3B). In accordance with flowcytometry results, Western blot demonstrated that SPAG9 overexpression could upregulate the protein expression level of cyclin D1 and CDK2 proteins, whereas its knockdown could downregulate the protein expression levels of cyclin D1, and CDK2 (Figure 3C). Taken together, these data indicated that SPAG9 gene expression plays a critical role in PCa cell proliferation via inducing cell cycle S phase entry.
Figure 3.
SPAG9 promotes the prostate cancer cells proliferation. A. Prostate cancer cells were treated as indicated, cells were seeded in 96-well plates for CCK-8 assays or seeded in 6-well plates for fluid cytology technology tests. CCK-8 assay in PC-3 and DU145 cells transfected with SPAG9 over-expression or SPAG9-ShRNA at the indicated time points; *P<0.05. B. SPAG9 over-expression was shown of Flow cytometric in PC-3 cell lines, Cell cycle were accelerated at S phase in SPAG9 over-expression transfected cells as compared with control transfected cells. SPAG9-ShRNA was shown of Flow cytometric in DU145 cell lines, Cell cycle were blocked at S phase compared with negative control transfected cells. *P<0.05. C. CyclinD1 and CDK2 expression were regulated by SPAG9 in PC-3 and DU145 cell lines.
SPAG9 promotes MAPK signaling pathway
In order to uncover the underlying mechanism of the important roles of SPAG9 on cell proliferation, migration and invasion, we investigate its correlation with MAPK signaling pathway MAPK signaling network, which is mainly composed of three signaling pathways, including ERK, JNK, and p38, to promote cell proliferation, differentiation and other biological process. To evaluate these signaling pathways independently, we detected the protein levels of ERK, JNK and P38 signaling pathway using Western blotting. Our experiments found that SPAG9 overexpression could upregulate the protein levels of p-ERK, p-p38 and p-JNK in PC-3 cell line, whereas its inhibition could downregulate the protein levels of p-ERK, p-p38 and p-JNK in DU145 cell line (Figure 4A). In addition, PC-3 cells transfected SPAG9 were treated with SP600125, an inhibitor of JNK signaling pathway, at 10 μm presence or absence for 12 hours, a downregulation of protein levels of SPAG9 and MMP9 were observed (Figure 4B and 4C).
Figure 4.
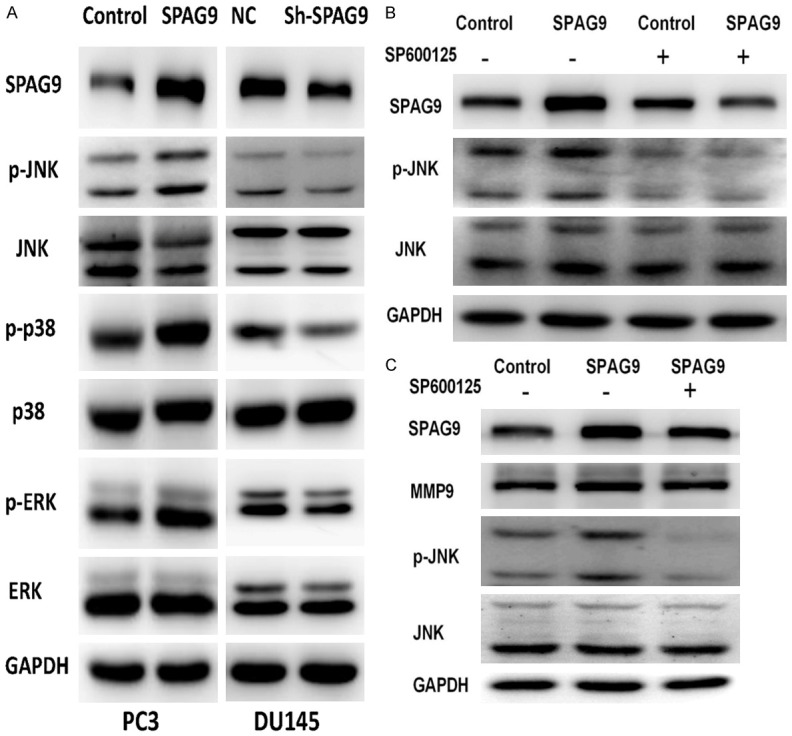
SPAG9 promotes expression of MAPK signaling pathway. A. Western blotting assay test the protein level of MAPK signaling pathway at PC-3 and DU145 cell lines. B, C. Western blotting assay showed that the overexpression of SPAG9 increased MMP9 protein expression, with up-regulation of JNK phosphorylation.
