Abstract
Background
Multi-drug resistant organisms are an increasingly important cause of neonatal sepsis.
Aim
This study aimed to review neonatal sepsis caused by multi-drug resistant Enterobacteriaceae (MDRE) in neonates in Johannesburg, South Africa.
Methods
This was a cross sectional retrospective review of MDRE in neonates admitted to a tertiary neonatal unit between 1 January 2013 and 31 December 2015.
Results
There were 465 infections in 291 neonates. 68.6% were very low birth weight (< 1500 g). The median age of infection was 14.0 days. Risk factors for MDRE included prematurity (p = 0.01), lower birth weight (p = 0.04), maternal HIV infection (p = 0.02) and oxygen on day 28 (p < 0.001). The most common isolate was Klebsiella pneumoniae (66.2%). Total MDRE isolates increased from 0.39 per 1000 neonatal admissions in 2013 to 1.4 per 1000 neonatal admissions in 2015 (p < 0.001). There was an increase in carbapenem-resistant Enterobacteriaceae (CRE) from 2.6% in 2013 to 8.9% in 2015 (p = 0.06). Most of the CRE were New Delhi metallo—β lactamase- (NDM) producers.
The all-cause mortality rate was 33.3%. Birth weight (p = 0.003), necrotising enterocolitis (p < 0.001) and mechanical ventilation (p = 0.007) were significantly associated with mortality. Serratia marcescens was isolated in 55.2% of neonates that died.
Conclusions
There was a significant increase in MDRE in neonatal sepsis during the study period, with the emergence of CRE. This confirms the urgent need to intensify antimicrobial stewardship efforts and address infection control and prevention in neonatal units in LMICs. Overuse of broad- spectrum antibiotics should be prevented.
Keywords: Neonatal Sepsis, Enterobacteriaceae, Carbapenem-resistant, Klebsiella pneumoniae
Background
Sepsis remains a major cause of morbidity and mortality in preterm infants [1]. There has been a significant increase in neonatal sepsis caused by multi-drug resistant organisms (MDRO) in the past decade [1, 2]. More than half the organisms causing bloodstream infections (BSI) in a neonatal unit in Johannesburg, South Africa were due to MDRO [3]. In a recent report from Jordan, two thirds of organisms causing neonatal sepsis were MDRO and most of the gram-negative organisms were extended-spectrum beta-lactamase (ESBL) producers [1]. Many preterm infants are colonised with MDRO - more than half the Klebsiella pneumoniae and Escherichia coli isolated from a group of preterm infants in Malaysia were MDRO [4].
Infections with multi-drug resistant gram-negative organisms, especially Enterobacteriaceae, are of concern in preterm infants. Neonatal sepsis caused by these pathogens is increasing and there are limited choices available for treatment [5]. Infections with multi-drug resistant Enterobacteriaceae (MRDE) are associated with poor outcome and high case fatality rates, especially in low and middle income countries (LMIC) [5]. Mechanisms of antibiotic resistance in Enterobacteriaceae include production of ESBLs or carbapenemases [5]. There are recent reports of colistin-resistant Enterobacteriaceae in neonates [4].
Carbapenem-resistant organisms were already described as a cause of neonatal septicaemia in India in 2007 [6]. It is not clear whether patterns of ESBL and carbapenem-resistant Enterobacteriaceae (CRE) reflect national resistance patterns or are specific to the neonatal units. The predominant CRE strain in Asia and the West Pacific is New Delhi metallo-beta-lactamase (NDM − 1), whereas that in Europe and the USA is Klebsiella pneumoniae carbapenemase (KPC) [5]. There is limited information on CRE in Africa. There is a report from Morocco where the predominant strain was OXA β-lactamase, i.e. OXA-48 carbapenemase [7].
{Magiorakos, 2012 #2052} NDM and KPC (KPC-2) were first described in adult patients in Johannesburg in 2011 [8]. The aim of this study is to describe the patterns of MDRE, including CRE, in a neonatal unit in Johannesburg, South Africa.
