Abstract
Axonal beading, or the formation of a series of swellings along the axon, and retraction are commonly observed shape transformations that precede axonal atrophy in Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative conditions. The mechanisms driving these morphological transformations are poorly understood. Here, we report controlled experiments that can induce either beading or retraction and follow the time evolution of these responses. By making quantitative analysis of the shape modes under different conditions, measurement of membrane tension, and using theoretical considerations, we argue that membrane tension is the main driving force that pushes cytosol out of the axon when microtubules are degraded, causing axonal thinning. Under pharmacological perturbation, atrophy is always retrograde, and this is set by a gradient in the microtubule stability. The nature of microtubule depolymerization dictates the type of shape transformation, vis-à-vis beading or retraction. Elucidating the mechanisms of these shape transformations may facilitate development of strategies to prevent or arrest axonal atrophy due to neurodegenerative conditions.
Significance
Understanding the mechanisms of neurodegeneration remains a challenge despite intense research activity in the past few decades. Although several disease-specific molecular/genetic factors have been reported, identifying such targets has not provided much traction in terms of prevention or cure. However, axons of neurons undergoing degeneration exhibit similar abnormal morphologies before becoming atrophied. The biomechanics of such shape evolution is poorly understood. This article explores the biophysical mechanisms that drive axonal atrophy via hallmark shape transformations like “beading” and “retraction.” We show that microtubule stability, F-actin-microtubule coupling, and membrane tension together dictate the shape evolution in affected axons. We believe that these results will contribute toward devising new strategies to prevent, arrest, or even reverse neurodegeneration.
Introduction
Neuronal cells do not divide and are rarely replenished when lost. As a consequence, they have to last an entire lifetime while maintaining their tubular extensions, namely dendrites and axons. This makes the nervous system particularly susceptible to degeneration causing debilitating conditions. Axons, owing to their extreme lengths, are particularly vulnerable. Abnormal morphological transformations like the appearance of varicosities along the axon or “beading” is a hallmark of a very wide range of neurodegenerative conditions of the peripheral as well as the central nervous system. Beading occurs under surprisingly diverse conditions (1) such as Alzheimer’s disease (2, 3, 4, 5), Parkinson’s disease (6, 7), autoimmune encephalomyelitis and multiple sclerosis (8), ageing (9, 10, 11), ischemia and resulting hypoxia or inflammation (12, 13, 14, 15), or in response to traumatic stretch injuries (16, 17, 18, 19, 20). Axonal varicosities are also observed in normal axons as en passant synapses or presynaptic boutons (21, 22).
Two distinct physical mechanisms have been proposed for axonal beading. In induced Alzheimer’s-like conditions or under other biochemical perturbations, organelles are found to accumulate in the swellings (2, 23). In cases in which traumatic injury is mimicked by applying sudden stretch, microtubule splaying or breakage within the swellings has been reported (16, 18, 20). These observations led to a “traffic jam” hypothesis that suggests that the beads are the result of local accumulation of organelles resulting from defects or breakage along the microtubule tracks (2). A different mechanism has been proposed for beading resulting from stretch injury of nerves or sudden changes in external osmolarity that could occur, for example, as a result of inflammation (24, 25, 26). In this model, increased membrane tension drives a periodic modulation of the axonal diameter by a mechanism akin to that previously studied in synthetic lipid membrane tubes and cell protrusions (27, 28). In the case of axons under hypoosmotic stress, theoretical analysis points toward an interplay between cytoskeletal elastic stresses and elevated membrane tension, which leads to a tubular-to-peristaltic shape transition, which agrees quantitatively with those experiments (25).
Axonal retraction, on the other hand, occurs extensively as a natural process during development, a process known as pruning, apart from being induced by injury (Wallerian degeneration) or diseases like peripheral neuropathies (29, 30, 31). It is also an essential process in maintaining neural plasticity in the brain. Axonal atrophy due to neurodegenerative conditions were initially thought to occur via damage to the soma (30). Recent studies, especially those using chemical isolation of the cell body, have, however, revealed a “dying-back” process initiating at the axon’s distal end (29, 30, 31). Microtubule breakdown occurs early during this process (17). The interplay between cortical F-actin and microtubules, axon-substrate interactions, or motor activity dictate the dynamics of axonal retraction (32, 33, 34). In addition, axonal degradation via retraction or beading can also be triggered by axonal crush or transection due to injury, a process known as Wallerian degeneration. Extensive degradation of microtubules, possibly due to elevated Ca2+ triggered by transection, is observed in these degenerating axons (17).
Recent studies have revealed that shape transformations occur early in diseased conditions and are not signatures of apoptosis (30). Despite a remarkable range of conditions leading to axonal atrophy via these shape modulations, the biomechanical factors driving axonal loss remain largely unknown. Although the molecular pathways responsible for neurodegeneration are diverse, cytoskeletal disruption, especially that of microtubules, occurs early and is responsible for the eventual loss of the axon through beading or retraction. Hence, understanding how cytoskeletal integrity influences axonal stability is critical in devising strategies to prevent, arrest, or even reverse neurodegeneration (29, 30, 31).
In this article, we report a time-resolved investigation aimed at understanding the biomechanical responses of axons that lead to axonal atrophy when the cytoskeleton is perturbed using either pharmacological agents or laser ablation. We first show that destabilized axons exhibit two distinct shape modes. Direct disruption of microtubules using nocodazole (noco) results in a peristaltic shape mode or beading characterized by the formation of axonal swellings arising progressively from the distal to the proximal side. The swellings drift in a retrograde direction on the average and coalesce over time, leading to eventual axonal atrophy. Perturbation of F-actin, on the other hand, leads to a retracting front, again initiated at the growth cone, but characterized by a single sharp variation in caliber. In both cases, the outer membrane tube remains intact over the entire length of the axon and only varies in caliber. By combining experimental data and theoretical considerations, we argue that these shape evolutions are driven by membrane tension. Further, we show that gradients in microtubule stability is responsible for setting the retrograde directionality of atrophy, and the nature of degradation of the microtubule core dictates whether atrophy occurs via beading or retraction. This study arrives at some general principles that dictate axonal shape stability and provide a framework to eventually connect molecular-level process to axonal biomechanical responses that drive axonal atrophy.
Materials and Methods
Cell culture
Dorsal root ganglia from day-8 chick embryo were incubated in 0.25% trypsin-EDTA (15400; Gibco, Gaithersburg, MD) for 10 min, dissociated in Hanks’ Balanced Salt Solution (without Ca2+, Mg2+; 14175-095; Gibco), and plated on clean, uncoated glass coverslips. For plating, L-15 medium (11415; Gibco) made viscous using Methocel E4M (ID 34516; Colorcon, Harleysville, PA) at 0.3 g to 50 mL, stirred overnight at 4°C, and supplemented with 10% heat inactivated fetal bovine serum (10100; Gibco), 33.3 μM D-Glucose (G6152; Sigma-Aldrich, St. Louis, MO), and Nerve Growth Factor 7S (13290-010; Invitrogen, Carlsbad, CA) at 20 ng per mL was used. A good growth of axons (∼150 μm or longer) is seen after 14 h of incubation at 37°C. Before experiments, cells were incubated for 20 min in growth medium as above but lacking Methocel.
Drugs
Noco (M1404; Sigma-Aldrich), Latrunculin-A (Lat-A) (L12370; Life Technologies, Carlsbad, CA), blebbistatin (blebbi) (B0560; Sigma-Aldrich), and Taxol (T7402; Sigma-Aldrich) were dissolved in dimethyl sulfoxide (DMSO) with final DMSO kept below 1% v/v. DMSO control was performed at 1% DMSO. Y-27632 (Y0503; Sigma-Aldrich) stock was prepared in water. All drugs were tested on chick fibroblasts for known responses.
Immunolabeling and Ca2+ imaging
Cells were fixed using 0.05% EM-grade glutaraldehyde (16200; Electron Microscopy Sciences, Hatfield, PA), permeabilized using 0.5% Triton X-100 (8787; Sigma-Aldrich), and exposed to 5% heat inactivated goat serum (ab7481; Abcam, Cambridge, United Kingdom) for 1 h at room temperature (all in PHEM buffer). After rinsing, the cells were exposed to the primary antibody, DM1A mouse monoclonal anti-α-tubulin (T6199; Sigma-Aldrich) or mouse antigen antibody against neurofilament (Developmental Studies Hybridoma Bank: 3A10), at 1:1000 overnight at 4°C. Cells were rinsed and exposed to the secondary antibody Alexa Fluor 488 (A-11001; Molecular Probes, Eugene, OR) at 1:10,000 for 1 h in the dark. After rinsing, the cells were imaged using Andor iXon 885 electron-multiplying CCD camera at 100× magnification. For F-actin labeling, cells were permeabilized using 1% saponin, exposed to rhodamine-phalloidin (77418; Fluka Sigma-Aldrich, Seelze, Germany) at 0.025 μg per mL for 20 min at room temperature, and then rinsed. Calcium levels in axons were imaged by preloading the cells with Fluo-4 AM (F14217; Invitrogen) at 0.1 μM concentration. Images were taken using a Leica TCS SP8 (Wetzlar, Germany) confocal system before and after ablation with exactly the same imaging parameters.
