Abstract
Recent work suggests that the circadian pacemaker responds optimally to millisecond flashes of light, not continuous light exposure as has been historically believed. It is unclear whether these responses are influenced by the physical characteristics of the pulsing. In the present study, Drosophila (n = 2199) were stimulated with 8, 16 or 120 ms flashes. For each duration, the energy content of the exposure was systematically varied by changing the pulse irradiance and the number of stimuli delivered over a fixed 15 min administration window (64 protocols surveyed in all). Results showed that per microjoule invested, 8 ms flashes were more effective at resetting the circadian activity rhythm than 16- and 120 ms flashes (i.e. left shift of the dose–response curve, as well as a higher estimated maximal response). These data suggest that the circadian pacemaker's photosensitivity declines within milliseconds of light contact. Further introduction of light beyond a floor of (at least) 8 ms leads to diminishing returns on phase-shifting.
Keywords: circadian, light, flash, millisecond, photostimulation, phototherapy
1. Introduction
The circadian pacemaker's responses to light have been largely studied with electric incandescent and gas-discharge lamps, emission sources that are rarely spectrally tuned or automated to control exposure duration [1]. The historical use of such lighting in circadian research has led to the assumption that the pacemaker acts in a dose-dependent fashion over 30–60 min of non-saturating light exposure (i.e. photon-for-photon, the reciprocity hypothesis) [2,3]. However, recent investigation has challenged this notion by showing that 1–2 s of light can trigger phase shifts in humans similar to those produced by hour-long stimulation if the exposure is organized as a series of brief millisecond stimuli [4]. Flash induction of phase resetting—stemming at least in part from restoration of the pacemaker's photosensitivity with intervening darkness [5]—suggests that the pacemaker integrates photic information optimally via a non-continuous process and might be influenced by the physical characteristics of individual flashes delivered within a stimulation sequence. Discrete episodes of light have three relevant physical-exposure variables: spectrum, intensity and duration. To date, none of these variables has been systematically examined at the millisecond timescale to assess its effects on the circadian system (though some consideration has been given to spectrum [6]). In the present study, we show that flash duration is an important factor controlling the magnitude of phase shifts in response to blue LED solid-state lighting in Drosophila. Shorter millisecond pulses are more effective at resetting the pacemaker's rhythm than longer millisecond pulses. These results suggest that the pacemaker's photosensitivity is reduced almost instantaneously upon contact with blue light, an important stimulus organizing entrainment to the solar cycle via photopigments that summate light exposure over time such as cryptochrome and melanopsin [7,8]. Future phototherapy techniques might benefit from devices that provide greater temporal control of light exposure at the resolution that semiconductor LED illuminants confer (i.e. in the millisecond and microsecond range).
2. Material and methods
Drosophila ananassae were derived from an isofemale line maintained at the Drosophila Species Stock Center (DSSC) at Cornell University (stock no. 14024-0371.16; NSF Award no. 1351502). Stocks were reared at 25°C in DigiTherm® incubators (Tritech Research, Los Angeles, CA, USA) and entrained to a 12 L : 12 D cycle. House lighting was provided by a broad-spectrum 4 W cold-cathode fluorescent light tube with a step-up inverter (freely mounted with no fixture, illuminance at rack level = 887.7 lux, irradiance = 309.5 µW cm−2; Tritech model DT2-LB-F12IN/CIRC-L-INV; lights-on at 07.00 h MST (Mountain Standard Time)). The stocks were transferred daily to generate a steady supply of offspring. For phase-shifting experiments, female flies were selected as late-stage, ‘pharate-adult’ pupae, moved onto fresh food (corn-flour–nutritional yeast–agar medium—0.8% agar, 3.5% sucrose, 1.7% glucose, 6% fine-grained masa and 1% yeast) and housed in groups of 5–6 in a secondary DigiTherm® incubator. This secondary incubator, in which the collected pupae eclosed, was programmed to run a 12 L : 12 D cycle with lights-on at 01.00 h, MST, to accommodate subsequent phase-delaying treatments at ZT13 (i.e. 14.00 h, the first hour after lights-off).