Subjects and methods
This is a retrospective descriptive cross-sectional study. All newborn neonates admitted to the neonatal unit between 01 January 2013 and 31 December 2015 were eligible for inclusion. The study group included all neonates with culture proven blood stream infection (BSI) caused by MDRE. A control group of 30% of all neonates without infection admitted to the neonatal unit during the study period was randomly generated from the neonatal database using SPSS IBM 24. Subjects were identified through the laboratory information system of the National Health Laboratory Service (NHLS). Patient characteristics were obtained from the neonatal computer database. Information was obtained from hospital records on discharge of each neonatal patient and was entered into a computerised database for the purpose of quality control. Data was managed using Research Electronic Data Capture (REDCAP), hosted by the University of the Witwatersrand [9]. Maternal information, demographic and clinical characteristics, as well as survival to hospital discharge, were described for each patient. Causative organisms and their antimicrobial sensitivity patterns were described. Organism identification and antimicrobial susceptibility testing was done on the Vitek 2® (bioMerieux, Marcy-I’Etoile, France). Vitek 2 breakpoint interpretation was based on the Clinical and Laboratory Standards Institute (CLSI) guidelines. Isolates were characterised as CRE based on carbapenem Etest® (bioMerieux, Marcy-I’Etoile, France) minimum inhibitory concentration (MIC) testing. Colistin broth micro-dilution testing was not performed and hence colistin susceptibility rates cannot be reported for all isolates. Multiplex PCR for the carbapenemase genes (for blaNDM, blaKPC, blaOXA-48 and its variants, blaGES, blaIMP and blaVIM; LightMix Modular kits, Roche Diagnostics, Basel, Switzerland) was performed on a subset of the CRE isolates. Typing of isolates was not performed.
Statistical analysis
IBM SPSS 24 was used to analyse the data.. Maternal and neonatal characteristics were described for each patient (not bacterial isolate). Microbiological information (resistance patterns, isolates over time) was analysed for each bacterial isolate. Mean and standard deviation or median and range, were used to describe central tendency in continuous variables, depending on the distribution of the data. Categorical variables were described using frequency and percentages. Only valid cases were analysed for each variable (i.e. missing cases were excluded). Two comparisons were performed. Firstly, survivors and non- survivors within the MDRE group were compared to determine risk factors for mortality. Secondly, the MDRE group and control group were compared to establish associations with MDRE infection. Frequencies were compared using Chi Square analysis, while unpaired t tests were used to compare continuous variables, as the data was normally distributed. A p value of 0.05 was considered to be statistically significant. Adjusted odds ratios were determined through binary logistic regression for significant associations with mortality and MDRE infection respectively.
Ethics
Ethics clearance was obtained from the Human Research Ethics Committee of the University of the Witwatersrand (Certificate M 151108). Permission was obtained to access the Laboratory information system from the NHLS.
Definitions
Early-onset sepsis (EOS) was defined as culture proven sepsis within the first 72 h of life, while late onset sepsis (LOS) was referred to as culture proven sepsis after 72 h of life [1]. Multidrug resistance was defined as the isolate being non-susceptible to ≥1 agent in ≥3 antimicrobial categories [10]. The presence of resistance to third generation cephalosporins was used as a marker for ESBL production. The presence of cefoxitin resistance was used as a marker for Amp C beta-lactamase production. Necrotising enterocolitis (NEC) was defined as modified Bell’s stages 2 or 3 [11]. Resuscitation at birth was defined as the need for bag mask ventilation. “Outborn” referred to all neonates born outside the study hospital. Very low birth weight indicated neonates with a birth weight below 1500 g. Mortality was defined as all-cause mortality during hospitalization.
Results
Characteristics associated with MDRE sepsis
There were a total of 465 MDRE infections in 291 neonates and 2146 control neonates without infection. The comparison between neonates with MDRE and controls is shown in Table 1. Control neonates weighed significantly more at birth than those neonates with MDRE sepsis 1878 g (SD 956) vs 1438 g (SD 660) (p < 0.001). Neonates with MDRE sepsis were significantly more preterm than controls − 30.4 weeks (SD 4.0) vs 32.9 weeks (SD 4.8) (p < 0.001) The all-cause mortality rate was higher in MDRE neonates than controls − 97/291 (33.3%) vs- 49/2146 (2.2%)) (p < 0.001). Most of the neonates with MDRE sepsis (199/291; 68.6%) were very low birth weight. The median age at presentation with MDRE was 14.0 days (IQR 20). Although more than one third of neonates with MDRE sepsis were HIV exposed, there was only one neonate (1/108; 0.9%) with a positive HIV polymerase chain reaction at birth. Adjusted odds ratios for those variables significantly associated with MDRE are shown in Table 2. Prematurity, lower birth weight, maternal HIV infection and oxygen on day 28 were all associated with MDRE infection.
Table 1.