Visualization of microtubule tracks
Two techniques were used for this. 1) Cells expressing tubulin-GFP were treated with 10 μM noco for 5–7 min, fixed (as above) to retain the overall beaded shape and permeabilized using 1% saponin for 1 min to get rid of free tubulin that otherwise causes strong background. 2) After noco treatment as above, cells were treated first with a mixture of 1% saponin and 10 μM Taxol for 1 min for complete removal of membrane and tubulin monomers without losing microtubules and then fixed. In both cases, images were acquired at 63×, 1.4 NA, using Z-stack feature of a Leica TCS SP8 confocal system.
Tubulin-GFP cells were obtained by transfecting cells with the plasmid using cuvette electroporation (NEPA21; Nepa Gene, Ichikawa, Japan). These cells were used after 48 h of incubation. The construct was tested using chick fibroblasts and growth cones where individual microtubules are clearly visible.
Laser ablation
Nd:YAG laser (Spotlight 600; InnoLas, Munich, Germany) with wavelength 355 nm (ultraviolet) and pulse width 6 ns was used for ablation. The beam was directed into the back port of an inverted microscope (iX71; Olympus, Tokyo, Japan) using steering mirrors and reflected by a dichroic mirror into the objective’s back aperture. Imaging was done using either a 40× (NA 0.7) or a 20× (NA 0.45) ultraviolet-compatible Olympus objective and a charge-coupled device camera (Optikon/PCO, Bavaria, Germany). An infrared lamp was used to keep the neuronal sample at 35 ± 2°C as measured using an immersed tiny platinum resistor. For Ca imaging, ablation was performed using a 355 nm, 25 μJ, 350 ps pulsed laser (PowerChip PNV-M02510-100; Teem Photonics, Meylan, France) coupled to a Leica TCS SP8 confocal microscope. The temperature was set to 37 ± 0.1°C using the microscope incubation chamber.
Image analysis
Kymograph was prepared using phase-contrast images recorded at 5 fps after the addition of noco at 16.6 μM and a custom program written in MATLAB (The MathWorks, Natick, MA). Shape analysis of the beads was performed using a custom edge detection algorithm written in MATLAB. Edges were detected by finding the peaks in intensity gradients across the axon. The detected edge was fitted to a seventh degree polynomial to get a smooth boundary before calculation of principle curvatures.
Results
Investigation of axonal beading caused by microtubule depolymerization
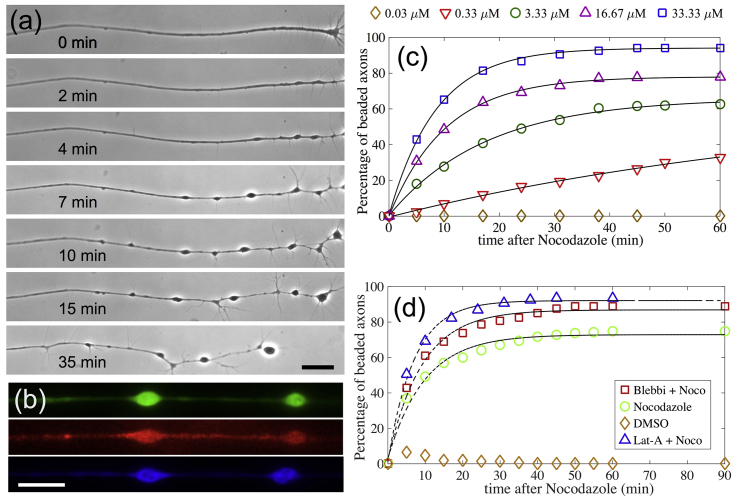
Typical in vitro cultures of chick dorsal root ganglia neurons show axonal growth of 100–150 μm overnight. When subjected to the drug noco, which disrupts microtubules, these axons develop a modulated shape with a series of bulges separated by thinned segments as shown in Fig. 1 a. This morphology is termed axonal beading. We observe that the process of shape change is always initiated near the growth cone and proceeds toward the soma (Fig. 1 a). Beads move along the axon bidirectionally at short timescales (tens of seconds), but there is a net drift toward the soma at the timescale of several minutes (Fig. 1 a). This movement of beads leads to coarsening via bead coalescence. At much longer times, of the order of 30–60 min, the net retrograde migration of beads results in complete axonal atrophy, leaving behind a trail of a thin membrane tub, as can be seen better in Videos S1 and S2. Fluorescence microscopy images revealing how cytoskeletal components are distributed in the beaded axons are shown in Fig. 1 b.
Figure 1.
(a) Progression of nocodazole (noco)-induced beading in an axon. The time after the addition of noco is indicated, and the scale bar represents 20 μm. Note that beading starts from the growth cone end (right) and progresses toward the soma (outside the field of view). This progression happens via retrograde bead migration, coarsening due to merging of beads, and formation of new beads. A thin membrane tube is left behind as beads migrate and coarsen (seen better in Videos S1 and S2). Further quantification of the bead distribution is provided in Fig. S1. (b) Fluorescence images show the distribution of tubulin (green), F-actin (red), and neurofilaments (blue) in beaded axons (scale bars, 20 μm). (c) The percentage of beaded axons over time for different noco concentrations is shown. About 150 axons were recorded for each concentration. The black lines are fits to the function This yields a parameter τ, the beading time, which characterizes the axonal susceptibility to beading. (d) The percentage of beaded axons for combinatorial drug treatments is shown. Latrunculin-A (Lat-A) was used at 1 μM and blebbistatin (blebbi) at 30 μM; these pretreatments were followed by noco at 16.67 μM.
The axon has not been completely transected and the retracting segments are connected by a thin tube. Time is shown in min:sec
The thickness of the axon is about a micron and the duration of the video is about 1 min. Time is in min:sec.
We then quantified the time evolution of noco-induced axon beading and what influence the actomyosin cortex has on these dynamics. The percentage of beaded axons as a function of time for different drug concentration is shown in Fig. 1 c. It can be seen from the exponential fits that the beading transition follows first-order kinetics with a threshold concentration between 0.03 and 0.3 μM. The choice of fit is justified by noting that the appearance of a bead after noco treatment is expected to follow Poisson statistics. No beading is seen at 0.03 μM for over an hour of exposure time. The exponential dependence allows for a precise quantification of timescale for beading, which is a measure of the axonal stability to treatments, and are 83.4 min (0.33 μM), 17.6 min (3.33 μM), 10.3 min (16.67 μM), and 8.5 min (33.33 μM). An analysis of this beading time plotted as a function of noco concentration yielding a measure of the rate of microtubule turnover in live axons is described in Appendix 1. The characterization in Fig. 1 c helps us to quantitatively investigate how disruption of F-actin or blocking of myosin-II affects beading. For this, axons were pretreated with either 1 μM Lat-A for >10 min or 30 μM blebbi for >20 min and then subjected to 16.67 μM noco. As can be seen from Fig. 1 d, these pretreatments resulted in an increase in the rate of beading, with a characteristic time of 7.0 min for Lat-A and 9.1 min for blebbi as compared to 10.3 min for control cells. Further quantification of the bead distribution is provided in Fig. S1. To cross-check the blebbi experiment, we pretreated cells with the Rho kinase inhibitor Y27632 to reduce myosin light chain phosphorylation. This pretreatment did not have any noticeable effect on the total number of beaded axons (Table S1). Note that blebbi alone did not cause any axonal beading (Table S2) and although Lat-A causes axonal retraction as discusses later, no significant retraction occurs at early stages, enabling us to quantify beading.
To understand why beading initiates from the growth cone side, we performed local application of noco using a dual micropipette technique (see Fig. S2). No beading could be observed when the midsections or the proximal sections were exposed to the drug (Fig. S2). However, when growth cones and the adjacent axonal shafts were exposed to noco, beading started within a minute. This suggests that there is a growth-cone-to-soma stability gradient for microtubules in the axon.
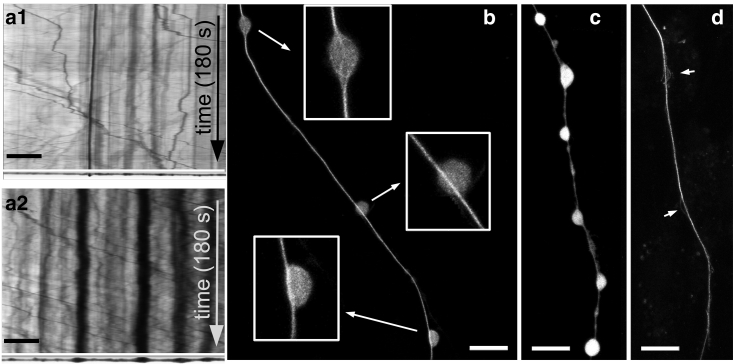
To test the prevailing hypothesis of axonal beading being caused by traffic jams in axonal transport (2, 20, 23), we recorded transport using both the phase-contrast method and using GFP-labeled synaptic vesicles (Videos S3, S4, and S5). Although there is a tendency for organelles to pause within the swellings, several organelles can be seen passing through the beads unhindered during the early stages of noco-induced beading as also shown in the kymograph (Fig. 2 a). Because phase-contrast images can only capture large and optically dense organelles like mitochondria, we also carried out imaging of tiny synaptic vesicles using synaptophysin-GFP. As can be seen from Video S5, these vesicles too do not show any significant accumulation within beads. In both cases, organelles occupied only a small fraction of the bead volume. Fluorescence imaging of microtubules in axons soon after beading clearly shows that continuous tracks of microtubules are left intact in most beads (n = 56 out of 75), as can be seen in Fig. 2, b–d. It has been reported previously that posttranslational modifications render additional stability to a fraction of microtubules in axons, which are depolymerized only upon prolonged exposure to noco (35). Taken together, these observations suggest that accumulation of organelles is not the primary cause for noco-induced beading but is rather a consequence of beading.
