Abstract
Juvenile hormone analogs (JHA) are known to interfere with growth and biosynthesis of insects with potential for insecticide action. However, there has been comparatively few data on morphological effects of JHA on insect organs. To determine pyriproxyfen effects on Aedes aegypti larvae, we conducted toxicity, behavioral bioassays and assessed ultrastructural effects of pyriproxyfen on midgut cells. A. aegypti larvae were exposed in aqueous solution of pyriproxyfen LC50 concentrations and evaluated for 24 h. This study fulfilled the toxic prevalence of pyriproxyfen to A. aegypti larvae (LC50 = 8.2 mg L−1). Behavioral observations confirmed that pyriproxyfen treatment significantly changes swimming behavior of larvae, limiting its displacement and speed. The pyriproxyfen causes remarkable histopathological and cytotoxic alterations in the midgut of larvae. Histopathological study reveals presence of cytoplasmic vacuolization and damage to brush border of the digestive cells. The main salient lesions of cytotoxic effects are occurrence of cell debris released into the midgut lumen, cytoplasm rich in lipid droplets, autophagosomes, disorganized microvilli and deformed mitochondria. Data suggest that pyriproxyfen can be used to help to control and eradicate this insect vector.
Keywords: Autophagy, Aedes aegypti, Ultrastructure, Juvenile Hormone, Locomotory behavior
Introduction
Among the main pathogen vectors in tropical regions, insects share a major part, causing serious diseases resulting in high mortality and economic loss (Sachs & Malaney, 2002; Shepard et al., 2011). Mosquitoes are major public health threats which transmit deadly and debilitating diseases throughout the world. Container-inhabiting mosquitoes, particularly Aedes aegypti Linnaeus (Diptera: Culicidae), are considered the most important vector of viral diseases in humans, including dengue fever (Jansen & Beebe, 2010; Rosen et al., 1983), urban yellow fever (Reiter, 2010), Chikungunya (Burt et al., 2012; Paupy et al., 2010) and more recently Zika virus (ZIKV) (Gardner, Chen & Sarkar, 2016; Marcondes & Ximenes, 2016; Thangamani et al., 2016). The lifespan of Aedes aegypti can range from two weeks to a month (Maricopa County Environmental Services, 2006), whereas larvae pass through four instars with a short time in first two, and up to 3 days in last instars (Foster & Walker, 2002). The above-mentioned diseases are increasingly becoming a global health concern, due to widespread distribution of their vectors, rapid geographical spread and high disease burden (Leta et al., 2018). During the last few years, mosquitoes have been responsible for transmitting ZIKV in Brazil and Colombia with 146,675 recognized cases (World Health Organization, 2015, 2017). There have been strong associations between existence of diseases and distribution of vectors, transmitting them (Carlson, Dougherty & Getz, 2016; Messina et al., 2016).
There is no cure for these diseases and regulating disease transmission relies mainly on vector management (Suman et al., 2018). To control this vector, immature stages must be considered a preliminary threat (Carvalho et al., 2017); this involves the use of chemical compounds which can prevent development of adult mosquitoes in aquatic environments, without damaging other organisms (Yang et al., 2017). Many insecticides are used to control Aedes aegypti populations, including organophosphate (Boyer et al., 2018) and pyrethroid group (World Health Organization, 2016) but their use is declining due to resistance development (Chediak et al., 2016; Goindin et al., 2017; Prophiro et al., 2011) and environmental pollutants (Eulaers et al., 2014; Jaspers et al., 2011) including air (Mäkinen et al., 2009; Yang et al., 2014), dust (Cao et al., 2014; Fang et al., 2013; Mizouchi et al., 2015), water (Cristale et al., 2013; Ding et al., 2015; Hu et al., 2014) and sediment (Chung & Ding, 2009; Cristale et al., 2013; Tan et al., 2016).
Pyriproxyfen, a juvenile hormone analog, has a unique mode of action that affects embryogenesis (Maharajan et al., 2018), metamorphosis (Barbosa et al., 2018) and reproduction of insects (Meng et al., 2018). Treatment from pyriproxyfen thus results in death typically at the pupal stage (Hustedt et al., 2017; Invest & Lucas, 2008; WHO Pesticide Evaluation Scheme (WHOPES), 2000). Additional advantage of pyriproxyfen, requiring low concentrations than other larvicides such as temephos and Bacillus thuringiensis var. israelensis (Oo et al., 2018), makes it suitable larvicide against Aedes aegypti. Thus, no resistance can be detected upon exposure up to 17 generations (Schaefer & Mulligan, 1991), which is a promising feature for mosquito control. Although pyriproxyfen affects metamorphosis of the insect, other insect organs may also be secondary targets (Catae et al., 2018). Among the non-target organs of the insects, the midgut has been reported to be severely damaged by xenobiotics (Gutiérrez et al., 2016; Catae et al., 2018; Fiaz et al., 2018a).
The objective of the study was to evaluate lethal and sublethal effects of pyriproxyfen against Aedes aegypti larvae. We investigated the toxicity, locomotory behavior, histological and ultrastructural changes of pyriproxyfen on the non-target midgut organ.
