Abstract
Background
Hepatocellular carcinoma (HCC) ranks fifth among malignancies globally. Previous studies have shown that systemic inflammatory response, platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) are associated with poor prognosis of various types of cancer.
Materials and methods
Radiofrequency ablation (RFA) was performed using an internal cooling electrode with a 2- or 3-cm exposed tip. The LMR was calculated as the ratio of lymphocytes to monocytes. In order to explore the influence of pretreatment with PLR and LMR on survival of HCC patients undergoing transcatheter arterial chemoembolization (TACE) and RFA, 204 cases with HCC which accepted RFA and TACE were retrospectively analyzed and assigned into 2 groups based on optimal cutoff values for LMR (low: ≤2.13 or high: >2.13) and PLR (low: ≤95.65 or high: >95.65).
Results
Patients with a lower PLR had a longer overall survival (OS) compared to those with a higher PLR (median OS, 20 versus 13 months), and patients with a higher LMR had a longer OS than those with a lower LMR (OS, 22 versus 10 months). Multivariate logistic regression analysis was performed using Cox proportional hazards regression analysis for multiple prognostic factors and identified PLR and LMR as prognostic factors for OS of HCC cases.
Conclusion
We conclude that PLR and LMR, whose detection is generally available and affordable, may be novel noninvasive circulating markers to potentially assist doctors assess the prognosis of patients.
Keywords: lymphocyte-to-monocyte ratio, platelet-to-lymphocyte ratio, prognosis, inflammation, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) ranks fifth among malignancies worldwide, which also ranks the third among causes of tumor-related deaths in our country.1,2 Despite a great progress observed in improving the treatment, the outcomes of patients with HCC are poor due to rapid proliferation, deterioration of liver function, increased intrahepatic transmission, and metastasis.3 Several HCC patients miss the chance of resection and transplantation because they have advanced stages of HCC or poor liver function or encounter with shortage of liver transplantation donors.4 Transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) (percutaneous radiofrequency ablation) remain the leading therapeutic approaches for unresectable HCC.5,6 Combined treatment of TACE with RFA has appeared as a promising strategy for improving survival.7
It is suggested that systemic inflammatory response (SIR) is significant for the tumor metastasis and progression.8,9 Recently, it has been reported that increased SIR is related to poor prognosis of different types of malignancies, including colorectal cancer, non-small cell lung cancer, and breast cancer.10–;12 Blood-based inflammatory biomarkers, eg, lymphocyte-to-monocyte ratio (LMR), C-reactive protein levels, platelet-to-lymphocyte ratio (PLR), and neutrophil-to-lymphocyte ratio (NLR), in certain cancers have remarkably attracted scholars’ attention. A number of clinical investigations have suggested that an elevated NLR or PLR may be associated with prognosis in normal simple HCC, advanced HCC, huge HCC, unresectable HCC, recurrent HCC undergoing TACE, and HCC treated by transplantation.13–18
To our knowledge, however, a limited number of studies have explored the association between PLR, NLR, LMR, and prognosis of HCC patients undergoing RFA and TACE. Hence, in this study, the value of NLR, PLR, and LMR in predicting prognosis of HCC cases undergoing RFA and TACE was investigated.
Materials and methods
Ethics statement
All the eligible patients signed the written informed consent form before treatment. Approval of the protocol was obtained from the Ethics Committee of our hospital. This study was conducted in accordance with the Declaration of Helsinki.
Patients
Two hundred and four patients who underwent TACE with RFA at Beijing Ditan Hospital between January 2009 and March 2017 were retrospectively analyzed, and the laboratory data were collected. HCC was diagnosed according to the criteria of American Association of the Study of Liver Disease using biopsy or two imaging techniques showing typical features of HCC.19
According to the patients’ medical record, the information below was harvested, including sex, age, maximum tumor diameter (cm), number of tumors, Child–Pugh grade, serum alpha-fetoprotein (AFP) levels (ng/dL), presence of liver cirrhosis, neutrophil count, platelet count, monocyte count, lymphocyte count, in addition to presence of portal vein tumor thrombosis.
Inclusion criteria were as follows: (a) patient’s age at the range of 18–80 years, (b) liver function indicated by Child–Pugh grade A or B cirrhosis, (c) preoperative NLR, PLR, and LMR obtained <1 week prior to treatment, (d) no other malignancies that may determine the prognosis, and (e) no previous treatment for HCC.
