Abstract
Background:
Previous cross-sectional findings from adolescents and adults with Bulimia Nervosa (BN) suggest disturbances in fronto-striatal and cingulo-opercular task control circuits that support self-regulatory processes, including the resolution of cognitive conflict. Herein, we used longitudinal data to examine the developmental trajectories of such disturbances and how the functioning of these circuits relates to changes in BN symptoms over adolescence.
Methods:
Thirty-two adolescent females with BN symptoms and 28 healthy control (HC) adolescents participated in the study. Functional magnetic resonance images (fMRI) during performance of a Simon task were acquired at three time points within 2-year intervals over adolescence. From the initial sample, 70% and 30% of the participants completed the second and third time points, respectively. Participants who completed all study time points did not differ from those lost to attrition on baseline demographic characteristics or any outcome measures. Using a region-of-interest approach, growth curve models tested group differences in the trajectory of conflict-related activation in task control circuits over time. Cross-lagged panel models examined transactional relationships between conflict-related activation in the same regions and BN symptoms over time.
Results:
Growth curve models revealed different trajectories of conflict-related activation in right task control regions across BN and HC adolescents, such that HC but not BN adolescents showed activation decreases over time. These group differences were greatest when including only the BN adolescents whose symptoms remitted over time. Cross-lagged panel models revealed that less frequent bulimic episodes at first follow-up predicted later increases in conflict-related activation in bilateral task control regions.
Conclusion:
These longitudinal findings suggest over-engagement of task control circuits in BN adolescents, especially those most resilient to persistent illness. Such over-engagement may compensate for regulatory disturbances, allowing them to regulate eating behaviors over development. Thus, task control circuits may constitute targets for early interventions that enhance self-regulatory control.
INTRODUCTION
Bulimia Nervosa (BN) typically emerges in adolescence, is more prevalent in females, and associated with substantial functional impairment (Stice, Marti, Spoor, Presnell, & Shaw, 2008). It is characterized by recurrent episodes of binge eating followed by inappropriate compensatory behaviors (such as self-induced vomiting) to avoid weight gain (American Psychiatric Association, 2013). BN is associated with deficits in self-regulatory control, a construct that encompasses executive control, emotional regulation, and the ability to delay gratification (Mischel, Shoda, & Rodriguez, 1989). Findings from healthy (Casey et al., 2011; Marsh et al., 2006) and ill (Emond, Joyal, & Poissant, 2009; Marsh, Zhu, Wang, Skudlarski, & Peterson, 2007) individuals suggest that fronto-striatal and cingulo-opercular task control circuits underlie self-regulatory processes including the resolution of cognitive conflict between response options. Previous cross-sectional findings from adolescents (Lock, Garrett, Beenhakker, & Reiss, 2011; Marsh et al., 2011) and adults (Marsh et al., 2009; Skunde et al., 2016) with BN suggest disturbances in these processes and circuits, perhaps contributing to their inability to regulate eating behaviors or resolve conflict between their urge to binge-eat and drive for thinness. However, little is known about the developmental trajectories of such disturbances and how the functioning of these circuits may influence or be influenced by BN symptoms over adolescence and into early adulthood. Thus, we used longitudinal functional magnetic resonance imaging (fMRI) data from adolescents with and without BN symptoms during their performance of a Simon Spatial Incompatibility task (Simon, 1969) to examine changes in the functioning of task control circuits during the engagement of control and conflict resolution over time. We also examined whether the functioning of these circuits predicted changes in BN symptoms, whether BN symptoms predicted changes in circuit function, or both.
Cross-sectional studies have examined the neural correlates of self-regulatory control in BN (Lock et al., 2011; Marsh et al., 2011; Marsh et al., 2009; Skunde et al., 2016), but only two have specifically assessed those associated with cognitive conflict (i.e., cognitive inhibition or interference control) (Marsh et al., 2011; Marsh et al., 2009). Findings from adults with BN compared to healthy adults suggested reduced activation in fronto-striatal (i.e., bilateral inferior frontal gyrus [IFG] and dorsal striatum) and cingulo-opercular (i.e., anterior cingulate cortex [ACC] and insula [in a cluster overlapping with the IFG]) regions during correct responding to conflict (incongruent) stimuli on a Simon task (Marsh et al., 2009). In addition, those who made the most errors had the most severe BN symptoms and the least activation in IFG, striatum, ACC, and insula during correct responding to conflict stimuli. Findings from BN compared to healthy adolescents also revealed reduced conflict-related activation in fronto-striatal and cingulo-opercular circuits on a Simon task (Marsh et al., 2011), including bilateral IFG, right putamen/insula and ACC. These patterns of deficient activation in BN adolescents were driven by their responses to post-congruent conflict (i.e., incongruent stimuli preceded by congruent stimuli), when conflict was maximal. Finally, those who engaged in the most frequent binge-eating episodes showed the least activation in task control circuits.
No study to date has investigated the developmental trajectory of self-regulatory disturbances in BN over adolescence or relationships between the functioning of task control circuits and BN symptoms from one time point to another. Understanding how these disturbances contribute to the persistence of BN symptoms may lead to early interventions designed to enhance the functioning of task control circuits. Thus, the current longitudinal study assessed conflict-related activation in task control circuits early after BN onset and over the course of adolescence. Specifically, growth curve models tested whether changes in activation over time differed in BN compared to age-matched healthy adolescents. Trajectories of BN symptoms over adolescence and early adulthood are heterogeneous (Abebe, Lien, & von Soest, 2012; Keel, Baxter, Heatherton, & Joiner, 2007) and many girls with early-emerging BN symptoms show significant symptom reductions during adolescence (Abebe, Lien, Torgersen, & von Soest, 2012; Pearson, Wonderlich, & Smith, 2015). Thus, we also examined activation changes in a subgroup of BN adolescents who fully or partially remitted over time.