Characteristics associated with multi-drug resistant Enterobacteriaceae infection in neonates
| Variable | Babies with MDRE sepsis n/N (%) | Babies without sepsis n/N (%) | p value |
|---|---|---|---|
| Inborn | 196/288 (68.1) | 1716/2096 (81.9) | < 0.001 |
| Attended antenatal care | 188/254 (74.0) | 1663/1971 (84.4) | < 0.001 |
| Maternal chorioamnionitis | 7/238 (2.9) | 47/1875 (2.5) | 0.689 |
| Maternal HIV | 108/279 (38.7) | 600/2009 (29.9) | 0.003 |
| Vaginal Delivery | 134/261 (51.3) | 902/2076 (43.4) | 0.016 |
| Male | 157/291 (54.0) | 1113/2135 (52.1) | 0.697 |
| Multiple gestation | 44/284 (15.5) | 267/2078 (12.8) | 0.216 |
| Resuscitation at birth | 112/269 (41.6) | 451/2086 (21.6) | < 0.001 |
| Oxygen on day 28 | 118/262 (45.0) | 161/1957 (8.2) | < 0.001 |
| Nasal CPAP | 168/191 (87.9) | 961/2146 (44.8) | < 0.001 |
| Mechanical ventilation | 85/196 (43.4) | 791/2101 (37.6) | 0.115 |
| Necrotising enterocolitis (Grade 2 and 3) | 61/288 (21.2) | 164/2080 (7.9) | < 0.001 |
| Surgery (excluding necrotising enterocolitis)` | 45/284 (15.8) | 71/2065 (3.4) | < 0.001 |
Table 2.
Adjusted odds ratios for characteristics associated with multi-drug resistant Enterobacteriaceae infection in neonates
| Characteristic | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Maternal HIV | 1.60 | 1.076–2.303 | 0.02 |
| Oxygen on day 28 | 5.855 | 3.976–8.621 | < 0.001 |
| Birth weight | 0.998 | 0.997–0.999 | 0.01 |
| Gestational age | 0.881 | 0.794–0.977 | 0.04 |
Bacterial isolates
There were 41/465 (8.8%) of EOS caused by MDRE. Most neonates (195/291; 67%) had a single episode of infection, 18.2% (53/291) had two episodes of infection and 14.8% (43/291) had three or more episodes. The most common isolate was Klebsiella pneumoniae (308/465; 66.2%), followed by Enterobacter cloacae. (49/465; 10.5%), Escherichia coli (45/465; 9.6%); Serratia marcescens (29/465; 6.2%), Klebsiella spp. (Klebsiella spp. other than K. pneumoniae) (27/465; 5.8%), Proteus mirabilis (3/465, 0.65%), Citrobacter freundii (2/465, 0.43%), Citrobacter koseri (1/465, 1.2%) and Salmonella spp. (1/465, 1.2%).
