Key Points
Heme induces complement-dependent thromboinflammation in human whole blood.
Heme-induced proinflammatory cytokines and tissue factor are C5 dependent.
Combined inhibition of C5 and CD14 attenuate prothrombin cleavage.
Abstract
Heme is a critical danger molecule liberated from hemeproteins in various conditions, including from hemoglobin in hemolytic diseases. Heme may cause thromboinflammatory damage by activating inflammatory and hemostatic pathways, such as complement, the TLRs, coagulation, and platelets. In this study, we explored the effect of single and dual inhibition of complement component C5 and TLR coreceptor CD14 on heme-induced thromboinflammation in an ex vivo human whole blood model. Heme induced a dose-dependent activation of complement via the alternative pathway. Single inhibition of C5 by eculizumab attenuated the release of IL-6, IL-8, TNF, MCP-1, MIP-1α, IFN-γ, LTB-4, MMP-8 and -9, and IL-1Ra with more than 60% (p < 0.05 for all) reduced the upregulation of CD11b on granulocytes and monocytes by 59 and 40%, respectively (p < 0.05), and attenuated monocytic tissue factor expression by 33% (p < 0.001). Blocking CD14 attenuated IL-6 and TNF by more than 50% (p < 0.05). In contrast to single inhibition, combined C5 and CD14 was required for a significantly attenuated prothrombin cleavage (72%, p < 0.05). Markers of thromboinflammation were also quantified in two patients admitted to the hospital with sickle cell disease (SCD) crisis. Both SCD patients had pronounced hemolysis and depleted plasma hemopexin and haptoglobin. Plasma heme and complement activation was markedly increased in one patient, a coinciding observation as demonstrated ex vivo. In conclusion, heme-induced thromboinflammation was largely attenuated by C5 inhibition alone, with a beneficial effect of adding a CD14 inhibitor to attenuate prothrombin activation. Targeting C5 has the potential to reduce thromboinflammation in SCD crisis patients.
Introduction
Heme is the prosthetic group for proteins, such as hemoglobin, myoglobin, and cytochromes, carrying out various biological functions, including oxygen transport and storage (1, 2). In hemolytic disorders, such as malaria and sickle cell disease (SCD), the release of hemoglobin and accumulation of heme in plasma may be deleterious (3). Normally, hemoglobin and heme are scavenged by the plasma proteins haptoglobin and hemopexin, respectively, but increased heme concentration may exceed the physiological defense capability (4). Heme induces endothelial injury and exacerbates vascular injury and vaso-occlusive crisis observed in patients with SCD (5). In human whole blood, heme is a potent inflammatory stimulus that induces complement activation, upregulation of proinflammatory cytokines and chemokines, adhesion molecules, neutrophil migration, and neutrophil extracellular trap formation (6–8). Recently, deposits of complement C3 and C5b-9 were demonstrated in kidney biopsy specimens of SCD nephropathy patients and in a mouse model of SCD, wherein the alternative pathway was identified as crucial for the complement activation (9, 10). C3b is also deposited in the membrane of sickle RBCs, which can adhere these cells to activated endothelial cells and possibly promote vaso-occlusive crisis (11). Studies have connected heme to TLR4 binding and a companioned activation of NF-κB, which caused endothelial cell activation and vasoconstriction (12, 13). Heme-induced endothelial injury may lead to adhesion and activation of platelets and leukocytes and tissue factor (TF) upregulation, subsequently activating coagulation and thrombosis (14). Microvascular endothelial cells, especially in the glomeruli, are sensitive to heme-induced stress (15). Furthermore, heme drives oxidative stress by scavenging NO (4). Thus, the liberation of heme overwhelming the neutralizing capacity of scavenger proteins may unleash a devastating thromboinflammatory reaction.
Complement and the TLRs are upstream branches of innate immunity that identify and eliminate pathogens and endogenous danger motifs by soluble or membrane-bound pattern recognition receptors for host protection and homeostasis (16, 17). Activation of complement occurs via three routes: the classical, the lectin, and the alternative pathway, all converging by cleavage of C3. Further activation results in the cleavage of C5 to C5a and C5b. C5a is a potent anaphylatoxin known to induce downstream proinflammatory effector functions and to increase thrombogenicity by upregulation of TF (18, 19). C5b induces the assembly of the terminal C5b-9 complement complex (TCC), which exists in two different forms, the membrane-inserted and the soluble form. When inserted into a membrane as the membrane attack complex, it might lyse certain Gram-negative bacteria and cells, such as erythrocytes, but nuclear and metabolically active cells might undergo a “sub-lytic attack” that is not able to lyse and kill the cell but rather activates it by calcium influx and exerts proinflammatory activity (20–22). The soluble form of TCC, frequently called sC5b-9, is a water-soluble macromolecule useful as an indicator of complement activation as measured in plasma but without hardly any biological function (23).
The TLRs are membrane-bound pattern recognition receptors found on nearly all cells, and they are critical for innate immunity signaling (24). Activation is dependent on the interaction with cofactors and accessory molecules, in which CD14 is specifically important, as it not only enhances LPS responsiveness and TLR4/MD2 signaling but is also implicated in the interaction with most of the other TLRs (25). Extensive crosstalk and mutual interactions between complement and the TLRs are essential parts of host defense, and several studies have demonstrated that combined inhibition of both has a pronounced attenuating effect on both endogenously and exogenously induced inflammation (26–29).
The aim of the current study was to explore the inhibitory effect of targeting C5, CD14, and the combination thereof, in a human whole blood model of heme-induced thromboinflammation. Additionally, two patients admitted with acute SCD crisis are discussed in relation to our ex vivo results.
Materials and Methods
Activators and inhibitors
Hemin is the ferric form of heme. The term heme is used as a generic name with no particular iron valence. Porcine heme (ferriprotoporphyrin IX, 98% purity; product number 51280; Sigma-Aldrich, St. Louis, MO) was dissolved in 50 mM NaOH and 145 mM NaCl and kept in the dark at 4°C until use. The heme was tested and confirmed as endotoxin free (<5 pg/ml) by limulus amebocyte lysate test (Pyrotell, E. Falmouth, MA). Complement C5 was inhibited by eculizumab (100 μg/ml) from Alexion Pharmaceuticals (Cheshire, CT). Inhibition of CD14 was obtained by using a recombinant anti-human CD14 IgG2/4 (clone r18D11, 15 μg/ml) produced in our own laboratory and previously described in detail elsewhere (30). Eritoran (E5564), was kindly provided by Eisai (Andover, MA) and used at a concentration 1 μM.