Materials and Methods
Insects
Late third instar (L3) Aedes aegypti larvae fed on cat food (Whiskas) previously, were obtained from mass rearing from the “Laboratório de Biologia Molecular de Insetos” of “Universidade Federal de Viçosa” (Viçosa, Minas Gerais, Brazil). The reason we chose late third instar larvae is to utilize early fourth instar developmental phase in our bioassays because larva spends short amount of time in the first three and up to three days in successive instar. All bioassays performed, and insect colonies were kept at 25 ± 2 °C, with a 12:12 (L:D) h photoperiod.
Pyriproxyfen
Pyriproxyfen (TIGER® 100 EC; Sumitomo Chemical Corporation, Chūō, Japan) 100 g L−1 was diluted in one mL water to produce a stock solution by adjusting one g L−1 to obtain the desired concentrations, as previously described in Fiaz et al. (2018a, 2018b).
Toxicity test
Efficacy of pyriproxyfen was determined by calculating lethal concentrations LC50 under laboratory conditions. Besides control, which was distilled water, six pyriproxyfen concentrations were adjusted in one mL stock solution: 0.3125, 0.625, 1.25, 2.5, 5 and 10 µg L−1. From the stock solution, aliquots were obtained for each treatment and mixed with distilled water in 30 mL glass vial. Different concentrations of treatments were mixed in 25 mL of distilled water, with completely randomized design having three replications, containing 20 larvae (L3) each. Mortality was assessed every hour from the start of experiment until total mortality.
Locomotory behavior of larvae
The behavioral recordings of Aedes aegypti were carried out 24 h after exposure to LC50 of pyriproxyfen to determine sublethal effects of treatments on larva. Bioassays were performed in a Petri dish (nine cm diameter × 1.5 cm height) with 25 mL of treatment solution diluted to the LC50 obtained for larvae. A single larva (L3) per petri dish was video-recorded for 10 min using a video camera (SD5 Superdynamic; modelWV-CP504; Spacecom lens 1/3″, 3–8 mm; Panasonic, Newark, NJ, USA), coupled to a computer. The measurements taken with the tracking system included distance swimmed and resting time spent in arenas. These bioassays were conducted at 25 ± 2 °C under artificial fluorescent light and each treatment had five biological replications.
Morphological analysis of the midgut
Aedes aegypti larvae were exposed to LC50 lethal concentration of pyriproxyfen by contact and ingestion in aqueous solution for 24 h. Ten collected larvae (L3), from treatment and control were dissected in saline solution (0.1 M NaCl, 0.1 M KH2PO4, 0.1 M Na2HPO4). Dissected midgut was transferred for fixation to Zamboni’s fixative solution (Stefanini, Martino & Zamboni, 1967) and kept for 12 h at 5 °C. Dehydration of the samples were done in a graded ethanol series (70%, 80%, 90% and 95%), later embedded in historesin Leica (Leica Biosystems Nussloch GmbH, Heildelberger, Germany) and sections of three μm thickness were cut in a microtome (Leica RM2255). The acquired sections were then stained with hematoxylin and eosin and analyzed with Leica DMLS light microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Another set of 10 midguts from each treatment and control Aedes aegypti larvae were evaluated with transmission electron microscopy and samples were fixed in 2.5% glutaraldehyde in 0.2 M sodium cacodylate buffer, pH 7.2 containing 0.2 M sucrose. Post fixation of the samples were done in 1% osmium tetroxide in the same buffer and kept for 2 h at room temperature. Samples were washed in the buffer followed by dehydration in a graded ethanol series (70%, 80%, 90% and 99%), and embedded in LR White Resin (Electron Microscopy Sciences, Fort Washington, PA, USA). Sections of 70–90 nm, obtained with glass knife in a Sorvall MT2-BMT2-B ultramicrotome (Sorvall Instruments, Wilmington, DE, USA) were stained with aqueous uranyl acetate (1%) and lead citrate (Reynolds, 1963). Those sections were then examined with Zeiss EM 109 transmission electron microscope (Carl Zeiss, Jena, Germany).
Immunofluorescence
Aedes aegypti larvae (L3) were exposed to LC50 pyriproxyfen concentration in aqueous solution for 24 h. Midguts from treatment and control were dissected in insect physiological solution to identify cell proliferation. Five-treated midguts were used in analysis. After dissection, midgut was transferred to Zamboni’s fixative solution for 2 h, subsequently washing in 0.1M sodium phosphate buffer pH 7.2 plus 1% Tween-20 (PBST) for 2 h. The samples were incubated for 12 h with the primary antibody anti-phospho-histone H3 (1:100) in PBST, that recognize proliferating cells in the midgut of A. aegypty larvae (Fernandes et al., 2014, 2015), following with washing in PBST and incubation for 12 h with a FITC-conjugated anti-rabbit IgG secondary antibody (1:500). Those samples were then dehydrated in a graded ethanol series (70%, 80%, 90% and 95%) and embedded in JB4 resin. Three μm thick sections were stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) (one μg/μL) for DNA staining and then slides were analyzed and photographed under light microscope (Olympus BX-60) using a digital camera (Q-Color, 3 Olympus).