Cross-lagged panel models also investigated relationships between conflict-related activation in task-control regions and BN symptoms over time. Specifically, we tested whether conflict-related activation at a given time point predicted BN symptoms at a later time point while controlling for symptoms at the previous time point, thereby predicting changes in BN symptoms. We also tested whether BN symptoms at a given time point predicted conflict-related activation at a later time point while controlling activation at the previous time point, thereby predicting changes in activation. These analyses also permitted examination of transactional relationships between BN symptoms and activation over time. Such relationships have never been assessed in BN or any eating disorder, since longitudinal data are necessary for these analyses.
Given the consistent findings of deficient activation in specific fronto-striatal (IFG, dorsal striatum) and cingulo-opercular (ACC, insula) regions in adolescents (Marsh et al., 2011) and adults (Marsh et al., 2009) with BN during Simon task performance, we hypothesized that these task control regions would show different functional trajectories over time across BN and healthy adolescents. Specifically, we hypothesized that deficient activation in these regions would persist over time in the BN group and explored changes in activation in those who remitted from the illness over time, predicting that activation would either remain reduced compared to healthy adolescents or increase (i.e., ‘normalize’) over time. We also hypothesized that conflict-related activation in these regions would inversely predict changes in BN symptoms over time, reflecting a predictive relationship between the functioning of these circuits in the service of conflict resolution and the ability to regulate eating behaviors.
METHODS
Participants
Participants were adolescent females with BN symptoms (n = 32) and healthy controls (HC; n = 28) group-matched for age, BMI, race and ethnicity. Participants were recruited through local and online advertisements. Participants with a history of neurological illness, past seizures, head trauma with loss of consciousness, mental retardation, pervasive developmental disorder, or current Axis I disorders (other than depressive or anxiety disorders in the BN group) were excluded. Controls had no lifetime Axis I disorders. Axis I disorders were assessed using the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (Kaufman et al., 1997).
BN symptom severity and prior diagnoses of Anorexia Nervosa were assessed using the Eating Disorders Examination (Fairburn, Cooper, & O’Connor, 2008). Participants in the BN group were included if they engaged in an average of one loss-of-control eating episode (including both objectively and subjectively large bulimic episodes) and one compensatory episode (self-induced vomiting, laxative/diuretic misuse, or compulsive exercise) per week within the past 3 months, with at least one loss-of-control eating and compensatory episode occurring in the past month. Two follow-up assessments (FU1 and FU2) were conducted, each within 2-year intervals over adolescence. BN symptom severity and the presence or absence of comorbid illnesses were assessed each time point.
Ethical Considerations
The research protocol was approved by the Institutional Review Board of the New York State Psychiatric Institute and all participants gave informed consent or assent before participating.
FMRI Paradigm
Participants completed the Simon task, as previously described (Marsh et al., 2011). Briefly, participants were presented with a leftward or rightward pointing arrow on each trial that was either congruent or incongruent with its position (left or right) on the screen. They were instructed to respond as quickly and accurately as possible to the direction of the arrow by pressing a button on a response box using the index finger for left and the middle finger for right. Stimulus duration was 1300 msec, with jittered intervals ranging from 4160 to 6960 msec (M=5350, SD=1159.98) between each trial. Each of 3 runs contained 55 stimuli, including 11 blank, 22 congruent, and 22 incongruent stimuli. E-prime software (Psychology Software Tools, Inc., Sharpsburg, Pa.) was used to program and run the experiment, and to record participants’ responses and reaction times (RTs).
Image Acquisition and Processing
Details regarding image acquisition and preprocessing are presented in the supplement. Images were collected using a GE Signa 3 Tesla LC scanner (Milwaukee, WI). Image preprocessing and first-level analyses were carried out using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) and MATLAB 9.0 (Mathworks, Natick, MA). For each participant, preprocessed time series data from all three Simon task runs (420 volumes) were modeled using a GLM with six conditions: 1) Incongruent correct trails preceded by congruent trials (cI), 2) Congruent correct trials preceded by incongruent trials (iC), 3) Incongruent correct trials preceded by incongruent trials (iI), 4) Congruent correct trials preceded by congruent trials (cC), 5) fixation trials, and 6) incorrect trials (incongruent or congruent), including those trials with RTs below the minimal RT of 200ms for stimulus detection and processing. These events were convolved with the canonical HRF and least-squares regression was used to estimate parameters for each independent variable for each participant. Consistent with previous studies using this task, runs in which a participant had more than 30% errors on the task were excluded from our analyses (Horga et al., 2011; Marsh et al., 2014; Marsh et al., 2011).
Because activation of task control regions is greatest when level of conflict is maximal (i.e., when incongruent stimuli are preceded by congruent stimuli) (Horga et al., 2011), we focused our analyses on post-congruent trials. Parameter estimates averaged across the three runs were used to produce a post-congruent Incongruent versus post-congruent Congruent (cI-cC) contrast for each participant to access brain activation associated with the engagement of self-regulatory control and resolution of maximal cognitive conflict.
Given our a priori hypotheses regarding fronto-striatal and cingulo-opercular regions based on their involvement in control processes and, in particular, the resolution of cognitive conflict (Nee, Wager, & Jonides, 2007), we defined two a priori task control masks (one per hemisphere) encompassing the IFG (pars opercularis, orbitalis and triangularis), dorsal striatum (i.e., putamen), the ACC, and insula, as defined by the Automated Anatomical Labeling (AAL) atlas. Mean cI-cC contrast betas were extracted from each mask.
Statistical Analyses
Stability of Conflict-related Activation and BN Symptoms over time.
Path models were computed using IBM SPSS Amos (version 23.0, IBM Corporation, Armonk, NY, USA) with Full Information Maximum Likelihood (FIML) estimation to examine the stability of conflict-related activation in task control regions within each group, as well as the stability of BN symptoms in the BN group from baseline to FU1, and from FU1 to FU2.