Whole blood experiments
Whole blood experiments were performed using healthy donors and carried out as previously described (31). In brief, blood drawn by venipuncture was immediately distributed into polypropylene tubes containing the thrombin inhibitor lepirudin (Refludan; Celgene, Uxbridge, U.K.) with a final concentration 50 μg/ml for anticoagulation during the whole incubation time. Lepirudin does not have any modulatory effects on complement activation allowing the inflammatory network upstream of thrombin formation to crosstalk freely (31). The tubes were preincubated with the inhibitors and PBS for 5 min at 37°C. PBS and heme, at final concentrations from 0 to 800 μM, were then added and incubated for 15 or 240 min at 37°C. Following incubation, the tubes were placed on ice, and EDTA was added (final concentration, 20 mM) to stop further activation, and the blood was centrifuged at 3000 × g for 15 min at 4°C. Plasma was immediately isolated and stored in aliquots at −80°C.
Enzyme immunoassays
The soluble TCC, sC5b-9, was quantified in an ELISA as previously described in detail (32, 33). Briefly, the mAb aE11, which reacts with the C9 neoepitope exposed after incorporation of C9 in the C5b-9 complex, was used as the capture Ab, and a biotinylated anti-C6 mAb (clone 9C4) was used as the detection Ab. The results were expressed in arbitrary units per milliliter using human serum activated with zymosan as a standard set to 1000 arbitrary units per milliliter. The C3bc concentration was evaluated by an ELISA based on the mouse anti-human C3bc Ab, mAb clone bH6, reacting with a neoepitope exposed in C3b and C3c after C3 activation (34). The alternative pathway activation was detected by quantifying the alternative pathway C3-convertase, C3bBbP, based on the monoclonal anti-factor P, clone number 2 (Quidel, San Diego, CA), binding the C3BbP complex and detected by anti-C3c when activated) (31, 33). LTB4 in plasma was quantified using a competitive enzyme immunoassay from R&D Systems (Minneapolis, MN). Prothrombin fragment 1+2 (PTF1.2) in plasma was evaluated using Enzygnost F1+2, an enzyme immunoassay from Dade Behring GmbH (Marburg, Germany). A 27-plex kit from Bio-Rad Laboratories (Hercules, CA) was used to measure 27 different cytokines, including chemokines and growth factors, and was performed according to the instructions from the manufacturer. Plasma matrix metalloproteinases (MMPs) were detected using a 9-Plex Panel Multiplex MMP assay from Bio-Rad Laboratories.
Heme assay
Total heme concentration was quantified in plasma from patients by using the chromogenic Heme Assay Kit (Sigma-Aldrich). The assay was performed according to the manufacturer’s instructions, and the colored product was detected at 405 nm by using a multiplate reader (Tecan Sunrise, Mannedorf, Switzerland). It is important to point out that this assay does not discriminate free heme in plasma from heme bound to scavenger- and other heme-binding proteins.
Hemopexin assay
An ELISA was established for the quantification of hemopexin levels in EDTA plasma, based on an mAb to hemopexin as the capture Ab (Thermo Fischer Scientific, Waltham, MA) and a biotinylated anti-human hemopexin Ab as the detection Ab (Dako A/S, Glostrup, Denmark). Hemopexin, purified from human serum as described previously (35), was used as a standard. Plasma samples from 50 healthy blood donors were analyzed individually and used to express a 95% reference range (1–3 mg/ml) of the method.
Flow cytometry
Expression of the activation marker CD11b on granulocytes and monocytes and CD62P on platelets after 15 min of incubation of heme in whole blood was detected using flow cytometry. For gating, monocytes were stained with mouse anti-human CD14 PerCP (BD Biosciences, San Jose, CA); granulocytes were stained with mouse anti-human CD15 eFluor 450 (Invitrogen, Waltham, MA), and platelets were stained with mouse anti-human CD42a FITC (BD Biosciences). Mouse anti-human CD11b APC/Fire 750 from (BioLegend, San Diego, CA) and mouse anti-human CD62P PE (BD Biosciences) were used for detection of activation markers on granulocytes, monocytes, and platelets, respectively. All Abs were incubated with the whole blood for 30 min. RBCs were thereafter lysed with high-yield fixative-free lysing solution (Invitrogen), leukocytes were fixed and resuspended with 0.5% (v/v) paraformaldehyde in PBSA (0.1% BSA) and then analyzed on an Attune NxT flow cytometer (Thermo Fisher Scientific) with the threshold set at forward scatter of 2.5 × 104 to remove debris. Platelets were gated as CD42a-positive on the side scatter plot. CD42a-negative granulocytes and monocytes were gated in a side scatter CD15 and CD14 dot plot, respectively. Expression of CD11b and CD42a are given as mean fluorescence intensity. TF expression was measured on monocytes after 4 h of incubation of heme in whole blood. Cells were stained with anti-CD15 450, anti-CD42a PE, anti-CD14 PerCP, and mouse anti-human TF FITC (Sekisui Diagnostics, Stamford, CT). Samples were prepared as described above. Monocytes were gated in the side scatter plot as CD14-positive and CD42a-negatitive. TF was expressed as mean fluorescence intensity. The data were analyzed by FlowJo X (Tree Star, Ashland, OR).