Resistance patterns of all isolates are shown in Table 3. High rates of resistance to ampicillin and amoxicillin -clavulanic acid were observed across most of the Enterobacteriaceae. Seventy-one percent (330/463) and 23% (107/456) of all MDRE isolates were ESBL- and Amp C β-lactamase-producers, respectively. Of the 29% of MDRE isolates that were not ESBL producers, 40% (53/133) were Amp C-producers. Non-ESBL, non-Amp C-producing isolates (80/133) remained susceptible to cefepime. Eighty-two percent (369/452) of MDRE isolates retained susceptibility to ciprofloxacin. Both imipenem (426/448) and meropenem (439/461), showed a high susceptibility rate of 95%. Amikacin displayed a slightly higher susceptibility rate at 96% (433/448).
Table 3.
Antimicrobial resistance patterns in different isolates (%)
| Antimicrobial agent | K.Pneumonia | Klebsiella sp. | Enterobacter | E.Coli | Serattia | Other |
|---|---|---|---|---|---|---|
| Ampicillin/Amoxil | 98,7 | 100 | 85,7 | 84,4 | 84,2 | 100 |
| Amox-Clavulanate | 75,9 | 55,5 | 95,9 | 26,6 | 100 | 14,2 |
| Piperacillin-tazobactam | 39,4 | 25,9 | 22,4 | 6,6 | 14,2 | 14,2 |
| Cefotaxime | 89,8 | 62,9 | 31,9 | 20 | 27,5 | 14,2 |
| Ceftazidime | 90,5 | 59,5 | 28,5 | 20 | 27,5 | 14,2 |
| Cefepime | 89,2 | 53,8 | 12,2 | 17,7 | 24,1 | 0 |
| Ciprofloxacillin | 22,9 | 18,5 | 0 | 0 | 0 | 0 |
| Ertapenem | 4,3 | 0 | 2,1 | 0 | 0 | 28 |
| Imipenem | 6,1 | 3,7 | 0 | 0 | 0 | 28,5 |
| Meropenem | 6,5 | 3,8 | 0 | 0 | 0 | 14,2 |
| Amikacin | 4,9 | 5 | 0 | 2,2 | 0 | 25 |
| Tobramycin | 66,6 | 50 | 4 | 25,9 | 25 | 30 |
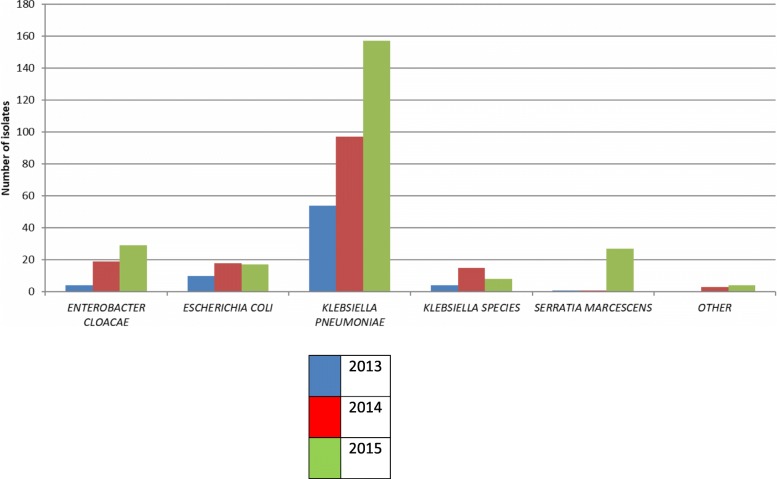
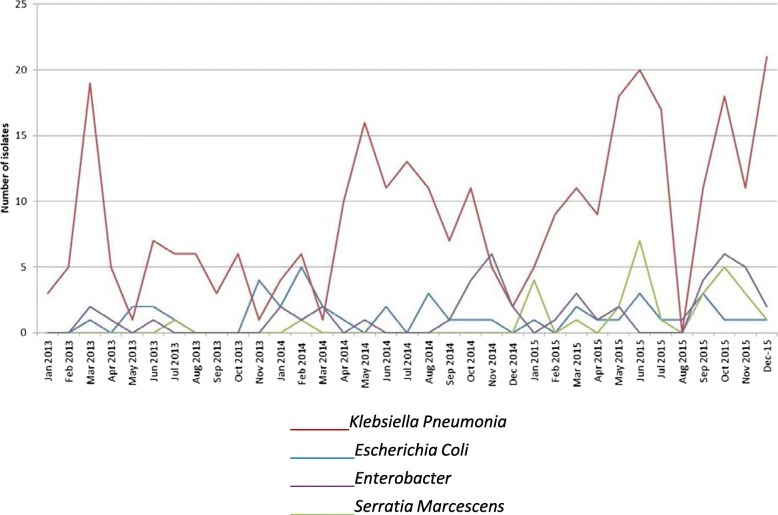
The variation in annual MDRE isolates is shown in Fig. 1. Total MDRE isolates increased from 0.39 per 1000 neonatal admissions in 2013 to 1.4 per 1000 neonatal admissions in 2015 (p < 0.001). Monthly variation in isolates of K. pneumoniae, E. cloacae, S. marcescens and E. coli is shown in Fig. 2.
Fig. 1.
Multi drug resistant bacterial isolates by year
Fig. 2.
Multi-drug resistant Enterobacteriaceae bacterial isolates by month
Carbapenem resistant isolates
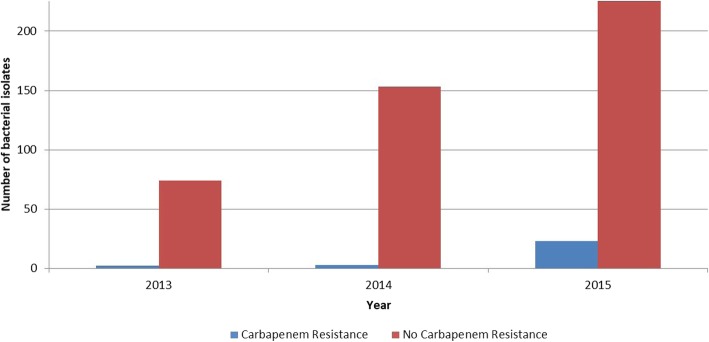
There were 26 CREs isolated from 24 neonates shown in Table 4. There was one isolate each of C. freundii and Klebsiella oxytoca and two E. cloacae. The remaining 22 isolates were K. pneumoniae. CRE isolates per year are shown in Fig. 3. There was an increase in CRE from 2.6% in 2013 to 8.9% in 2015 (p = 0.06). One Klebsiella pneumoniae isolate produced Verona Integron-Mediated Metallo-β-lactamase (VIM) and fourteen produced NDM. The K. oxytoca produced VIM and the C. freundii produced NDM. One isolate was negative for CPE genes and 8 isolates were not tested. Ninety-two percent (24/26) and 62% (16/26) of the CRE isolates were ciprofloxacin and amikacin susceptible, respectively.
