Abstract
In this work, we perform a comprehensive theoretical study on adsorption of representative 10-electron molecules H2O, CH4 and NH3 onto defective single-walled carbon nanotubes. Results of adsorption energy and charge transfer reveal the existence of both chemical adsorption (CA) and physical adsorption (PA). While PA processes are common for all molecules, CA could be further achieved by the polar molecule NH3, whose lone-pair electrons makes it easier to be bonded with the defective nanotube. Our systematic work could contribute to the understanding on intermolecular interactions and the design of future molecular detectors.
Keywords: physical adsorption, chemical adsorption, defective carbon nanotube, gas molecule
1. Introduction
Carbon nanotubes (CNTs), which were discovered in 1991 [1,2], have attracted enduring attention due to their unique structural, thermal, electronic and dynamic properties, which facilitate the use of CNTs in promising medical and biochemical applications. In fact, CNTs have already found applications in many fields, including hydrogen storage [3], free-radicals scavenging activity [4], chemical sensors [5], nanobiology electronics [6], functional groups adsorption substrates [7], capacitors [8] and the like.
The properties of CNTs can be affected strongly by the presence of various defects, which are usually formed during their growth process or caused by the environmental factors. The properties of defective CNTs have been systematically explored in many theoretical and experimental research projects [9–13]. By using scanning gate microscopy (SGM) and scanning impedance microscopy (SIM), workers have studied the defects in semiconducting single-walled carbon nanotubes (SWCNTs) [12]. Theoretical calculations have shown that local structural defects, such as topological defects, vacancies, impurities and deformations can substantially modify the electronic and transport properties of SWCNTs [13]. Covalent functionalization of SWCNTs with multiplicative biological groups [14–18] based on the effects of the nanotubes' conductive properties, have also been reported [19]. For instance, oxidation-induced defects have been introduced to enhance the sensitivity of an SWCNT to chemical vapours [20]. A five-membered or otherwise configured biological group inserted into the hexagon ring in the SWCNTs has generated apparent chemical reactivity [21]. Also, in the one-dimensional topological defect consisting of octagonal and pentagonal carbon rings inserted into the SWCNT's hexagon, charge transfer [22] and field-effect doping [23] can be applied to manipulate charge carrier concentrations, enabling it to act as a conducting wire [24].
Adsorption of small molecules—such as Xe [25], CF4 [26], H2 [27], CH4 [28], NO [29], NH3 [30], NO2 [31] and O2 [32]—onto SWCNTs, as well as adsorption of H2O onto graphene [33] or SWCNTs, has been studied theoretically. The sensitivity of SWCNTs to these various small molecules, combined with their own inherent characteristics—such as small size, favourable electrical and mechanical properties, and high surface-area-to-volume ratios, type of defect—have led to many applications for them in various roles as functionalized materials in sensors and in many biological fields. Nevertheless, the complicated, weak interactions between these molecules and carbon materials have been widely reported, but a systematic investigation on the nature of those interactions has been lacking.
A thorough and fundamental theoretical study on small molecules adsorbing on SWCNTs, with a more advanced dispersion force correction, is needed. When a strongly oxidizing defective nanotube interacts with various molecules, it has been found to exhibit different properties from those of the perfect tube. Liu et al. reported that reactions replacing reactive sites arise among the 5–1DB defects with the oxynitride on the CNT [34]. Actually, it is easy to chemically bind an acetone onto a Stone–Wales defect or a vacancy of the SWCNT [35]. Such chemical adsorptions (CAs) change the electronic processes and transmission characteristics of the imperfect tube. However, research efforts into the physical adsorption (PA) which is a weak interaction of small molecules, such as H2O, CH4 and NH3, on defective tubes are seldom reported (e.g. the adsorption energy, charge transfer, gap value, dipole moment, etc.).
Previous research studies have examined the adsorption of various molecules on graphene and revealed obviously different characteristics in Raman vibrational and ultraviolet–visible adsorption spectra of the molecules [36]. However, a systematic study on the interaction of specific molecules with different types of defective SWCNTs has yet to be conducted. In this paper, we theoretically study three commonly found representative 10-electron molecules (H2O, CH4 and NH3) adsorbed onto four common defective SWCNT structures. Our results include the various adsorption structures, adsorption energy and charge transfer with the defective structures, compared with those values from the perfect SWCNT. The case of NH3 is found to be exceptional, because it shows distinct chemical characteristics when it is adsorbed on two types of defective SWCNTs. To elucidate our results, a brief discussion has also been made on the polarity of the small gas molecules in relation to their PA or CA on SWCNTs.