Statistics
Lethal concentrations and their confidence limits (Finney, 1964) were subjected to probit analysis using the SAS user (v.9.0) program for Windows (SAS Institute, Cary, NC, USA). Data about behavior response were analyzed by one-way ANOVA and for mean comparisons in the bioassays Tukey’s Honestly Significant test was used at 5% significance level.
Results
Toxicity
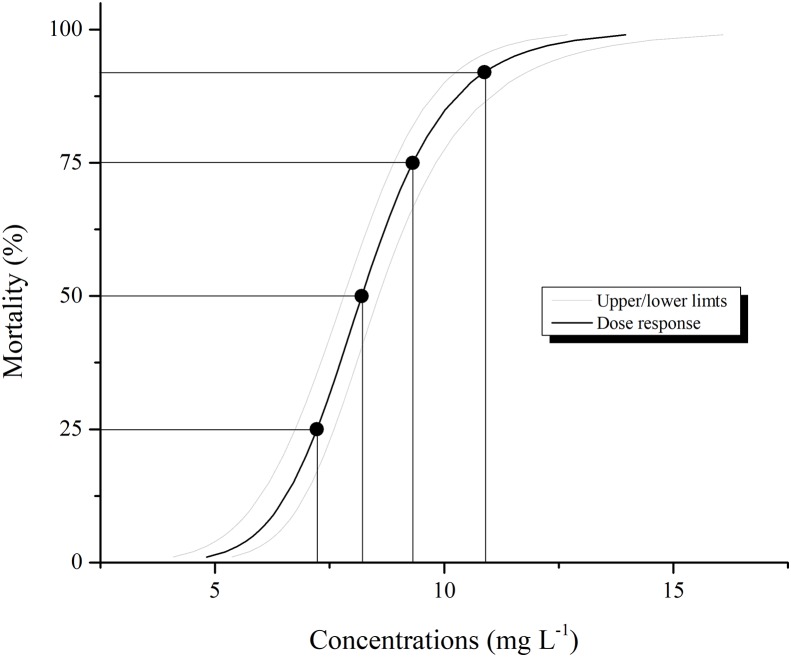
Larval mortality of the Aedes aegypti population treated with pyriproxyfen was different from that the control, as expected. Larval mortality increased with pyriproxyfen concentration in the aqueous solution with highest value obtained with 8.20 mg L−1 of this insecticide (Table 1). The estimated LC50 for pyriproxyfen obtained with the probit model was 8.20 mg L−1 (Fig. 1). Mortality observed in control was always <1%.
Table 1. Lethal pyriproxyfen concentrations to Aedes aegypti larvae after 24 h exposure.
| Lethal Concentration (LC) |
Estimated value (mg L−1) | Confidence limits | χ2 | |
|---|---|---|---|---|
| Inferior | Superior | |||
| LC25 | 7.22 | 6.75 | 7.60 | 20.20 |
| LC50 | 8.20 | 7.81 | 8.57 | |
| LC75 | 9.31 | 8.90 | 9.82 | |
| LC90 | 10.5 | 10.0 | 11.4 | |
Note:
χ2, Chi-squared value for lethal concentrations and fiducial limits based on a log scale with significance level at P < 0.001.
Figure 1. Larval mortality of Aedes aegypti caused by aqueous solution of pyriproxyfen.
Lethal concentrations were estimated based on concentration-mortality assays using Probit analysis (χ2 = 20.20; df = 5; P < 0.001). Lines denote 95% confidence intervals. Black point represents LC25, LC50, LC75 and LC90 concentrations, while LC50 was selected to evaluate histological, ultrastructural changes and immunofluorescence.
Locomotory behavior
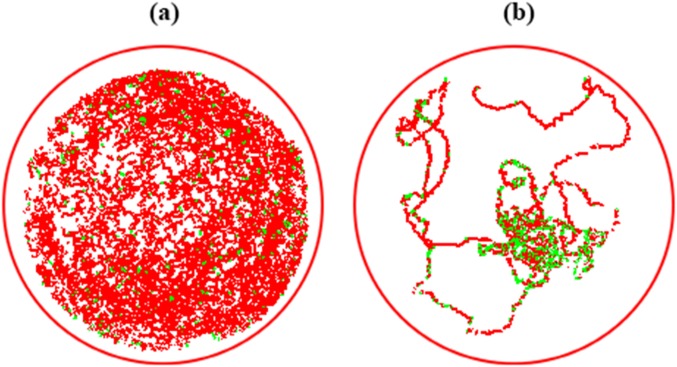
Swimming behavior of Aedes aegypti larvae, when released in aqueous solution are illustrated in Fig. 2. It was observed that larval average swimming speed from control (Fig. 2A) was different from treated larvae (Fig. 2B). Immediately after contact, pyriproxyfen reduced the displacement of larvae and increased resting time with difference in number of meanders and angular velocity from the control.
Figure 2. Displacement trails of Aedes aegypti larvae from control (A) and exposure to LC50 concentrations of pyriproxyfen (B).
Red tracks indicate high swimming speed; green tracks indicate low (initial) velocity.