Exclusion criteria were as follows: (a) severe diseases (eg, heart failure or hepatic failure), (b) previous chemotherapy and/or radiotherapy, (c) previous use of anti-inflammatory medicines within 1 week, (d) esophageal or gastric variceal bleeding, (e) hepatic encephalopathy, (f) severe coagulation disorders, (g) active infection at the time of blood sampling to establish NLR and LMR, (h) severe coagulation disorders, (i) chemotherapy after TACE and RFA, or (j) lymphatic metastasis.
In addition, PLR, LMR, and ratios of platelets to lymphocytes and lymphocytes to monocytes in blood samples taken before initiation of the first treatment were calculated. The receiver operating characteristic (ROC) curve was employed, and optimal cutoff values for PLR and LMR were determined. The patients were classified into 2 groups for each ratio based on the best cutoff values of the two ratios.
TACE procedure
Indications of TACE were as follows: (1) preoperative chemoembolization, (2) incomplete resection or postoperative recurrence, (3) HCC that could not be resected due to various reasons or was unwilling to receive surgical treatment, (4) bleeding from ruptured HCC, which is difficult to control cancer pain without undergoing surgery, and (5) diffuse or bilateral multinodular HCC.
Selective celiac and superior mesenteric arteriography was performed to estimate the pathological features (size, number, shape, and feeding artery) of the tumors. Using a selective/super-selective technique, the flexible coaxial microcatheter previously placed approximately in the hepatic artery was selectively inserted into the tumor-feeding artery.
After microcatheter placement, a mixture of pirarubicin (15 mg/m2), hydroxycamptothecin (8 mg/m2), and iodine oil (5–10 mL) was infused. Next, embolization with embolic materials, eg, gelatin sponge particles or polyvinyl alcohol particles, was administered until complete stasis in the tumor-feeding vessels was achieved. To attenuate the related symptoms, patients generally received premedication (eg, antiemetic agents) prior to undergoing TACE.
RFA procedures
Indications of RFA were as follows: (1) one tumor had a diameter not more than 5 cm or many tumors had the maximum diameter not more than 3 cm. RFA can be used alone or selectively after undergoing TACE/transcatheter arterial embolization (TAE), (2) one tumor had a diameter more than 5 cm or many tumors had the maximum diameter more than 3 cm (TACE was first followed by RFA treatment), (3) residual/recurrence/new tumor after surgical resection, RFA, TACE/TAE, etc., and (4) to control tumor growth before liver transplantation, as well as recurrence and metastasis after transplantation.
In this study, RFA was conducted using an internal cooling electrode with a 2-cm or 3-cm exposed tip (Olympus Winter & Ibe GmbH, Hamburg, Germany). After administration of local anesthesia, the electrode needles were percutaneously introduced into the tumor under CT. Radiofrequency treatment was performed until the tumor was fully ablated, associated with a close attention to avoid emerging large blood vessels and the intrahepatic bile duct. The patients received RFA treatment within 1 week after TACE.
Follow‑up
The primary endpoint of the present study was overall survival (OS), which referred to the interval between the date of HCC diagnosis and death or the time of the last follow-up.
Statistical analysis
In the current study, SPSS 21.0 software (provided by IBM Corp., Armonk, NY, USA) was employed for statistical analysis. Baseline continuous variables were presented as the mean ± standard error or the median (SEM). Two groups were compared by using Student’s t-test. Categorical data were analyzed by using the chi-square test. The correlation between the two groups was analyzed via Pearson’s and Spearman’s correlation analyses. Kaplan–Meier analysis and the log-rank test were used to calculate cumulative survival rates. Cox proportional hazards regression analysis and multivariate logistic regression analysis were conducted. The survival curves, correlation, and ROC curves were plotted via GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA). ROC measured diagnostic accuracy and illustrated true or false positives. For determination of the “size” (range 0.5–1.0) of the curve of a prediction model composed of graphic display between “sensitivity” and “1–specificity” relationship, AUC was employed. The AUC values of the four groups were <0.60, 0.60–0.80, 0.8–0.9, and >0.9, respectively. We also attempted to address potential bias through sensitivity analysis. P<0.05 indicated a significant difference.