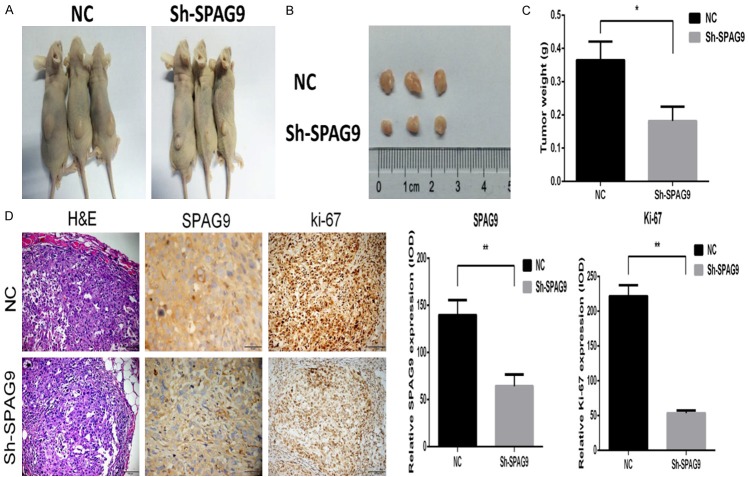
Silencing of SPAG9 inhibits tumor cell growth in vivo
To determine whether silencing of SPAG9 could suppress tumor cell growth in vivo, we establish subcutaneous tumor on athymic mice model. Compared with the control group, SPAG9-knockdown DU145 cells in experimental groups induced a lower tumor volume and weight (0.3693±0.01157 vs 0.2157±0.01097, P=0.0006) (Figure 5A-C). In tumor tissues, a strong correlation between the expression levels of SPAG9 and ki-67 was also observed by IHC assay (SPAG9, 123.9±13.890 vs 49.17±9.325, P<0.0111); ki-67, 158.2±4.956 vs 68.61±1.877, P<0.0001) (Figure 5D).
Figure 5.
Silencing of SPAG9 inhibits tumor cell growth in vitro. DU145 cells (1×106) stably expressing SPAG9 shRNA and Control shRNA were injected subcutaneously into the right backside of the nude mice. A. The representative photo of showing nude mice with tumor (arrows) treated with control shRNA or SPAG9 shRNA. B, C. The average volume and weight of was shown in the SPAG9-silenced DU145 compared with the control group (n=3, *P<0.05). D. H&E staining of the mice tumor and immunohistochemical staining expression of SPAG9 and ki-67 (*P<0.05, **P<0.01, ***P<0.001).
Discussion
In the present studies, IHC have revealed that SPAG9 protein exhibited higher expression level in PCa tissue compared with para-carcinoma tissue. With statistical analysis, SPAG9 expression was positively correlated with postoperative Gleason score, suggesting its potential involvement in tumor invasion, metastasis, adhesion and interstitial morphological alterations. In addition, we also found SPAG9 protein upregulation in PCa tissue closely correlated to pathological clinical stage. Although, SPAG9 expression showed no significant correlation with pathologic stage, lymph node metastasis and distant metastasis, which may be caused by selective bias since most patients with advanced stage have lost opportunity for operation.
The correlation among SPAG9 protein expression, PCa postoperative Gleason score and pathological clinical stage revealed that it may promote PCa development by participating in the process of cell adhesion, migration, invasion and proliferation. Metastasis has been traditionally thought as an event of advanced cancer. However, more and more studies have confirmed that cancer cell can acquire metastatic ability in early stage. Tumor metastasis is a complex, multi-step process. Tumor cells need to get the escape signal and transfer to distance through blood. This process was associated with tumor related matrix, cardiovascular, immune system, and multiple interactions between cells and tumor cells. PC-3 cells invasive ability enhancement was confirmed by migration and scratch experiments after overexpressing SPAG9. In addition, DU145 cell movement ability significantly reduced after SPAG9 downregulation. It is suggested that SPAG9 participated in PCa cell invasion and metastatic process, and promoted disease progression.
Particularly, cell proliferation plays a predominant role in most solid tumors. SPAG9 was significantly related to breast cancer grading and lymph node metastasis [11]. The cyclin protein and cyclin-dependent protein kinases play an important role in the regulation of cell proliferation. Cyclin D1 and CDK2 were represented as the key regulator of G1/S phase in cell cycle [18]. On the contrary, suppressing SPAG9 markedly inhibited DU145 cell proliferation. Thus, SPAG9 can promote PCa cell proliferation, and was significantly correlated with tumor size. Some scholars proposed a new target for tumor therapy as inducing cancer cell apoptosis, blocking unlimited proliferation and differentiation [19]. Our research showed that PC-3 cells accumulate in S and G2 phase in response to SPAG9 downregulation and decrease in G1 phase. SPAG9 overexpression can upregulate Cyclin D1 and CDK2 protein expression and accelerate cell cycle.