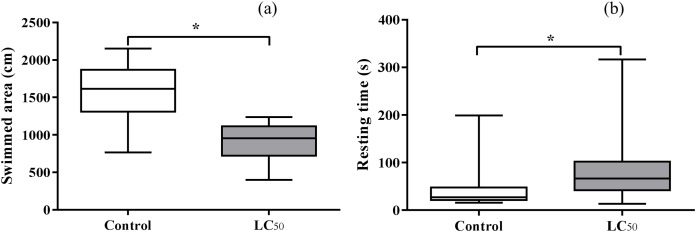
Significant difference was observed between distance traveled in pyriproxyfen exposure LC50 aqueous solution and control (t = 6.40, df = 1.32, P < 0.05). Distance traveled in control was higher than pyriproxyfen exposure LC50 aqueous solution (Fig. 3A). An increase in larval resting time (t = 2.09, df = 1.38, P < 0.05) was found for those exposed to pyriproxyfen (Fig. 3B).
Figure 3. Means ± SD of swimmed area (A) and resting time (B) of third instar Aedes aegypti larvae exposed in aqueous solution for 24 h to LC50 pyriproxyfen concentrations.
Bars followed by different letters differ at P < 0.05 (Tukey’s mean separation test). The bars represent mean values and the error bars are standard errors of the mean, asterisks (*) indicate significant differences between treatments.
Morphological analysis of the midgut
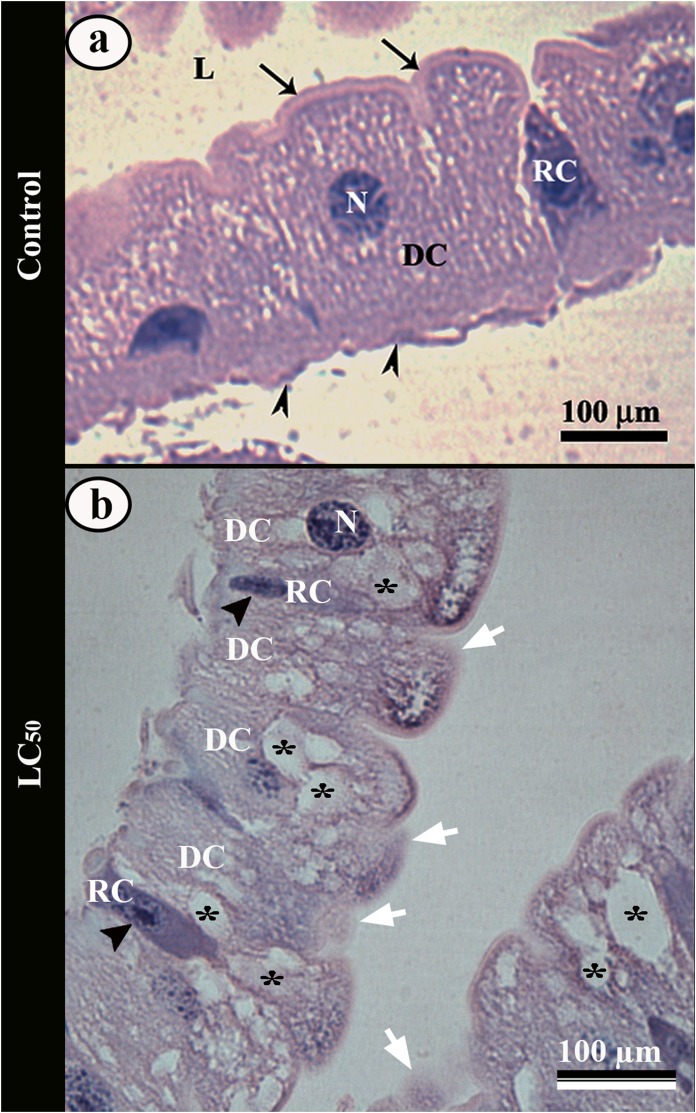
The midgut of control Aedes aegypti larvae had a single layered columnar epithelium with median spherical nucleus and well-developed apical brush border (Fig. 4A). Some regenerative cells were in differentiation process for digestive ones, characterized by a basophil cytoplasm (Fig. 4A).
Figure 4. Light micrographs of the midgut of third instar Aedes aegypti larva.
(A) Single layered epithelium with columnar digestive cells (DC) from control larvae, with spherical nucleus (N), well-developed brush border (arrows) and basal membrane (arrowheads). Note a regenerative cell (RC) in differentiation. (B) Midgut epithelium of larvae exposed to aqueous solution of LC50 pyriproxyfen showing digestive cells (DC) with cytoplasmic vacuoles (asterisks) and disorganized brush border (arrows). Regenerative cells (RC) from the larvae exposed in concentrations. Note many regenerative cells (RC) in differentiation with large nucleus (black arrow head). L – lumen.
The ingestion of pyriproxyfen caused disorganization of the midgut epithelium in the larvae, with increase in the number of cytoplasmic vacuoles, clear areas and disorganized brush border (Fig. 4B). In these larvae there was an increase in the amount of regenerative cells differentiation characterized by many regenerative cells with large nucleus (Fig. 4B).
Ultrastructural analysis of the midgut cells in Aedes aegypti larvae from control showed digestive cells with many apical microvilli and rough endoplasmic reticulum (Fig. 5A) and basal plasma membrane with infoldings (Fig. 5B). Perinuclear cytoplasm was rich in lipid droplets, glycogen (Fig. 5C) mitochondria and rough endoplasmic reticulum (Figs. 5C–5D).