Figure 2.
(a) Phase-contrast kymographs of a control axon (a1) and a beaded axon (a2). The images of the axon after 180 s of recordings are shown at the bottom of the respective kymographs. The beaded axon too shows frequent transport of organelles across beads. Time is as indicated and the scale bar represents 10 μm (also see Videos S3, S4, and S5). (b) Cells with tubulin-GFP were fixed, and free monomers were removed by mild saponin treatment to reveal inner details (see Materials and Methods). Contiguous microtubule bundles are often visible across beads (enlarged in lower insets) (n = 56 beads out of 75). A bead with disrupted microtubules is also shown (top inset). (c and d) Continuous microtubule tracks in beaded axons are also seen after a complete removal of the membrane using saponin + Taxol and then fixing. Images (c) and (d) were taken before and after this procedure on the same axon, respectively. Arrows indicate splaying of microtubules seen in some beads. Scale bars, 10 μm.
Time is in hr:min:sec.
The recording started 1 min after Noco was added to the dish. The onset of beading can be seen around 2 min 24 s for both the axons, starting at their respective growth cone ends. Beading progresses with coarsening and retrograde migration (t ∼ 20 min onwards). At 31 min 42 s the FoV is changed to the location of the soma. Time is shown in min:sec.
The recording was started at 35 min after Noco was added to the dish. Thus, beading can be seen already in the beginning of the movie. A clear retrograde migration of the beads can be seen throughout the movie, leaving behind a thin membrane trail. Time is shown in min:sec, and the scale bar is 10 micron.
We next looked at whether noco treatment increases membrane tension and thus would be the reason for beading (see Appendix S1 for reasoning). Indeed, a rapid increase in axonal membrane tension in conditions of hypoosmotic shock has been shown to trigger axonal beading (25, 26). In noco-treated axons, such an increase in the membrane tension may occur because of generation of tubulin subunits leading to osmotic stress. The method of pulling tethers from axonal membranes allows us to estimate the membrane tension for noco-treated and beaded axons (n = 12) to be 7.7 ± 4.2 μNm−1 in comparison with the control (n = 15) 5.6 ± 1.8 μNm−1 (see Fig. S3 for data) (36). In contrast, the critical membrane tension required to cause beading of an axon with unperturbed cytoskeleton is estimated to be of the order of 3 × 10−4 N/m (25). Thus, the small rise in membrane tension caused by noco treatment alone is insufficient to cause axonal beading. However, as will be elaborated later, microtubule disruption drastically lowers the threshold tension because of a reduction of the bulk elastic modulus.
Actin disruption causes microtubule-dependent axonal retraction
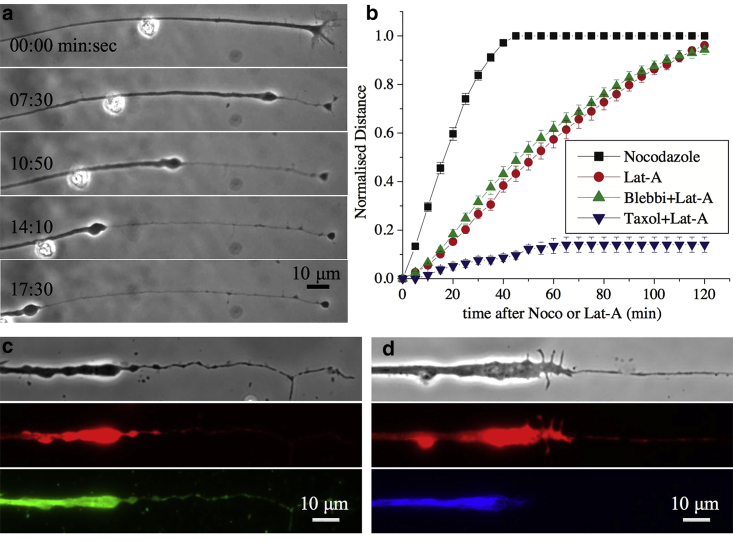
To see how F-actin disruption affects axon response, cells were treated with 1 μM of Lat-A. In this case, axons exhibit dynamics very different from noco-induced beading, as can be seen from Fig. 3 a. Initially, the growth cone collapses, and its contents gradually accumulate and develop into a retracting front characterized by an abrupt change in radius. This single front propagates toward the soma, leaving behind a thin trail of membrane (Fig. 3 a; Video S6). In this respect, these dynamics may differ from the usual axon retraction in which the growth cone detaches and retreats. Occasionally, small beads appear close to the retracting front, but these quickly merge with the front (Video S6). The retraction speed, as can be deduced from Fig. 3 b, remains almost constant until the entire axons becomes atrophied. The relative motion of the front and the extraneous particle seen in Fig. 3 a suggests an extended flow field with velocity decreasing away from the front. Fluorescence microscopy images of F-actin, microtubules and neurofilaments in partially retracted axons show that all three cytoskeletal components are depleted in the thinned-out segment of the axon (Fig. 3, c and d). This shows that either cytoskeletal filaments are depolymerized or are being squeezed back along with the retraction.
Figure 3.
(a) A sequence of images showing axonal retraction in response to treatment with F-actin-disrupting drug Latrunculin-A (Lat-A) at 1 μM. The retraction front characterized by a sharp change in radius propagates toward the soma, leaving a thin tube behind. Note that frames two to five have the same time gap. The relative movement of the front and the extraneous particle indicate a flow profile with decreasing velocity away from the front (also see Video S6). (b) Plots show the retracted length (thinned segment) measured from the growth cone end and normalized with total axon length for control axons, those pretreated with the myosin-II inhibitor blebbistatin (blebbi), and those pretreated with the microtubule stabilizer Taxol. The length of the axonal segment over which beading can be seen after treatment with noco, also measured from the growth cone, is shown for comparison. Each curve is an average taken over n > 20 axons, and the error bars are standard errors of the mean. The nonnormalized and nonaveraged data for Lat-A and noco are shown in Fig. S4. (c and d) Phase-contrast (grayscale) and corresponding fluorescence images show F-actin (red) and microtubule (green) on the left side and F-actin (red) and neurofilament (blue) on the right side.
The recording was started 5 min after adding 16.6 μM of Noco. Images were recorded using an 100X objective and the image histogram was adjusted by setting a minimum and maximum cutoffs to highlihgth the dense particles. Time in hr:min:sec.
To check if microtubules are being depolymerized during retraction, neurons were pretreated with the microtubule-stabilizing drug Taxol. Surprisingly, when axons (n = 27) were treated with Taxol at 10 μM for 15 min before the addition of Lat-A, it prevented axonal retraction except for a collapse of the growth cone (Fig. 3 b; Video S7). This suggests that Lat-A treatment also affects the microtubule stability in axons via an unknown signaling mechanism (37, 38). Retraction involves microtubule disassembly rather than intact filaments being squeezed out along with the front, unlike in retraction induced by nitric oxide (33). F-actin filaments, however, do not undergo complete disassembly and may be partly squeezed back, as can be seen from rhodamine-phalloidin labeling, which marks only the polymer form of the protein (Fig. 3, c and d).
Time is in hr:min:sec:m-sec, and the scale bar is 10 microns.
We next explored the influence of myosin-II in Lat-A-induced axon retraction. We found that the retraction rate is slightly enhanced by treatment with blebbi (Fig. 3 b). Blebbi alone did not cause any retraction events (Table S2). Reducing myosin light chain phosphorylation by pretreating cells with Y27632 also did not affect the occurrence of retraction events (Table S1). Thus, as in the case of beading, myosin-II activity does not play a major role in this type of axon retraction, although contractility has been implicated in spontaneous axonal shortening (39). We note that others have reported Lat-A causing growth cone collapse but without any retraction. These differences may be due to the duration of treatment or to the differences in culture conditions (34, 40).
Noco- and Lat-A-induced morphological transitions, vis-à-vis beading and retraction, show interesting concentration dependences. When neurons were treated with a much higher dose of Lat-A (10 μM), axons started to show more frequent beaded structures near the retraction front (Table S2). In some rare cases, beading was extensive (Fig. S5). Conversely, when cells were treated with very low concentrations of noco, a small fraction of axons showed retraction, without clear beading (Fig. S5; Table S2). Taken together with the fact that Taxol arrests Lat-A-induced retraction, these results suggest a common microtubule-dependent mechanism for both retraction and beading, and this will be elaborated in the Discussion.