An Aschoff Type II paradigm was used to quantify the effects of blue millisecond pulses on phase resetting of the flies' locomotor activity rhythms. Animals were entrained to the 12 L : 12 D schedule under which they enclosed for 3 days. Prior to lights-off on the last day of the schedule, the flies were grouped into disposable cotton-plugged Pyrex tubes (approx. 8–10 flies per tube, 13 mm outside diameter, 100 mm long). For light administration at ZT13, 2–4 of these tubes were placed side-by-side onto a titanium dioxide paint-coated platform and exposed to one of the 64 blue (λmax = 452 nm, half-bandwidth ≤21 nm) LED protocols described in electronic supplementary material, tables S1 and S2. In all cases, light was delivered within a 15 min window using a ColorDome LED Ganzfeld lamp (Diagnosys, Lowell, MA, USA). Stimulation instructions were sent by Diagnosys’ software-interfaced Espion E3, an amplifier console capable of producing PWM (pulse width modulation) intensity-controlled LED flashes as short as 8 ms at the irradiances that were tested. Lamp output was specified in candelas per square metre (cd m−2; i.e. a photometric measure of luminous intensity) and quantified—photometrically (lux, lumens m−2) and radiometrically (µW cm−2)—with the ILT950 spectroradiometer (International Light Technologies, Peabody, MA, USA). Irradiance measures were used to calculate the energy content of each stimulation protocol, as well as photon flux (photons cm−2 s−1) according to the equation: photon flux = irradiance (µW cm−2)/energy per photon (hc/λmax). All flash protocols began precisely at ZT13, ended by ZT13.25, and were conducted in complete darkness with the aid of night vision headgear. Independent sets of naive animals were used for each of the 64 protocols.
Following photic treatment, flies were transferred without anaesthesia to single housing in glass chambers (5 mm outside diameter, 65 mm long) containing a plug of food medium (2% agar and 5% sucrose) and loaded into Trikinetics DAM2 Drosophila Activity Monitors (TriKinetics, Inc., Waltham, MA, USA). Their motion was independently tracked for the next 3–4 days under constant darkness (DD) by cross-sectioned infrared beams that transmitted movement information to computer acquisition software every 30 s. DAM2 units were situated in climate-controlled vivariums identical to the ones used in colony management and under the same ambient conditions.
Phase shifts of behaviour were calculated for each fly (total n = 2199) by determining the horizontal distance between the time of lights-on in the previous LD schedule (ZT0, 01.00 h) and the software-called activity onset on the second day after millisecond flash exposure (ClockLab Analysis Version 6, Actimetrics, Wilmette, IL, USA). Our previous work has shown that the activity onsets of D. ananassae are always phase-locked to the timing of lights-on within an LD schedule [9]. Post-pulse in DD, transients are observed for a day, but the flies' behavioral rhythms invariably reset by the second DD cycle [9] (see also electronic supplementary material, figure S1 for a few representative actograms from this study demonstrating phase stability of the activity rhythms 2–4 days after flash exposure). A control group was transferred into DD without any light treatment at ZT13 to correct for phase movements produced by light schedule transitions from LD to DD. The final values for flash-induced phase shifts were calculated by subtracting out the average phase movement exhibited by the no-light-exposure group (mean ± s.e.m., 1.53 ± 0.13 h phase delay, n = 28; see electronic supplementary material, figure S1A,B). The general protocol for our assessment is consistent with the standard (long-held) practices of the Drosophila literature. Here, rather than exposing animals to light pulses in the middle of a free-running cycle (Aschoff Type I protocol), an Aschoff II or ‘anchored’ protocol is routinely used to measure the effect of night-time light presented during the dark phase of the last night of an entraining LD cycle feeding into DD. The position of the phase reference point 2 days post-pulse is often the benchmark for quantifying the magnitude of a shift [10,11].
To compare the reset efficacy of stimulation protocols using 8, 16 and 120 ms pulses, the average phase shift recorded in each variant condition (created by systematically varying the pulse irradiance and the interstimulus interval) was plotted according to the light energy (µJ) accumulated to produce it. Using least-squares regression, the 8, 16 and 120 ms datasets were then fitted with three-parameter (sigmoidal) dose–response curves according to the model: Y = Baseline + X*(Top − Baseline)/(EC50 + X) (GraphPad Prism 8, San Diego, CA, USA). Here, the Baseline term refers to the estimated response of the system to 0 µJ of light, the Top term refers to the asymptotic maximal response of the system and the EC50 term refers to the µJ value at which 50% of the maximal response is achieved. Note that when fitting phase shift data with this model, we constrained the Baseline term to 0; estimated drift (phase movements unrelated to the experimental delivery of a light protocol) was already accounted for by standardizing the magnitude of each phase shift against the movements seen with transitions from LD to free-running in DD. Each model's EC50 and asymptotic response was statistically compared with the extra-sum-of-squares F-test. Significance was set at p < 0.05.