Table 4.
Carbapenem resistant Enterobacteriaceae determinants and susceptibility profiles
| Number | Organism | Carbapenemase Gene | Antibiotic Susceptibility | ||||
|---|---|---|---|---|---|---|---|
| Amikacin | Ciprofloxacin | Ertapenem | Meropenem | Imipenem | |||
| 1. | K. pneumoniae | *NT | **R | ***S | R | R | S |
| 2. | K. pneumoniae | NT | ****I | S | R | I | S |
| 3. | K. pneumoniae | NDM | R | S | NT | R | R |
| 4. | K. pneumoniae | NDM | R | R | R | R | R |
| 5. | K. pneumoniae | NDM | S | S | NT | R | R |
| 6. | K. pneumoniae | NDM | R | S | R | R | R |
| 7. | K. pneumoniae | VIM | S | S | S | R | R |
| 8. | K. pneumoniae | NT | S | S | R | R | R |
| 9. | K. pneumoniae | NT | S | S | R | R | R |
| 10. | K. pneumoniae | NDM | S | S | NT | R | R |
| 11. | K. pneumoniae | NDM | S | S | R | R | R |
| 12. | K. pneumoniae | NDM | R | S | NT | R | R |
| 13. | K. pneumoniae | NDM | R | S | NT | R | R |
| 14. | K. pneumoniae | NDM | S | S | NT | R | R |
| 15. | K. pneumoniae | NDM | S | S | R | R | R |
| 16. | K. pneumoniae | NDM | R | S | NT | R | R |
| 17. | K. pneumoniae | NDM | R | S | R | R | R |
| 18. | K. pneumoniae | NDM | S | S | NT | R | R |
| 19. | K. pneumoniae | NDM | S | S | NT | R | R |
| 20. | K. pneumoniae | NT | S | S | R | R | R |
| 21. | K. pneumoniae | NEG | S | S | R | R | R |
| 22. | K. pneumoniae | NT | S | R | R | S | I |
| 23. | K. oxytoca | VIM | S | S | NT | R | R |
| 24. | E. cloacae | NT | S | S | S | S | R |
| 25. | E. cloacae | NT | S | S | R | S | S |
| 26. | C. freundii | NDM | R | S | R | R | R |
NT- not tested
**R- resistant
***S- susceptible
****I- intermediately-susceptible
The susceptibility to Amikacin and/or Ciprofloxacin allows these antibiotics to be considered as treatment options either as (1) monotherapy or (2) in combination with each other or (3) in combination with a carbapenem, which spares colistin use
Fig. 3.
Carbapenem resistance by year
Mortality in the MDRE neonates
All-cause mortality in the neonates with MDRE sepsis was 33.3% (97/291). Adjusted odds ratios for variables significantly associated with mortality were birth weight (OR 0.997; 95%CI 0.996–0.999, p = 0.003), NEC (OR 4.644; 95%CI 2.012–1-.715, p < 0.001) and mechanical ventilation (2.496; 95%CI 1.282–4.860, p = 0.007). Mortality rate by isolate is shown in Table 5. The highest mortality rate was seen in neonates with S. marcescens BSI (55.2%) (p < 0.017). Neither ESBL production nor carbapenem resistance was significantly associated with mortality.
Table 5.
Mortality by bacterial isolate
| Bacteria | Total | Died | % Mortality |
|---|---|---|---|
| Enterobacter cloacae | 46 | 20 | 43.5 |
| Escherichia coli | 42 | 17 | 40.5 |
| Klebsiella pneumoniae | 306 | 89 | 29.1 |
| Klebsiella spp | 25 | 5 | 20.0 |
| Serratia marcescens | 29 | 16 | 55.2 |
| Other | 6 | 2 | 33.0 |
Discussion
This is the first report on the increase in MDRE, in particular CRE, in neonates in South Africa. The most common isolate was K. pneumoniae. These results are in keeping with other reports from LMICs where Klebsiella spp. have also been the predominant pathogen causing neonatal sepsis [12, 13]. The Study for Monitoring Antimicrobial resistance (SMART), which studied bacterial resistance patterns globally between 2002 to 2011 from isolates in urinary tract infections and intra-abdominal sepsis, reported an increase in ESBLs in all continents except Africa [14]. Unfortunately, this is no longer the case - ESBL isolates are not uncommon in Africa. A report form Burkina Faso found that almost 60% of Enterobacteriaceae were ESBL producers, [15]. while more than half of all Klebsiella isolates in South Africa were reported to produce ESBLs [16]
In the present study, there was a marked increase in all MDRE over the study period, but particularly in isolates of Klebsiella spp., which more than doubled. This increase in MDRE in neonates is in keeping with global trends [2, 5]. Carbapenem-resistant K. pneumoniae was first reported in 2008 in neonates in Karachi, Pakistan [2]. and by 2011, 72% of K. pneumoniae isolates were resistant to carbapenems. The first case of carbapenem-resistant K. pneumoniae in a neonate in South Africa was described in 2015 in KwaZulu Natal [17], while invasive CRE was isolated in paediatric patients in Cape Town in 2012 [18]. Neonatal CRE was first noted in the present study in 2013 and by 2015, 7.3% of the MDRE isolates were carbapenem resistant.