Results
Patients’ characteristics
In the present study, all 204 patients died [170 males (83.3%) and 34 females (16.7%)]. The median age of the patients was 59 years (range 39–77 years). Of the 204 HCC patients, the median pretreatment peripheral blood monocyte count, lymphocyte count, LMR, platelet count, and PLR were 1.12×109/L (0.20–5.12), 0.40×109/L (0.10–2.01), 2.77 (0.58–5.06), 96.45×109/L (22.0–483.90), and 83.5 (20.4–515.30), respectively (Table 1). The median TACE period was 3 (range 1–10). The median RFA period was 2 (range 1–15) (Table S1, Figure S1).
Table 1.
Counts of platelets, lymphocytes, and monocytes; the ratio of lymphocyte/monocyte; and the ratio of platelet/lymphocyte in HCC
| Blood components | Mean | Median | Minimum | Maximum | Normal values |
|---|---|---|---|---|---|
| Platelet count (×109/L) | 113.71±12.81 | 96.45 | 22.0 | 483.9 | 100.00–300.00 |
| Lymphocyte count (×109/L) | 1.24±0.69 | 1.12 | 0.20 | 5.12 | 1.00–5.00 |
| Monocyte count (×109/L) | 0.45±0.14 | 0.4 | 0.10 | 2.01 | 0.20–0.80 |
| LMR | 3.11±1.80 | 2.77 | 0.58 | 5.06 | |
| PLR | 101.99±17.93 | 83.5 | 20.4 | 515.3 |
Abbreviations: LMR, the ratio of lymphocyte/monocyte; PLR, the ratio of lymphocyte/monocyte.
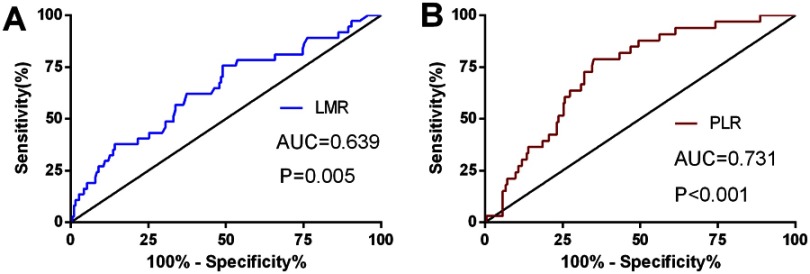
For determination of the cutoff values for the preoperative LMR and PLR, ROC curve was used (Figure 1). The ROC curves defined the recommended cutoff points for the LMR and PLR equal to 2.13 (sensitivity, 0.802; specificity, 0.417; AUC, 0.639) and 95.65 (sensitivity, 0.620; specificity, 0.767; AUC, 0.731), respectively. The AUC value (NLR) was 0.617 (P=0.08) (Figure S2).
Figure 1.
ROC curves with LMR (A) and PLR (B).
The low-PLR group (PLR ≤95.65) included more patients with liver cirrhosis (90.1% versus 74.4%), a Child–Pugh grade (45.1% versus 25%), and a small tumor diameter compared with the high-PLR group (PLR >95.65). The low-LMR group (LMR ≤2.13) included more patients with a high Child–Pugh grade (50.7% versus 29.9%), a high serum AFP level (28.3% versus 16.1%), and portal vein tumor thrombosis (40.3% versus 15.3%) compared with the high-LMR group (LMR >2.13) (Table 2).
Table 2.