Trajectories of Conflict-related Activation and RTs over time.
Growth curve models were estimated within a multilevel modeling framework using SAS PROC MIXED (version 9.3, SAS Institute Inc, Carry, NC, USA). This approach permits the analysis of unbalanced data (i.e., unequal numbers of data from participants at each time point) and makes use of all available data via maximum-likelihood estimation (Schafer & Graham, 2002). Models regressed conflict-related activation on time (months from baseline), group status (HC vs. BN), their interaction, and age at baseline (in years), thereby controlling for the effects of maturational differences on brain activity. These models included a random effect for the intercept and fixed effects for predictors. To assess the trajectory of conflict-related activation in adolescents who fully or partially remitted from BN over time (i.e., “BN remitters”), analyses were repeated including only BN adolescents who showed more than a 50% symptom reduction in the frequency of their core BN symptoms between their baseline and last assessments, calculated as the sum of the frequency of objective bulimic (OBEs) and self-induced vomiting episodes over the past 28 days. Additional growth curve models explored group differences in the trajectories of RTs to conflict over time.
Relationship between Conflict-related Activation and BN Symptoms over time.
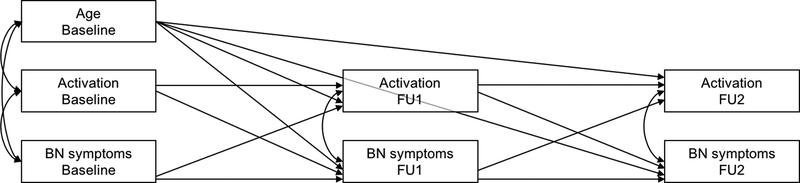
Cross-lagged path models were computed in the BN group using IBM SPSS Amos 23.0 (Figure 1). Such models provide estimates of the extent to which a variable at time t (e.g. ActivationBaseline) predicts another variable at time t+1 (e.g., OBEFU1), above and beyond variability attributable to scores of that second variable at time t (e.g., OBEBaseline) (Finkel, 1995). Thus, by controlling for the effects of each variable at time t on the same variable at time t+1 (e.g., FU1), t variables then predict the residual, or change in that variable from t to t+1. Separate models were estimated for activation in left and right hemispheres, and for OBEs and vomiting episodes. All models adjusted for age at baseline, illness duration, and variables within each time point were covaried to adjust for shared variance. Goodness of fit measures are described in the Supplement.
Figure 1. Schematic of the initial path models relating conflict-related activation and BN symptoms at baseline, FU1 and FU2.
Covariances on endogenous variables refer to covariances on error terms of those variables. Abbreviations: BN, bulimia nervosa; FU1, first follow-up; FU2, second follow-up.
Effects of Potential Confounds.
Both comorbid depression or anxiety and the use of selective serotonin reuptake inhibitors (SSRIs) may influence changes in brain activity (Silton et al., 2011; Wagner et al., 2010) and BN symptoms (Flament, Bissada, & Spettigue, 2012) over time. Thus, separate growth curve and cross-lagged panel models were conducted including these additional time-varying covariates (i.e., comorbid depression, comorbid anxiety and current SSRI use) at each time point. Additional models also included prior history of anorexia nervosa (AN) as a baseline covariate. Finally, additional models examined the potential confounding effects of premenarche on our results.
RESULTS
Participants
Demographic and clinical characteristics are shown in Table 1. Three HC (but no BN) participants were premenarcheal at baseline, and one was still premenarcheal at FU1. Twenty-one of the 32 BN adolescents met DSM-5 criteria for BN at baseline. The remaining 11 met criteria for Other Specified Feeding or Eating Disorder (OSFED) with subjective or objective loss of control eating episodes and compensatory behaviors to avoid weight gain (i.e., OSFED-BN). BN adolescents with this latter presentation were included, given that the loss of control over eating is more characteristic of binge-eating behavior than the amount of food consumed in adolescents (Fitzsimmons-Craft et al., 2014) and that many adolescents with less severe BN symptoms tend to engage in more frequent binge-eating and purging behaviors over time (Kotler, Cohen, Davies, Pine, & Walsh, 2001; Pearson et al., 2015). Figure S1 depicts each assessment point and corresponding age of each participant over the course of the study. Baseline demographic and clinical characteristics across BN sub-types are presented in Table S1. Across groups and time points, 25 runs (including all three runs from two participants) were excluded from our imaging analyses because error rates on the task were greater than 30%. Two additional runs from two different participants were excluded because of severe motion. After quality control, baseline MRI data was available from 30 adolescents with BN and 26 healthy adolescents. FU1 MRI and clinical/demographic data were available from 23 BN and 19 HC adolescents (BN: Meantime from baseline=17.0 months, SDtime from baseline=7.5; HC: Meantime from baseline=14.1 months, SDtime from baseline=3.6; t(40)=−1.617, p=.113), and FU2 data from 15 BN and 12 HC adolescents (BN: Meantime difference from FU1=15.0 months, SDtime difference from FU1=4.0; HC: Meantime difference from FU1=14.5 months, SDtime difference from FU1=2.1; t(25)=−0.415, p=.681). Participants with any missing data (n = 36) (i.e., any variable at any time point) did not differ significantly from those with complete data from all time points (n = 24) on baseline demographic characteristics, task performance, conflict-related activation within task control regions, or the frequency of OBEs or vomiting episodes in the BN group (all ps>.05). Data were thus missing at random. Seventeen of the 32 BN adolescents included in the study showed more than a 50% reduction in the frequency of their core BN symptoms between their baseline and last assessments, and were therefore considered ‘BN remitters’.
Table 1.