Factors associated with MDRE sepsis in the present study included prematurity, oxygen on day 28 of life and lower birth weight. This is in agreement with other reports where prematurity, low birth weight, prolonged hospitalization, surgical procedures, mechanical ventilation and use of invasive devices were reported as risk factors for MDRE [19–22]. However, the risk factors for all late onset neonatal sepsis include lower birth weight and gestational age, necrotising enterocolitis and bronchopulmonary dysplasia [23]. In order to determine risk factors that are specific for MDRE infection, a control group of neonates with late onset sepsis without MDRE organisms should be used, rather than neonates without sepsis. The case fatality rate in the current study was 33.3% which is slightly higher than that reported from a review of MDRE in Taiwan [24]. Mortality in the present study was significantly associated with lower birth weight, NEC and mechanical ventilation.
Most of the MDRE isolates in the current study were resistant to the penicillins and cephalosporins; the most common mechanism of resistance being production of ESBLs, particularly in Klebsiella spp. The frequency of ESBL producers in the current study resulted in widespread use of carbapenems (usually meropenem) for empiric treatment of presumed sepsis in neonates. This compounds the problem of resistant organisms as widespread use of broad-spectrum antibiotics is an important mechanism in the development and spread of MDRE [5, 20, 21, 25]. Previous exposure to carbapenems is a risk factor for subsequent CRE [18]. Treatment options for confirmed MDRE sepsis were also limited. Fluoroquinolones and aminoglycosides were not frequently used, due to significant side effects. Cefepime remains a treatment option for the isolates that were non-ESBL and non-Amp C producers. Meropenem was the appropriate antibiotic choice in the majority of isolates. Several of the CRE isolates were susceptible to Amikacin and/or Ciprofloxacin. As a result, these antibiotics could be considered as treatment options either as (1) monotherapy or (2) in combination with each other or (3) in combination with a carbapenem. This would result in sparing Colistin use. The emergence of colistin resistance in neonatal sepsis has been reported in other studies [5, 24]. The lack of appropriate colistin susceptibility testing precludes the determination of the presence colistin resistance in this study. Previous reports have suggested a seasonal incidence in Klebsiella infections in neonates [2]. Although there was marked variation in the number of MDRE isolate (including Klebsiella spp.) in the current study, there was no evidence of a seasonal variation.
The mortality rate was highest in neonates infected with S. marcescens in the present study. S. marcescens is an important nosocomial pathogen, especially in neonatal intensive care units (NICU) where several outbreaks have been described globally. It causes serious infections, including bacteremia, pneumonia, urinary tract infections and meningitis with significant morbidity and mortality rates among newborns. Risk factors for acquisition of nosocomial infections caused by S. marcescens in NICUs are low birth weight, long duration of hospitalization and receiving of critical care [26]. Although we found a higher mortality rate in S. marcescens infections, we did not analyze possibly confounding variables such as the severity of illness related to prematurity in these infants.
Prevention of MDRE infections includes screening for colonization, antibiotic stewardship, and stringent infection control practices including, hand hygiene practices, use of appropriate personal protective equipment, and decreased use of invasive devices [20, 25]. The importance of the neonatal gut microbiome has been underestimated. A recently published systematic review showed that supplementation with probiotics reduced the incidence of LOS in preterm infants [27]. Many neonates colonized with MDRE develop infection with the same organism and the presence of components of the gut normal flora, such as Lactobacillus plantarum and Bifidobacterium breve, can have a protective effect by exhibiting antimicrobial activity against gut pathogens [25]
Limitations of the study
This was a retrospective review of an existing database, so timing of complications and antibiotic therapy for BSI could not be evaluated. In addition, many possible risk factors for sepsis, such as catheterization, time to the onset of feeding, and total parenteral nutrition had not been captured and were therefore not available for analysis.