Relationship between pretherapy LMR, PLR, and clinico-pathological features in HCC
| Characteristics | Case | LMR | P | PLR | P | ||
|---|---|---|---|---|---|---|---|
| ≤2.13 | >2.13 | ≤95.65 | >95.65 | ||||
| Age (years) | 0.777 | 0.568 | |||||
| ≤55 | 82 | 26 | 56 | 51 | 31 | ||
| >55 | 122 | 41 | 81 | 71 | 51 | ||
| Sex | 0.053 | 0.307 | |||||
| Male | 170 | 51 | 119 | 99 | 71 | ||
| Female | 34 | 16 | 18 | 23 | 11 | ||
| Liver cirrhosis | 0.457 | 0.003 | |||||
| Presence | 171 | 58 | 113 | 110 | 61 | ||
| Absence | 33 | 9 | 24 | 12 | 21 | ||
| Number of tumors | 0.123 | 0.249 | |||||
| 1 | 122 | 35 | 87 | 69 | 53 | ||
| ≥2 | 82 | 32 | 50 | 53 | 29 | ||
| Tumor diameter (cm) | 0.208 | 0.005 | |||||
| ≤5 | 134 | 40 | 94 | 90 | 44 | ||
| >5 | 70 | 27 | 43 | 32 | 38 | ||
| Child–Pugh grade | 0.004 | 0.003 | |||||
| A | 129 | 33 | 96 | 67 | 62 | ||
| B | 75 | 34 | 41 | 55 | 20 | ||
| AFP (ng/mL) | 0.040 | 0.864 | |||||
| ≤400 | 163 | 48 | 115 | 97 | 66 | ||
| >400 | 41 | 19 | 22 | 25 | 16 | ||
| Portal vein thrombosis | 0.001 | 0.113 | |||||
| Yes | 48 | 27 | 21 | 24 | 24 | ||
| No | 156 | 40 | 116 | 98 | 58 | ||
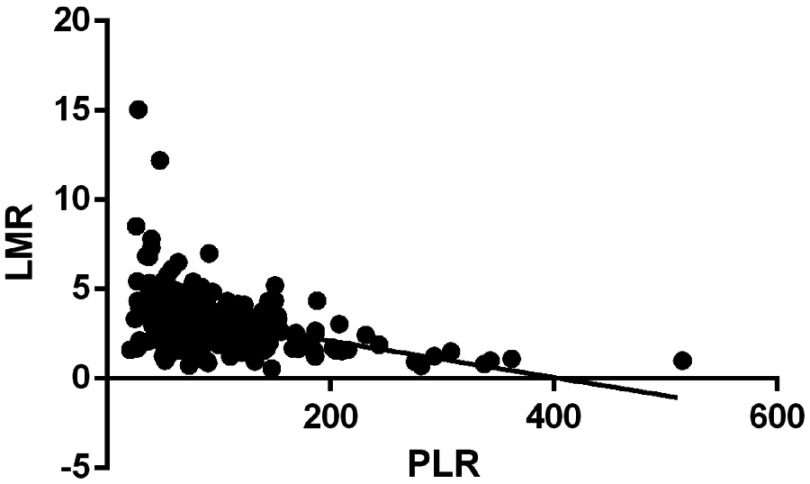
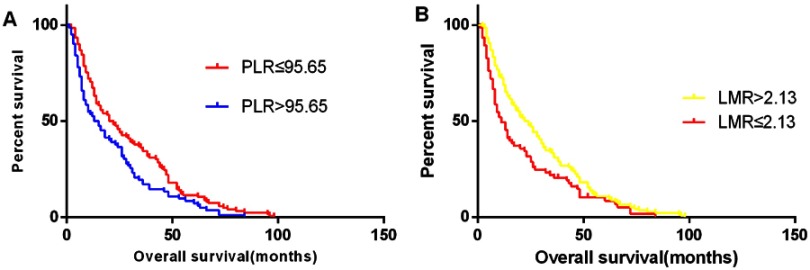
Moreover, the LMR was negatively correlated with PLR (r=−0.383; Figure 2). The OS was significantly longer in the low-PLR group than that in the high-PLR group (median OS, 20 versus 13 months, respectively, P<0.05), which was also found in the high-LMR group compared to the-low LMR group (OS, 22 vs 10 months, respectively, P=0.006) (Figure 3).
Figure 2.
Correlation between LMR and PLR.
Figure 3.
The overall survival according to the ratios of platelet to lymphocyte (A) and lymphocyte to monocyte (B).
Univariate logistic regression analysis indicated that OS was significantly related to liver cirrhosis, tumor diameter, Child–Pugh grade, PLR, LMR, and portal vein tumor thrombosis. Besides, multivariate logistic regression analysis identified PLR (95% confidence interval: 1.005–1.006, hazard ratio: 1.006) and LMR (95% confidence interval: 1.133–1.178, hazard ratio: 1.155) as prognostic factors for OS of HCC cases. Moreover, tumor diameter, Child–Pugh grade, and portal vein tumor thrombosis were also prognostic factors for OS (Table 3).
Table 3.