3. Results
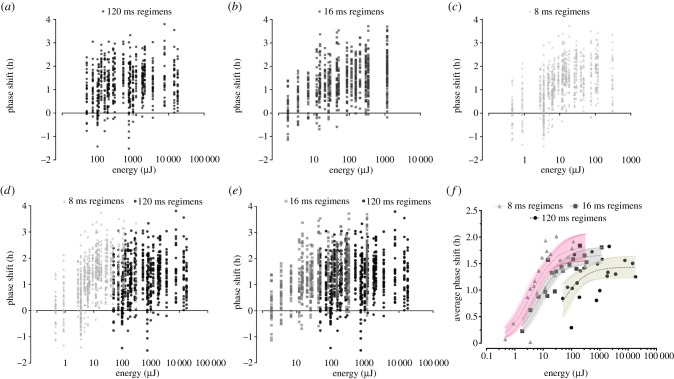
To investigate the role of flash duration in phase-shifting of the circadian activity rhythm, we exposed independent groups of D. ananassae to one of the 64 blue LED protocols described in electronic supplementary material, tables S1 and S2 (survey compiled from 2199 flies). For each condition, the total energy content of the light exposure was systematically varied by changing the pulse irradiance and the number of stimuli delivered over a fixed 15 min administration window; the stimulus number in the 15 min window was altered by changing the interstimulus interval of the flash delivery from 0.06 to 1.0 Hz. The results from these experiments are provided on a logarithmic scale in figure 1, with the size of each animal's phase shift (h) shown in the scatter plot as a function of the light energy (µJ) accumulated to produce it. On average and irrespective of pulse width, animals treated with blue millisecond flashes exhibited significant delays in locomotor onset that quickly asymptoted within 100 µJ of light treatment (figure 1a–c), consistent with previous reports in flies, rodents and humans that phase-shifting can be triggered through circumscribed stimulation with xenon flashes [4,12,13]. Overlays of the raw scatter plot from the 120 ms pulse regimen with those from the 8 and 16 ms regimens (figure 1d–e) raised the possibility that the EC50 and peak response values for circadian resetting by light were altered as a result of the pulse shortening. After averaging the individual responses to each protocol in our matrix (n = 64), we plotted the data from the three flash duration conditions together and fitted sigmoidal dose–response curves to each (Sy.x or standard deviation of the residuals = 0.18–0.32 h; figure 1f). This visualization revealed a pronounced sensitization (left shift) of the dose–response curve for 8 ms flash induction of circadian resetting relative to 120 ms (mean ± s.e., 8 ms EC50 = 3.7 ± 1.1 µJ; 120 ms EC50 = 40.3 ± 20.3 µJ) as well as an increase in the response ceiling (8 ms responsemax = 1.8 ± 0.1 h; 120 ms responsemax = 1.4 ± 0.1 h). For the 16 ms dose–response curve, the sensitivity and asymptotic functions were similarly affected but to a degree roughly proportional to the reductions in pulse duration (16 ms EC50 = 7.1 ± 1.7 µJ; 16 ms responsemax = 1.7 ± 0.1 h). An extra-sum-of-squares F-test confirmed that each model's parameters were significantly different (Fs2,58 > 3.7, p < 0.03).
Figure 1.
Phase shifts of locomotor activity (h) are quantified for individual flies (n = 2199) stimulated with one of the 64 blue LED protocols for 15 min starting at ZT13, 1 h after lights-off on the last day of the light–dark schedule (12 L : 12 D, lights-on at 01.00 h, MST). Protocols employed 120 ms (a), 16 ms (b) or 8 ms flashes (c). Note that any colour variations within (a–c) result from the overlay of semi-transparent data points (i.e. darker areas reflect the concentration of more data in the scatter plot). (d,e) Individual data from the 120 ms and 8 or 16 ms flash conditions are reproduced/superimposed to facilitate visual comparisons between the regimens. (f) Averaged data from each family of protocols are plotted against one another, fitted with three-parameter dose–response curves (dotted lines) and overlaid with prediction bands reflecting the 95% confidence intervals for the fits (8 ms = pink; 16 ms = grey; 120 ms = beige). (Online version in colour.)
4. Discussion
LED lighting technology offers more opportunities for the clinical application of light's major physical-exposure variables [1]. Electroluminescence from these semiconductor chips produces fast narrowband light emissions whose intensity can be linearly controlled over an extended dynamic range by PWM [14]. Given the historical assumptions about the importance of overall light exposure in circadian timekeeping, little thought has been devoted to whether the flexible parameter space that LEDs afford at the microsecond-to-millisecond timescale would be meaningful to how phototherapy is applied in conditions commonly thought to have an underlying circadian problem such as seasonal affective disorder or manic depression. To our knowledge, these results are the very first to suggest that one aspect of this parameter space—pulse duration—matters and hints at the likelihood that the circadian pacemaker habituates quickly to light stimuli; comparisons between the 8 and 16 ms flash protocols tested in the current study suggest that light's effects on the circadian activity rhythm begin to lose efficiency within just a few milliseconds of continuous administration. Future work will be necessary to establish an empirical lower floor for what durations of light exposure are necessary to incur negligible photohabituation from the pacemaker and to begin to unpack the changes in pacemaker responses that might be achieved through a combined manipulation of duration, intensity and spectrum within the delivery of a flash sequence. Such data are likely to be the building blocks for next-generation phototherapies.