Laser ablation causes bidirectional beading or retraction of axons
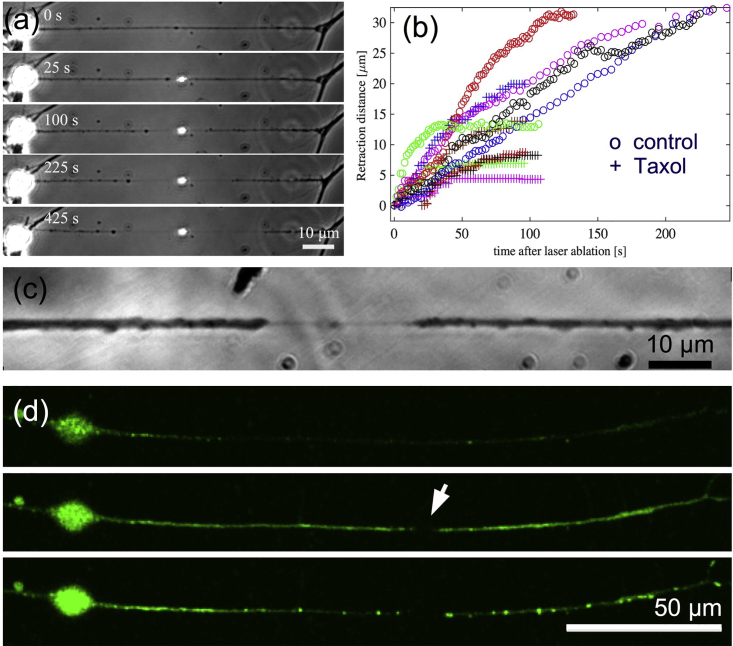
As mentioned earlier, biochemical perturbations always trigger shape changes in axons from the growth cone end. As shown by local drug treatment (see Fig. S2), this breaking of symmetry arises because of a gradient in cytoskeletal stability. Because axonal microtubules are arranged in a polar fashion, a directionality arising from axonal polarity also needs to be investigated. Because local application of drugs to midsections did not evoke any response, we resorted to point laser ablation. When ablated, most axons undergo complete transection and buckling (Fig. S6, A and B). Every transected axon exhibited a rapid (<1 s) shortening of length, which we call “snapping,” followed by a slower retraction. The snapping response, quantified in Fig. S7, and buckling show that axons are under tension before ablation. Interestingly, a subset of ablated axons does not undergo complete transection, presumably because of a lower laser power resulting from variations in focusing of the laser on the axon. In such “partial cuts,” axonal segments on either side of the ablation point undergo thinning via either retraction or beading. This thinning response is initiated at the point of ablation and widens with time (Fig. 4, a and c; Videos S8 and S9).
Figure 4.
(a) Image sequence of an axon subjected to a single-shot “partial laser ablation,” which leaves the membrane tube intact. The membrane tube starts thinning down, starting from the point of ablation, and this section widens with time. Beading is visible on the left side and retraction on the right in this example. The bright spot is due to the scattering of microscope light from the laser damage of the glass coverslip. (b) A plot of retraction distance measured from the point of cut as a function of time for control axons (open circles) and for axons treated with Taxol (+), obtained using partial cuts, is shown. (c) An image of another partially cut axon recorded at higher magnification shows the thin tube between the two retracting fronts. (d) Ca2+ imaging in partially cut axons (n = 6 out of 6) shows an increase in Ca2+ levels immediately after ablation (midframe with arrow indicating the ablation point) and subsequent concentration of Ca2+ in puncta along the axon after ∼1 min, presumably due to sequestering of Ca2+ into stores. To see this figure in color, go online.
Beading progresses during the course of the movie. Time is in hr:min:sec, and the scale bar is 10 microns.
In the first few frames the growth cone deforms and shrinks. Later the contents begin to retract leaving behind a much thinner trail of membrane. In another vertically oriented axon in the same movie, the retraction front can be very clearly seen from 16 min onwards. The time is shown in hr:min:sec, and the scale bar is 10 microns.
To proceed further, we defined a retraction front based on the change in axon caliber, as in the case of Lat-A-induced retraction. This retraction behavior for control axons is quantified in Fig. 4 b. Most axons undergo complete retraction. Interestingly, pretreatment with Taxol, which stabilizes microtubules, halts the retraction much earlier compared to untreated cells, as is shown in Fig. 4 b. This suggests that ablation affects microtubule stability in axons, presumably because of the creation of unprotected ends and membrane-activated Ca2+ influx as reported in literature (19, 41, 42). Indeed, fluorescence imaging of Ca2+ shows a marked elevation in Ca2+ levels subsequent to ablation (Fig. 4 d). These experiments show that even though axonal microtubules are arranged in a polar fashion with their plus ends pointed toward the growth cone, this polarity is not responsible for setting the propagation direction of beading or retraction. Instead, microtubule stability gradients, due to cut ends and Ca2+ influx in this case, dictate the direction of propagation of axonal atrophy.
Axonal actin-spectrin skeleton affects beading and retraction dynamics
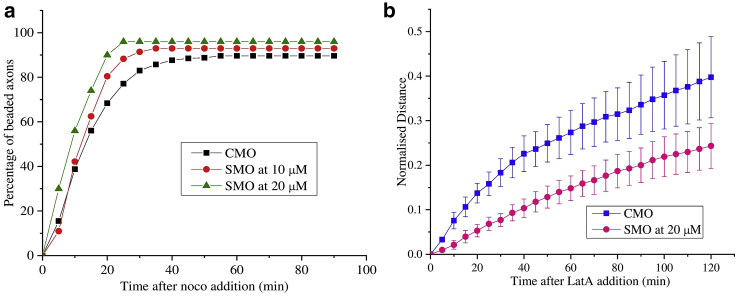
Next, we explore the effect of the recently discovered axonal actin-spectrin membrane skeleton on beading and retraction dynamics (43). This skeleton forms a one-dimensional periodic scaffold and is known to influence axonal caliber (44), circumferential tension (45), and longitudinal viscoelasticity (46). For this, we perform spectrin knockdown experiments by treating the cells with β-II spectrin specific morpholino (SMO) for 48 h (see (46) for details). Axons thus treated were subjected to cytoskeletal perturbations using either 16.6 μM of noco or 1 μM of Lat-A. For comparison, experiments were also done using a nonspecific control morpholino. All knockdown experiments were performed on 2-DIV cells because the spectrin lattice was seen to be more prevalent in them (see (46) for quantification). The results obtained are shown in Fig. 5, a and b.
Figure 5.
(a) Percentage of beading exhibited by neurons pretreated with either β-II spectrin knockdown morpholino (n = 128) for 10 μM SMO and n = 50 for 20 μM SMO or 20 μM control morpholino (n = 154) and then exposed to 16.6 μM noco. (b) Averages of the retraction distances (length of the thin segments) normalized with the corresponding initial axon lengths for spectrin knockdown axons (n = 5) and control axons (n = 5) exposed to 1 μM Lat-A (error bars are standard errors) are shown. To see this figure in color, go online.
It can be seen from Fig. 5 a that axons with disrupted spectrin skeleton show a relatively higher percentage of beading for a given exposure time to noco compared to the control. The total number of beaded axons at saturation (long time) too increases with a higher extent of knockdown. When spectrin knockdown treatment was applied to axons before treating with Lat-A, a marked reduction in the retraction speed is observed (Fig. 5 b). These experiments clearly show that the axonal spectrin skeleton does play a role in axonal stability against beading and retraction induced by these drug treatments.
Discussion
Microtubule disassembly modifies axon shape
Axonal beading and retraction are hallmarks of a wide variety of neurodegenerative conditions that lead to axonal atrophy. Such commonality suggests a general mechanism behind these morphological changes. However, aside from the hypothesis that traffic jams of organelles are responsible for beading, there is no mechanistic understanding of such axonal shape modifications. Nonetheless, because cytoskeletal changes are known to be implicated in a variety of cell deformations, we inquired into how different types of perturbations to the axonal cytoskeleton might affect axon shape.
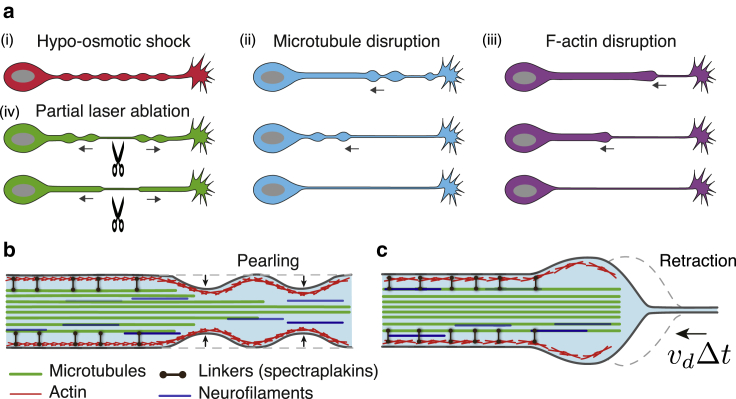
The three types of axonal shape change experiments performed in this study are summarized in Fig. 6 a. These experiments (noco-induced beading, Lat-A-induced retraction, and laser ablation) all involve modifications to the axonal cytoskeleton. Moreover, they all illuminate a key role played by microtubules in maintaining axon integrity, as revealed by Taxol pretreatments. In the case of noco treatment, which directly disassembles microtubules, loss of axon integrity occurs through a sequence of shape transformations involving the appearance of “beads” (or “pearls”). In contrast, when axons are treated with Lat-A, whose primary target is F-actin, they undergo a retraction process involving a front moving from the growth cone toward the soma that separates a thick, cytoskeleton-filled axon from a cytoskeleton-devoid membrane tube. Disassembly of microtubules after Lat-A is an indirect consequence, possibly arising from an interdependence between F-actin and microtubule mediated by other molecular players like tip-binding proteins, spectraplakins, etc. (37, 47, 48). It is also known that freshly formed tyrosinated microtubules depolymerize within 10 min of treatment with Lat-A (38). Consistent with these results, retraction is significantly impeded by Taxol treatment, which is known to stabilize microtubules against disassembly. Moreover, axon retraction after partial laser ablation can also be largely blocked by previous Taxol treatment. Taken together, these experiments point to a key role played by microtubules in maintaining axon shape and caliber.