Univariate and multivariate analyses of the relationship of risk factors and OS
| Risk factor | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Sex (male/female) | 0.881 | 0.703–1.104 | 0.272 | |||
| Age (≤55/>55 years) | 0.992 | 0.927–1.051 | 0.785 | |||
| Tumor diameter (≤5/>5 cm) | 1.917 | 1.908–1.926 | 0.005 | 1.884 | 1.872–1.896 | 0.000 |
| Number of tumors (1/≥2) | 1.001 | 0.991–1.10 | 0.991 | |||
| Child–Pugh grade | 1.154 | 1.090–1.222 | 0.000 | 1.279 | 1.202–1.361 | 0.000 |
| AFP: ≤400/>400 ng/mL | 1.548 | 1.412–1.697 | 0.065 | |||
| Liver cirrhosis (presence/absence) | 1.864 | 1.805–1.98 | 0.014 | |||
| Portal vein thrombosis (yes/no) | 1.509 | 1.465–1.557 | 0.001 | 1.734 | 1.652–1.826 | 0.000 |
| LMR (≤2.13/>2.13) | 1.123 | 1.104–1.142 | 0.000 | 1.155 | 1.133–1.178 | 0.000 |
| PLR (≤95.65/>95.65) | 1.003 | 1.002–1.003 | 0.001 | 1.006 | 1.005–1.006 | 0.000 |
Discussion
It is usually recognized that the inflammatory tumor microenvironment plays a substantial role in tumor occurrence and progression8 and may affect the survival of malignancy patients.20 Several potential mechanisms for this correlation between tumor and inflammatory response have been suggested. First, inflammation, which can remove or neutralize injurious substances and promote healing and regeneration, has been proposed for a portion of the normal but complex biological responses to aseptic necrosis or foreign infection.21 Second, the tumor inflammatory response is significant for initiation, promotion, malignant conversion, growth, and metastasis of the progression of various malignancies. When tumor growth and metastasis occur, inflammatory cytokines, including tumor necrosis factor, chemokines, interleukin-1, vascular endothelial growth factor (VEGF), interleukin-6, and interleukin-8, are drastically induced by malignant cells themselves and/or malignancy-infiltrating leukocytes, eg, neutrophils, lymphocytes, and platelets, which can remarkably promote proliferation, invasion, epithelial–mesenchymal transition (EMT), and angiogenesis, and also facilitate cancer growth.22 Due to the inexpensive and objective nature of blood tests used in clinical laboratories worldwide, lymphocytes, monocytes, and platelets are common inflammatory markers that form composite indices of LMR and PLR and reflect host inflammatory status as well. Additionally, LMR and PLR, as common inflammatory markers to evaluate SIRs, have been shown to be prognostic factors in certain types of malignancies.23–26 To date, a limited number of studies have explored association between PLR, LMR, and prognosis of HCC patients undergoing TACE and RFA. The findings of the current study demonstrated that LMR and PLR are associated with tumor growth and metastasis and can be considered independent prognostic biomarkers of poor prognosis in HCC patients undergoing treatment with TACE and RFA.
A complex malignant microenvironment is an important prognostic factor in tumors. Numerous studies have revealed that interactions between cancer and SIR may lead to development and progression of tumors.27 The leading features of the cancer-related inflammatory responses are production of cytokines, infiltration of leukocytes, remodeling of tissues, and angiogenesis.28 The majority of HCC patients in our country suffer hepatitis B virus infection, causing persistent inflammation that affects the development and progression of tumors.29 A change in PLR and LMR can be explained by increased platelets, elevated monocytes, and decreased lymphocytes, in which several explanations for this change exist.
First, clinical and experimental studies have reported that malignancies are often associated with thrombocytosis. Platelets not only are involved in coagulation, but also secrete certain growth factors, such as TGF-β, platelet-derived growth factor, VEGF, PF4, and thrombospondin-1.30,31 Tumor cell proliferation, tumor growth, and metastasis are stimulated by these growth factors.32 The direct signaling between tumor cells and platelets can induce an EMT and promote metastasis as well.33–;36
In addition, lymphocytes, a type of inflammatory cells, are also responsible for antitumor immunity, especially increased number of lymphocytes. The involvement of lymphocytes, eg, T cells, in tumor infiltration is often related to the improved prognosis of cancer patients and has been used for targeted cancer therapy.37,38 However, SIR from cancer cells is a significant cause of immunosuppression, leading to lymphocytopenia, and associates with decreased CD4+ helper lymphocytes and increased CD8+ suppressor lymphocytes.39 A decreased number of lymphocytes can be suggestive for abnormal immune mechanisms and a decline in immune surveillance to remove tumor cells that may affect tumor microenvironment, causing tumor metastasis and invasion.40 The prognostic value of elevated or decreased levels of lymphocytes in tumor patients should be further confirmed in future trials.