Demographic and clinical characteristics at each assessment point.
| Baseline | FU1 | FU2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BNa (n=32) | HC (n=28) | Analysis | BNa (n=23) | HC (n=19) | Analysis | BNa (n=15) | HC (n=12) | Analysis | ||||||||||||
| Characteristic | Mean(SD) | Mean(SD) | t(df) | p | Mean(SD) | Mean(SD) | t(df) | p | Mean(SD) | Mean(SD) | t(df) | p | ||||||||
| Age | 16.7(1.3) | 16.3(2.0) | −0.93(56) | 0.47 | 18.3(1.4) | 17.5(2.1) | −1.55(40) | 0.18 | 18.0(1.5) | 17.6(2.2) | −0.58(25) | 0.57 | ||||||||
| BMI (kg/m) | 22.1(2.9) | 21.5(3.6) | −0.68(56) | 0.39 | 22.8(2.3) | 22.8(3.6) | <0.01(40) | 1.00 | 23.6(2.8) | 23.9(4.9) | 0.22(25) | 0.83 | ||||||||
| WAIS IQ score (Full) | 106.4(11.0) | 106.0(16.6) | −0.11(54) | 0.91 | ||||||||||||||||
| Illness duration (months) | 25.7(19.4) | |||||||||||||||||||
| EDE ratings | ||||||||||||||||||||
| OBEs | 15.1(18.2) | 8.9(12.6) | 7.13(20.3) | |||||||||||||||||
| SBEs | 20.2(24.8) | 3.2(4.2) | 3.8(5.9) | |||||||||||||||||
| Vomiting episodes | 35.0(28.8) | 10.5(15.2) | 8.8(19.9) | |||||||||||||||||
| LOCs | 35.3(28.5) | 12.1(12.8) | 10.9(25.4) | |||||||||||||||||
| Comorbid MDD (%) | 43.8 | 13.0 | 6.7 | |||||||||||||||||
| Comorbid Anxiety (%) | 21.9 | 21.7 | 6.7 | |||||||||||||||||
| Medication (%) | 28.1 | 39.1 | 33.3 | |||||||||||||||||
| Prior AN (%) | 12.5 | 13.0 | 6.7 | |||||||||||||||||
| Treatmentb | ||||||||||||||||||||
| Inpatient (%) | 37.5 | |||||||||||||||||||
| Outpatient (%) | 28.1 | |||||||||||||||||||
In the BN group, the number BN remitters (i.e., participants whose BN symptoms improved by more than 50% between their baseline and last assessments) was 17 at baseline, 17 at FU1 and 12 at FU2.
Treatment seeking participants received inpatient or outpatient treatment in the Eating Disorders Clinic at the New York State Psychiatric Institute only following their baseline assessment/scan. Abbreviations: AN, anorexia nervosa; BMI, body mass index; BN, bulimia nervosa; EDE, Eating Disorder Examination; HC, healthy controls; LOC, loss-of-control eating episodes of any size; MDD, major depressive episode; OBE, objective bulimic episode; SBE, subjective bulimic episode; WAIS, Weschler Adult Intelligence Scale.
Bivariate Correlations and Stability of Conflict-related Activation and BN Symptoms over Time
Bivariate correlations between study variables and their descriptive statistics are presented in Table S2. Path models revealed that conflict-related activation in task control regions was not significantly stable across time points in either group (ßs=−0.400–0.180, ps>.05; Table S3). In the BN group, the frequency of OBEs was not stable between baseline and FU1 (ß=0.240, p=.237), but was highly stable between FU1 and FU2 (ß=0.872, p<.001), and the frequency of vomiting episodes was moderately stable across all time points (Baseline to FU1: ß=0.630, p=.004; FU1 to FU2: ß=0.489, p=.019 Table S4).
Behavioral Analyses
Table S5 presents descriptive statistics and group comparisons at each time point on task performance for each condition (cC, cI, iC, iI) and conflict effect (i.e., post-congruent conflict [cI-cC] and global conflict [I-C]). No group differences in performance were detected at any time point (ps>.05; Table S5). Growth curve findings from models predicting RT to post-congruent conflict (cI-cC) and global conflict (I-C) are summarized in Table S6. No Group effects or Group-by-Time interactions were detected in either model when all BN or only BN remitters were included in analyses (ps>.05).
Trajectories of Conflict-related Activation over Time
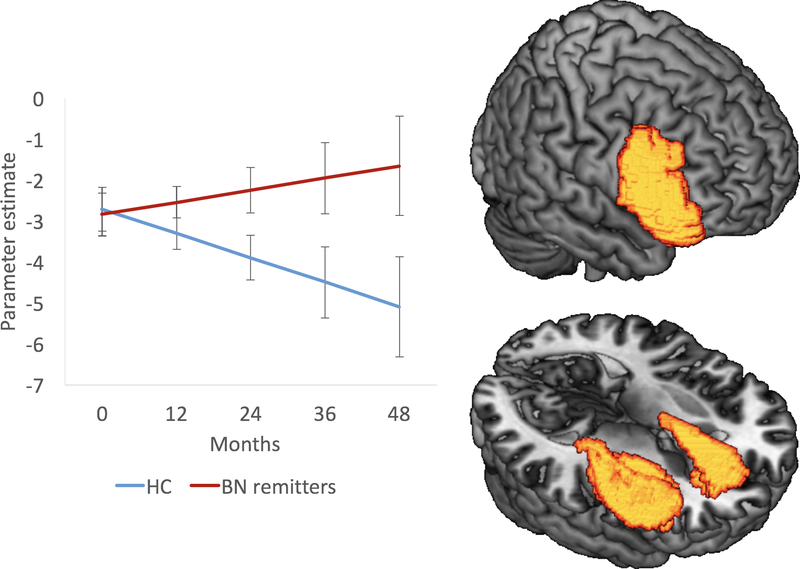
Findings from growth curve models predicting activation in response to post-congruent conflict (cI-cC) are summarized in Table 2, Figure S2 (HC vs. all BN), and Figure 2 (HC vs. BN remitters). In models including all BN participants, Group effects (i.e., average group differences in conflict-related activation at baseline) were not detected in either a priori task control mask. A significant Group-by-Time interaction in the right hemisphere (p=.045; Table 2) revealed decreasing activation over time in HC (p=.037) but not BN adolescents. Including only the BN remitters, main effects of group were also not detected in either mask, but a significant Group-by-Time interaction in the right hemisphere (p=.024; Table 2) again revealed decreasing activation over time in HC (p=.036) but not BN adolescents. Point slope estimates of right hemisphere activation were computed yearly from baseline (0, 12, 24, 36 and 48 months) and contrast comparisons of activation revealed the magnitude of the group differences over time (Table 2B). Finally, findings from exploratory growth curve models predicting activation in response to global conflict (I-C) are presented in Table S7.