2. Computational methods
The interactions between three representative small molecules (H2O, CH4 and NH3) and four kinds of defective CNTs were studied with the DFTB+ code [37]. Density functional tight-binding (DFTB) is based on DFT and derived from the second-order expansion of the Kohn–Sham total energy functional as calculated within DFT under the standard tight-binding theory [38]. In order to describe the van der Waals (vdW) interaction between the small molecules and defective CNTs, we adopted the DFTB-D method, which is augmented by the empirical London dispersion energy term into the DFTB total energy [39].
The London dispersion energy is given by
| 2.1 |
| 2.2 |
and
| 2.3 |
where Nα is the Slater–Kirkwood effective number of electrons. The 1/R6 dependence is truncated for small interatomic distances with an appropriate damping function [40]. This method has been successfully used to study organic molecules interacting with materials [41], the adsorption of water clusters on a graphene surface [42] and so forth.
To ensure the reliability of calculations, the cases of defective nanotubes with and without the cap at both ends were also considered. All of our results for the capped defective and perfect nanotubes adsorbed with CH4, H2O and NH3 calculated with DFTB were verified with density functional theory (DFT) calculations using PBE0-D3 functional [43,44] and 6–31 g (d, p) basis set. Conventional DFT fails in the study of weak interactions, but a newly developed method––density functional theory with empirical dispersion corrections (DFT-D)—can yield more trustworthy results. The results with DFT-D are superior because the method can compute the long-range force part, which gives more accurate data in dealing with the point charge Coulomb interaction among long-range parts; thus, it can describe PA more accurately. However, DFTB-D has an efficiency advantage in comparison with DFT-D, which is conducive to the study of more complex systems [45].
3. Results and discussion
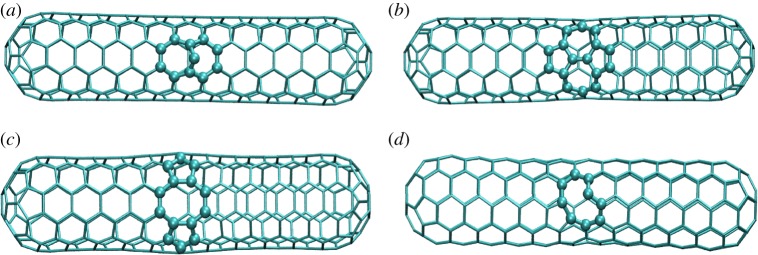
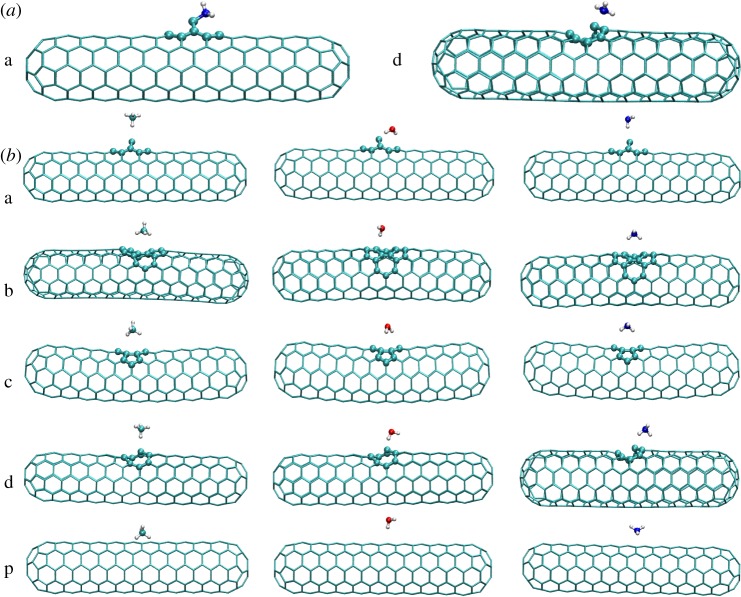
In this work, we consider the interactions of H2O, CH4 and NH3 with the pure (5, 5) SWCNT (marked with letter ‘p') and four types of defective CNTs. The denotations of the four defective nanotubes are ‘a' for ad-atom, ‘b' for a chiral Stone–Wales (SW) defect, ‘c' for the case of two missing C-atoms and ‘d' for a monovacancy defect, respectively. The configurations of the CNTs with defects are presented in figure 1. There are several conformations of the same molecule adsorbed on the various CNTs. With different initial molecular-CNT conformations, we obtained 15 stable structures that are based on a large number of different initial conformations and are shown in figure 2. The results show that CA occurred when NH3 adsorbed on ‘a' and ‘d' defective nanotubes, as shown in figure 2a. In figure 2b, the conformations for the PA case are shown. We can see several stable structures with a hydrogen atom pointing to the nanotube, which is similar with the anion-π interaction reported in the literature [46]. It should be noted that, for all the adsorbates, it is the proton instead of the heavy atom (N or O) that is pointing to the CNTs. This is because their electrons are sp3 hybridized, indicating that electron lone pairs exist in the ‘proton-free' directions. Such electron-negative lone pair would repulse either with the lone pairs of the dangling C or the π electrons, causing instability of the system, contrary to the electron-relative protons.