Eventually, monocytes, a type of inflammatory cells, secrete monocyte chemoattractant protein 1 to mediate and stimulate tumor-related monocyte infiltration in malignant solid tumors, and several types of chemokines, including tumor necrosis factor-α, interleukin-6, transforming growth factor-α, and interleukin-1, are produced. Chemokines can also promote distant metastasis, angiogenesis, and tumorigenesis of malignancies.41
It is noteworthy that PLR and LMR, which are ratios of the absolute numbers of two types of cells, are relatively stable. Increased PLR and LMR are typically related to an imbalance of the two cells, indicating that dynamic balance between immune status and tumor inflammation has been destroyed. The tipping of this balance in favor of the tumor inflammatory response promotes malignancy, cell proliferation, and tumor metastasis, while attenuated antitumor protection is associated with patients’ prognosis.
Under normal conditions, LMR and PLR both play an extremely important role in this relative dynamic balance. A decreased LMR and an increased PLR do not suggest an imbalance of any single factor among monocytes, platelets, or lymphocytes. Once this relative dynamic balance is destroyed (for example, a relative increase in monocytes and platelets or lymphocytopenia), the balance between the tumor-related inflammatory response and the antitumor-related inflammatory response will be broken, antitumor immunity will be impaired, and the patient’s immune system will fall. The patient will thus be in a state of systemic immunosuppression, and the inflammatory response will promote tumor progression, leading to poor prognosis patients.42
In the present study, multivariate logistic regression analysis identified PLR and LMR as independent predictive factors for OS. Furthermore, the cutoff value for LMR was herein 2.13; however, in another study, Yang et al43 achieved a cutoff value of 4.01 for LMR. They also included less patients with the Child–Pugh class B (5.1% vs 36.8%) than the present study. The cutoff value for PLR was 95.65 in the current study; however, Chen et al42 obtained the cutoff value of 131.78 for PLR. In the present study, the AUC value for PLR was 0.731; however, in another study,42 that value was 0.701; Fan et al17 reported the value of 0.791 for AUC. The findings of the current study confirmed that inflammation can promote development and progression of HCC.44 Our comparisons of OS and multivariate logistic regression analysis also demonstrated that other factors, such as tumor diameter, Child–Pugh grade, and portal vein tumor thrombosis, are independent unfavorable factors for OS, which is consistent with the results of previous studies.45,46 These results added further support to the fact that high PLR and low LMR are associated with poor outcomes in patients due to decreased number of lymphocytes, increased number of monocytes and platelets, or both. Besides, HCC patients with high PLR and/or low LMR should be closely followed up after treatment with TACE and RFA.
Our study is a retrospective, single-center one of a relatively limited number of HCC patients. To eliminate the limitations of this study, larger sample sizes are required to confirm and update preoperative prognostic score model for prediction of OS in HCC cases undergoing RFA and TACE. Short-term outcomes of cases with HCC undergoing RFA and TACE should be further confirmed in future trials.
Conclusion
In conclusion, the readily available indicators (PLR and LMR), whose detection is generally available and affordable, may be novel noninvasive circulating markers to potentially assist physicians assess the patient’s prognosis.