Patients
We refer to two patients of African origin with known SCD who were admitted to the hospital with sickle cell crisis. Patient 1 (male, 32 y) developed rapid acute chest syndrome with increased fever, hypoxemia, chest pain, and increased opacities on chest x-rays. Biochemical analyses revealed anemia, an increased fraction of hemoglobin S (68%), and a low level of hemoglobin A (21%), whereas haemolysis was demonstrated by low levels of haptoglobin <0.1 g/l, significantly increased levels of lactate dehydrogenase (LDH) (2168 U/l), and bilirubin (99 μmol/l) (Table I). Ferritin (6574 μg/l) and procalcitonin (3.2 μg/l) were markedly increased. Patient 2 (male, 31 y) was clinically less affected and did not have acute chest syndrome. Biochemical analyses revealed low levels of haptoglobin (<0.1 g/l), moderately increased LDH (722 U/l) and bilirubin (56 μmol/l). Increased fractions of hemoglobin S (68%) and low levels of hemoglobin A (17.8%) were observed (Table I). Both patients received exchange transfusion according to the hospitals protocol (3500 ml red cell concentrate and 1000 ml plasma [patient 1] and 4000 ml red cell concentrate and 1000 ml plasma [patient 2]). Additionally, crystalloid solutions were administered. Both patients recovered without any sequel. Blood samples were taken on K2EDTA tubes prior to exchange transfusion, placed directly on ice, and centrifuged for 15 min at 3500 × g and 4°C before being stored at −70°C. A pool of normal human plasma (NHP) from six healthy donors was used as control.
Table I. Patient characteristics of two male patients, 32 and 31 y of age, respectively, with known SCD, admitted to the hospital with sickle cell crisis.
| Patients | Prophylactic Transfusion | Hydroxyurea Treatment | Hemoglobin Sickle % | Hemoglobin Fetal % | Hemoglobin Adult % | Bilirubin μmol/l | Haptoglobin g/l | LDH U/l | Ferritin μg/l |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 (32 y) | |||||||||
| At admittance | No | Noa | 68 | 6 | 24 | 99 | <0.1 | 2168 | 6574 |
| 3 d after exchange transfusion | 16 | 1.4 | 77 | 53 | <0.1 | 1716 | 5950 | ||
| Patient 2 (31 y) | |||||||||
| At admittance | No | Yes | 68 | 18 | 10 | 56 | <0.1 | 772 | 260 |
| 3 d after exchange transfusion | 15 | 1.6 | 78 | 19 | 0.2 | 363 | 195 |
Biochemical data from admittance and 3 d after exchange transfusion.
Hydroxyurea was removed 14 d prior to the admittance because of leg ulcers.
Statistics
The data were analyzed using GraphPad (San Diego, CA) Prism version 6 for Mac by ordinary or repeated-measures one-way ANOVA with Dunnett multiple-comparison posttest for the comparison of multiple columns and paired t tests for the comparison of two columns. A p value < 0.05 was considered statistically significant.
Ethics
Informed written consent was obtained from each donor. The local ethical committee approved the study.
Results
Heme-induced complement activation and inhibition by targeting C5
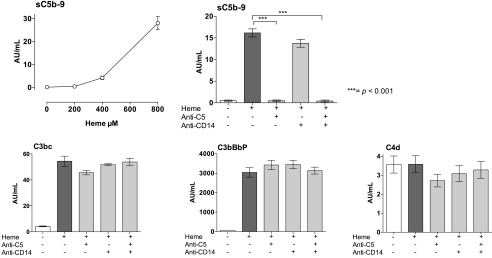
To evaluate heme-induced complement activation, we incubated whole blood with incremental doses of heme for 15 min and measured sC5b-9 in plasma. The formation of sC5b-9 increased modestly by increased heme concentration up to 400 μM, whereas an abrupt increased formation of sC5b-9 was observed when 800 μM of heme was used (Fig. 1, upper left). The C5 inhibitor eculizumab completely inhibited heme-induced sC5b-9 formation, whereas no effect was observed by anti-CD14 (Fig. 1, upper right panel). Heme at 800 μM induced increased levels of C3bc and C3bBbP, whereas the level of the classical and lectin pathway-dependent C4d remained unchanged compared with background. This indicates that complement activation occurred via direct activation of the alternative pathway independently of the classical and the lectin pathway. Neither anti-C5 nor anti-CD14 had any effect on the proximal complement activation markers (Fig. 1, lower panels). The hemopexin levels were measured in all donors and found to be in the normal range (i.e., 1.4 to 2.8 mg/ml).
FIGURE 1.
Heme-induced complement activation. Human whole blood was incubated with different concentrations of heme for 15 min at 37°C, and complement activation was evaluated by measuring the formation of sC5b-9 (upper left panel). Human whole blood was incubated with heme, 800 μM, for 240 min at 37°C in the absence or presence of anti-C5 (eculizumab, 100 μg/ml), aCD14 (15 μg/ml), or the combination thereof. The terminal complement complex, sC5b-9 (upper right panel), and the complement activation products C3bc and C3bBbP, reflecting alternative pathway activation, and C4d, reflecting classical/lectin pathways activation (lower panels) are shown. The data are presented as mean ± SEM (n = 6) for all experiments. Statistical comparisons were performed between the abovementioned groups and controls. ***p < 0.001.
The effect of C5 and CD14 inhibition and the combination thereof on heme-induced inflammation in whole blood
Cytokines.
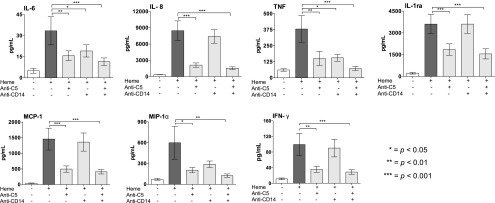
Whole blood incubated with heme for 240 min induced a robust release of inflammatory cytokines, including ILs, chemokines, and IFNs, demonstrated by an increased formation of IL-6, IL-8, TNF, MCP-1, MIP-1α, IFN-γ, and IL-1Ra. C5 inhibition by eculizumab significantly attenuated the formation of IL-6 by 60%, IL-8 by 85%, TNF by 85%, MCP-1 by 75%, MIP-1α by 66%, IFN-γ by 71%, and IL-1Ra by 70% (p < 0.05 for all, Fig. 2). Inhibition of CD14 reduced the formation of IL-6 by 47% and TNF by 60% (p < 0.05 for both). The reduction in MIP-1α (52%) was NS, and no inhibitory effects were observed on IL-8, IL-1Ra, MCP-1, and IFN-γ (Fig. 2). Compared to single C5 inhibition, combined inhibition of C5 and CD14 had a more pronounced attenuating effect on the formation of IL-6, TNF, MIP-1α, and IFN-γ, reaching a higher level of statistical significance (Fig. 2). No significant effect was observed in heme-induced release of cytokines IL-1b, IL-2, IL-4, IL-5, IL-7, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin, FGF-basic, G-CSF, GM-CSF, IP-10, MIP-1β, PDGF-BB, RANTES, and VEGF. The LPS analog eritoran, competitively binding TLR4-MD2, was additionally tested. Eritoran didn’t have any significant attenuating effect on IL-6, IL-8, TNF, IL-1Ra, MCP, MIP-1α, or IFN-γ (Supplemental Fig. 1).