Figure 6.
Shape transformations observed in axons under different induced conditions. (a) Axon beading or retraction occurs after four types of perturbation: (i) hypoosmotic shock produces a transient, periodic, nonpropagating peristaltic mode (25). Moreover, this shape transformation occurs simultaneously along the entire axon and is transient (Video S10). (ii) After microtubule disruption using noco, beading always begins from the growth cone end and proceeds toward the soma. Finally, only a thin tube is left behind. (iii) In the case of F-actin disruption, a retracting front forms near the growth cone and proceeds toward the soma, finally leaving a thin tube behind. (iv) In the case of partial laser ablation, either beading or retraction occurs. In both cases, the ablation point thins down and expands with time, causing atrophy. Beading induced by Lat-A and laser ablation are prevented by Taxol pretreatment. (b) Experiments suggest that noco treatment results in a radially shrinking microtubule bundle, separating it from the actin-spectrin-membrane skeleton. Membrane tension renders the resulting fluid contained between the membrane and remaining microtubule core susceptible to Rayleigh-Plateau instability. Axon beading thus occurs, which propagates from the thinner end of the bundle (near the growth cone), to the thicker one (near the soma). (c) Upon Lat-A treatment, axon cytoskeleton is disassembled in a front-like manner. This front separates a thin tube from a cytoskeleton-filled axon nearer to the soma.
Microtubule stability gradients set direction of beading and retraction
In both noco-induced beading and Lat-A-induced retraction, axonal shape change is initiated near the growth cone and then proceeds toward the soma, accompanied by a flow of axoplasm and organelles (Figs. 1 a and 3 a). Is this retrograde directionality set by microtubule polarity or by microtubule stability? Local application of noco using the dual micropipette arrangement shows that the cell shape is more readily affected at the growth cone end when compared to the rest of the axon, showing the existence of a stability gradient or a lower stability at the distal end, which probably initiates a depolymerization process (Fig. S2). These findings are consistent with studies reporting a correlation between microtubule stability and levels of posttranslational modification or turnover rate, with less stable microtubules occurring closer to the growth cone (38, 49). They are also consistent with our observations that Taxol treatment arrests Lat-A-induced retraction from the growth cone to the soma, indicating that microtubule stability gradient plays a role in setting the direction of front motion. Finally, upon partial ablation in the middle of the axon, retraction proceeds equally on both sides of the cut, and this retraction can be opposed by Taxol treatment. In this case, additional stability gradients are generated by the ablation via the creation of a large number of unstable microtubule ends and an influx of Ca2+ as shown in Fig. 4 d and reported by others (19, 41, 42). Taken together, these experiments show that microtubule stability gradients dictate the direction of propagation of beading or retraction.
Axon beading is not a result of traffic jams
Based on the observation of accumulated organelles and hindered transport, it has been proposed that localized disruption of transport or traffic jams may be the cause for beading in some cases (2). This is an attractive model considering the fact that microtubule breaks or disorganization has been observed in axons with mechanically induced injury-like conditions (18, 19, 20). However, we note the following from our experiments. 1) Continuous microtubule tracks are seen in a large fraction of beads (n = 56 out of 75). 2) Consistent with this, imaging of organelles shows little hindrance at the early stages of beading. No accumulation of synaptic vesicles is seen in beads at early stages. 3) Bead locations are not fixed and beads migrate along the axon as they form. When beads move, fresh beads do not appear in place of the old ones, as is expected if microtubule defects dictate the location of beads. 4) As will be elaborated below, the bead shapes (lemon like at early stages and clamshell like at late stages) are typical of a surface maintained under tension as opposed to a shape defined by a collection of organelles. All these observations suggest that accumulation of organelles may not be the primary cause for beading but rather a consequence of beading. Indeed, we observe splaying out of microtubule in beads, which may be caused by the expansion of the surrounding cortex (Fig. 2 d). In the next section, we argue that beading and retraction can be explained via mechanical balance between membrane tension and cytoskeletal elasticity.
Axon beading is a tension-driven shape instability
Shape transitions from cylindrical to beaded shape, known in the physics community as “pearling instability,” have been studied previously in synthetic membrane tubes (50) and have also been observed in in vitro membrane tubes with a destabilized microtubule core (51). These systems lack molecular motors, and beading is driven by membrane tension. Beading occurs also in cellular protrusions containing only F-actin (28) and in neurites subjected to osmotic shock (25, 52) (Video S10). In these systems, quantitative analyses have shown that beading occurs when membrane tension exceeds a threshold value and the periodicity of beads increases linearly with radius, as is expected for pearling instability. We observed that neurites of mouse PC12 cells exhibit beading when treated with either noco or Lat-A. After noco treatment, these neurites show a periodic shape modulation at early stages and a linear relation between modulation wavelength and initial axon radius, as expected for pearling instability (25, 52) (Fig. S8; Video S11). Thus, in all these cases, the beading process is related to the Rayleigh-Plateau instability of liquid jets (25, 52) (see Appendix S1 for a simplified explanation).
The recording was started soon after adding Lat-A. The growth cone begin to collapse as soon as Lat-A is added but no further retraction is observed. Time is in hr:min:sec.
The appearance of a bright spot indicate the point where the laser pulse has hit. The bright spot persists because the laser had caused micro-cracks on the upper surface of the coverslip resulting in scattering of light. Note that the axon is not completely transected. There is a very thin tube of membrane connecting the two thicker 11 segments on both sides. Until the thin connection between the two retracting segments is intact, the two retracting segments lie along a line. In cases where the cut is complete, the axon snaps back suddenly and also buckles (see Document S1. Supporting Materials and Methods, Figs. S1–S9, Tables S1 and S2, and Appendices S1 and S2, Video S1. Time Lapse Recording of Two Axons with their Growth Cones Located Towards the Bottom of the Field of View, Video S2. Time Lapse Recording of an Axon with the Soma on the Right as Indicated by the Arrow in the First Frame, Video S3. Phase Contrast Imaging of Transport in an Axon as it Undergoes Beading, Video S4. Time Lapse Recording of the Movement of GFP-Synaptophysin-Tagged Synaptic Vesicles in a Control Axon, Video S5. Time Lapse Recording of the Movement of GFP-Synaptophysin-Tagged Synaptic Vesicles in a Noco Treated Axon, Video S6. Time Lapse Recording of a Lat-A-Treated Axon with a Healthy Growth Cone Visible in the Beginning, Video S7. Time Lapse Recording Showing an Axon Which Was Pretreated with Taxol and Then With Lat-A, Video S8. Time Lapse Recording of Laser Ablation and Ensuing Retraction of an Axon, Video S9. Time Lapse Recording of Laser Ablation of an Axon Which Exhibit Beading in the Retracting Segments, Video S10. Time Lapse Recording of a PC12 Neurite Undergoing Pearling Instability When Subjected to an Osmotic Shock, Video S11. Time Lapse Recording of a PC12 Neurite Treated with 10 μg/ml Noco, Document S2. Article plus Supporting Material). Time is shown in min:sec.
As further evidence that axon beading is not the result of organelle traffic jams but rather results from membrane tension, we analyzed the bead shapes after noco treatment. At early stages of beading, lemon-shaped, axisymmetric beads are often seen (Figs. 1 b and S9; Videos S1 and S2). At later stages, asymmetric, “clamshell” beads are also observed (Fig. 2 b). These shapes are strikingly similar to the quasistationary liquid beads, or pearls, that arise because of the Rayleigh-Plateau instability that dewets a liquid film initially coating a cylindrical fiber (53). In this case, the shape of the pearl derives from Laplace’s law, which states that the mean curvature of the drop is given by H = P/(2σ), where P is the internal pressure. At equilibrium, P is constant, and hence so is H.