Figure 5. Transmission electron micrographs of the digestive cells from midgut of control third instar Aedes aegypti larvae.
(A) General view of digestive cell, showing microvilli (MV) and rough endoplasmic reticulum (ER). (B) Basal region of digestive cell showing rough endoplasmic reticulum (ER), glycogen island (GL), regular basal labyrinth and muscle (M). (C) Perinuclear cytoplasm showing lipid droplets (asterisks), rough endoplasmic reticulum (ER), glycogen (GL), and mitochondria (arrowhead). (D) Detail of rough endoplasmic reticulum (ER), mitochondria (arrowhead) and glycogen (GL).
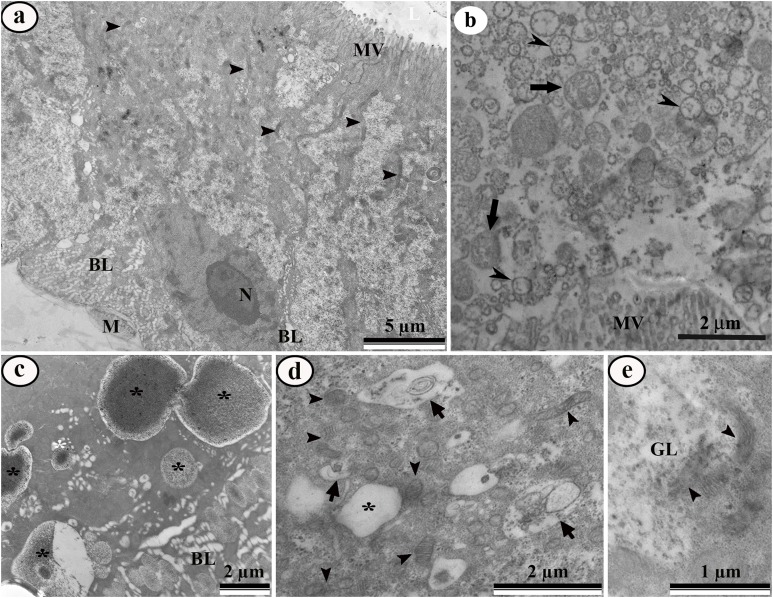
Aedes aegypti larvae exposed to LC50 pyriproxyfen aqueous solution represented damaged midgut digestive cells, including microvilli fragmentation and cytoplasm disorganization characterized by extensive electron-lucent areas (Fig. 6A). The midgut lumen had plenty of cell debris (Fig. 6B). The basal cytoplasm was rich in enlarged lipid droplets, some of them coalescing to form big droplets (Figs. 6C–6D). The basal labyrinth had enlarged extracellular space (Fig. 6C). The cytoplasm had many deformed mitochondria (Figs. 6D–6E), autophagic vacuoles (Fig. 6D), almost empty glycogen deposits (Figs. 6E and 7), and depletion of endoplasmic reticulum characterized by change of flattened to vesicular cisternae (Fig. 7).
Figure 6. Transmission electron micrographs of the digestive cells from midgut of third instar Aedes aegypti larvae exposed to LC50 pyriproxyfen aqueous solution.
(A) General view showing damaged microvilli (MV), Nucleus (N), mitochondria (arrowhead) and enlarged basal labyrinth (BL) and muscle (M). (B) Midgut lumen showing cell debris similar to mitochondria (arrows) and rough endoplasmic reticulum (arrowheads). (C) Basal cell region showing big lipid droplets (asterisks) and enlarged basal labyrinth (BL). (D) Perinuclear cytoplasm with autophagic vacuoles (arrows), lipid droplet (asterisk) and damaged mitochondria (arrowheads). (E) Details of damaged mitochondria (arrowheads) and empty glycogen deposit (GL).
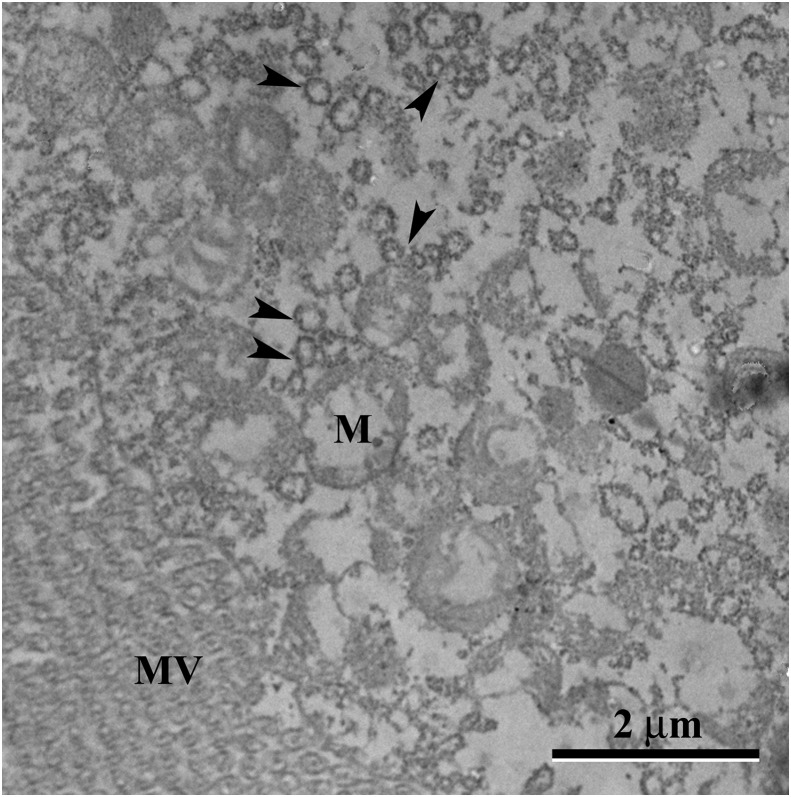
Figure 7. Transmission electron micrograph of the digestive cell form midgut of third instar Aedes aegypti larvae exposed to LC50 pyriproxyfen aqueous solution.