FIGURE 2.
The attenuating effect of targeting C5, CD14, and the combination thereof on heme-induced cytokine release. Human whole blood was incubated with heme, 800 μM for 240 min at 37°C, in the absence or presence of anti-C5 (eculizumab, 100 μg/ml), aCD14 (15 μg/ml), or the combination thereof. Plasma was then analyzed, and the concentration of IL-6, IL-8, TNF, IL-1Ra, MCP-1, MIP-1α, and IFN-γ are shown. The data are presented as mean ± SEM (n = 10). Statistical comparisons were performed between the abovementioned groups and controls (T240). *p < 0.05, **p < 0.01, ***p < 0.001.
MMPs and LTB4.
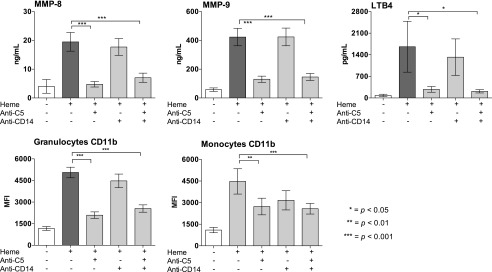
Single C5 inhibition attenuated heme-induced release of MMP-8 and -9 by more than 60% (p < 0.001) and attenuated the formation of LTB4 by 84% (p < 0.001) (Fig. 3, upper panel). No effect was observed for single CD14 inhibition, and no additive effect was obtained by the combined regimen when compared with single C5 inhibition (Fig. 3, upper panel). No significant heme-induced release of MMP-1, -2, -3, -4, -5, -6, and -7 was observed.
FIGURE 3.
The attenuating effect of targeting C5, CD14, and the combination thereof on heme-induced MMPs, LTB4, and CD11b. Human whole blood was incubated with heme, 800 μM for 15 min at 37°C, in the absence or presence of anti-C5 (eculizumab, 100 μg/ml), aCD14 (15 μg/ml), or the combination thereof. The release of MMP-8, -9, and LTB4 (upper panels) were evaluated by flow cytometry; the expression of CD11b on granulocytes and monocytes is shown (lower panels). The data are presented as mean ± SEM (n = 6) for all experiments. Statistical comparisons are as in Fig. 2. *p < 0.05, **p < 0.01, ***p < 0.001.
CD11b expression on granulocytes and monocytes.
In whole blood, single inhibition of C5 attenuated heme-induced upregulation of the cell surface activation marker CD11b on granulocytes and monocytes with 59 and 40%, respectively (p < 0.001) (Fig. 3, lower panel). No significant attenuating effect was obtained for single inhibition of CD14, and the effect observed for the combined regimen was similar to what was obtained with single C5 inhibition (Fig. 3, lower panel).
The effect of C5 and CD14 inhibition and the combination thereof on heme-induced thrombogenicity in human whole blood
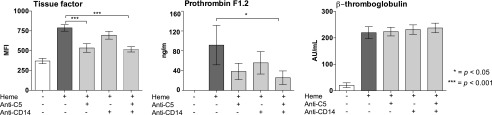
Single C5 inhibition attenuated TF expression on monocytes by 33% (p < 0.001) (Fig. 4, left). No effect was observed by single CD14 inhibition, and no additive effect was obtained for the combined regimen when compared with the effect of eculizumab alone. The release of PTF1.2, a marker for prothrombin cleavage, was attenuated by 72% by the combined inhibition of C5 and CD14 (p < 0.05) (Fig. 4, middle). Single inhibition of either C5 or CD14 attenuated the release of PTF1.2 significantly. Heme-induced platelet activation was measured as increased plasma levels of α-granule β-thromboglobulin, but neither C5 nor CD14 inhibition or the combination thereof had any attenuating effect (Fig. 4, right panel).
FIGURE 4.
The attenuating effect of targeting C5, CD14, and the combination thereof on heme-induced thrombogenicity. Human whole blood was incubated with 800 μM heme for 240 min (TF) or with 100 μM heme for 120 min (prothrombin fragment 1.2, PTF1.2) at 37°C, in the absence or presence of anti-C5 (eculizumab, 100 μg/ml), aCD14 (15 μg/ml), or the combination thereof. TF expression was evaluated by flow cytometry. Plasma PTF1.2 and β-thromboglobulin were quantified by ELISA. The data are presented as mean ± SEM (n = 6) for all experiments. Statistical comparisons were performed as for Fig. 2. *p < 0.05, ***p < 0.001.
Patients with SCD crisis
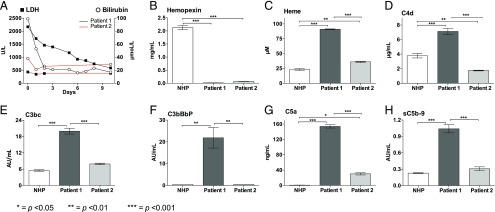
Patients 1 and 2 (Table I) both had SCD and were admitted to the hospital with sickle cell crisis and in need of an exchange transfusion. Patient 1 was clinically more affected, and routine biochemical analyses revealed a more severe hemolysis, measured as higher level of LDH and bilirubin, compared with patient 2 (Fig. 5A). Further analyses were performed and compared with a pool of NHP.
FIGURE 5.
Heme, hemopexin, and complement activation in two patients with acute SCD crisis. Two patients with acute SCD crisis were admitted to the hospital in need of exchange transfusion. Blood samples from both patients were obtained prior to the transfusion and stored for evaluation of heme, hemopexin, and complement activation. Patient 1 had acute chest syndrome with increased fever, hypoxemia, chest pain, and increased opacities on chest x-rays. Patient 2 was clinically less affected. LDH and bilirubin were measured in both patients before exchange transfusion on the following days (A). The exchange transfusions were started day 0. Plasma concentration of hemopexin (B) and heme (C) were measured as well the complement activation products C4d (D), C3bc (E), C3bBbp (F), C5a (G), and sC5b-9 (H). A pool of NHP from six healthy donors was used as control. The analyses were performed in technical triplicates, and the data are presented as mean ± SEM. Statistical comparisons were performed between NHP, patient 1 and patient 2. *p < 0.05, **p < 0.01, ***p < 0.001.