Table 2. Growth curve models predicting activation in response to post-congruent conflict in task control regions over time in HC vs. BN.
Table 2A shows the statistics for each predictor in the models and Table 2B shows contrast comparisons of activation at 0, 12, 24, 36 and 48 months from baseline based on activation point slope estimates from the growth curve models.
| Hemisphere | Term | HC (n=26) vs. all BN (n=32) | HC (n=26) vs. BN remitters (n=17) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Analysis | 95% CI | Estimate | SE | Analysis | 95% CI | |||||||||||
| t | p | Lower | Upper | t | p | Lower | Upper | |||||||||||
| Left | Age | 0.186 | 0.106 | 1.76 | .084 | −0.026 | 0.397 | 0.217 | 0.112 | 1.95 | .057 | −0.007 | 0.441 | |||||
| Groupa | −0.054 | 0.483 | −0.11 | .911 | −1.020 | 0.911 | 0.188 | 0.559 | 0.34 | .738 | −0.935 | 1.311 | ||||||
| Time | −0.034 | 0.024 | −1.40 | .166 | −0.083 | 0.015 | −0.034 | 0.024 | −1.41 | .163 | −0.083 | 0.014 | ||||||
| Group*Time | 0.042 | 0.030 | 1.37 | .176 | −0.019 | 0.102 | 0.049 | 0.033 | 1.46 | .149 | −0.018 | 0.116 | ||||||
| Right | Age | 0.172 | 0.100 | 1.72 | .091 | −0.028 | 0.372 | 0.176 | 0.104 | 1.69 | .097 | −0.033 | 0.386 | |||||
| Groupa | −0.199 | 0.458 | −0.44 | .665 | −1.115 | 0.716 | −0.129 | 0.530 | −0.24 | .809 | −1.193 | 0.935 | ||||||
| Time | −0.050 | 0.023 | −2.15 | .036 | −0.096 | −0.003 | −0.050 | 0.023 | −2.15 | .037 | −0.096 | −0.003 | ||||||
| Group*Time | 0.059 | 0.029 | 2.05 | .045 | 0.001 | 0.117 | 0.074 | 0.032 | 2.33 | .024 | 0.010 | 0.138 | ||||||
| a The effect of Group on conflict-related activation in left task control regions remained non-significant (p>.1) after removing the Group-by-Time interaction term from the model. Abbreviations: BN, bulimia nervosa; CI, confidence interval; HC, healthy control. | ||||||||||||||||||
| Time from baseline | HC (n=26) vs. all BN (n=32) | HC (n=26) vs. BN remitters (n=17) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ba | SE | t | p | ESb | Ba | SE | t | p | ESb | ||
| 0 month | −0.199 | 0.458 | −0.440 | .665 | −0.109 | −0.129 | 0.530 | −0.240 | .809 | −0.070 | |
| 12 months | 0.509 | 0.338 | 1.510 | .137 | 0.278 | 0.763 | 0.379 | 2.010 | .049 | 0.415 | |
| 24 months | 1.217 | 0.507 | 2.400 | .020 | 0.664 | 1.654 | 0.546 | 3.030 | .004 | 0.900 | |
| 36 months | 1.925 | 0.799 | 2.410 | .019 | 1.051 | 2.546 | 0.864 | 2.950 | .005 | 1.385 | |
| 48 months | 2.633 | 1.122 | 2.350 | .022 | 1.438 | 3.437 | 1.219 | 2.820 | .007 | 1.870 | |
| a B estimates represent group difference in CT, where group was coded as HC = 0 and BN = 1. | |||||||||||
| b ESs were calculated as the (MeanCT BN – MeanCT HC)/residual standard deviation. | |||||||||||
| Abbreviations: BN, bulimia nervosa; CI, confidence interval; HC, healthy control. | |||||||||||
Figure 2. Trajectories of conflict-related activation in right task control regions (i.e., inferior frontal gyrus, dorsal striatum, anterior cingulate cortex and insula) over time in HC (n=26) versus BN remitters (n=17).
Line graph showing each group’s yearly conflict-related activation based on the growth curve model’s point slope estimates at 0, 12, 24, 36, and 48 months (left panel) and surface-rendered brain maps of Group-by-Time interaction in right task control regions (right panel).
Error bars represent ± SEM. Abbreviations: BN, Bulimia Nervosa; HC, healthy control.