Apical region showing damaged mitochondria (M) and vesicular rough endoplasmic reticulum (arrowheads). MV – microvilli.
Immunofluorescence
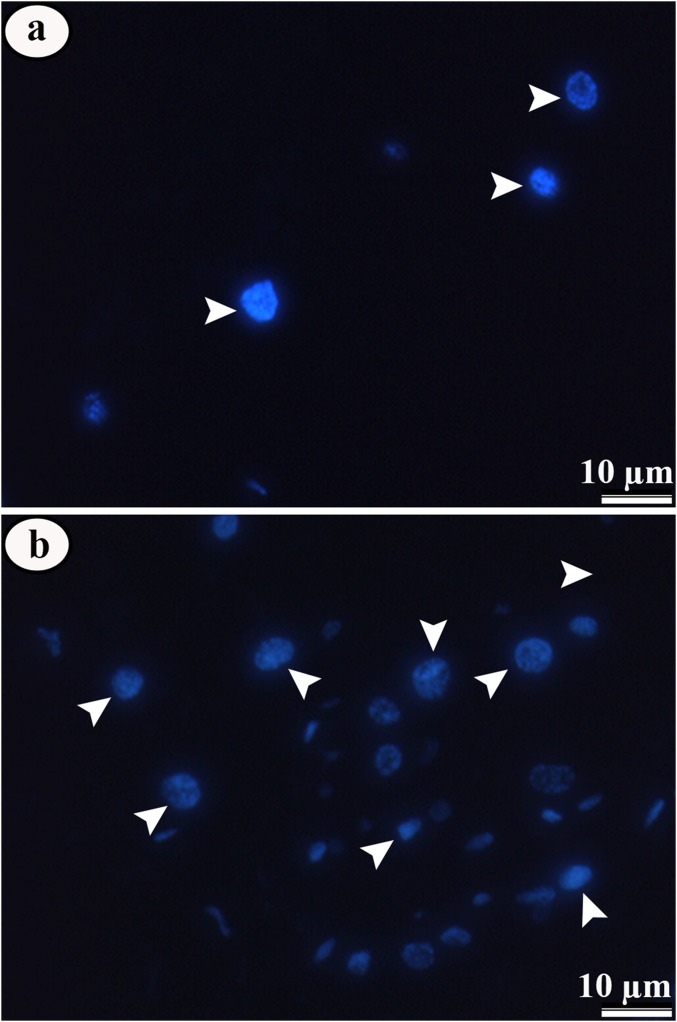
The immunofluorescence to identify cell proliferation showed negative results for phosphorylate histone-H3 in both control and treated larvae, but there was an increase in the number of cell nucleus DAPI-stained in larvae exposed to pyriproxyfen (Figs. 8A–8B).
Figure 8. Micrographs of midgut epithelium of third instar Aedes aegypti larvae in the control and exposed to LC50 pyriproxyfen.
Micrographs of midgut epithelium of third instar Aedes aegypti larvae in the control (A) and exposed to LC50 pyriproxyfen (B) showing negative staining for phosphorylate histone-H3, but with increase in the number of cell nucleus (blue) in treated ones.
Discussion
Our toxicological findings strongly suggest that pyriproxyfen is toxic to Aedes aegypti larvae supporting the potential use of this compound for the control this insect vector. Similar level of efficacy against mosquito larvae have been found for monoterpenes (Silva et al., 2018), squamocin (Costa et al., 2014) and essential oils combined with permethrin (Gross et al., 2017).
The pyriproxyfen efficacy against Aedes aegypti larvae is proved by the concentration killing 99% of larvae almost equal to other bioinsecticides like squamocin (Costa et al., 2014), essential oils (Aguiar et al., 2015), and imidazolium salts (Goellner et al., 2018). In addition to have lower lethal doses as compared to other insecticides, pyriproxyfen is found to be suitable insecticide for autodissemination (Suman et al., 2018; Tuten et al., 2016) and its larvicide effects remains up to 8 months in field (Oo et al., 2018).