Acknowledgment
The current research was financially supported by the Research and Application of Clinical Characteristics of Capital City (Grant Nos. 161100000516141 and 181100001718131). Yanjun Shen and Huige Wang are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4(2):439–474. doi: 10.1007/s12072-010-9165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chianchiano P, Pezhouh MK, Kim A, et al. Distinction of intrahepatic metastasis from multicentric carcinogenesis in multifocal hepatocellular carcinoma using molecular alterations. Hum Pathol. 2018;72:127–134. doi: 10.1016/j.humpath.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu MD, Jia LH, Liu HB, Zhang KH, Guo GH. Sorafenib in combination with transarterial chemoembolization for hepatocellular carcinoma: ameta-analysis. Eur Rev Med Pharmacol Sci. 2016;20:64–74. [PubMed] [Google Scholar]
- 6.Nouso K, Kariyama K, Nakamura S, et al. Application of radiofrequency ablation for the treatment of intermediate-stage hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;32(3):695–700. doi: 10.1111/jgh.13586 [DOI] [PubMed] [Google Scholar]
- 7.Song MJ, Bae SH, Lee JS, et al. Combination transarterial chemoembolization and radiofrequency ablation therapy for early hepatocellular carcinoma. Korean J Intern Med. 2016;31(2):242–252. doi: 10.3904/kjim.2015.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611 [DOI] [PubMed] [Google Scholar]
- 9.Kimura Y, Nagai N, Tsunekawa N, et al. IL-17A-producing CD30(+) Vδ1 T cells drive inflammation-induced cancer progression. Cancer Sci. 2016;107(9):1206–1214. doi: 10.1111/cas.13005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minami S, Ogata Y, Ihara S, Yamamoto S, Komuta K. Neutrophil-to-lymphocyte ratio predicts overall survival of advanced non-small cell lung cancer harboring mutant epidermal growth factor receptor. World J Oncol. 2017;8(6):180–187. doi: 10.14740/wjon1069w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimura T, Shibata M, Gonda K, et al. Prognostic impact of preoperative lymphocyte-to-monocyte ratio in patients with colorectal cancer with special reference to myeloid-derived suppressor cells. Fukushima J Med Sci. 2018;64(2):64–72. doi: 10.5387/fms.2018-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113(1):150–158. doi: 10.1038/bjc.2015.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabir T, Ye M, Mohd Noor NA, Woon W, Junnarkar SP, Shelat VG. Preoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes after curative resection for hepatocellular carcinoma. Int J Hepatol. 2019;2019:4239463. doi: 10.1155/2019/4239463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Chen ZH, Xing YF, et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol. 2015;36:2263–2269. doi: 10.1007/s13277-014-2833-9 [DOI] [PubMed] [Google Scholar]
- 15.Xue TC, Jia QA, Ge NL, et al. The platelet-to-lymphocyte ratio predicts poor survival in patients with huge hepatocellular carcinoma that received transarterial chemoembolization. Tumour Biol. 2015;36:6045–6051. doi: 10.1007/s13277-015-3281-x [DOI] [PubMed] [Google Scholar]
- 16.McNally M, Martinez A, Khabiri H, et al. Inflammatory markers are associated with outcome in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. Ann Surg Oncol. 2013;20(3):923–928. doi: 10.1245/s10434-012-2639-1 [DOI] [PubMed] [Google Scholar]
- 17.Fan W, Zhang Y, Wang Y, Yao X, Yang J, Li J. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PLoS One. 2015;10:e0119312. doi: 10.1371/journal.pone.0119312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia W, Ke Q, Wang Y, et al. Predictive value of pre-transplant platelet to lymphocyte ratio for hepatocellular carcinoma recurrence after liver transplantation. World J Surg Oncol. 2015;13:60. doi: 10.1186/s12957-015-0472-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M. American association for the study of liver diseases: management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125(9):3347–3355. doi: 10.1172/JCI80323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Chen S, Zheng C, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17(1):285. doi: 10.1186/s12885-017-3220-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun F, Ge X, Liu Z, Du S, Ai S, Guan W. Postoperative C-reactive protein/albumin ratio as a novel predictor for short-term complications following gastrectomy of gastric cancer. World J Surg Oncol. 2017;15(1):191. doi: 10.1186/s12957-017-1258-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia J, Zheng X, Chen Y, et al. Stage-dependent changes of preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in colorectal cancer. Tumour Biol. 2015;36:9319–9325. doi: 10.1007/s13277-015-3667-9 [DOI] [PubMed] [Google Scholar]