Relationship between Conflict-related Activation and BN Symptoms over Time
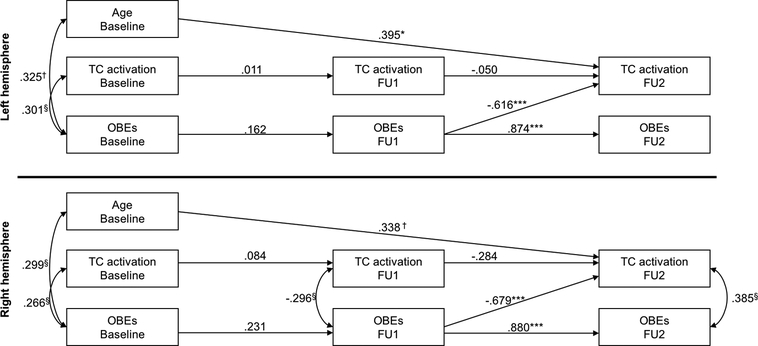
Separate models were tested for activation in left and right hemispheres, and for OBEs and vomiting episodes. Non-significant paths were removed one at a time within each model, and a chi-square difference test examined whether each removal significantly reduced model fit (order of removal and fit indices for the initial and final models are presented in the supplement). Non-significant stability paths were retained to adjust for the effects of prior levels of each variable. Other non-significant paths were retained in the model if their removal significantly reduced model fit. Illness duration correlated with age at baseline (r=.675, p<.001) but did not contribute to any other significant path and was therefore removed from the final models. Figure 3 presents the standardized parameters for the final models testing the relationships between OBEs and activation in left (top panel) and right (bottom panel) task control regions (based on a priori ‘task control’ masks). Less frequent OBEs at FU1 predicted increased activation at FU2 in both hemispheres (left: p=.002; right: p<.001), although activation did not predict change in symptoms at any time point. Older age at baseline also predicted increased activation at FU2 (left: p=.040; right: p=.051). Detailed statistics for these models are reported in Table S8. Models with vomiting episodes did not reveal significant relationships between activation and symptoms over time.
Figure 3. Cross-lagged panel results relating task control activation in left (top panel) and right (bottom panel) hemispheres and the frequency of OBEs at baseline, FU1 and FU2 in the BN group (n=32).
§p>.1, †p<.1, *p<.05, **p<.01, ***p<.001. Regression parameters represent standardized estimates. Covariances between endogenous terms refer to covariances on the error terms of those variables. Values on covariances represent correlations. Abbreviations: FU1, first follow-up; FU2, second follow-up; OBEs, objective bulimic episodes; TC, task control.
Effects of Potential Confounds
The effects of current SSRI use and comorbidities in our growth curve and cross-lagged panel models are presented in Table S9 and Figure S3, respectively. The inclusion of a lifetime history of AN, current SSRI use, or comorbid anxiety as covariates in the growth curve models did not appreciably affect our findings. Adding comorbid depression as a covariate rendered the Group-by-time interaction in right task control regions non-significant when including all BN participants, and marginally significant when including only the BN remitters, although depression status was not significant itself. None of these covariates affected the significance of our cross-lagged panel results. Finally, including premenarche as a time varying covariate in our growth curve models did not affect the significance of Group-by-Time interactions.
DISCUSSION
This longitudinal study is first to examine the developmental trajectory of self-regulatory disturbances in BN over adolescence and relationships between the functioning of task control circuits and BN symptoms over time. Growth curve models revealed decreasing activation in right task control regions over time in HC but not BN adolescents. These group differences in functional trajectories were more robust when including only the BN adolescents whose symptoms improved over time (i.e. ‘BN remitters’). Cross-lagged panel models in the BN group further revealed that BN symptoms inversely predicted conflict-related activation over time. These findings are a first step towards understanding how fronto-striatal and cingulo-opercular circuits may be targets for early interventions for BN that reduce symptoms by enhancing self-regulatory capacity.
Stability analyses suggested that conflict-related activation in task control regions was unstable in both groups over time, perhaps reflecting changes that naturally occur during this developmental period (e.g., myelination and synaptic pruning). To our knowledge, this is the first study to examine the stability of conflict-related activation over several years of the adolescent period in healthy or ill individuals, thereby adding to our understanding of the functioning of task control circuits over adolescence. BN adolescents also showed low stability in the frequency of OBEs between baseline and FU1, but high stability between FU1 and FU2, perhaps reflecting the progressive crystallization of BN symptoms with advancing age (Allen, Crosby, Oddy, & Byrne, 2013). The frequency of vomiting episodes, in contrast, was moderately stable across time points and did not relate to changes in activation over time.
In contrast to our hypothesis, based on our previous cross-sectional findings from another sample of adolescents with BN (Marsh et al., 2011), those in the current study did not show deficient activation that persisted over time. Instead, activation did not differ across BN and HC groups at baseline, and only the healthy adolescents showed decreased activation over time. The BN adolescents in the current study were younger and less severely ill than those in the previous cross-sectional study, thereby likely contributing to these discrepant findings across the previous and current studies. Deficient activation of task control circuits may be associated with persistently severe BN symptoms, consistent with findings from the previous study of adolescents and from adults with BN (Marsh et al., 2011; Marsh et al., 2009). The BN adolescents, and particularly the BN remitters, seemed to maintain their engagement of task control regions over time, perhaps allowing them to better regulate their eating behaviors over time. Although tentative and beyond the scope of our data, this interpretation is consistent with evidence that self-regulatory control processes develop gradually, with susceptibility to cognitive interference/conflict decreasing over adolescence (Casey, Thomas, Davidson, Kunz, & Franzen, 2002; Goldman-Rakic, 1987). For example, cross-sectional fMRI data suggest that children make more errors and display greater prefrontal activation than adults during performance of tasks that require response inhibition or the resolution of cognitive conflict (Casey et al., 2002; Tamm, Menon, & Reiss, 2002). Thus, decreased activation of right task control regions over time in the HC group may reflect normative increases in self-regulatory capacity and less reliance on these circuits to resolve cognitive conflict. In contrast, BN adolescents may need to recruit task control regions consistently over time to compensate for their persistent difficulty engaging control in the service of resolving conflict (Marsh et al., 2009), consistent with the absence of group differences in Simon task performance.