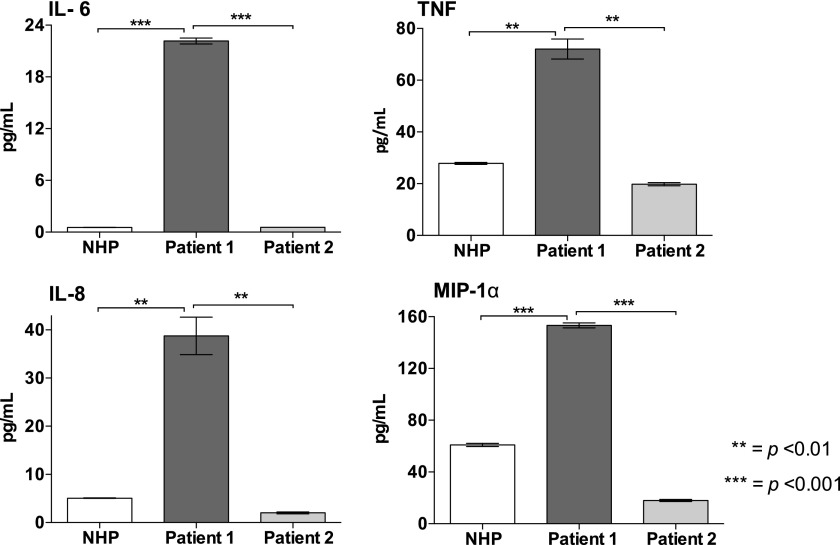
The low level of haptoglobin (<0.1 g/l) coincided with an equivalently low level of hemopexin measured in both patients, which was almost ablated compared with the pool of NHP (p < 0.001 for both) (Fig. 5B). Notably, the level of heme was significantly higher in both patients versus NHP, whereas the heme concentration was considerably higher in patient 1 compared with patient 2, indicating more severe hemolysis (p < 0.001) (Fig. 5C). Systemic complement activation, measured as increased formation of C4d, C3bc, C3bBbP, C5a, and sC5b-9, was observed in patient 1 (p < 0.01 for all), whereas only C5a was increased in patient 2 (p < 0.05) (Fig. 5D–H). Increased formation of C3bBbP and C4d was demonstrated in patient 1, indicating both alternative pathway activation and indicating the involvement of the classical and/or lectin pathway. Compared with NHP, increased formation of IL-6, IL-8, TNF, and MIP-1α was observed in patient 1 (p < 0.01 for all), whereas the levels in patient 2 were similar to what was observed in the control plasma pool (Fig. 6).
FIGURE 6.
Plasma cytokines in the patients with acute SCD crisis. Concentrations of IL-6, TNF, IL-8, and MIP-1α were measured in plasma collected from the patients described in Fig. 5. A pool of NHP from six healthy donors was used as control. The analyses were performed in technical duplicates, and the data are presented as mean ± SEM. Statistical comparisons were performed between NHP, patient 1, and patient 2. **p < 0.01, ***p < 0.001.
To compare the level of heme used in the ex vivo experiments with the heme level measured in the patients, we investigated whether the measured heme concentrations corresponded to the added amount of heme. Human whole blood was incubated 15 min at 37°C with incremental concentrations of heme, 0–800 μM, before being measured. Additionally, human plasma and PBS was incubated 15 min at 37°C with heme at 800 μM. Notably, the amount of heme was consistently lower compared with what was added (Supplemental Fig. 2, left panel). Heme added at 400 μM corresponded to a measured concentration of 80 μM, which is comparable to the level measured in patient 1, whereas heme added at 100 μM corresponded to a measured concentration of 35 μM, comparable to level measured in patient 2. Heme added at 800 μM in human plasma blood was measured as 140 μM, whereas a significantly higher level, 290 μM, was measured in PBS (p < 0.001) (Supplemental Fig. 2, right panel).
Discussion
To the best of our knowledge, this study demonstrates for the first time that inhibition of complement component C5 efficiently attenuated the whole spectrum of heme-induced thromboinflammation markers when investigated in human whole blood. Additionally, inhibition of CD14 reduced several of the markers, in particular the cytokines TNF and IL-6, but complement inhibition was clearly more powerful. C5 inhibition alone counteracted a broad proinflammatory response unleashed by heme and attenuated TF upregulation. Interestingly, the combined inhibition of C5 and CD14 was required to significantly attenuate the heme-induced prothrombin cleavage. A sufficient concentration of heme in whole blood was required to attain complement activation, an ex vivo observation that coincided with the clinical observation; in the two patients admitted with SCD crisis, only the patient who showed elevated levels of heme in plasma presented with systemic complement activation.
Excess liberation of heme is a characteristic feature in hemolytic pathologies, but, in healthy individuals, liberation of heme in plasma is efficiently counteracted by the defense capability of haptoglobin and hemopexin (4). In the ex vivo human whole blood experiments, a high concentration of heme was required to induce a moderate but adequate activation of complement. However, when measured after addition, the concentration was much lower and comparable to what can be seen in vivo. Furthermore, locally, in the microcirculation, when RBC are lysed during a vaso-occlusive crisis, the concentration of heme might be much higher than what is measured systemically. Still, heme in the range of 200–300 μM corresponds to lysis of <0.1% of the circulating erythrocytes (36). Furthermore, the increased C3bc and C3bBbP observed ex vivo confirm previous data showing that heme activates complement by the alternative pathway (10, 36). The mechanism for heme-induced alternative pathway activation is today not known, but it is proposed that the hydrophobic free heme would directly bind C3 adjacent to the thioester bond and promote C3 hydrolysis into C3(H2O). Fluid phase C3H2O is able to form the alternative pathway C3 convertase C3(H2O)Bb for cleavage of fluid phase C3 (10). This is also in line with a recent observation documenting that RBC-derived microvesicles from SCD patients heme dependently activate complement by the alternative pathway (9). In the current study, the clinically most affected SCD patient had elevated C4d plasma levels, in addition to C3bc and C3bBbP, indicating an additional activation of the classical and/or the lectin pathway. Heme did not elevate C4d in our experimental model, which is in line with previous results (37), so this may imply alternative mechanisms of complement activation in SCD, such as chronic continuous activation, or part of a delayed hemolytic transfusion reaction involving alloantibodies and classical pathway activation (38, 39). Thereby, the classical and lectin pathway may recognize damaged self-molecules released in vivo, which are not generated in whole blood ex vivo.