We then inquired whether axon bead shapes could also be understood from a similar analysis. We first mathematically characterized the surface of a typical axon bead by determining the radial distance, R(z), from the bead centerline to the bead contour in the image plane as a function of distance along the axon, z (see Document S1. Supporting Materials and Methods, Figs. S1–S9, Tables S1 and S2, and Appendices S1 and S2, Video S1. Time Lapse Recording of Two Axons with their Growth Cones Located Towards the Bottom of the Field of View, Video S2. Time Lapse Recording of an Axon with the Soma on the Right as Indicated by the Arrow in the First Frame, Video S3. Phase Contrast Imaging of Transport in an Axon as it Undergoes Beading, Video S4. Time Lapse Recording of the Movement of GFP-Synaptophysin-Tagged Synaptic Vesicles in a Control Axon, Video S5. Time Lapse Recording of the Movement of GFP-Synaptophysin-Tagged Synaptic Vesicles in a Noco Treated Axon, Video S6. Time Lapse Recording of a Lat-A-Treated Axon with a Healthy Growth Cone Visible in the Beginning, Video S7. Time Lapse Recording Showing an Axon Which Was Pretreated with Taxol and Then With Lat-A, Video S8. Time Lapse Recording of Laser Ablation and Ensuing Retraction of an Axon, Video S9. Time Lapse Recording of Laser Ablation of an Axon Which Exhibit Beading in the Retracting Segments, Document S2. Article plus Supporting Material). Then, by fitting the contour data (Document S1. Supporting Materials and Methods, Figs. S1–S9, Tables S1 and S2, and Appendices S1 and S2, Video S1. Time Lapse Recording of Two Axons with their Growth Cones Located Towards the Bottom of the Field of View, Video S2. Time Lapse Recording of an Axon with the Soma on the Right as Indicated by the Arrow in the First Frame, Video S3. Phase Contrast Imaging of Transport in an Axon as it Undergoes Beading, Video S4. Time Lapse Recording of the Movement of GFP-Synaptophysin-Tagged Synaptic Vesicles in a Control Axon, Video S5. Time Lapse Recording of the Movement of GFP-Synaptophysin-Tagged Synaptic Vesicles in a Noco Treated Axon, Video S6. Time Lapse Recording of a Lat-A-Treated Axon with a Healthy Growth Cone Visible in the Beginning, Video S7. Time Lapse Recording Showing an Axon Which Was Pretreated with Taxol and Then With Lat-A, Video S8. Time Lapse Recording of Laser Ablation and Ensuing Retraction of an Axon, Video S9. Time Lapse Recording of Laser Ablation of an Axon Which Exhibit Beading in the Retracting Segments, Document S2. Article plus Supporting Material), we are able to calculate the bead curvature H(z) (see Appendix S2 for details). Interestingly, there is a central region of the bead where H is approximately constant, suggesting that the bead is liquid and with a surface shape governed by a competition between internal pressure and membrane tension. However, the mean curvature increases by roughly a factor of two near the bead edges (where the axon surface is expected to interact with the remaining microtubule core), a behavior that does not occur for liquid droplets wetting a fiber. This difference may be attributed to the bending stiffness of the axonal membrane, a property not present for a liquid droplet interface (see Appendix S2).
We have seen here that noco drives a beading instability in axons, and an analysis of the bead shape and distribution suggests a tension-driven process. Broadly, there are two ways that this could be triggered. First, noco increase the membrane tension through osmotic effects arising from tubulin monomer generation, thus favoring beading. However, our optical tweezer measurements show no significant increase in membrane tension after noco treatment (Fig. S3). This, then, points to the more likely scenario, which is that once microtubules are largely depolymerized, the elastic resistance to membrane tension-driven beading is lowered. More precisely, the critical tension for pearling instability is σc ∼ ER0 (25, 28); hence, a sharp reduction in the elastic modulus E caused by microtubule depolymerization would lower the critical membrane tension, thus favoring beading.
Beading versus retraction
Although noco treatment causes axon beading, Lat-A treatment, in contrast, resulted in axon thinning via a retracting front. What might be the physical reason for this difference? Below, we argue that under the influence of an effective membrane tension, the shape mode that develops should depend on the nature of depolymerization dynamics of the cytoskeleton and how these dynamics compare to those associated with the tension-driven Rayleigh instability.
-
1)
Based on Fig. 2, b–d, which show a remnant microtubule trail after noco treatment, we propose that noco-induced disassembly occurs via radial thinning of the microtubule bundle. Because beads first appear near the growth cone, the radial thinning should occur fastest there and slowest near the soma, resulting in a shrinking conical microtubule core, with the point facing the growth cone. We furthermore assume that microtubule disassembly leaves behind in its wake a viscous fluid enclosed between the axonal membrane with its associated cortical skeleton and an intact microtubule core (see Figs. 2 b and 6 b). This fluid is assumed to contain cytosol, depolymerized cytoskeletal fragments, and neurofilaments (Figs. 1 b and 3 d show that neurofilaments are being squeezed into the beads or toward the soma, respectively). As a result of membrane tension, this fluid is unstable to pearling.
-
2)
There is a typical speed associated with the growth of axon beads. It is well known that a liquid film of thickness e and viscosity η coating a fiber core of radius ra − e is unstable for wavelengths , and these modes grow with speed (53). This is the characteristic speed associated with flow of the cytosol. Furthermore, assuming a radially tapered microtubule core and therefore a thickness of the fluid film that increases from the soma to the growth cone, beads will develop first near the growth cone and then progressively appear near the soma (54). This also accounts for the observation of bead drift (see Videos S1, S2, and S11): because beads are more developed near the growth cone and the necks that connect them are consequently thinner, the Laplace pressure increases from growth cone to soma, thus driving bead motion and coarsening (merging of fast-moving beads that are nearer to the growth cone with slower neighbors that are farther away from the growth cone). This situation is reminiscent of the dewetting of a radially tapered wire, as studied in (54).
In contrast, under Lat-A treatment, experiments suggest that actin and microtubules depolymerize completely, starting at the growth cone; hence, no remaining microtubule core is left behind (see Fig. 3, c and d). We therefore consider that Lat-A initiates a propagating depolymerization front with speed vd away from the growth cone. This depolymerization fluidizes actin and microtubules, and as a result, the depolymerized region of the axon is vulnerable to pearling instability. The velocity scale associated with the development of the instability depends on the membrane tension and the viscosity of the enclosed fluid (cytosol and fluidized cytoskeleton) and is given by . Assuming a bare membrane tension σ = 5 × 10−6 N/m and η ∼ Pa.s (55), we find v1′ ∼ 100 μm/min, which is faster than the depolymerization speed implied by Fig. 3 b (i.e., vd ∼ 100 μm/100 min ∼ 1 μm/min). This means that beading is happening very quickly compared to depolymerization and in such a way that beads coalesce fast enough to give rise to a single retracting front. The enclosed volume is then expulsed toward the soma, forming a bulge just behind the depolymerization front (see Fig. 6 c). We note further that the remaining thin tube does not undergo beading because of the stabilizing effect of the membrane bending rigidity at this small radius (56).
The role of the actin-spectrin membrane scaffold in axon shape stability
We observe that axons of spectrin knockdown neurons bead more easily compared to the control and retracts slower (Fig. 5, a and b). A passive spectrin skeleton is expected to stabilize axons against beading due to elastic effects (46). This is because the axonal spectrin tetramers are attached to the membrane by ankyrins and arranged longitudinally and hence are expected to get stretched during beading. This can explain the results shown in Fig. 5 a. Recent experiments have shown that the actin rings that interconnect spectrin tetramers influence axonal caliber (44) and exert a circumferential stress via myosin-II mediated contraction (45). It is conceivable that a circumferential stress arising out of the actin rings can contribute to the squeezing out of cytoplasm during axonal thinning. However, we do not observe any significant change in the retraction speed when myosin-II is pharmacologically inhibited (Fig. 3 b). Therefore, understanding the precise biomechanics of the actin-spectrin skeleton during beading and retraction would require further experiments and theoretical analysis that invoke its anisotropic elasticity and active response.
Conclusions
We argue that axonal beading and retraction are membrane tension-driven instabilities occurring when microtubule integrity is compromised. The rate and manner in which microtubules disassemble (radial thinning with a spatial gradient versus a decaying front) dictate the shape evolution, vis-à-vis beading or retraction. Moreover, we show that it is the lower stability of microtubules at the distal end, and not polarity, that sets the retrograde flow of material during atrophy. These morphological transitions can be understood based on membrane and cytoskeletal mechanics. Furthermore, these shape changes bear similarities to inanimate systems but with related underlying physics. For instance, both beading-like and retraction-like modes with directional propagation have been observed in the surface tension-driven dewetting of tapered, liquid-coated wires (54).
Although microtubule stability has been long recognized as a major factor affecting neurons undergoing neurodegeneration, preventing or arresting axon loss using microtubule-stabilizing drugs has been met with limited success (57). Although Taxol seems to prevent axonal loss in short-term treatments like the one reported here, it is well known that prolonged anticancer treatment using Taxol and other microtubule-stabilizing agents causes chemotherapy-induced peripheral neuropathy (58). Our study shows that axonal beading occurs even when a contiguous microtubule core remains. This is because the core becomes disconnected from the surrounding F-actin and therefore also from the membrane, making the membrane unstable to beading. Moreover, we show that destabilization of F-actin triggers axonal atrophy by disrupting microtubules via unknown signaling pathways. Therefore, despite the central role played by microtubules in dictating the shape evolution, it is necessary to consider the stability of the microtubule-actin-membrane composite and not just microtubules in isolation. These interactions are mediated by a large number of other proteins (37). Thus, understanding the biomechanics of axonal shape evolution under conditions that perturb microtubules, as discussed in this article, provides a framework to connect diverse molecular-level processes to general biomechanical principles that drive axonal atrophy.
Author Contributions
A.D. and P.A.P. designed research. A.D., J.A., A.B., R.S., A.M., and R.B. performed research and analysis. J.P., A.C.-J., and P.A.P. did theoretical analysis/interpretation of data. A.D., A.C.-J., and P.A.P. wrote the article.