Significant changes in swimming behavior, including displacement and speed, found here is the evidence of sublethal effect of pyriproxyfen to Aedes aegypti larvae. Some insecticides have tendency to modify behavioral responses in insects as soon as toxic compound is detected on the body (Barson, Fleming & Allan, 1992; Watson & Barson, 1996). Verheggen et al. (2007) proved that plant volatiles and their constituents effectively disrupt the recognition process of the host substrate also influence the walking behavior of insect. Similar swimming behavior was found for Aedes aegypti exposed in aqueous solution of monoterpene bioinsecticides (Silva et al., 2018) and deltamethrin (Marriel et al., 2016).
Life history attributes, such as behavior, morphology and physiology may represent the adaptations to deal with predators. Highlighting these behaviors, swimming speed allows them in avoiding detection by predators (Engström-Öst & Lehtiniemi, 2004) and competing with feed sources (Alvarez Costa et al., 2018; Janssens & Stoks, 2012). In our experiment, pyriproxyfen acted quickly, compromising larval displacement and ultimately making them detectible to predators in natural conditions. The relationship of this behavior generated by sublethal concentrations of pesticides is already observed in Aedes aegypti and Anopheles pseudopunctipennis (Diptera: Culicidae) larvae treated with temephos, permethrin, and Eucalyptus nitens essential oil (Alvarez Costa et al., 2018).
Although midgut is not a target organ to pyriproxyfen it is the first region that molecule interact and needs cross to be widespread into the hemocoel. To date, how insecticide molecules overcome the midgut epithelium barrier is poorly understood and it is an important subject matter. Here, we show that pyriproxyfen is not only transported from the midgut lumen to the hemolymph, because it causes histological changes in digestive cells of Aedes aegypti larvae characterizing brush border disorganization, intense cytoplasmic vacuolization and differentiation of regenerative cells. Vacuolization and damage to brush border suggests that digestive cells are dying, due to the insecticide toxicity (Costa et al., 2014; Hazarika et al., 2018; Vasantha-Srinivasan et al., 2018). The same histopathological effect was observed in midgut cells of Aedes aegypti (Gaban et al., 2015; Costa et al., 2014) and other insects when treated with different bio and chemical insecticide (Fiaz et al., 2018a, 2018b; Martínez et al., 2018a, 2018b).
Ultrastructural analyses show that pyriproxyfen had a cytotoxic effect on midgut cells of Aedes aegypti larvae. Midgut of treated larvae has damaged epithelial layer, with cell debris released in the midgut lumen, similar to found in this insect exposed to other new chemistry insecticides (Gaban et al., 2015). Cytoplasm vacuolization followed by cell breakdown releasing cell debris into the midgut lumen has been associated with digestive cell death caused by xenobiotics in different insects (Costa et al., 2014; Fiaz et al., 2018a; Suresh Kumar et al., 2013; Mangia et al., 2018; Martínez et al., 2018b).
Presence of abundant lipid droplets in treated midgut of Aedes aegypti with pyriproxyfen in our experiment, is consistent with previous findings (Valzania et al., 2018). Neutral lipid droplets contain triacylglycerols and cholesteryl esters which provide great energy reserves to the cell (Brasaemle, 2007). Lipid droplets are exploited during cell stresses (Henne, Reese & Goodman, 2018) releasing energy in response to the demands of insects (Arrese & Soulages, 2010) as well signaling for immune response (Cheon et al., 2006; Dettloff et al., 2001; Mullen & Goldsworthy, 2003). Glycogen and lipids both are energy reservoirs, however, glycogen is stored in polymers, readily degraded on demand to be used as glycolytic fuel (Steele, 1982), whereas lipids are used as energy source through β-oxidation (Athenstaedt & Daum, 2006).
The increase in the occurrence of autophagosomes in midgut digestive cells exposed by pyriproxyfen indicates that these cells may undergo cytoplasm reorganization, since autophagy is a constitutive process of cell compounds turnover (Nagata, 2018) and can be triggered in response to overcome energy depletion, oxidative stress, organelles damage, hypoxia or DNA damage (Kroemer, Mariño & Levine, 2010). Similar effects occur in the midgut cells of insects exposed to other toxic molecules (Costa et al., 2014; Fiaz et al., 2018b; Martínez et al., 2018a).
Mitochondrial deformation in the midgut cells found in this study is an indication of pyriproxyfen toxicity to Aedes aegypti. Mitochondrial functions are linked to their morphology and membrane ultrastructure (Vincent et al., 2016), and deformation may result in mitochondrial disfunction leading to cell death (Fiaz et al., 2018b). Similar features occur in the midgut of Anitcarsia gemmatalis (Lepidoptera: Noctuidae) exposed to squamocin (Fiaz et al., 2018a) and tebufenozide (Fiaz et al., 2018b).
An intriguing finding in Aedes aegypti larvae exposed to pyriproxyfen is depletion of rough endoplasmic reticulum fragmented in to small vesicles, suggesting that main function of midgut digestive cells in synthesizing protein (Fialho et al., 2009) almost cease. Perhaps much of energy expended for protein synthesis (Alberts et al., 2014) may be used for cell detoxification. In fact, detoxificant cytochrome P450 monooxygenases have been found in the midgut cells of insects exposed to xenobiotics (Liu et al., 2018). In addition, pyriproxyfen operates at molecular level by altering or inhibiting protein translation (Nisbet, 2000).