Figure 1.
The structures of the four types of defective CNTs studied. The SWCNTs had the following defects: (a) an ad-atom (a), (b) a chiral Stone–Wale defect (b), (c) two missing C atoms (c) and (d) a monovacancy defect (d).
Figure 2.
(a) The optimized configurations of NH3 adsorbed on the ‘a' and ‘d' defective nanotubes for the chemical case. (b) The optimized configurations of the small molecules (CH4, NH3, H2O) adsorbed on the ‘a' ‘b' ‘c' ‘d' defective and perfect nanotubes for the physical case.
However, NH3 adsorbed onto the perfect CNT reveals different properties from those of the other molecules, with the N atom staying close to the nanotube. That observation is concordant with the findings of Shirvani et al. [47]. In these structures, we readily found that one C atom is not coplanar with other atoms on the same six-membered rings in the ‘a' and ‘d’ types of defective tubes, unlike the case with the ‘b' and ‘c' types. The structure of CH4 differs in that one H atom pointing to the CNT appears in the ‘a' and ‘d' tubes and three H stable structures, and when H2O adsorbs on ‘a' defective nanotube, the distance is the smallest. Atoms pointing to the CNT appear in the ‘b' and ‘c' tubes, as is the case with the ‘p' tube. To summarize, we obtain different stable structures when the same molecule is adsorbed onto the various CNTs. Comparing the stable structures of the PA cases, we concluded that the distance of the CH4 to ‘c' defective nanotubes is the largest in all of the angles which measure the deviations of the small molecules from the x-axis. When H2O and NH3 adsorb on ‘b’ defective nanotubes, the angles are larger; however, when CH4 and H2O adsorb on ‘d’ defective and perfect nanotubes, the angles are the smallest. The data of all stable structures are provided in the electronic supplementary material.
The results of our calculations indicate that the NH3 molecule behaves differently from the others. The NH3 molecule exhibits both PA and CA adsorption on the ‘a’ and ‘d' type CNTs for the different structures of the ‘a' and ‘d' defective nanotubes from other types, with the lone-pair electrons of the top defective C-atoms in ‘a' and ‘d' being more easily bonded with small molecules. By contrast, in the other defective ‘b' and ‘c' defective nanotubes, three electrons in the outer shell of the defective C-atoms bond with other C-atoms and the last electron forms π bonds. As for the polar molecule NH3, the N-atom in the NH3 takes the inequivalent sp3 hybridization forming three σ bonds and one lone-pair electrons, making it is easier to bond with ‘a' and ‘d' defective nanotubes to form the stable structure as shown in figure 2a. However, the H2O and CH4 molecules show only PA interactions. Among them, CH4 is non-polar molecule with the C-atom taking sp3 equivalent hybridization and involving no lone-pair electrons while there are two lone-pair electrons in H2O, making the structures of ‘a' and ‘d' defective nanotubes less stable.
Figure 3 reveals the direction of the charge transfer clearly for the CA case in ‘a' and ‘d' defective nanotubes. We can see in figure 3a the electron density difference of ‘a' and ‘d' defective nanotubes adsorbed with NH3.
Figure 3.
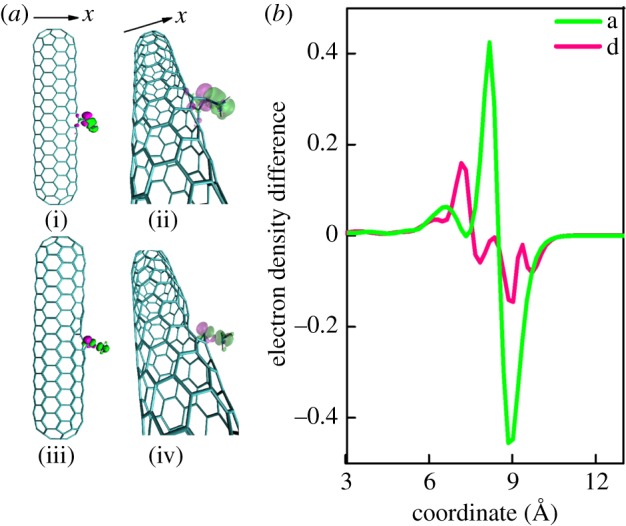
(a) The electron density difference of the ‘a' and ‘d' defective nanotubes adsorbed with NH3 molecule for the CA case. (i and ii) The front and side figures of the electron density difference when NH3 adsorbed on ‘a’ defective nanotube. (iii and iv) The front and side figures of the electron density difference when NH3 is adsorbed on ‘d' defective nanotube. (b) The electron density difference integral for the chemical adsorption that ‘a' and ‘d' defective nanotubes adsorbed with NH3.