Acknowledgments
We thank Mohammad Ashraf Arsalan for the preparation of the kymographs, Serene Rose David for help with experiments, Manoj Mathew and Sandhya Kaushika for facilitating the usage of the laser ablation setup, Veena Chatti for help with transfection protocol, and Thomas Bornschlögl and Patricia Bassereau for facilitating usage of the laser tweezer setup. A.C.-J. thanks Adrien Daerr and Joshua McGraw for helpful discussions.
R.S. was supported by a research grant from the Department of Biotechnology, Government of India (BT/PR13310/GBT/27/245/2009). P.A.P. acknowledges the Department of Science and Technology, Government of India, for support via the Ramanujan Fellowship. R.B. acknowledges funding from DICYT-USACH project 041731BV.
Footnotes
Alka Bhat’s present address is Laboratory of Cell Physics, Institut de génétique et de biologie moléculaire et cellulaire, University of Strasbourg, Strasbourg, France.
Editor: Jochen Guck.
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2019.07.046.
Contributor Information
Andrew Callan-Jones, Email: andrew.callan-jones@univ-paris-diderot.fr.
Pramod A. Pullarkat, Email: pramod@rri.res.in.
Appendix 1: Quantification of Microtubule Depolymerization Dynamics
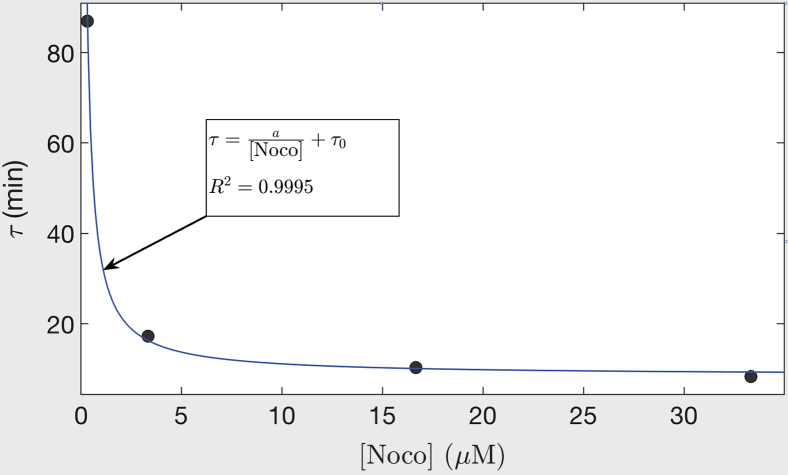
The time constant τ([noco]), corresponding to the rate of beading at different noco concentrations and obtained from the fits shown in Fig. 1 c, is plotted against noco concentration in Fig. 7. These data fit well to the equation , where a = 26 and τ0 = 8.5 s are constants. Saturation of the curve to τ0 indicates that there is a rate-limiting step in the noco-induced depolymerization process. The diffusion of noco into the axon happens within a minute as per the response of the growth cone end of the axon observed in local drug experiments. This is fast compared to the time taken for beading, which is of the order of 10 min at the highest noco concentration (see Fig. 1 c). Therefore, we conclude that the rate-limiting step has its origin in the turnover rate of microtubules in the axons. This is because noco binds to tubulin in a 1:1 ratio, and at high concentration, all αβ-subunits are bound to noco and incapable in participating in the polymerization process. In such a scenario, the beading rate is dictated by microtubule depolymerization rate alone. This allows us to obtain an estimate for the microtubule turnover rate in live axons, which is otherwise difficult to quantify. If one supposes that the average length of microtubules is L ∼ 5 μm (59) and the bare shrinkage rate (i.e., without any noco) is Ls ∼ 500 nm/s (60), then koff should be of the order of Ls/L ∼ 0.1 s−1. The inverse of this gives 10 s, which is close to the value of τ0 obtained from the fit in Fig. 7. Thus, quantifying the beading percentage under the action of noco provides a powerful assay to quantify the stability of microtubules in axons. It can be used to study the effect of posttranslational modifications to microtubules on their stability or to screen anticancer drugs for their potential to cause neuropathy.
Figure 7.
Plot of the beading time constant τ obtained from the fits shown in Fig. 1c plotted against noco concentration ([Noco]). The data are fitted to the equation shown in the figure and discussed in the main text. To see this figure in color, go online.
Supporting Material
References
- 1.Yagishita S. Morphological investigations on axonal swellings and spheroids in various human diseases. Virchows Arch. A Pathol. Anat. Histol. 1978;378:181–197. doi: 10.1007/BF00427359. [DOI] [PubMed] [Google Scholar]
- 2.Stokin G.B., Lillo C., Goldstein L.S. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 3.Tseng J.H., Xie L., Cohen T.J. The deacetylase HDAC6 mediates endogenous neuritic tau pathology. Cell Rep. 2017;20:2169–2183. doi: 10.1016/j.celrep.2017.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohgami T., Kitamoto T., Tateishi J. Alzheimer’s amyloid precursor protein accumulates within axonal swellings in human brain lesions. Neurosci. Lett. 1992;136:75–78. doi: 10.1016/0304-3940(92)90651-m. [DOI] [PubMed] [Google Scholar]
- 5.Gowrishankar S., Yuan P., Ferguson S.M. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proc. Natl. Acad. Sci. USA. 2015;112:E3699–E3708. doi: 10.1073/pnas.1510329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Liu W., Li C. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat. Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tagliaferro P., Burke R.E. Retrograde axonal degeneration in Parkinson disease. J. Parkinsons Dis. 2016;6:1–15. doi: 10.3233/JPD-150769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikić I., Merkler D., Kerschensteiner M. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 2011;17:495–499. doi: 10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]
- 9.Marangoni M., Adalbert R., Conforti L. Age-related axonal swellings precede other neuropathological hallmarks in a knock-in mouse model of Huntington’s disease. Neurobiol. Aging. 2014;35:2382–2393. doi: 10.1016/j.neurobiolaging.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Geula C., Nagykery N., Wu C.K. Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2008;67:309–318. doi: 10.1097/NEN.0b013e31816a1df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan C.L., Peng C.Y., McIntire S. Genetic analysis of age-dependent defects of the Caenorhabditis elegans touch receptor neurons. Proc. Natl. Acad. Sci. USA. 2011;108:9274–9279. doi: 10.1073/pnas.1011711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy T.H., Li P., Liu R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J. Neurosci. 2008;28:1756–1772. doi: 10.1523/JNEUROSCI.5128-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budde M.D., Frank J.A. Neurite beading is sufficient to decrease the apparent diffusion coefficient after ischemic stroke. Proc. Natl. Acad. Sci. USA. 2010;107:14472–14477. doi: 10.1073/pnas.1004841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu B., Luo L., Cynader M.S. Dendritic and synaptic pathology in experimental autoimmune encephalomyelitis. Am. J. Pathol. 2003;162:1639–1650. doi: 10.1016/S0002-9440(10)64298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christianson M.G., Lo D.C. Differential roles of Aβ processing in hypoxia-induced axonal damage. Neurobiol. Dis. 2015;77:94–105. doi: 10.1016/j.nbd.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochs S., Pourmandt R., Friedman R.N. The origin and nature of beading: a reversible transformation of the shape of nerve fibers. Prog. Neurobiol. 1997;52:391–426. doi: 10.1016/s0301-0082(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhai Q., Wang J., He Z. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 18.Kilinc D., Gallo G., Barbee K.A. Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp. Neurol. 2008;212:422–430. doi: 10.1016/j.expneurol.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y., Jukkola P., Gu C. Polarity of varicosity initiation in central neuron mechanosensation. J. Cell Biol. 2017;216:2179–2199. doi: 10.1083/jcb.201606065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang-Schomer M.D., Johnson V.E., Smith D.H. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp. Neurol. 2012;233:364–372. doi: 10.1016/j.expneurol.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepherd G.M., Raastad M., Andersen P. General and variable features of varicosity spacing along unmyelinated axons in the hippocampus and cerebellum. Proc. Natl. Acad. Sci. USA. 2002;99:6340–6345. doi: 10.1073/pnas.052151299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L., Dong J., Cioffi G.A. Varicosities of intraretinal ganglion cell axons in human and nonhuman primates. Invest. Ophthalmol. Vis. Sci. 2003;44:2–9. doi: 10.1167/iovs.02-0333. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs J.R., Stevens J.K. Experimental modification of PC12 neurite shape with the microtubule-depolymerizing drug Nocodazole: a serial electron microscopic study of neurite shape control. J. Cell Biol. 1986;103:907–915. doi: 10.1083/jcb.103.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markin V.S., Tanelian D.L., Ochs S. Biomechanics of stretch-induced beading. Biophys. J. 1999;76:2852–2860. doi: 10.1016/S0006-3495(99)77439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pullarkat P.A., Dommersnes P., Ott A. Osmotically driven shape transformations in axons. Phys. Rev. Lett. 2006;96:048104. doi: 10.1103/PhysRevLett.96.048104. [DOI] [PubMed] [Google Scholar]
- 26.Fernández P., Pullarkat P.A. The role of the cytoskeleton in volume regulation and beading transitions in PC12 neurites. Biophys. J. 2010;99:3571–3579. doi: 10.1016/j.bpj.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bar-Ziv R., Moses E. Instability and “pearling” states produced in tubular membranes by competition of curvature and tension. Phys. Rev. Lett. 1994;73:1392–1395. doi: 10.1103/PhysRevLett.73.1392. [DOI] [PubMed] [Google Scholar]