SPAG9 promotes PCa proliferation, invasion and metastasis, and accelerates cell cycle mainly through JNK signaling pathway. In PCa, the exact molecular mechanism of SPAG9 underlying its biological function remains to be uncover. Several studies have indicated that JNK signaling pathway is closely related to tumor metastasis [20], which can promote tumor metastasis by expression and interaction with other cells. As a scaffold protein involved in JNK signaling pathway, SPAG9 overexpression can activate JNK signaling pathway to play its oncogene effect [21]. Finally, the interaction between the tumor cells and their surrounding tissues further promote cancer metastasis. MAPK signaling pathway mainly contains three pathways including ERK, JNK, and p38 that promote cell proliferation, differentiation, etc. [22]. ERK signaling pathway not only regulates cell proliferation, differentiation, and survive, but also plays an intermediary and amplification role in tumor metastasis and invasion [23]. ERK overexpression can upregulate the protein expression of transcriptional factor AP-1 and NF-κB to regulate biological behavior in oral cancer, melanoma, and breast cancer [24]. Our study confirmed that SPAG9 overexpression can activate ERK signaling pathway and promote PCa biological behavior through its intermediary and magnified effect. As an important pathway in MAPK signal, JNK plays an important role in cancer proliferation and apoptosis process [25]. Our study suggested that SPAG9 overexpression in PC-3 can activate JNK signaling pathway. On the contrary, SPAG9 interference in DU145 cells obviously downregulate p-JNK. It has been reported that JNK promote cancer proliferation by negatively regulating p53 expression. P38 pathway also participates in multiple biological processes through different mechanisms especially in cell cycle. P38 can cause cell resistance in chemotherapy. For SPAG9-overexpressed PC-3 cell treated with SP600125, the expression of MMP9 was decreased compared to control group. Our investigation found that SPAG9 can activate p38 signaling pathway. Thus, SPAG9 may promote prostate cancer formation, survival, angiogenesis and metastasis through MAPK signaling pathway including ERK, JNK and p38.
Conclusions
In PCa patients, our study has demonstrated that upregulated expression of SPAG9 in PCa tissue was correlated with postoperative Gleason scores and pathological T stage, and SPAG9 was also a significant independent prognostic factor for BCR in PCa patients. In vitro experiments have confirmed that SPAG9 promoted cell proliferation, migration, invasion and cell cycle via MAPK signaling pathway, while in vivo xenograft studies further emphasize the role of SPAG9 in PCa. In sum, therapeutic inhibition of SPAG9 may be of great significance in the prevention of PCa progression.
Acknowledgements
This work was supported by the Science and Technology Planning & Social Development Project of Guangdong Province of China (Grant number: 2017A020215027); Guangzhou Municipal Scientific and Technological Program, (Grant number: 201604020006); Science and Technology Project of Guangdong Province (Grant number: 2017B020227008); National Natural Science Foundation of China (Grant number: 81572503, 81772722); The National Key Basic Research Program (Grant number: 2017YFC0908004).
References
- 1.Gillard M, Tom WR, Antic T, Paner GP, Lingen MW, VanderWeele DJ. Next-gen tissue: preservation of molecular and morphological fidelity in prostate tissue. Am J Transl Res. 2015;7:1227–35. [PMC free article] [PubMed] [Google Scholar]
- 2.Durand X, Moutereau S, Xylinas E, de la Taille A. Progensa PCA3 test for prostate cancer. Expert Rev Mol Diagn. 2011;11:137–44. doi: 10.1586/erm.10.122. [DOI] [PubMed] [Google Scholar]
- 3.Schalken JA, Hessels D, Verhaegh G. New targets for therapy in prostate cancer: differential display code 3 (DD3(PCA3)), a highly prostate cancer-specific gene. Urology. 2003;62:34–43. doi: 10.1016/s0090-4295(03)00759-3. [DOI] [PubMed] [Google Scholar]
- 4.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–21. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann S, Greiner J. Immunogenic antigens as therapeutic targets against myeloid leukaemic cells. Leuk Res. 2010;34:850–1. doi: 10.1016/j.leukres.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Garg M, Chaurasiya D, Rana R. Sperm-associated antigen 9, a novel cancer testis antigen, is a potential target for immunotherapy in epithelial ovarian cancer. Clin Cancer Res. 2007;13:1421–1428. doi: 10.1158/1078-0432.CCR-06-2340. [DOI] [PubMed] [Google Scholar]