The isovalues of the isosurfaces are 0.004 and −0.004, respectively, for the pink and green area; the pink areas on the defective CNTs represent the electron increase but the green area expresses the charge decrease. The figures show that near the ‘a' and ‘d' defective CNTs the pink area is larger, revealing the direction of charge transfer to be from the defective CNTs to NH3. That result is consistent with the electron density difference integral as shown in figure 3b, which is obtained by integrating the electron density in the y and z direction onto the x-direction, following . In figure 3b, the vertical axis represents the integrated electron density difference and the horizontal axis is the coordinate x, ranging from 3 to 13 Å. The N atom is at x = 9.06 Å for ‘a' defective nanotube and 9.27 Å for ‘d' as shown in the electronic supplementary material, figure S1. The vertical coordinates of the points above zero represent the increment of the electron density and below zero the decrement of the electron density. We obtain the same conclusion that the charge transfer is from ‘a' and ‘d’ defective nanotubes to NH3.
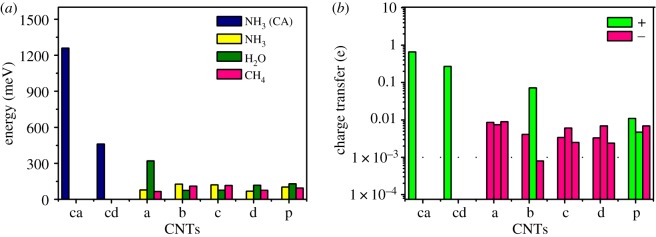
In table 1, the CA values are larger than the PA values for both the adsorption energy and the charge transfer of NH3 adsorbed on the ‘a' and ‘d' type CNTs. More specifically, the charge transfer for the CA case is of the order of 10−1 e, showing a significant contrast with 10−3 e for the PA. Both of the adsorption energies for the CA cases of ‘a' and ‘d' defects are of the order of 103 meV and 102 meV, whereas the adsorption energy of both the ‘a' and ‘d' defects is of the order of 101 meV for the PA case. However, the adsorption energy and charge transfer for adsorption on ‘a' defective nanotube is larger than ‘d' defective nanotube, especially for CA, for the difference of the defective C-atoms that the top defective C-atom of ‘a' is most distant from the principle axis of the nanotube among the defective and perfect nanotubes, which makes NH3 more easily polarized and bonded when adsorbed on ‘a’ defective nanotube.
Table 1.
The binding energies ΔE, the charge transfer Q of NH3 adsorbed on ‘a’ and ‘d’ type defect CNTs for the PA and the CA, respectively.
| PA |
CA |
|||
|---|---|---|---|---|
| tube | ΔE (meV) | Q (e) | ΔE (meV) | Q (e) |
| a | −78 | −0.01 | −1260 | 0.66 |
| d | −68 | 0 | −461 | 0.27 |
Compared with the CA, the adsorption energy and the charge transfer of H2O, CH4 and NH3 on the CNTs we calculated are much less, as shown in figure 4. The calculated PA energies vary within 400 meV. Moreover, all of them are around 100 meV except the case when H2O adsorbed on ‘a' defective nanotube (321 meV). To verify the differences of this phenomenon, further electronic density and the local density of state (LDOS) analyses are carried out. It turns out that not only the electron density of the H2O adsorption in the intermolecular area is obviously larger than those of NH3 and CH4, but its LDOS also presents delocalization of the orbitals through the region. It could be concluded that the interaction feature of this system is not simply van de Waals intermolecular interaction (detailed information is included in the electronic supplementary material). Figure 4 also presents the charge transfers for each of the configurations. The green values represent the direction of the charge transfer that shows an electron transfer from the defective or perfect nanotubes to the small molecules (i.e. CH4, H2O and NH3).
Figure 4.
(a) The comparison of the physical and chemical adsorption energies, CA structures (blue), PA structures NH3 (yellow), H2O (olive), CH4 (pink). (b) The comparison of the charge transfer of the PA and CA. The green (or pink) colour denotes the electron decrease (increase) or the charge increase (decrease).
Actually, the activation energy of desorption and the binding energies for small molecules adsorbed on carbon-based materials have been systematically observed in the previous experimental research [48]. The binding energy of small molecules with SWCNT is of similar magnitude to that of the PA cases above. This could also prove the reliability of our results.
