Abstract
INTRODUCTION
Chronic liver disease resulting in fibrosis, and ultimately cirrhosis, is a significant cause of morbidity and mortality worldwide. None of the conventional imaging techniques are able to detect early fibrosis and compare its grade with the histopathologic scale. Liver biopsy, as the diagnostic standard for liver fibrosis, also has limitations and is not well accepted by patients. Magnetic resonance elastography is a well-established technique for evaluating liver stiffness and may replace invasive procedures. Detection of liver fibrosis in its early stages, however, requires a detailed knowledge of normal liver stiffness.
OBJECTIVES
This study aimed to determine normal liver stiffness values in healthy volunteers.
PATIENTS AND METHODS
A total of 102 volunteers (mean age, 21.6 years; range, 20–28 years) with no history of gastrointestinal, hepatobiliary, or cardiovascular disease were enrolled in the study. Liver stiffness was evaluated by magnetic resonance elastography with a 1.5T clinical magnetic resonance scanner. Images of the induced transverse wave propagation were obtained and converted to tissue stiffness maps (elastograms).
RESULTS
The mean (SD) liver stiffness for the entire group of volunteers was 2.14 (0.28) kPa (range, 1.37–2.66 kPa). For women, the mean (SD) stiffness value was 2.14 (0.30) kPa (range, 1.37–2.66 kPa), and for men, 2.14 (0.25) kPa (range, 1.54–2.54 kPa).
CONCLUSIONS
Liver stiffness in a healthy adult cohort did not exceed 2.7 kPa and is not influenced by sex, body mass index, or fat content.
Keywords: healthy population, liver stiffness, magnetic resonance elastography
INTRODUCTION
Chronic liver disease is an important problem in modern medicine. In most cases, chronic liver disease progresses from the stage of fibrosis to liver cirrhosis, ultimately resulting in hepatic failure. Risk factors for the development of cirrhosis include hepatitis B and C virus infection, chronic alcohol abuse, and nonalcoholic fatty liver disease, which is closely related to the rapidly growing obesity epidemic.1–3 Despite the implementation of new forms of therapy for some chronic liver diseases such as hepatitis C, the number of deaths from cirrhosis and its complications remains high.4,5 The search for new drugs that could inhibit and reverse the ongoing process of fibrosis in the liver tissue requires reliable, sensitive, and repeatable tools that allow a frequent and safe assessment of the fibrotic process in hepatic tissue. Available imaging and laboratory methods enable a diagnosis of cirrhosis, but their diagnostic performance in detecting and assessing earlier stages of fibrosis is unsatisfactory. Liver biopsy as a diagnostic standard in hepatology is of limited value in the monitoring of the fibrotic process, owing to its invasive nature and potentially serious complications. For these reasons, patients are reluctant to undergo liver biopsy and repeat it frequently.6–13 Therefore, many centers are searching for new diagnostic methods that could reliably replace liver biopsy.
Magnetic resonance elastography (MRE) is a dynamically developing noninvasive method that allows an assessment of liver fibrosis stages.14–16 This test enables a quantitative evaluation of the mechanical properties of tissues by mechanical wave propagation analysis. The images are obtained during breath holding lasting about 15 seconds. The images reveal the propagation of the generated low-frequency shear waves (60 Hz) in the liver tissue and are automatically processed to create quantitative tissue stiffness maps called elastograms. Chronic liver damage causes progressive fibrosis of the organ, which increases its stiffness. The stiffness measurement closely parallels the progression of the fibrotic process. Multiple studies have demonstrated that MRE is a reliable, safe, and noninvasive diagnostic tool that can be used to monitor the extent of liver damage in patients with chronic liver disease.14–16 However, a credible assessment of an elastogram in a sick person is possible only by referring to normal liver stiffness values obtained by elastography in healthy individuals.
Given the substantial existing literature on MRE, there are surprisingly few published studies of normative liver stiffness values in healthy populations. A study of liver tissue stiffness in healthy Asian volunteers was reported.17 However, published data on liver stiffness in healthy non-Asian populations are based only on small study groups.18 Therefore, we aimed to assess the MRE-based liver tissue stiffness in a healthy European population.