- 7.Engstrom W, Ward A, Moorwood K. The role of scaffold proteins in JNK signalling. Cell Prolif. 2010;43:56–66. doi: 10.1111/j.1365-2184.2009.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg M, Kanojia D, Khosla A. Sperm-associated antigen 9 is associated with tumor growth, migration, and invasion in renal cell carcinoma. Cancer Res. 2008;68:8240–8248. doi: 10.1158/0008-5472.CAN-08-1708. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Dong Q, Miao Y, Fu L, Lin X, Wang E. Clinical significance and biological roles of SPAG9 overexpression in non-small cell lung cancer. Lung Cancer. 2013;81:266–272. doi: 10.1016/j.lungcan.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Garg M, Kanojia D, Suri S, Suri A. Small interfering RNA-mediated down-regulation of SPAG9 inhibits cervical tumor growth. Cancer. 2009;115:5688–5699. doi: 10.1002/cncr.24658. [DOI] [PubMed] [Google Scholar]
- 11.Sinha A, Agarwal S, Parashar D. Down regulation of SPAG9 reduces growth and invasive potential of triple-negative breast cancer cells: possible implications in targeted therapy. J Exp Clin Cancer Res. 2013;19:32–69. doi: 10.1186/1756-9966-32-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg M, Kanojia D, Suri S, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9: a novel diagnostic marker for thyroid cancer. J Clin Endocrinol Metab. 2009;94:4613–4618. doi: 10.1210/jc.2009-0703. [DOI] [PubMed] [Google Scholar]
- 13.Kanojia D, Garg M, Saini S, Agarwal S, Kumar R, Suri A. Sperm associated antigen 9 expression and humoral response in chronic myeloid leukemia. Leuk Res. 2010;34:858–863. doi: 10.1016/j.leukres.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Baser E, Togrul C, Ozgu E. Sperm-associated antigen 9 is a promising marker for early diagnosis of endometrial cancer. Asian Pac J Cancer Prev. 2013;14:7635–7638. doi: 10.7314/apjcp.2013.14.12.7635. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Peng Y, Niu H, Wu B, Zhang Y, Zhang Y, Bai X, He P. SPAG9 is overexpressed in human prostate cancer and promotes cancer cell proliferation. Tumour Biol. 2014;35:6949–6954. doi: 10.1007/s13277-014-1947-4. [DOI] [PubMed] [Google Scholar]
- 16.Chung MS, Lee SH, Lee DH, Chung BH. Evaluation of the 7th American Joint Committee on cancer TNM staging system for prostate cancer in point of classification of bladder neck invasion. Jpn J Clin Oncol. 2013;43:184–188. doi: 10.1093/jjco/hys196. [DOI] [PubMed] [Google Scholar]
- 17.Kanojia D, Garg M, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9 is a novel biomarker for colorectal cancer and is involved in tumor growth and tumorigenicity. Am J Pathol. 2011;178:1009–1020. doi: 10.1016/j.ajpath.2010.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan KC, Wang CJ, Ho HH, Chen HM, Huang CN. Simvastatin inhibits cell cycle progression in glucose-stimulated proliferation of aortic vascular smooth muscle cells by up-regulating cyclin dependent kinase inhibitors and p53. Pharmacol Res. 2008;58:247–256. doi: 10.1016/j.phrs.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Wang SF, Yen JC, Yin PH, Chi CW, Lee HC. Involvement of oxidative stress-activated JNK signaling in the methamphetamine-induced cell death of human SH-SY5Y cells. Toxicology. 2008;246:234–241. doi: 10.1016/j.tox.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Luo DH, Chen QY, Liu H. The independent, unfavorable prognostic factors endothelin A receptor and chemokine receptor 4 have a close relationship in promoting the motility of nasopharyngeal carcinoma cells via the activation of AKT and MAPK pathways. J Transl Med. 2013;11:203. doi: 10.1186/1479-5876-11-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26:3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- 22.Whyte J, Bergin O, Bianchi A, McNally S, Martin F. Key signalling nodes in mammary gland development and cancer. Mitogen-activated protein kinase signalling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res. 2009;5:201–209. doi: 10.1186/bcr2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duhamel S, Hebert J, Gaboury L. Self-downregulation by Ras causes MEK1/2 to become aberrantly nuclear localized leading to polyploidy and neoplastic transformation. Cancer Res. 2012;72:626–635. doi: 10.1158/0008-5472.CAN-11-2126. [DOI] [PubMed] [Google Scholar]
- 24.Kreiseder B, Holper-Schichl YM, Muellauer B. Alpha-catulin contributes to drug-resistance of melanoma by activating NF-kappaB and AP-1. PLoS One. 2015;10:e0119402. doi: 10.1371/journal.pone.0119402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]