Correspondingly, the pink values denote the opposite direction. It shows that the charge transfer from the perfect nanotube to NH3 is of the order of 10−2 e. The same amount of charge transfer number is found from the NH3 to a defective nanotube, whereas for other systems the charge transfer is almost negligible. The charge transfer from ‘a' defective nanotube to H2O is approximately 10−1 e. It is because of the lone-pair electrons of the top defective C-atom of ‘a' which is the most distant from the principle axis of the nanotube among the defective and perfect nanotubes. Also, H2O is polar molecular with sp3 non-equivalent hybridization and the two lone-pair electrons make it easily adsorbed on the defective nanotubes especially on ‘a' defective nanotube.
Furthermore, calculation of the cases for CNTs without the ‘cap' were also carried out. The results show that when the ‘cap' of the defective nanotubes is removed, the calculations still support the similar conclusions for the chemical case and PA cases, except for the ‘c' defective nanotube whose band gap becomes larger when the cap is removed.
In order to show the electronic density-based properties of the adsorption structure, electronic density difference analyses under DFT method were carried out. As expected, electronic density differences of the CA structures are much larger than those of the PA ones, consistent with the results of charge transfer analyses above. The details of electronic density difference are provided in the electronic supplementary material.
Furthermore, we also calculated the energies of all the dissociative adsorption cases with DFTB-D. By comparing the energies of all dissociative adsorptions stable structures with the corresponding coordination complexes, we concluded that dissociative adsorptions for ‘a' and ‘d' defective nanotubes are possible and further calculated their adsorption energies. The comparison of their local density states is also provided, from which we can see that the distribution of the density states is more uniform in the dissociative adsorption ones than the coordination complexes, and the delocalization of the orbital is weaker. We also performed molecular dynamic simulations of the possible dissociative adsorption cases. All the simulations were performed at room temperature. However, we have not observed any dissociative adsorptions, indicating that they do not easily occur. All the figures of the structures and energy data are provided in the electronic supplementary material.
Besides, there are many studies in the literature certifying the accuracy of the DFTB method which is compared favourably with DFT [36,45,49,50]. And the ‘d' defective nanotubes adsorbed chemically with NH3 calculated with DFT have been reported [30], which confirms the results of our work further. The comparisons of the adsorption energies and charge transfer values of the CA cases in which NH3 adsorbs on the ‘a' and ‘d' defective nanotubes calculated with DFTB and DFT are provided in the electronic supplementary material, tables S1 and S2. We obtained the same conclusions that there are obvious charge transfer and much larger adsorption energy when NH3 adsorbs on the ‘a' and ‘d’ defective nanotubes chemically. Because of the similarity in the results obtained with DFT and DFTB (the calculation speed of DFTB is three magnitudes faster than DFT), it can be used in the simulation and calculation of large molecules system [41,51] in the future.
4. Conclusion
In this work, we systematically studied the adsorption between common 10-electron small molecules and CNTs with different defects. The reliability of the results is ensured by comparing the DFTB and DFT methods. Our calculated adsorption energy, charge transfer, energy gap and dipole moment of the studied systems reveal that PA is the common process, except with NH3, which undergoes both PA and CA due to its large polarity and apparent charge transfer. CA occurs for NH3 on the defective ‘a' and ‘d' defective nanotube involving considerable adsorption energies and charge transfer because NH3 can be easily polarized. On the contrary, PAs take place for other small molecules such as CH4, H2O and NH3 on the ‘a', ‘b’, ‘c', ‘d' defective and perfect nanotubes with negligible adsorption energies and charge transfer.
The interactions that we determine between those gas molecules and the defective nanotubes are very important for identifying a specific gas such as NH3 based on the obvious different charge transfer and adsorption energies of the CA and PA. The mechanism can be used to fabricate gas sensors, as the conductivity of defective CNTs affected by the CA. Furthermore, the obtained consistency of DFTB and DFT calculations has proved the reliability of the highly efficient DFTB method. This would be helpful for us to conduct large-scale calculations and extend research to more complicated systems.
Supplementary Material
Acknowledgements
We thank Miss Sonam Wangmo, Mr Jianpeng Wang, Weiyu Xie, Drs Yan Meng, Minsi Xin, Ruixia Song and Bolong Huang for the stimulating discussions. We also acknowledge the High Performance Computing Center (HPCC) of Jilin University for computation resources.
Data accessibility
Additional data are available in the electronic supplementary material.
Authors' contributions
D.L. and F.W. performed simulations. Z.W. and R-Q.Z. conceived this project. D.L., Z.Z., W.J. and Y.Z. analysed results. D.L. produced the figures. D.L., Z.Z., R-Q.Z. and Z.W. wrote the paper. All authors commented on the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The work described in this paper is supported by grants from the National Science Foundation of China (under grant no. 11674123).