PATIENTS AND METHODS
Study population
A total of 102 white healthy volunteers (mean [SD] age, 21.5 [1.66] years; range, 20–28; 66 women [64.7%] and 36 men [35.3%]) were included in the study. The inclusion criteria were as follows: no contraindications to magnetic resonance imaging (MRI), no history of liver disease, no family history of chronic liver disease, normal liver enzyme levels, and no medication. All participants were on a normal diet and declared an alcohol consumption of less than 140 g/wk (men) and 70 g/wk (women). All volunteers provided written informed consent to participate in the study.
Magnetic resonance elastography tests were performed in the MRI laboratory of the Centre for Innovative Research in Medical and Natural Sciences (Medical Faculty of the University of Rzeszów, Poland) from December 2017 to May 2018.
Magnetic resonance elastography
A 1.5T whole--body magnetic resonance scanner (Optima, GE Healthcare, Milwaukee, Wisconsin, United States) was used to perform the MRE scans. Elastography examinations were performed on an empty stomach or 6 hours after the last meal. Volunteers were allowed to drink plain clean water up to 2 hours before the examination. The test was performed with patients in a supine position.
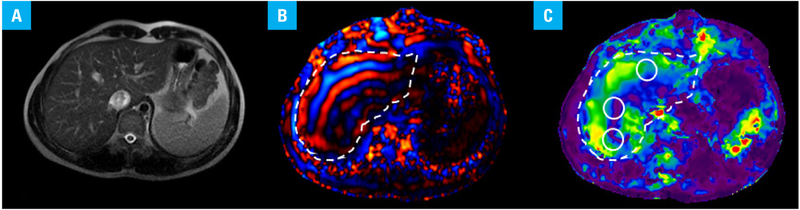
The MRE system includes special acquisition and processing software, as well as hardware consisting of an active and passive driver. The passive driver is a small plastic drum-like device that is placed against the body to transmit mechanical waves into the tissue. In this study, the passive driver was placed on the upper abdomen overlying the right lobe of the liver, with the center of the driver at the level of the xiphisternum. The passive driver was held in place with an elastic abdominal binder strap. The position of the passive driver was chosen so that the largest part of the liver was directly under the driver. Axial MRE slices were acquired (FIGURE 1A); slices were planned on the axial and coronal scout and T2-weighted images such that slices were obtained from the largest cross-section of the liver. For consistency, all sequences were acquired in breath-hold and in end-expiration. Volunteers were instructed to hold their breath in end-expiration for reproducibility of the position of the slice. No intravenous contrast was given.
FIGURE 1.
Magnetic resonance elastography of the liver in a healthy white individual. Axial magnitude image (A), wave image (B), and stiffness map (C) of one slice from the magnetic resonance elastography sequence. The liver is outlined in the wave image and the stiffness map (B and C). Three regions of interest are located in the right lobe of the liver (white circles) (C).
The passive driver was connected via flexible tubing to the active driver unit, which was located outside the MRI room. The active driver unit generates pressure pulses (at a 60-Hz frequency in this study) that are conducted to the passive driver via flexible tubing.
The MRE images were acquired using a 16-channel abdominal phase array coil. Shear-wave imaging was conducted using a modified 2-dimensional gradient-recalled echo-based pulse sequence with the following parameters: matrix size, 256 × 256; slice thickness, 10 mm; repetition time, 33 ms; echo time, 20 ms; flip angle, 30°; and time steps, 4. The resulting wave images (FIGURE 1B) were then automatically processed by the scanner, using a manufacturer-provided 2-dimensional direct inversion algorithm to generate quantitative images depicting tissue stiffness (elastograms) (FIGURE 1C).14,19
Liver stiffness was measured by placing the regions of interest (on magnitude stiffness maps) at 4 anatomically different slices of the liver. Three regions of interest were placed in each slice, avoiding large vessels, gallbladder, liver edge, and motion artefacts areas, and the stiffness measurement was recorded (FIGURE 1C). The mean liver stiffness value was calculated automatically and given in kPa.
Fat fraction was measured using a special technique provided by the manufacturer, called “IDEAL-IQ”, which slices through the entire liver during a single breath-hold at 6 different echo times. The data are automatically processed to generate quantitative maps showing fat fraction.
Statistical analysis
Liver stiffness values were presented as mean (SD). The Pearson correlation test was performed to assess the correlation of liver stiffness with age, estimated fat fraction percent of the liver, and body mass index (BMI). The χ2 test was performed to identify significant differences between male and female volunteers. Statistical significance was assumed at a P value of less than 0.05. Statistical analysis was performed using the MedCalc software (version 18.9.1; Mariakerke, Belgium).