Cross-lagged panel modeling revealed a significant influence of BN symptoms on conflict-related activation, but not of activation on BN symptoms over time. The frequency of OBEs at FU1 inversely predicted activation changes in both left and right task control masks from FU1 to FU2, suggesting that adolescents with less frequent OBEs during mid- to late adolescence showed greater increases in activation through late adolescence and early adulthood. Together with our growth curve results, these findings suggest that more ‘resilient’ BN adolescents may, over time, engage task control regions to compensate for putative deficits in self-regulatory control. Alternatively, binge-eating behaviors may influence changes in brain activation through unidentified mechanisms, independent of self-regulatory control. Future longitudinal studies could explore these potential mechanisms.
This study is limited by its modest sample size, which precluded investigating functional trajectories and relationships with symptoms in sub-groups of BN adolescents displaying different symptom trajectories than partial remission (e.g., full remission, symptoms worsening, and subclinical or severe symptoms remaining stable over time). To retain as may participants as possible in our analyses restricted to BN remitters (n=16), we defined remission as 50% symptom reduction, since few BN participants met criteria for a more stringent definition (i.e., only 12 BN participants showed more than 75% symptom reduction, and only 3 fully remitted). In addition, 34% of our BN sample met DSM5 criteria for OSFED-BN at baseline, with subjective or objective loss of control eating episodes and compensatory behaviors to avoid weight gain. This clinical presentation is consistent with the presentation of BN symptoms in early adolescence (Fitzsimmons-Craft et al., 2014), but our sample size precluded testing the differential effects of BN and OSFED-BN on our outcome measures. Moreover, given the low power due to our modest sample, we did not correct for multiple tests and our findings should therefore be interpreted with caution prior to replication in a larger sample. In addition, to reduce the number of statistical models, a single “task control” mask was defined in each hemisphere, thereby precluding the examination of regional specificity within the masks. Future studies should therefore examine whether the effects observed herein are homogeneous across regions within task control circuits and whether such effects are present in other brain regions. Although this study is limited by attrition, an inevitable consequence for longitudinal studies of adolescents (Young, Powers, & Bell, 2006), participants with any missing data at any time point did not significantly differ from those with complete data on baseline demographics, activation or BN symptoms. Thus, data was missing at random. Furthermore, growth curve models make use of all available data, while cross-lagged models estimate means and intercepts of missing data. These approaches thus include gold-standard methods to deal with attrition in longitudinal designs (Schafer & Graham, 2002).
Potential confounds are also worth noting. First, the absence of a clinical control group precluded examining whether findings are BN-specific or instead generalize to other eating disorders or psychopathology. Although controlling for prior history of AN, SSRI use and comorbid anxiety did not appreciably affect our findings, controlling for comorbid depression affected our growth curve findings within right task control regions. Thus, comorbid depression may partially contribute to functional changes over time in BN, but did not itself predict activation over and above naturalistic changes and group status. Second, we did not control for hunger, which can affect attentional and executive processes (Green & Rogers, 1998; Kemps, Tiggemann, & Marshall, 2005; Shaw & Tiggemann, 2004). Thus, future studies should control for satiety. Finally, we did not account for menstrual status, which might impact neural functioning in women (Dreher et al., 2007). However, it is unlikely that menstrual status differed systematically across BN and healthy adolescents to confound our results.
This is the first longitudinal study to assess changes in the engagement of task control circuits and BN symptoms over adolescence. Our findings may have important implications for understanding the developmental trajectory of brain functioning in BN and how conflict-related activation relates to BN symptoms over time. In contrast to healthy adolescents, those with BN, and particularly those who remitted over time, maintained their engagement of right task control regions over time. In addition, less frequent OBEs during mid-adolescence predicted activation increases in bilateral task control regions in late adolescence. Together, these findings suggest compensatory engagement of task control circuits in the most resilient adolescents. Future studies should investigate whether task control circuits constitute useful targets for early interventions that work by enhancing self-regulatory processes.
Supplementary Material
Acknowledgements:
The authors wish to thank Seonjoo Lee, PhD, of Columbia University, for her advice on statistical modeling of longitudinal data.
Funding: This work was supported by NIMH grant R01MH090062 (RM).