References
- 1.Iijima S. 1991. Helical microtubules of graphitic carbon. Nature 354, 56–58. ( 10.1038/354056a0) [DOI] [Google Scholar]
- 2.Iijima S, Ichihashi T. 1993. Single-shell carbon nanotubes of 1-nm diameter. Nature 363, 603–605. ( 10.1038/363603a0) [DOI] [Google Scholar]
- 3.Liu C, Fan YY, Liu M, Cong HT, Cheng HM, Dresselhaus MS. 1999. Hydrogen storage in single-walled carbon nanotubes at room temperature. Science 286, 1127–1129. ( 10.1126/science.286.5442.1127) [DOI] [PubMed] [Google Scholar]
- 4.Martínez A, Galano A. 2010. Free radical scavenging activity of ultrashort single-walled carbon nanotubes with different structures through electron transfer reactions. J. Phys. Chem. C 114, 8184–8191. ( 10.1021/jp100168q) [DOI] [Google Scholar]
- 5.Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H. 2000. Nanotube molecular wires as chemical sensors. Science 287, 622–625. ( 10.1126/science.287.5453.622) [DOI] [PubMed] [Google Scholar]
- 6.Katz E, Willner I. 2004. Biomolecule-functionalized carbon nanotubes: applications in nanobioelectronics. Chemphyschem 5, 1084–1104. ( 10.1002/cphc.200400193) [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, et al. 2017. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 8, 14675 ( 10.1038/ncomms14675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong W, et al. 2018. Carbon nanotubes and manganese oxide hybrid nanostructures as high performance fiber supercapacitors. Commun. Chem. 1, 16 ( 10.1038/s42004-018-0017-z) [DOI] [Google Scholar]
- 9.Hashimoto A, Suenaga K, Gloter A, Urita K, Iijima S. 2004. Direct evidence for atomic defects in graphene layers. Nature 430, 870–873. ( 10.1038/nature02817) [DOI] [PubMed] [Google Scholar]
- 10.Lusk MT, Carr LD. 2008. Nanoengineering defect structures on graphene. Phys. Rev. Lett. 100, 175 503–175 506. ( 10.1103/PhysRevLett.100.175503) [DOI] [PubMed] [Google Scholar]
- 11.Meyer JC, Kisielowski C, Erni R, Rossell MD, Crommie MF, Zettl A. 2008. Direct imaging of lattice atoms and topological defects in graphene membranes. Nano Lett. 8, 3582–3586. ( 10.1021/nl801386m) [DOI] [PubMed] [Google Scholar]
- 12.Freitag M, Johnson AT, Kalinin SV, Bonnell DA. 2002. Role of single defects in electronic transport through carbon nanotube field-effect transistors. Phys. Rev. Lett. 89, 216801 ( 10.1103/PhysRevLett.89.216801) [DOI] [PubMed] [Google Scholar]
- 13.Chico L, López Sancho MP, Muñoz MC. 1998. Carbon-nanotube-based quantum dot. Phys. Rev. Lett. 81, 1278–1281. ( 10.1103/PhysRevLett.81.1278) [DOI] [Google Scholar]
- 14.Chen J, Hamon MA, Hu H, Chen Y, Rao AM, Eklund PC, Haddon RC. 1998. Solution properties of single-walled carbon nanotubes. Science 282, 95–98. ( 10.1126/science.282.5386.95) [DOI] [PubMed] [Google Scholar]
- 15.Bahr JL, Yang J, Kosynkin DV, Bronikowski MJ, Smalley RE, Tour JM. 2001. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: a bucky paper electrode. J. Am. Chem. Soc. 123, 6536–6542. ( 10.1021/ja010462s) [DOI] [PubMed] [Google Scholar]
- 16.Holzinger M, Vostrowsky O, Hirsch A, Hennrich F, Kappes M, Weiss R, Jellen F. 2001. Sidewall functionalization of carbon nanotubes. Angew. Chem. Int. Ed. 40, 4002–4005. () [DOI] [PubMed] [Google Scholar]
- 17.Georgakilas V, Kordatos K, Prato M, Guldi DM, Holzinger M, Hirsch A. 2002. Organic functionalization of carbon nanotubes. J. Am. Chem. Soc. 124, 760–761. ( 10.1021/ja016954m) [DOI] [PubMed] [Google Scholar]
- 18.Dyke CA, Tour JM. 2003. Solvent-free functionalization of carbon nanotubes. J. Am. Chem. Soc. 125, 1156–1157. ( 10.1021/ja0289806) [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Park H, Han J, Lu JP. 2004. Electronic properties of carbon nanotubes with covalent sidewall functionalization. J. Phys. Chem. B 108, 4227–4230. ( 10.1021/jp036814u) [DOI] [Google Scholar]