Ethics
The aim of the study was explained to all volunteers, and written informed consent was obtained from every individual participating in the study. The study protocol was approved by the Ethics Committee at the Medical Faculty of the University of Rzeszów (No. 8/10/2016) and conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008).
RESULTS
All MRE exams were successfully completed. None of the volunteers reported any discomfort during the procedure.
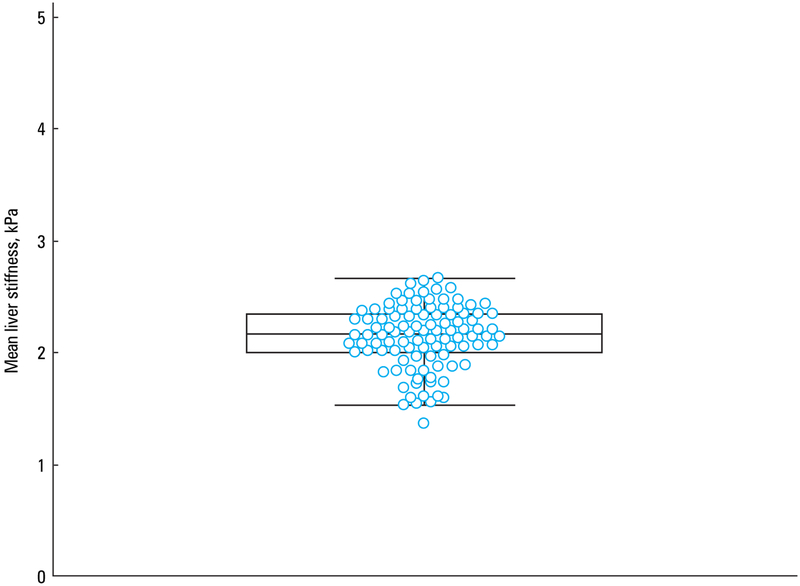
The liver stiffness value in healthy volunteers ranged from 1.37 to 2.66 kPa, with a mean (SD) of 2.14 (0.28) kPa (95% CI, 2.09–2.20) (FIGURE 2).
FIGURE 2.
A box‑and‑whisker plot showingthe distribution of mean liver stiffness values in 102 healthy white volunteers. Dots represent the mean values of each volunteer.
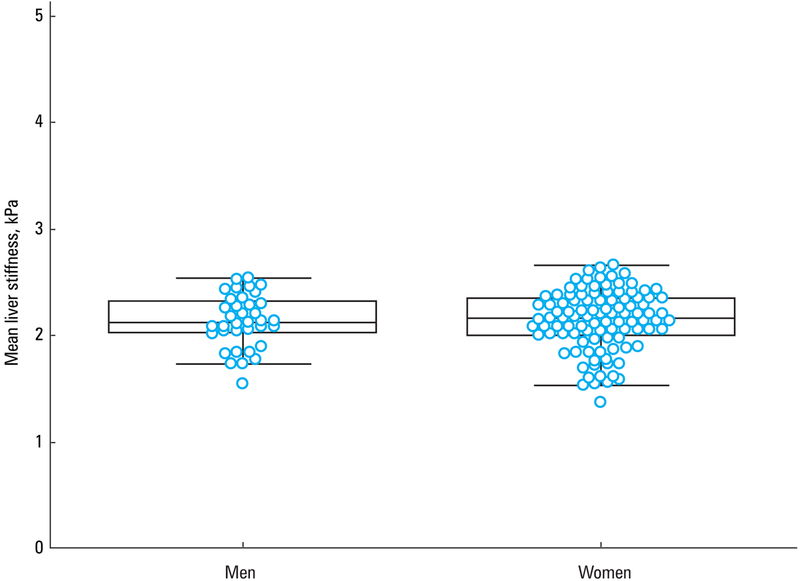
Liver stiffness values were similar between female and male volunteers (mean [SD], 2.14 [0.3] kPa; 95% CI, 2.07–2.22 and mean [SD], 2.13 [0.25] kPa; 95% CI, 2.05–2.22; respectively; P = 0.22) (FIGURE 3).
FIGURE 3.