In the current study, the heme-induced inflammatory response was found to be predominantly complement dependent and less CD14 dependent. Single inhibition of C5 attenuated a broad range of cytokines and attenuated upregulation of the important cell surface molecule CD11b, thus underscoring the profound anti-inflammatory potential in targeting this upstream sensor and effector system of innate immunity (27). Additionally, LTB4, which is a potent inducer of chemotaxis of neutrophil infiltration and an important mediator in heme-induced inflammation (40), was attenuated by C5 inhibition. This supports existing evidence that LTB4 synthesis is C5a dependent and, thus, blocked by targeting C5 (41). Heme-induced oxidative stress has previously been shown to activate MMPs, leading to blood–brain barrier dysfunction (42). MMPs, predominantly derived from degranulation of neutrophils, are prominent inflammatory actors in several diseases. Ex vivo, the inhibition of C5 attenuated MMP-8 and MMP-9 plasma levels, which is in accordance with a mouse study linking increased complement activity and C5a formation to increased release of MMPs (43).
The attenuating effect of blocking CD14 was limited mainly to IL-6 and TNF in the current study. Nevertheless, the results confirmed that heme may also act in a CD14-dependend manner, supposedly via TLR4 (13). In a murine model of SCD, heme-induced TLR4 signaling activated endothelial cells, resulting in vaso-occlusion, highlighting the significance of the TLR4 inflammatory pathway in heme-dependent inflammation (12). In this study, however, eritoran alone did not exert any inhibitory effects, suggesting that heme-induced inflammation in whole blood is not TLR4 dependent.
Heme induced a vast upregulation of TF and increased the formation of PTF1.2. TF is a transmembrane glycoprotein expressed within the pool of circulating cells, primarily on monocytes, and is a key initiator of coagulation (44). PTF1.2 is released upon prothrombin cleavage by factor Xa to yield thrombin (45). In rodent models of SCD, free heme was shown to trigger activation of the extrinsic coagulation pathway through TF upregulation on endothelial cells and monocytes, which promotes thrombosis (46, 47). Furthermore, in SCD mice, the anaphylatoxin C5a appears to play a key role in vaso-occlusive injuries, promoting inflammation and upregulation of adhesion molecules, such as VCAM-1, ICAM-1, and E-selectin (48). The crosstalk and mutual interaction between the complement and coagulation systems is well described. C5a enhances upregulation of TF on various cell types (19, 49), and TF upregulation is attenuated by targeting C5 and by the dual inhibition of C5 and CD14 (26, 50). Heme-induced thromboinflammation may amplify these detrimental reactions, as heme is a strong inducer of both inflammation and coagulation (14). In the current study, TF expression was reduced significantly by single inhibition of C5, whereas both TF expression and the level of PTF1.2 were attenuated by the combined C5- and CD14-blocking regimen. Thus, both C5 inhibition alone and combined inhibition of C5 and CD14 have vast propensities to attenuate heme-induced thromboinflammation, which is the motivation for future in vivo studies and clinical trials.
Importantly, the two patients with SCD crisis included in this study mirrored and partly corroborated the ex vivo results. Although both patients were considered in need of exchange transfusion, patient 1 had substantially more aggravated clinical symptoms and pathological biochemistry, higher concentration of heme, and, importantly, more pronounced systemic complement activation as compared with patient 2. Moreover, the degree of heme release observed in the patients was also proportional to the inflammatory response, with increased formation of IL-6, IL-8, TNF, and MIP-1α. Because only two patients were included in this study, future clinical studies are necessary to establish whether the magnitude of complement activation in SCD crisis really is dependent on and correlates to the concentration of heme.
A limitation to this study is the high heme concentrations used in our experimental model. Importantly, the use of concentrated heme was based on the titration of heme and correspondingly adequate complement activation. Although heme at 800 μM is higher compared with what was measured in the two patients, the corresponding measured level of heme was within patient-comparable range, as demonstrated in Supplemental Fig. 2. We cannot explain this discrepancy in detail, but we speculate that the plasma environment interfered with the signal for detection, as suggested by the significantly higher amount of heme measured in PBS versus plasma. However, as long as the heme assay used does not discriminate free heme in plasma from heme bound to proteins, we cannot claim any certainty on this point.
In conclusion, the present data indicate that heme is a strong activator of complement through alternative pathway activation, inducing a potent thromboinflammation in human whole blood. Single inhibition of C5 efficiently attenuated the thromboinflammatory response. Although the additive effect of the combined inhibition of C5 and CD14 was small compared with single inhibition of C5, the study documents the significance of TLRs/CD14 in heme-induced thromboinflammaton. Thus, single inhibition of C5 and dual inhibition of C5 and CD14 are therapeutic approaches that should be considered and explored in future studies of SCD crisis and other conditions of heme-induced thromboinflammation.
Supplementary Material
This work was supported by the Norwegian Council on Cardiovascular Disease, the Odd Fellow Foundation, and the Simon Fougner Hartmann Family Fund.
The online version of this article contains supplemental material.