Supplementary Material
Data accessibility
Original data have been uploaded as the electronic supplementary material. Electronic supplementary material information includes two data tables and one figure. The tables contain the parameters for all the stimulation protocols tested as well as the individual results represented in figure 1 of the main text as means (s.e.m.).
Authors' contributions
F.F. developed the study concept, oversaw its experimental design, drafted the paper, revised it critically for important intellectual content, and procured funding for all aspects of the work. Behavioural testing and data collection were done by D.C.N. and S.K.. F.F. and J.M.Z. developed strategies for analysing the dataset. All the authors contributed to writing and to interpreting the findings and approved the final version of the manuscript for submission. F.F. agrees to be accountable for all aspects of the work in ensuring that questions related to its accuracy are appropriately investigated and resolved.
Competing Interests
We declare we have no competing interests.
Funding
We are indebted to Science Foundation Arizona (SFAz) for their financial support.
References
- 1.Pattison PM, Tsao JY, Brainard GC, Bugbee B. 2018. LEDs for photons, physiology and food. Nature 563, 493–500. ( 10.1038/s41586-018-0706-x) [DOI] [PubMed] [Google Scholar]
- 2.Nelson DE, Takahashi JS. 1991. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). J. Physiol. 439, 115–145. ( 10.1113/jphysiol.1991.sp018660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dkhissi-Benyahya O, Sicard B, Cooper HM. 2000. Effects of irradiance and stimulus duration on early gene expression (Fos) in the suprachiasmatic nucleus: temporal summation and reciprocity. J. Neurosci. 20, 7790–7797. ( 10.1523/JNEUROSCI.20-20-07790.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najjar RP, Zeitzer JM. 2016. Temporal integration of light flashes by the human circadian system. J. Clin. Invest. 126, 938–947. ( 10.1172/JCI82306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comas M, Beersma DG, Spoelstra K, Daan S. 2007. Circadian response reduction in light and response restoration in darkness: a ‘skeleton’ light pulse PRC study in mice (Mus musculus). J. Biol. Rhythms 22, 432–444. ( 10.1177/0748730407305728) [DOI] [PubMed] [Google Scholar]
- 6.Negelspach DC, Kaladchibachi S, Fernandez F. 2018. The circadian activity rhythm is reset by nanowatt pulses of ultraviolet light. Proc. R. Soc. B 285, 20181288 ( 10.1098/rspb.2018.1288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinayak P, Coupar J, Hughes SE, Fozdar P, Kilby J, Garren E, Yoshii T, Hirsh J. 2013. Exquisite light sensitivity of Drosophila melanogaster cryptochrome. PLoS Genet. 9, e1003615 ( 10.1371/journal.pgen.1003615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emanuel AJ, Do MT. 2015. Melanopsin tristability for sustained and broadband phototransduction. Neuron 85, 1043–1055. ( 10.1016/j.neuron.2015.02.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaladchibachi S, Negelspach D, Fernandez F. 2018. Responses to intermittent light stimulation late in the night phase before dawn. Clocks Sleep 1, 26–41. ( 10.3390/clockssleep1010004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutila JE, Maltseva O, Rosbash M. 1998. The timSL mutant affects a restricted portion of the Drosophila melanogaster circadian cycle. J. Biol. Rhythms 13, 380–392. ( 10.1177/074873098129000200) [DOI] [PubMed] [Google Scholar]
- 11.Suri V, Qian Z, Hall JC, Rosbash M. 1998. Evidence that the TIM light response is relevant to light-induced phase shifts in Drosophila melanogaster. Neuron 21, 225–234. ( 10.1016/S0896-6273(00)80529-2) [DOI] [PubMed] [Google Scholar]
- 12.Kaladchibachi S, Negelspach DC, Fernandez F. 2018. Circadian phase-shifting by light: beyond photons. Neurobiol. Sleep Circadian Rhythms 5, 8–14. ( 10.1016/j.nbscr.2018.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Den Pol AN, Cao V, Heller HC. 1998. Circadian system of mice integrates brief light stimuli. Am. J. Physiol. Regul. Integr. Comp. Physiol. 275, R654–R657. ( 10.1152/ajpregu.1998.275.2.R654) [DOI] [PubMed] [Google Scholar]
- 14.Teikari P, Najjar RP, Malkki H, Knoblauch K, Dumortier D, Gronfier C, Cooper HM. 2012. An inexpensive Arduino-based LED stimulator system for vision research. J. Neurosci. Methods 211, 227–236. ( 10.1016/j.jneumeth.2012.09.012) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data have been uploaded as the electronic supplementary material. Electronic supplementary material information includes two data tables and one figure. The tables contain the parameters for all the stimulation protocols tested as well as the individual results represented in figure 1 of the main text as means (s.e.m.).