Box‑and‑whisker plots showing the distribution of mean liver stiffness values in men and women
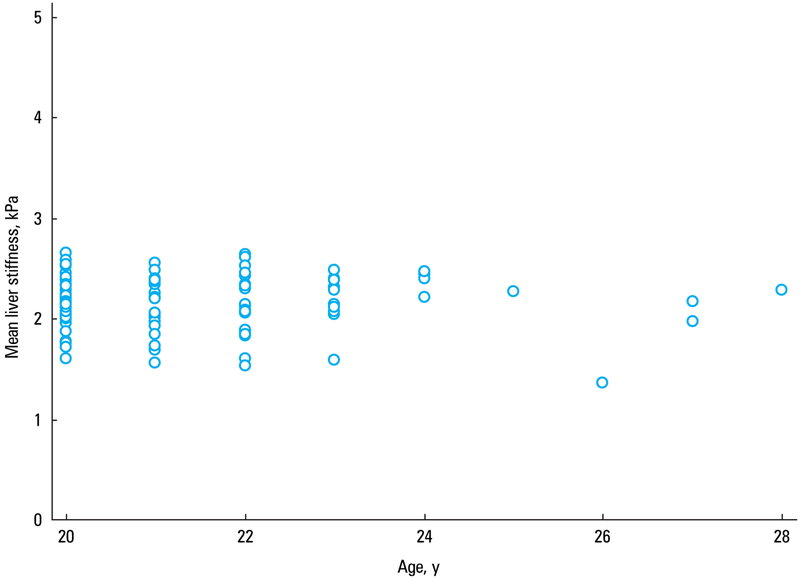
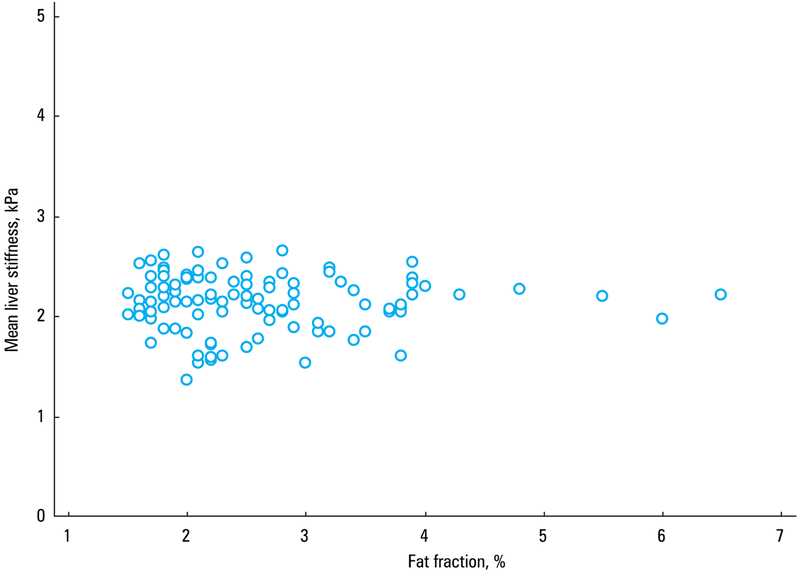
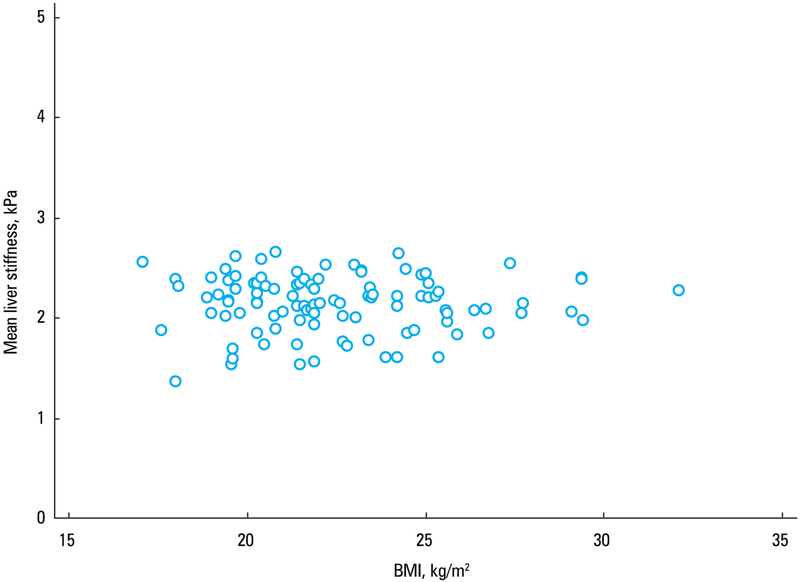
Liver stiffness values did not correlate with age (r = −0.03, P = 0.76) (FIGURE 4). The estimated fat fraction in the population ranged from 1.5% to 6.5%, and there was no correlation between the estimated fat content in the liver and mean liver stiffness (r = −0.02; 95% CI, −0.21 to 0.18; P = 0.85) (FIGURE 5). Similarly, liver stiffness did not correlate with BMI (r = 0.001; 95% CI, −0.19 to 0.20; P = 0.99) (FIGURE 6).