- LDH
- lactate dehydrogenase
- MMP
- matrix metalloproteinase
- NHP
- normal human plasma
- SCD
- sickle cell disease
- TCC
- terminal C5b-9 complement complex
- TF
- tissue factor.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Beri R., Chandra R. 1993. Chemistry and biology of heme. Effect of metal salts, organometals, and metalloporphyrins on heme synthesis and catabolism, with special reference to clinical implications and interactions with cytochrome P-450. Drug Metab. Rev. 25: 49–152. [DOI] [PubMed] [Google Scholar]
- 2.Wagener F. A., Volk H. D., Willis D., Abraham N. G., Soares M. P., Adema G. J., Figdor C. G. 2003. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol. Rev. 55: 551–571. [DOI] [PubMed] [Google Scholar]
- 3.Arruda M. A., Rossi A. G., de Freitas M. S., Barja-Fidalgo C., Graça-Souza A. V. 2004. Heme inhibits human neutrophil apoptosis: involvement of phosphoinositide 3-kinase, MAPK, and NF-kappaB. J. Immunol. 173: 2023–2030. [DOI] [PubMed] [Google Scholar]
- 4.Reiter C. D., Wang X., Tanus-Santos J. E., Hogg N., Cannon R. O., III, Schechter A. N., Gladwin M. T. 2002. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 8: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 5.Camus S. M., De Moraes J. A., Bonnin P., Abbyad P., Le Jeune S., Lionnet F., Loufrani L., Grimaud L., Lambry J. C., Charue D., et al. 2015. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood 125: 3805–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg A., Otterdal K., Patel S., Gonca M., David C., Dalen I., Nymo S., Nilsson M., Nordling S., Magnusson P. U., et al. 2015. Complement activation correlates with disease severity and contributes to cytokine responses in Plasmodium falciparum malaria. J. Infect. Dis. 212: 1835–1840. [DOI] [PubMed] [Google Scholar]
- 7.Graça-Souza A. V., Arruda M. A. B., de Freitas M. S., Barja-Fidalgo C., Oliveira P. L. 2002. Neutrophil activation by heme: implications for inflammatory processes. Blood 99: 4160–4165. [DOI] [PubMed] [Google Scholar]
- 8.Chen G., Zhang D., Fuchs T. A., Manwani D., Wagner D. D., Frenette P. S. 2014. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 123: 3818–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merle N. S., Grunenwald A., Rajaratnam H., Gnemmi V., Frimat M., Figueres M.-L., Knockaert S., Bouzekri S., Charue D., Noe R., et al. 2018. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight 3: 96910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frimat M., Tabarin F., Dimitrov J. D., Poitou C., Halbwachs-Mecarelli L., Fremeaux-Bacchi V., Roumenina L. T. 2013. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood 122: 282–292. [DOI] [PubMed] [Google Scholar]
- 11.Lombardi E., Matte A., Risitano A. M., Ricklin D., Lambris J. D., De Zanet D., Jokiranta S. T., Martinelli N., Scambi C., Salvagno G., et al. 2019. Factor H interferes with the adhesion of sickle red cells to vascular endothelium: a novel disease-modulating molecule. Haematologica 104: 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belcher J. D., Chen C., Nguyen J., Milbauer L., Abdulla F., Alayash A. I., Smith A., Nath K. A., Hebbel R. P., Vercellotti G. M. 2014. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 123: 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueiredo R. T., Fernandez P. L., Mourao-Sa D. S., Porto B. N., Dutra F. F., Alves L. S., Oliveira M. F., Oliveira P. L., Graça-Souza A. V., Bozza M. T. 2007. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 282: 20221–20229. [DOI] [PubMed] [Google Scholar]
- 14.Roumenina L. T., Rayes J., Lacroix-Desmazes S., Dimitrov J. D. 2016. Heme: modulator of plasma systems in hemolytic diseases. Trends Mol. Med. 22: 200–213. [DOI] [PubMed] [Google Scholar]
- 15.May O., Merle N. S., Grunenwald A., Gnemmi V., Leon J., Payet C., Robe-Rybkine T., Paule R., Delguste F., Satchell S. C., et al. 2018. Heme drives susceptibility of glomerular endothelium to complement overactivation due to inefficient upregulation of heme oxygenase-1. Front. Immunol. 9: 3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Köhl J. 2006. The role of complement in danger sensing and transmission. Immunol. Res. 34: 157–176. [DOI] [PubMed] [Google Scholar]
- 17.Matzinger P. 2002. The danger model: a renewed sense of self. Science 296: 301–305. [DOI] [PubMed] [Google Scholar]
- 18.Ward P. A. 2010. The harmful role of c5a on innate immunity in sepsis. J. Innate Immun. 2: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritis K., Doumas M., Mastellos D., Micheli A., Giaglis S., Magotti P., Rafail S., Kartalis G., Sideras P., Lambris J. D. 2006. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 177: 4794–4802. [DOI] [PubMed] [Google Scholar]
- 20.Ricklin D., Hajishengallis G., Yang K., Lambris J. D. 2010. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11: 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walport M. J. 2001. Complement. First of two parts. N. Engl. J. Med. 344: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 22.Mollnes T. E., Song W.-C., Lambris J. D. 2002. Complement in inflammatory tissue damage and disease. Trends Immunol. 23: 61–64. [DOI] [PubMed] [Google Scholar]
- 23.Harboe M., Thorgersen E. B., Mollnes T. E. 2011. Advances in assay of complement function and activation. Adv. Drug Deliv. Rev. 63: 976–987. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T., Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11: 373–384. [DOI] [PubMed] [Google Scholar]
- 25.Lee C. C., Avalos A. M., Ploegh H. L. 2012. Accessory molecules for Toll-like receptors and their function. Nat. Rev. Immunol. 12: 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barratt-Due A., Thorgersen E. B., Egge K., Pischke S., Sokolov A., Hellerud B. C., Lindstad J. K., Pharo A., Bongoni A. K., Rieben R., et al. 2013. Combined inhibition of complement C5 and CD14 markedly attenuates inflammation, thrombogenicity, and hemodynamic changes in porcine sepsis. J. Immunol. 191: 819–827. [DOI] [PubMed] [Google Scholar]