Conclusions
The present study confirms that there is an alarming increase in MDRE with the emergence of CRE. This confirms the urgent need to intensify antimicrobial stewardship efforts and address infection control and prevention in neonatal units in LMICs. In particular, the overuse of broad-spectrum antibiotics should be discouraged. In addition, other treatment and prevention modalities, such as strategies targeting the neonatal gut microbiome, should be investigated.
Acknowledgements
The authors gratefully acknowledge that assistance of Ms. Melanie Mcree in collecting data for the study.
Abbreviations
- BSI
Blood stream infection
- CRE
Carbapenem resistant Enterobacteriaceae
- EOS
Early onset sepsis
- ESBL
Extended βlactamase
- LMIC
low and middle income country
- LOS
Late onset sepsis
- MDRE
Multi drug resistant Enterobacteriaceae
- MDRO
Multidrug resistant organisms
- MIC
Minimum inhibitory concentration
- NDM 1
New Delhi metallo β lactamase
- NEC
Necrotizing enterocolitis
- REDCAP
Research Electronic Data Capture
Authors’ contributions
DEB – conceptualized the paper, obtained ethics clearance, analyzed data, wrote drafts and submitted the manuscript. RB- data collection and analysis, revision of various drafts. TN, NB, TT – data collection and analysis, revision of various drafts. VAD, PAC, MM and JL – reviewed and advised on study design and methodology, revision of various drafts. All authors reviewed and approved the final submission.
Funding
None to declare.
Availability of data and materials
Data will be made available upon reasonable request to the corresponding author.
Ethics approval and consent to participate
The study was approved by the Human Research Ethics Committee of the University of the Witwatersrand (Clearance Number M 15 1108). This was a retrospective record review of de-identified data so consent to participate was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daynia E. Ballot, Email: daynia.ballot@wits.ac.za
Rosella Bandini, Email: Rosella.bandini@wits.ac.za.
Trusha Nana, Email: trusha.nana@nhls.ac.za.
Noma Bosman, Email: noma.bosman@nhls.ac.za.
Teena Thomas, Email: teena.thomas@wits.ac.za.
Victor A. Davies, Email: victor.davies@wits.ac.za
Peter A. Cooper, Email: peter.cooper@wits.ac.za
Mervyn Mer, Email: mervyn.mer@wits.ac.zaJ.
Jeffrey Lipman, Email: jlipman@uq.edu.au.
References
- 1.Yusef D, Shalakhti T, Awad S, Algharaibeh H, Khasawneh W. Clinical characteristics and epidemiology of sepsis in the neonatal intensive care unit in the era of multi-drug resistant organisms: a retrospective review. Pediatr Neonatol. 2017. [DOI] [PubMed]
- 2.Saleem AF, Qamar FN, Shahzad H, Qadir M, Zaidi AK. Trends in antibiotic susceptibility and incidence of late-onset Klebsiella pneumoniae neonatal sepsis over a six-year period in a neonatal intensive care unit in Karachi, Pakistan. Int J Infect Dis. 2013;17(11):e961–e965. doi: 10.1016/j.ijid.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Ballot DE, Nana T, Sriruttan C, Cooper PA. Bacterial bloodstream infections in neonates in a developing country. ISRN pediatrics. 2012;2012:508512. doi: 10.5402/2012/508512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap PS, Ahmad Kamar A, Chong CW, Yap IK, Thong KL, Choo YM, Md Yusof MY, Teh CS. Intestinal carriage of multidrug-resistant gram-negative bacteria in preterm-infants during hospitalization in neonatal intensive care unit (NICU) Pathogens Global health. 2016;110(6):238–246. doi: 10.1080/20477724.2016.1229884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folgori L, Bielicki J, Heath PT, Sharland M. Antimicrobial-resistant gram-negative infections in neonates: burden of disease and challenges in treatment. Curr Opin Infect Dis. 2017;30(3):281–288. doi: 10.1097/QCO.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 6.Datta S, Roy S, Chatterjee S, Saha A, Sen B, Pal T, Som T, Basu S. A five-year experience of carbapenem resistance in Enterobacteriaceae causing neonatal septicaemia: predominance of NDM-1. PLoS One. 2014;9(11):e112101. doi: 10.1371/journal.pone.0112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barguigua A, El Otmani F, Talmi M, Zerouali K, Timinouni M. Emergence of carbapenem-resistant Enterobacteriaceae isolates in the Moroccan community. Diagn Microbiol Infect Dis. 2012;73(3):290–291. doi: 10.1016/j.diagmicrobio.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Brink AJ, Coetzee J, Clay CG, Sithole S, Richards GA, Poirel L, Nordmann P. Emergence of New Delhi metallo-beta-lactamase (NDM-1) and Klebsiella pneumoniae carbapenemase (KPC-2) in South Africa. J Clin Microbiol. 2012;50(2):525–527. doi: 10.1128/JCM.05956-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 11.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dramowski A, Madide A, Bekker A. Neonatal nosocomial bloodstream infections at a referral hospital in a middle-income country: burden, pathogens, antimicrobial resistance and mortality. Paediatrics Int Child Health. 2015;35(3):265–272. doi: 10.1179/2046905515Y.0000000029. [DOI] [PubMed] [Google Scholar]