FIGURE 4.
A scatterplot diagram showing the correlation between age and mean liver stiffness
FIGURE 5.
A scatterplot diagram showing the correlation between estimated fat fraction percent of the liver and mean liver stiffness values in 102 healthy white volunteers
FIGURE 6.
A scatterplot diagram showing the correlation between body mass index (BMI) and mean liver stiffness values in 102 healthy white volunteers
DISCUSSION
In order to correctly interpret liver stiffness values in patients, it is important to determine the reference range in a healthy population, taking into account potential population differences. Ultrasound elastography studies have suggested that normal liver stiffness values may be slightly lower in Asian populations compared with white populations.20–23
The present study of 102 volunteers is the largest study performed to date assessing liver stiffness in a healthy white European population. Liver stiffness values in our healthy volunteers ranged from 1.37 to 2.66 kPa, with a mean value of 2.14 kPa. In studies from the United States, the values ranged from 2.1 to 2.44 kPa.14,19,24,25 Hines et al24,25 reported higher liver stiffness in an American population (mean [SD], 2.44 [0.06] kPa) than that observed in our study. However, Rouviere et al,26 who investigated 12 healthy volunteers, reported a mean (SD) liver stiffness value of 2.0 (0.3) kPa, which is consistent with our results.
Liver stiffness examinations in Asian volunteers were performed in small groups.27,28 In a study performed in 5 living liver transplant donors, the stiffness was 2.20 kPa.28 This value is comparable to that obtained in the present study. Venkatesh et al17 reported a slightly lower mean liver stiffness value in Asians (2.09 kPa; range, 1.68–2.48 kPa). In another study, which included 10 Asian volunteers, no stiffness value was reported.27
Corpechot et al29 demonstrated lower ultra-sound elastography liver stiffness in women. Lee et al30 examined a group of 49 liver donors and found no correlation between liver stiffness and sex. In our study, we found no significant differences in liver stiffness between men and women. We also found no correlation between liver stiffness and age, which is consistent with other reports.24,25,31
A limitation of our study is that the volunteers were within a narrow age range (20–28 years). Although we found no correlation between liver stiffness and age, future studies should include individuals within a wider age range to confirm the results.
The strength of the study is its objectivity. Normal liver enzyme values were part of the inclusion criteria, which significantly contributes to the reliability of our results. In addition, we included the largest study group compared with all other reports available in the literature.
In conclusion, liver stiffness in a healthy European population did not exceed 2.66 kPa and is not influenced by sex, BMI, or fat content. We believe that our results will contribute to the establishment of normative liver stiffness values, which will translate into a wider application of MRE in clinical practice.
ACKNOWLEDGMENTS
The study was performed as part of the project “Centre for Innovative Research in Medical and Natural Sciences” conducted by the University of Rzeszów, cofinanced within the Regional Operational Program for the Podkarpackie Province for the years 2007–2013 (contract number UDA-RPPK.01.03.00-18-004/12–00).
Footnotes
CONFLICTS OF INTEREST RLE and Mayo Clinic have intellectual property rights and a financial interest in magnetic resonance elastography technology. RLE serves as CEO of Resoundant, Inc. Other authors declare no conflict of interest.