REFERENCES
- Abebe DS, Lien L, Torgersen L, & von Soest T (2012). Binge eating, purging and non-purging compensatory behaviours decrease from adolescence to adulthood: A population-based, longitudinal study. BMC Public Health, 12, 32. doi: 10.1186/1471-2458-12-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abebe DS, Lien L, & von Soest T (2012). The development of bulimic symptoms from adolescence to young adulthood in females and males: a population-based longitudinal cohort study. Int J Eat Disord, 45(6), 737–745. doi: 10.1002/eat.20950 [DOI] [PubMed] [Google Scholar]
- Allen KL, Crosby RD, Oddy WH, & Byrne SM (2013). Eating disorder symptom trajectories in adolescence: effects of time, participant sex, and early adolescent depressive symptoms. J Eat Disord, 1, 32. doi: 10.1186/2050-2974-1-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, … Shoda Y (2011). Behavioral and neural correlates of delay of gratification 40 years later. Proc Natl Acad Sci U S A, 108(36), 14998–15003. doi: 10.1073/pnas.1108561108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Davidson MC, Kunz K, & Franzen PL (2002). Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. J Neurosci, 22(19), 8647–8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, & Berman KF (2007). Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A, 104(7), 2465–2470. doi: 10.1073/pnas.0605569104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond V, Joyal C, & Poissant H (2009). [Structural and functional neuroanatomy of attention-deficit hyperactivity disorder (ADHD)]. Encephale, 35(2), 107–114. doi: 10.1016/j.encep.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, & O’Connor M (2008). Eating Disorder Examination (Edition 16.0D) In Fairburn CG (Ed.), Cognitive Behavior Therapy and Eating Disorders. New York: Guilford Press. [Google Scholar]
- Finkel SE (1995). Causal analysis with panel data. Beverly Hills: Sage Publications. [Google Scholar]
- Fitzsimmons-Craft EE, Ciao AC, Accurso EC, Pisetsky EM, Peterson CB, Byrne CE, & Le Grange D (2014). Subjective and objective binge eating in relation to eating disorder symptomatology, depressive symptoms, and self-esteem among treatment-seeking adolescents with bulimia nervosa. Eur Eat Disord Rev, 22(4), 230–236. doi: 10.1002/erv.2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament MF, Bissada H, & Spettigue W (2012). Evidence-based pharmacotherapy of eating disorders. Int J Neuropsychopharmacol, 15(2), 189–207. doi: 10.1017/S1461145711000381 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1987). Development of cortical circuitry and cognitive function. Child Dev, 58(3), 601–622. [PubMed] [Google Scholar]
- Green MW, & Rogers PJ (1998). Impairments in working memory associated with spontaneous dieting behaviour. Psychol Med, 28(5), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Horga G, Maia TV, Wang P, Wang Z, Marsh R, & Peterson BS (2011). Adaptation to conflict via context-driven anticipatory signals in the dorsomedial prefrontal cortex. J Neurosci, 31(45), 16208–16216. doi: 10.1523/JNEUROSCI.2783-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). The Schedule for Affective Disorders and Schizophrenia for School Aged Children: Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry, 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Keel PK, Baxter MG, Heatherton TF, & Joiner TE Jr. (2007). A 20-year longitudinal study of body weight, dieting, and eating disorder symptoms. J Abnorm Psychol, 116(2), 422–432. doi: 10.1037/0021-843X.116.2.422 [DOI] [PubMed] [Google Scholar]
- Kemps E, Tiggemann M, & Marshall K (2005). Relationship between dieting to lose weight and the functioning of the central executive. Appetite, 45(3), 287–294. doi: 10.1016/j.appet.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Kotler LA, Cohen P, Davies M, Pine DS, & Walsh BT (2001). Longitudinal relationships between childhood, adolescent, and adult eating disorders. J Am Acad Child Adolesc Psychiatry, 40(12), 1434–1440. doi: 10.1097/00004583-200112000-00014 [DOI] [PubMed] [Google Scholar]
- Lock J, Garrett A, Beenhakker J, & Reiss AL (2011). Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. The American journal of psychiatry, 168(1), 55–64. doi: 10.1176/appi.ajp.2010.10010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Horga G, Parashar N, Wang Z, Peterson BS, & Simpson HB (2014). Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biol Psychiatry, 75(8), 615–622. doi: 10.1016/j.biopsych.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Horga G, Wang Z, Wang P, Klahr KW, Berner LA, … Peterson BS (2011). An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. The American journal of psychiatry, 168(11), 1210–1220. doi: 10.1176/appi.ajp.2011.11010094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Steinglass JE, Gerber AJ, Graziano O’Leary K, Wang Z, Murphy D, … Peterson BS (2009). Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Archives of general psychiatry, 66(1), 51–63. doi: 10.1001/archgenpsychiatry.2008.504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, & Peterson BS (2006). A developmental fMRI study of self-regulatory control. Hum Brain Mapp, 27(11), 848–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Wang Z, Skudlarski P, & Peterson BS (2007). A Developmental fMRI Study of Self-Regulatory Control in Tourette’s Syndrome. The American journal of psychiatry, 164(6), 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, & Rodriguez MI (1989). Delay of gratification in children. Science, 244(4907), 933–938. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, & Jonides J (2007). Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci, 7(1), 1–17. [DOI] [PubMed] [Google Scholar]
- Pearson CM, Wonderlich SA, & Smith GT (2015). A risk and maintenance model for bulimia nervosa: From impulsive action to compulsive behavior. Psychol Rev, 122(3), 516–535. doi: 10.1037/a0039268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: our view of the state of the art. Psychol Methods, 7(2), 147–177. [PubMed] [Google Scholar]
- Shaw J, & Tiggemann M (2004). Dieting and working memory: preoccupying cognitions and the role of the articulatory control process. Br J Health Psychol, 9(Pt 2), 175–185. doi: 10.1348/135910704773891032 [DOI] [PubMed] [Google Scholar]
- Silton RL, Heller W, Engels AS, Towers DN, Spielberg JM, Edgar JC, … Miller GA (2011). Depression and anxious apprehension distinguish frontocingulate cortical activity during top-down attentional control. J Abnorm Psychol, 120(2), 272–285. doi: 10.1037/a0023204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR (1969). Reactions toward the source of stimulation. J Exp Psychol, 81(1), 174–176. [DOI] [PubMed] [Google Scholar]
- Skunde M, Walther S, Simon JJ, Wu M, Bendszus M, Herzog W, & Friederich HC (2016). Neural signature of behavioural inhibition in women with bulimia nervosa. J Psychiatry Neurosci, 41(5), E69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Marti CN, Spoor S, Presnell K, & Shaw H (2008). Dissonance and healthy weight eating disorder prevention programs: long-term effects from a randomized efficacy trial. J Consult Clin Psychol, 76(2), 329–340. doi: 10.1037/0022-006X.76.2.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, & Reiss AL (2002). Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry, 41(10), 1231–1238. doi: 10.1097/00004583-200210000-00013 [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Sobanski T, Reichenbach JR, Sauer H, & Schlosser RG (2010). Differential effects of serotonergic and noradrenergic antidepressants on brain activity during a cognitive control task and neurofunctional prediction of treatment outcome in patients with depression. J Psychiatry Neurosci, 35(4), 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AF, Powers JR, & Bell SL (2006). Attrition in longitudinal studies: who do you lose? Aust N Z J Public Health, 30(4), 353–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.