- 13.Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr. 2015;61(1):1–13. doi: 10.1093/tropej/fmu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A review of ten years of the study for monitoring antimicrobial resistance trends (SMART) from 2002 to. Pharmaceuticals (Basel, Switzerland) 2013. 2011;6(11):1335–1346. doi: 10.3390/ph6111335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouedraogo AS, Sanou M, Kissou A, Sanou S, Solare H, Kabore F, Poda A, Aberkane S, Bouzinbi N, Sano I, et al. High prevalence of extended-spectrum ss-lactamase producing enterobacteriaceae among clinical isolates in Burkina Faso. BMC Infect Dis. 2016;16:326. doi: 10.1186/s12879-016-1655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasaikar S, Obi L, Morobe I, Bisi-Johnson M. Molecular characteristics and antibiotic resistance profiles of Klebsiella isolates in Mthatha, eastern Cape Province, South Africa. Int J Microbiol. 2017;2017:8486742. doi: 10.1155/2017/8486742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radhika Singh PM. Koleka Mlisana, Kimesh Naidoo and Prashini Moodley: isolation of New Delhi Metallo-ßeta- Lactamase-1(NDM-1) producing Klebsiella pneumoniae in a neonate in Kwazulu-Natal, South Africa. J Paediatr Neonatal Care. 2015;2(4):2. [Google Scholar]
- 18.Malande OO, Du Plessis A, Rip D, Bamford C, Eley B. Invasive carbapenem-resistant Enterobacteriaceae infection at a paediatric hospital: a case series. S Afr Med J. 2016;106(9):877–882. doi: 10.7196/SAMJ.2016.v106i9.11028. [DOI] [PubMed] [Google Scholar]
- 19.Pragosa H, Marcal M, Goncalves E, Martins F, Lopo-Tuna M. Multi-drug-resistant Enterobacteriaceae in a Portuguese neonatal intensive care unit. J Hospital Infect. 2017;96(2):130–131. doi: 10.1016/j.jhin.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Brink A, Coetzee J, Clay C, Corcoran C, van Greune J, Deetlefs JD, Nutt L, Feldman C, Richards G, Nordmann P, et al. The spread of carbapenem-resistant Enterobacteriaceae in South Africa: risk factors for acquisition and prevention. S Afr Med J. 2012;102(7):599–601. doi: 10.7196/SAMJ.5789. [DOI] [PubMed] [Google Scholar]
- 21.Lukac PJ, Bonomo RA, Logan LK. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in children: old foe, emerging threat. Clin Infect Dis. 2015;60(9):1389–1397. doi: 10.1093/cid/civ020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akturk H, Sutcu M, Somer A, Aydin D, Cihan R, Ozdemir A, Coban A, Ince Z, Citak A, Salman N. Carbapenem-resistant Klebsiella pneumoniae colonization in pediatric and neonatal intensive care units: risk factors for progression to infection. Braz J Infect Dis. 2016;20(2):134–140. doi: 10.1016/j.bjid.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD neonatal research network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 24.Tsai MH, Chu SM, Hsu JF, Lien R, Huang HR, Chiang MC, Fu RH, Lee CW, Huang YC. Risk factors and outcomes for multidrug-resistant gram-negative bacteremia in the NICU. Pediatrics. 2014;133(2):e322–e329. doi: 10.1542/peds.2013-1248. [DOI] [PubMed] [Google Scholar]
- 25.Delerue T, De Pontual L, Carbonnelle E, Zahar JR. The potential role of microbiota for controlling the spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) in neonatal population. F1000Research. 2017;6:1217. doi: 10.12688/f1000research.10713.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chemsi M, Hamid HHS, Zerouali K, Lehlimi M, et al. An outbreak of Serratia marcescens on the neonatal unit: description and investigations. J Infect Dis Ther. 2016;4:306. [Google Scholar]
- 27.Aceti A, Maggio L, Beghetti I, Gori D, Barone G, Callegari ML, Fantini MP, Indrio F, Meneghin F, Morelli L, et al. Probiotics Prevent Late-Onset Sepsis in Human Milk-Fed, Very Low Birth Weight Preterm Infants: Systematic Review and Meta-Analysis. Nutrients. 2017:9(8). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request to the corresponding author.