- 20.Bower C, Rosen R, Jin L, Han J, Zhou O. 1999. Deformation of carbon nanotubes in nanotube–polymer composites. Appl. Phys. Lett. 74, 3317–3319. ( 10.1063/1.123330) [DOI] [Google Scholar]
- 21.Hirsch A. 2002. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 41, 1853–1859. () [DOI] [PubMed] [Google Scholar]
- 22.Jung N, Kim N, Jockusch S, Turro NJ, Kim P, Brus L. 2009. Charge transfer chemical doping of few layer graphenes: charge distribution and band gap formation. Nano Lett. 9, 4133–4137. ( 10.1021/nl902362q) [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Tang T-T, Girit C, Hao Z, Martin MC, Zettl A, Crommie MF, Shen YR, Wang F. 2009. Direct observation of a widely tunable bandgap in bilayer graphene. Nature 459, 820–823. ( 10.1038/nature08105) [DOI] [PubMed] [Google Scholar]
- 24.Lahiri J, Lin Y, Bozkurt P, Oleynik II, Batzill M. 2010. An extended defect in graphene as a metallic wire. Nat. Nanotechnol. 5, 326–329. ( 10.1038/nnano.2010.53) [DOI] [PubMed] [Google Scholar]
- 25.Kuznetsova A, Yates JT Jr, Liu J, Smalley RE. 2000. Physical adsorption of xenon in open single walled carbon nanotubes: observation of a quasi-one-dimensional confined Xe phase. J. Chem. Phys. 112, 9590–9598. ( 10.1063/1.481575) [DOI] [Google Scholar]
- 26.Byl O, Kondratyuk P, Forth ST, FitzGerald SA, Chen L, Johnson JK, Yates JT. 2003. Adsorption of CF4 on the internal and external surfaces of opened single-walled carbon nanotubes: a vibrational spectroscopy study. J. Am. Chem. Soc. 125, 5889–5896. ( 10.1021/ja020949g) [DOI] [PubMed] [Google Scholar]
- 27.Dillon AC, Heben MJ. 2001. Hydrogen storage using carbon adsorbents: past, present and future. Appl. Phys. A 72, 133–142. ( 10.1007/s003390100788) [DOI] [Google Scholar]
- 28.Lubezky A, Chechelnitsky L, Folman M. 1996. IR spectra of CH4, CD4, C2H4, C2H2, CH3OH and CH3OD adsorbed on C60 films. J. Chem. Soc. Faraday Trans. 92, 2269–2274. ( 10.1039/ft9969202269) [DOI] [Google Scholar]
- 29.Fastow M, Kozirovski Y, Folman M, Heidberg J. 1992. IR spectra of carbon monoxide and nitric oxide adsorbed on fullerene (C60). J. Phys. Chem. 96, 6126–6128. ( 10.1021/j100194a008) [DOI] [Google Scholar]
- 30.Oliva C, Strodel P, Goldbeck-Wood G, Maiti A. 2008. Understanding the interaction of ammonia with carbon nanotubes. NSTI Nanotech 3, 655–658. [Google Scholar]
- 31.Porto AB, de Oliveira LFC, Dos Santos HF. 2016. Exploring the potential energy surface for reaction of SWCNT with NO2+: a model reaction for oxidation of carbon nanotube in acid solution. Comput. Theor. Chem. 1088, 1–8. ( 10.1016/j.comptc.2016.05.002) [DOI] [Google Scholar]
- 32.Li J, Lu Y, Ye Q, Cinke M, Han J, Meyyappan M. 2003. Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 3, 929–933. ( 10.1021/nl034220x) [DOI] [Google Scholar]
- 33.Zhanpeisov NU, Zhidomirov GM, Fukumura H. 2009. Interaction of a single water molecule with a single graphite layer: an integrated ONIOM study. J. Phys. Chem. C 113, 6118–6123. ( 10.1021/jp810460b) [DOI] [Google Scholar]
- 34.Liu LV, Tian WQ, Wang YA. 2006. Chemical reaction of nitric oxides with the 5-1DB defect of the single-walled carbon nanotube. J. Phys. Chem. B 110, 1999–2005. ( 10.1021/jp053931b) [DOI] [PubMed] [Google Scholar]
- 35.Chakrapani N, Zhang YM, Nayak SK, Moore JA, Carroll DL, Choi YY, Ajayan PM. 2003. Chemisorption of acetone on carbon nanotubes. J. Phys. Chem. B 107, 9308–9311. ( 10.1021/jp034970v) [DOI] [Google Scholar]
- 36.Meng Y, et al. 2013. Signatures in vibrational and UV-visible absorption spectra for identifying cyclic hydrocarbons by graphene fragments. Nanoscale 5, 12 178–12 184. ( 10.1039/c3nr02933f) [DOI] [PubMed] [Google Scholar]