- 27.Barratt-Due A., Pischke S. E., Nilsson P. H., Espevik T., Mollnes T. E. 2017. Dual inhibition of complement and Toll-like receptors as a novel approach to treat inflammatory diseases-C3 or C5 emerge together with CD14 as promising targets. J. Leukoc. Biol. 101: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas A. M., Schjalm C., Nilsson P. H., Lindenskov P. H. H., Rørtveit R., Solberg R., Saugstad O. D., Berglund M. M., Strömberg P., Lau C., et al. 2018. Combined inhibition of C5 and CD14 attenuates systemic inflammation in a piglet model of meconium aspiration syndrome. Neonatology 113: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holst B., Raby A. C., Hall J. E., Labéta M. O. 2012. Complement takes its Toll: an inflammatory crosstalk between Toll-like receptors and the receptors for the complement anaphylatoxin C5a. Anaesthesia 67: 60–64. [DOI] [PubMed] [Google Scholar]
- 30.Lau C., Gunnarsen K. S., Høydahl L. S., Andersen J. T., Berntzen G., Pharo A., Lindstad J. K., Ludviksen J. K., Brekke O. L., Barratt-Due A., et al. 2013. Chimeric anti-CD14 IGG2/4 hybrid antibodies for therapeutic intervention in pig and human models of inflammation. J. Immunol. 191: 4769–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mollnes T. E., Brekke O.-L., Fung M., Fure H., Christiansen D., Bergseth G., Videm V., Lappegård K. T., Köhl J., Lambris J. D. 2002. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. [Published erratum appears in 2002 Blood 100: 2691.] Blood 100: 1869–1877. [PubMed] [Google Scholar]
- 32.Mollnes T. E., Lea T., Frøland S. S., Harboe M. 1985. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand. J. Immunol. 22: 197–202. [DOI] [PubMed] [Google Scholar]
- 33.Bergseth G., Ludviksen J. K., Kirschfink M., Giclas P. C., Nilsson B., Mollnes T. E. 2013. An international serum standard for application in assays to detect human complement activation products. [Published erratum appears in 2014 Mol. Immunol. 60: 115.] Mol. Immunol. 56: 232–239. [DOI] [PubMed] [Google Scholar]
- 34.Garred P., Mollnes T. E., Lea T., Fischer E. 1988. Characterization of a monoclonal antibody MoAb bH6 reacting with a neoepitope of human C3 expressed on C3b, iC3b, and C3c. Scand. J. Immunol. 27: 319–327. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K., Kato H., Sakuma Y., Namiki H. 2001. Hemopexins suppress phorbol ester-induced necrosis of polymorphonuclear leucocytes. Cell Struct. Funct. 26: 235–241. [DOI] [PubMed] [Google Scholar]
- 36.Pawluczkowycz A. W., Lindorfer M. A., Waitumbi J. N., Taylor R. P. 2007. Hematin promotes complement alternative pathway-mediated deposition of C3 activation fragments on human erythrocytes: potential implications for the pathogenesis of anemia in malaria. J. Immunol. 179: 5543–5552. [DOI] [PubMed] [Google Scholar]
- 37.Roumenina L. T., Radanova M., Atanasov B. P., Popov K. T., Kaveri S. V., Lacroix-Desmazes S., Frémeaux-Bacchi V., Dimitrov J. D. 2011. Heme interacts with c1q and inhibits the classical complement pathway. J. Biol. Chem. 286: 16459–16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mold C., Tamerius J. D., Phillips G., Jr 1995. Complement activation during painful crisis in sickle cell anemia. Clin. Immunol. Immunopathol. 76: 314–320. [DOI] [PubMed] [Google Scholar]
- 39.Merle N. S., Boudhabhay I., Leon J., Fremeaux-Bacchi V., Roumenina L. T. 2019. Complement activation during intravascular hemolysis: implication for sickle cell disease and hemolytic transfusion reactions. Transfus. Clin. Biol. 26: 116–124. [DOI] [PubMed] [Google Scholar]
- 40.Monteiro A. P., Pinheiro C. S., Luna-Gomes T., Alves L. R., Maya-Monteiro C. M., Porto B. N., Barja-Fidalgo C., Benjamim C. F., Peters-Golden M., Bandeira-Melo C., et al. 2011. Leukotriene B4 mediates neutrophil migration induced by heme. J. Immunol. 186: 6562–6567. [DOI] [PubMed] [Google Scholar]
- 41.Barratt-Due A., Thorgersen E. B., Lindstad J. K., Pharo A., Lissina O., Lambris J. D., Nunn M. A., Mollnes T. E. 2011. Ornithodoros moubata complement inhibitor is an equally effective C5 inhibitor in pigs and humans. J. Immunol. 187: 4913–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsu M., Niizuma K., Yoshioka H., Okami N., Sakata H., Chan P. H. 2010. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood-brain barrier dysfunction in vivo. J. Cereb. Blood Flow Metab. 30: 1939–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez J. M., Franzke C. W., Yang F., Romero R., Girardi G. 2011. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am. J. Pathol. 179: 838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bach R. R. 1988. Initiation of coagulation by tissue factor. CRC Crit. Rev. Biochem. 23: 339–368. [DOI] [PubMed] [Google Scholar]
- 45.Ota S., Wada H., Abe Y., Yamada E., Sakaguchi A., Nishioka J., Hatada T., Ishikura K., Yamada N., Sudo A., et al. 2008. Elevated levels of prothrombin fragment 1 + 2 indicate high risk of thrombosis. Clin. Appl. Thromb. Hemost. 14: 279–285. [DOI] [PubMed] [Google Scholar]
- 46.Sparkenbaugh E. M., Chantrathammachart P., Wang S., Jonas W., Kirchhofer D., Gailani D., Gruber A., Kasthuri R., Key N. S., Mackman N., Pawlinski R. 2015. Excess of heme induces tissue factor-dependent activation of coagulation in mice. Haematologica 100: 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chantrathammachart P., Mackman N., Sparkenbaugh E., Wang J. G., Parise L. V., Kirchhofer D., Key N. S., Pawlinski R. 2012. Tissue factor promotes activation of coagulation and inflammation in a mouse model of sickle cell disease. Blood 120: 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vercellotti G. M., Dalmasso A. P., Schaid T. R., Jr., Nguyen J., Chen C., Ericson M. E., Abdulla F., Killeen T., Lindorfer M. A., Taylor R. P., Belcher J. D. 2019. Critical role of C5a in sickle cell disease. Am. J. Hematol. 94: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markiewski M. M., Nilsson B., Ekdahl K. N., Mollnes T. E., Lambris J. D. 2007. Complement and coagulation: strangers or partners in crime? Trends Immunol. 28: 184–192. [DOI] [PubMed] [Google Scholar]
- 50.Brekke O. L., Waage C., Christiansen D., Fure H., Qu H., Lambris J. D., Østerud B., Nielsen E. W., Mollnes T. E. 2013. The effects of selective complement and CD14 inhibition on the E. coli-induced tissue factor mRNA upregulation, monocyte tissue factor expression, and tissue factor functional activity in human whole blood. Adv. Exp. Med. Biol. 735: 123–136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.