REFERENCES
- 1.Jou JH, Muir AJ. In the clinic. Hepatitis C. Ann Intern Med. 2012; 157: ITC6-1–ITC6-16. [DOI] [PubMed] [Google Scholar]
- 2.Vippalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009; 49: 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008; 371: 838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Habior A Liver diseases‑the threat for Europe in 21th Century [in Polish]. Progress in Medicine. 2009; 2: 77–83. [Google Scholar]
- 5.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013; 57: 1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001; 344: 495–500. [DOI] [PubMed] [Google Scholar]
- 7.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000; 32: 477–481. [DOI] [PubMed] [Google Scholar]
- 8.Castera L, Negre I, Samii K, Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology. 1999; 30: 1529–1530. [DOI] [PubMed] [Google Scholar]
- 9.Poynard T, Ratziu V, Bedossa P. Appropriateness of liver biopsy. Can J Gastroenterol. 2000; 14: 543–548. [DOI] [PubMed] [Google Scholar]
- 10.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003; 39: 239–244. [DOI] [PubMed] [Google Scholar]
- 11.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002; 97: 2614–2618. [DOI] [PubMed] [Google Scholar]
- 12.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003; 38: 1449–1457. [DOI] [PubMed] [Google Scholar]
- 13.Siddique I, El ‑Naga HA, Madda JP, et al. Sampling variability on per‑ cutaneous liver biopsy in patients with chronic hepatitis C virus infection. Scand J Gastroenterol. 2003; 38: 427–432. [DOI] [PubMed] [Google Scholar]
- 14.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastoenterol Hepatol. 2007; 5: 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for noninvasive staging of liver fibrosis. Gastoenterology. 2008; 135: 32–40. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta‑analysis of individual participant data. Clin Gastroenterol Hepatol. 2015; 13: 440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatesh SK, Wang G, Teo LL, Ang BW. Magnetic resonance elastography of liver in healthy Asians: normal liver stiffness quantification and reproducibility assessment. J Magn Reson Imaging. 2014; 39: 1–8. [DOI] [PubMed] [Google Scholar]
- 18.Mannelli L, Godfrey E, Graves MJ, et al. Magnetic resonance elastography: feasibility of liver stiffness measurements in healthy volunteers at 3T. Clin Radiol. 2012; 67: 258–262. [DOI] [PubMed] [Google Scholar]
- 19.Venkatesh SK, Ehman RL. Magnetic resonance elastography of abdomen. Abdom Imaging. 2015; 40: 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Son CY, Kim SU, Han WK, et al. Normal liver elasticity values using acoustic radiation force impulse imagine: a prospective study in healthy living liver and kidney donors. J Gastroenterol Hepatol. 2012; 27: 130–136. [DOI] [PubMed] [Google Scholar]
- 21.Kim SU, Choi GH, Han WK, et al. What are “true normal” liver stiffness values using FibroScan?: a prospective study in healthy living liver and kidney donors in South Korea. Liver Int. 2010; 30: 268–274. [DOI] [PubMed] [Google Scholar]
- 22.Colombo S, Belloli L, Zaccanelli M et al. Normal liver stiffness and its determinants in healthy blood donors. Dig Liver Dis. 2011; 43: 231–236. [DOI] [PubMed] [Google Scholar]
- 23.Herzka DA, Kotys MS, Sinkus R, et al. Magnetic resonance elastography in the liver at 3 Tesla using a second harmonic approach. Magn Reson Med. 2009; 62: 284–291. [DOI] [PubMed] [Google Scholar]
- 24.Hines C, Bley T, Lindstrom M, Reeder S. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. J Magn Reson Imaging. 2010; 31: 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hines CD, Lindstrom MJ, Varma AK, Reeder SB. Effects of postprandi‑ al state and mesenteric blood flow on the repeatability of MR elastography in asymptomatic subjects. J Magn Reson Imaging. 2011; 33: 239–244. [DOI] [PubMed] [Google Scholar]
- 26.Rouvière O, Yin M, Dresner MA, et al. MR elastography of liver: preliminary results. Radiology. 2006; 240: 440–448. [DOI] [PubMed] [Google Scholar]
- 27.Motosugi U, Ichikawa T, Sano K, et al. Magnetic resonance elastography of the liver: preliminary results and estimation of interrater reliability. Jpn J Radiol. 2010; 28: 623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim BH, Lee JM, Lee YJ, et al. MR elastography for noninvasive assessment of hepatic fibrosis: experience from a tertiary center in Asia. J Reson Imaging. 2011; 34: 1110–1116. [DOI] [PubMed] [Google Scholar]
- 29.Corpechot C, El Naggar A, Poupon R. Gender and liver: is the liver stiffness weaker in weaker sex? Hepatology. 2006; 44: 513–514. [DOI] [PubMed] [Google Scholar]
- 30.Lee DH, Lee JM, Han JK, Choi BI. MR elastography of healthy liver parenchyma: normal value and reliability of the liver stiffness value measurement. J Magn Reson Imaging. 2013; 38: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 31.Roulot D, Czernichow S, Le Clésiau H, et al. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008; 48: 606–613. [DOI] [PubMed] [Google Scholar]