- 37.Elstner M, Porezag D, Jungnickel G, Elsner J, Haugk M, Frauenheim T, Suhai S, Seifert G. 1998. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 58, 7260–7268. ( 10.1103/PhysRevB.58.7260) [DOI] [Google Scholar]
- 38.Frauenheim T, Seifert G, Elsterner M, Hajnal Z, Jungnickel G, Porezag D, Suhai S, Scholz R. 2000. A self-consistent charge density-functional based tight-binding method for predictive materials simulations in physics, chemistry and biology. Phys. Status Solidi B 217, 41–62. () [DOI] [Google Scholar]
- 39.Elstner M, Hobza P, Frauenheim T, Suhai S, Kaxiras E. 2001. Hydrogen bonding and stacking interactions of nucleic acid base pairs: a density-functional-theory based treatment. J. Chem. Phys. 114, 5149–5155. ( 10.1063/1.1329889) [DOI] [Google Scholar]
- 40.Lin CS, Zhang RQ, Niehaus TA, Frauenheim T. 2007. Geometric and electronic structures of carbon nanotubes adsorbed with flavin adenine dinucleotide: a theoretical study. J. Phys. Chem. C 111, 4069–4073. ( 10.1021/jp068846y) [DOI] [Google Scholar]
- 41.Elstner M, Frauenheim T, Kaxiras E, Seifert G, Suhai S. 2000. A self-consistent charge density-functional based tight-binding scheme for large biomolecules. Phys. Status Solidi B 217, 357–376. () [DOI] [Google Scholar]
- 42.Lin CS, Zhang RQ, Lee ST, Elstner M, Frauenheim T, Wan LJ. 2005. Simulation of water cluster assembly on a graphite surface. J. Phys. Chem. B 109, 14 183–14 188. ( 10.1021/jp050459l) [DOI] [PubMed] [Google Scholar]
- 43.Perdew JP, Burke K, Ernzerhof M. 1996. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 ( 10.1103/PhysRevLett.77.3865) [DOI] [PubMed] [Google Scholar]
- 44.Adamo C, Barone V. 1999. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170. ( 10.1063/1.478522) [DOI] [Google Scholar]
- 45.Zheng G, Irle S, Morokuma K. 2005. Performance of the DFTB method in comparison to DFT and semiempirical methods for geometries and energies of C20–C86 fullerene isomers. Chem. Phys. Lett. 412, 210–216. ( 10.1016/j.cplett.2005.06.105) [DOI] [Google Scholar]
- 46.Ma JC, Dougherty DA. 1997. The cation−π interaction. Chem. Rev. 97, 1303–1324. ( 10.1021/cr9603744) [DOI] [PubMed] [Google Scholar]
- 47.Shirvani BB, Beheshtian J, Parsafar G, Hadipour NL. 2010. DFT study of NH3(H2O)n=0,1,2,3 complex adsorption on the (8, 0) single-walled carbon nanotube. Comput. Mater. Sci. 48, 655–657. ( 10.1016/j.commatsci.2010.02.035) [DOI] [Google Scholar]
- 48.Ulbricht H, Zacharia R, Cindir N, Hertel T. 2006. Thermal desorption of gases and solvents from graphite and carbon nanotube surfaces. Carbon 44, 2931–2942. ( 10.1016/j.carbon.2006.05.040) [DOI] [Google Scholar]
- 49.Elstner M, Jalkanen KJ, Knapp-Mohammady M, Frauenheim T, Suhai S. 2001. Energetics and structure of glycine and alanine based model peptides: approximate SCC-DFTB, AM1 and PM3 methods in comparison with DFT, HF and MP2 calculations. Chem. Phys. 263, 203–219. ( 10.1016/S0301-0104(00)00375-X) [DOI] [Google Scholar]
- 50.Kotakoski J, Krasheninnikov AV, Nordlund K. 2006. Energetics, structure, and long-range interaction of vacancy-type defects in carbon nanotubes: atomistic simulations. Phys. Rev. B 74, 245420 ( 10.1103/PhysRevB.74.245420) [DOI] [Google Scholar]
- 51.Li H-B, Page AJ, Hettich C, Aradi B, Kohler C, Frauenheim T, Irle S, Morokuma K. 2014. Graphene nucleation on a surface-molten copper catalyst: quantum chemical molecular dynamics simulations. Chem. Sci. 5, 3493–3500. ( 10.1039/C4SC00491D) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data are available in the electronic supplementary material.