- 24.Sidaway P. Prostate cancer: platelet-to-lymphocyte ratio predicts prostate cancer prognosis. Nat Rev Urol. 2015;12:238. doi: 10.1038/nrurol.2015.69 [DOI] [PubMed] [Google Scholar]
- 25.Wang Q-X, Li S-H, Ji B-Y, et al. Lymphocyte/monocyte ratio is a novel predictor for early stage extranodal natural killer/T-cell lymphoma,nasal type. J Cancer. 2017;8:1030–1037. doi: 10.7150/jca.17400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z, Xu L, Zhuang L, et al. Role of monocyte-to-lymphocyte ratio in predicting sorafenib response in patients with advanced hepatocellular carcinoma. Onco Targets Ther. 2018;11:6731–6740. doi: 10.2147/OTT.S173275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeNardo DG, Johansson M, Coussens LM. Immune cells asmediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0 [DOI] [PubMed] [Google Scholar]
- 28.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127 [DOI] [PubMed] [Google Scholar]
- 29.Tu T, Bühler S, Bartenschlager R. Chronic viral hepatitis and its association with liver cancer. Biol Chem. 2017;398(8):817–837. doi: 10.1515/hsz-2017-0118 [DOI] [PubMed] [Google Scholar]
- 30.Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood. 1979;53:604–618. [PubMed] [Google Scholar]
- 31.Assoian RK, Sporn MB. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986;102:1217–1223. doi: 10.1083/jcb.102.4.1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin RJ, Afshar-Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood. 2014;124(2):184–187. doi: 10.1182/blood-2014-03-562538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Divella R, Daniele A, Savino E, et al. Circulating levels of transforming growth factor-βeta (TGF-β) and chemokine (C-X-C motif) ligand-1 (CXCL1) as predictors of distant seeding of circulating tumor cells in patients with metastatic breast cancer. Anticancer Res. 2013;33:1491–1497. [PubMed] [Google Scholar]
- 34.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majeti BK, Lee JH, Simmons BH, Shojaei F. VEGF is an important mediator of tumor angiogenesis in malignant lesions in a genetically engineered mouse model of lung adenocarcinoma. BMC Cancer. 2013;13:213. doi: 10.1186/1471-2407-13-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via p2y2 receptor. Cancer Cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 37.Bonini C, Mondino A. Adoptive T-cell therapy for cancer: the era of engineered T cells. Eur J Immunol. 2015;45(9):2457–2469. doi: 10.1002/eji.201545552 [DOI] [PubMed] [Google Scholar]
- 38.Duong CP, Yong CS, Kershaw MH, Slaney CY, Darcy PK. Cancer immunotherapy utilizing gene-modified T cells: from the bench to the clinic. Mol Immunol. 2015;67(2):46–57. doi: 10.1016/j.molimm.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 39.Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med. 1999;27(4):733–740. doi: 10.1097/00003246-199912000-00014 [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Hu Q, Hu K, et al. Increased CD8+CD28+ T cells independently predict better early response to stereotactic ablative radiotherapy in patients with lung metastases from non-small cell lung cancer.J. Transl Med. 2019;17(1):120. doi: 10.1186/s12967-019-1872-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Liao Q, Li X, et al. HYOU1, regulated by LPLUNC1, is up-regulated in nasopharyngeal carcinoma and associated with poor prognosis. J Cancer. 2016;7(4):367–376. doi: 10.7150/jca.13695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen K, Zhan MX, Hu BS, et al. Combination of the neutrophil to lymphocyte ratio and the platelet to lymphocyte ratio as a useful predictor for recurrence following radiofrequency ablation of hepatocellular carcinoma. Oncol Lett. 2018;15:315–323. doi: 10.3892/ol.2017.7291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang YT, Jiang JH, Yang HJ, Wu ZJ, Xiao ZM, Xiang BD. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival compared to established biomarkers in HCC patients undergoing liver resection. Sci Rep. 2018;8:2535. doi: 10.1038/s41598-018-20199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nirei K, Kanda T, Nakamura H, et al. Persistent hepatic inflammation plays a role in hepatocellular carcinoma after sustained virological response in patients with HCV infection. Int J Med Sci. 2018;15(5):466–474. doi: 10.7150/ijms.23147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He C, Zhang Y, Cai Z, Lin X. The prognostic and predictive value of the combination of the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma who receive transarterial chemoembolization therapy. Cancer Manag Res. 2019;11:1391–1400. doi: 10.2147/CMAR.S190545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai CY, Lin CY, Tsai PC, et al. Impact of tumor size on the prognosis of hepatocellular carcinoma in patients who underwent liver resection. J Chin Med Assoc. 2018;81(2):155–163. [DOI] [PubMed] [Google Scholar]