- 28.Bar-Ziv R., Tlusty T., Bershadsky A. Pearling in cells: a clue to understanding cell shape. Proc. Natl. Acad. Sci. USA. 1999;96:10140–10145. doi: 10.1073/pnas.96.18.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 30.Luo L., O’Leary D.D. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 31.Neukomm L.J., Freeman M.R. Diverse cellular and molecular modes of axon degeneration. Trends Cell Biol. 2014;24:515–523. doi: 10.1016/j.tcb.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baas P.W., Ahmad F.J. Force generation by cytoskeletal motor proteins as a regulator of axonal elongation and retraction. Trends Cell Biol. 2001;11:244–249. doi: 10.1016/s0962-8924(01)02005-0. [DOI] [PubMed] [Google Scholar]
- 33.He Y., Yu W., Baas P.W. Microtubule reconfiguration during axonal retraction induced by nitric oxide. J. Neurosci. 2002;22:5982–5991. doi: 10.1523/JNEUROSCI.22-14-05982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallo G., Yee H.F., Jr., Letourneau P.C. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J. Cell Biol. 2002;158:1219–1228. doi: 10.1083/jcb.200204140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baas P.W., Black M.M. Individual microtubules in the axon consist of domains that differ in both composition and stability. J. Cell Biol. 1990;111:495–509. doi: 10.1083/jcb.111.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hochmuth F.M., Shao J.Y., Sheetz M.P. Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys. J. 1996;70:358–369. doi: 10.1016/S0006-3495(96)79577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coles C.H., Bradke F. Coordinating neuronal actin-microtubule dynamics. Curr. Biol. 2015;25:R677–R691. doi: 10.1016/j.cub.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Dent E.W., Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J. Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutalik S.P., Joseph J., Ghose A. Cytoskeletal mechanisms of axonal contractility. Biophys. J. 2018;115:713–724. doi: 10.1016/j.bpj.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad F.J., Hughey J., Baas P.W. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat. Cell Biol. 2000;2:276–280. doi: 10.1038/35010544. [DOI] [PubMed] [Google Scholar]
- 41.Lingor P., Koch J.C., Bähr M. Axonal degeneration as a therapeutic target in the CNS. Cell Tissue Res. 2012;349:289–311. doi: 10.1007/s00441-012-1362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho Y., Sloutsky R., Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu K., Zhong G., Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 2013;339:452–456. doi: 10.1126/science.1232251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leite S.C., Sampaio P., Sousa M.M. The actin-binding protein α-adducin is required for maintaining axon diameter. Cell Rep. 2016;15:490–498. doi: 10.1016/j.celrep.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan A., Tofangchi A., Saif T. Coupled circumferential and axial tension driven by actin and myosin influences in vivo axon diameter. Sci. Rep. 2017;7:14188. doi: 10.1038/s41598-017-13830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubey S., Bhembre N., Pullarkat P. The axonal actin-spectrin lattice acts as shock absorbers to protect neurons from stretch-induced damage. bioRxiv. 2019 [Google Scholar]
- 47.Bartolini F., Moseley J.B., Gundersen G.G. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J. Cell Biol. 2008;181:523–536. doi: 10.1083/jcb.200709029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suozzi K.C., Wu X., Fuchs E. Spectraplakins: master orchestrators of cytoskeletal dynamics. J. Cell Biol. 2012;197:465–475. doi: 10.1083/jcb.201112034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown A., Slaughter T., Black M.M. Newly assembled microtubules are concentrated in the proximal and distal regions of growing axons. J. Cell Biol. 1992;119:867–882. doi: 10.1083/jcb.119.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spira M.E., Oren R., Gitler D. Critical calpain-dependent ultrastructural alterations underlie the transformation of an axonal segment into a growth cone after axotomy of cultured Aplysia neurons. J. Comp. Neurol. 2003;457:293–312. doi: 10.1002/cne.10569. [DOI] [PubMed] [Google Scholar]
- 51.D’Onofrio T.G., Hatzor A., Weiss P.S. Controlling and measuring the interdependence of local properties in biomembranes. Langmuir. 2003;19:1618–1623. [Google Scholar]
- 52.Chandrasekhar S. Hydrodynamic and hydromagnetic stability. Phys. Uspekhi. 1981;179:652. [Google Scholar]
- 53.Quéré D. Fluid coating on a fiber. Annu. Rev. Fluid Mech. 1999;31:347–384. [Google Scholar]
- 54.Lorenceau E., Quéré D. Drops on a conical wire. J. Fluid Mech. 2004;510:29–45. [Google Scholar]
- 55.Haak R.A., Kleinhans F.W., Ochs S. The viscosity of mammalian nerve axoplasm measured by electron spin resonance. J. Physiol. 1976;263:115–137. doi: 10.1113/jphysiol.1976.sp011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gurin K.L., Lebedev V.V., Muratov A.R. Dynamic instability of a membrane tube. Sov. Phys. JETP. 1996;83:321–326. [Google Scholar]
- 57.Baas P.W., Ahmad F.J. Beyond taxol: microtubule-based treatment of disease and injury of the nervous system. Brain. 2013;136:2937–2951. doi: 10.1093/brain/awt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fukuda Y., Li Y., Segal R.A. A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Front. Neurosci. 2017;11:481. doi: 10.3389/fnins.2017.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu W., Ahmad F.J., Baas P.W. Microtubule fragmentation and partitioning in the axon during collateral branch formation. J. Neurosci. 1994;14:5872–5884. doi: 10.1523/JNEUROSCI.14-10-05872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brugués J., Nuzzo V., Needleman D.J. Nucleation and transport organize microtubules in metaphase spindles. Cell. 2012;149:554–564. doi: 10.1016/j.cell.2012.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The axon has not been completely transected and the retracting segments are connected by a thin tube. Time is shown in min:sec
The thickness of the axon is about a micron and the duration of the video is about 1 min. Time is in min:sec.
Time is in hr:min:sec.
The recording started 1 min after Noco was added to the dish. The onset of beading can be seen around 2 min 24 s for both the axons, starting at their respective growth cone ends. Beading progresses with coarsening and retrograde migration (t ∼ 20 min onwards). At 31 min 42 s the FoV is changed to the location of the soma. Time is shown in min:sec.
The recording was started at 35 min after Noco was added to the dish. Thus, beading can be seen already in the beginning of the movie. A clear retrograde migration of the beads can be seen throughout the movie, leaving behind a thin membrane trail. Time is shown in min:sec, and the scale bar is 10 micron.
The recording was started 5 min after adding 16.6 μM of Noco. Images were recorded using an 100X objective and the image histogram was adjusted by setting a minimum and maximum cutoffs to highlihgth the dense particles. Time in hr:min:sec.
Time is in hr:min:sec:m-sec, and the scale bar is 10 microns.
Beading progresses during the course of the movie. Time is in hr:min:sec, and the scale bar is 10 microns.
In the first few frames the growth cone deforms and shrinks. Later the contents begin to retract leaving behind a much thinner trail of membrane. In another vertically oriented axon in the same movie, the retraction front can be very clearly seen from 16 min onwards. The time is shown in hr:min:sec, and the scale bar is 10 microns.
The recording was started soon after adding Lat-A. The growth cone begin to collapse as soon as Lat-A is added but no further retraction is observed. Time is in hr:min:sec.
The appearance of a bright spot indicate the point where the laser pulse has hit. The bright spot persists because the laser had caused micro-cracks on the upper surface of the coverslip resulting in scattering of light. Note that the axon is not completely transected. There is a very thin tube of membrane connecting the two thicker 11 segments on both sides. Until the thin connection between the two retracting segments is intact, the two retracting segments lie along a line. In cases where the cut is complete, the axon snaps back suddenly and also buckles (see Document S1. Supporting Materials and Methods, Figs. S1–S9, Tables S1 and S2, and Appendices S1 and S2, Video S1. Time Lapse Recording of Two Axons with their Growth Cones Located Towards the Bottom of the Field of View, Video S2. Time Lapse Recording of an Axon with the Soma on the Right as Indicated by the Arrow in the First Frame, Video S3. Phase Contrast Imaging of Transport in an Axon as it Undergoes Beading, Video S4. Time Lapse Recording of the Movement of GFP-Synaptophysin-Tagged Synaptic Vesicles in a Control Axon, Video S5. Time Lapse Recording of the Movement of GFP-Synaptophysin-Tagged Synaptic Vesicles in a Noco Treated Axon, Video S6. Time Lapse Recording of a Lat-A-Treated Axon with a Healthy Growth Cone Visible in the Beginning, Video S7. Time Lapse Recording Showing an Axon Which Was Pretreated with Taxol and Then With Lat-A, Video S8. Time Lapse Recording of Laser Ablation and Ensuing Retraction of an Axon, Video S9. Time Lapse Recording of Laser Ablation of an Axon Which Exhibit Beading in the Retracting Segments, Video S10. Time Lapse Recording of a PC12 Neurite Undergoing Pearling Instability When Subjected to an Osmotic Shock, Video S11. Time Lapse Recording of a PC12 Neurite Treated with 10 μg/ml Noco, Document S2. Article plus Supporting Material). Time is shown in min:sec.