Since the pyriproxyfen causes damages in the midgut digestive cells, would be expected that these should be replaced by regenerative ones in response to infections (Buchon et al., 2009) and xenobiotics (Forkpah et al., 2014). Surprisingly, we did not detect proliferation in the midgut regenerative cells by immunofluorescence with anti-phospho-histone H3, an antibody that recognizes cell proliferation in the midgut of Aedes aegypti larvae (Fernandes et al., 2014, 2015). This finding indicates that Aedes aegypti larvae may not trigger the cell proliferation program after exposed to pyriproxyfen. Similar results are reported in Spodoptera frugiperda (Lepidoptera: Noctuidae) with Azadirachtin (Nisbet, 2000), cultured invertebrate and vertebrate cells (Salehzadeh et al., 2002) and cultured mosquito cells Aedes albopictus C6/36 (Diptera: Culicidae) with 20-hydroxyecdysone and non-steroidal ecdysone agonist (Smagghe et al., 2003). However, we find that the midgut of treated larvae has more digestive cells, as evidenced by increase in the amount of DAPI-labeled nucleus, suggesting that although regenerative cells did not undergo mitoses, they are in differentiation process to replace damaged digestive cells. The regenerative cells occur in nests with six to eight undifferentiated cells scattered along the midgut epithelium (Nardi, Bee & Miller, 2010; Nardi & Bee, 2012) and in some insects they are claimed to replace other midgut cells by differentiation other than proliferation (Martins et al., 2006; Rost-Roszkowska et al., 2010; Rocha et al., 2014).
Conclusions
Overall, the present study leads to a useful strategy in contribution to the development of control strategies against Aedes aegypti. Despite being a juvenile hormone analog, pyriproxyfen acted quickly provoking toxicity not only limited to behavioral changes, but also elicited morphological damage to midgut cells. Thus, further investigations aiming to evaluate other non-target organs of this chemical would contribute to a better understanding of the potential of pyriproxyfen for an insect control program.
Funding Statement
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq (Brazil) (305165/2013-5), The World Academy of Sciences TWAS (Italy), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CAPES (Brazil), Fundação de Amparo a Pesquisa do Estado de Minas Gerais FAPEMIG (Brazil) and Núcleo de Microscopia e Microanálise of Universidade Federal de Viçosa. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Muhammad Fiaz, Email: muhammad.fiaz@ufv.br.
José Eduardo Serrão, Email: jeserrao@ufv.br.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Muhammad Fiaz conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Luis Carlos Martínez conceived and designed the experiments, analyzed the data, prepared figures and/or tables, approved the final draft, statistical analysis.
Angelica Plata-Rueda performed the experiments, contributed reagents/materials/analysis tools.
Wagner Gonzaga Gonçalves performed the experiments, analyzed the data, prepared figures and/or tables.
Debora Linhares Lino de Souza performed the experiments, contributed reagents/materials/analysis tools.
Jamile Fernanda Silva Cossolin performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, preparation of figures.
Paulo Eduardo Gomes Rodrigues Carvalho performed the experiments.
Gustavo Ferreira Martins conceived and designed the experiments, contributed reagents/materials/analysis tools, approved the final draft.
José Eduardo Serrão conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data is available at Figshare:
Fiaz, Muhammad (2019): Fig 1 S1.xlsx. figshare. Dataset. https://doi.org/10.6084/m9.figshare.7462082.v1.
Fiaz, Muhammad (2019): Fig 3 S2.xlsx. figshare. Dataset. https://doi.org/10.6084/m9.figshare.7462094.v1.
References
- Aguiar et al. (2015).Aguiar RWS, Dos Santos SF, Da Silva Morgado F, Ascencio SD, De Mendonça Lopes M, Viana KF, Didonet J, Ribeiro BM. Insecticidal and repellent activity of Siparuna guianensis Aubl. (Negramina) against Aedes aegypti and Culex quinquefasciatus. PLOS ONE. 2015;10(2):e0116765. doi: 10.1371/journal.pone.0116765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts et al. (2014).Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P. Molecular biology of the cell. Sixth Edition. New York: Garland Science; 2014. [Google Scholar]
- Alvarez Costa et al. (2018).Alvarez Costa A, Gonzalez PV, Harburguer LV, Masuh HM. Effects of Temephos, Permethrin, and Eucalyptus nitens essential oil on survival and swimming behavior of Aedes aegypti and Anopheles pseudopunctipennis (Diptera: Culicidae) Larvae. Journal of Medical Entomology. 2018;55(5):1098–1104. doi: 10.1093/jme/tjy086. [DOI] [PubMed] [Google Scholar]
- Arrese & Soulages (2010).Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annual Review of Entomology. 2010;55(1):207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athenstaedt & Daum (2006).Athenstaedt K, Daum G. The life cycle of neutral lipids: synthesis, storage and degradation. Cellular and Molecular Life Sciences. 2006;63(12):1355–1369. doi: 10.1007/s00018-006-6016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa et al. (2018).Barbosa PRR, Oliveira MD, Barros EM, Michaud JP, Torres JB. Differential impacts of six insecticides on a mealybug and its coccinellid predator. Ecotoxicology and Environmental Safety. 2018;147:963–971. doi: 10.1016/j.ecoenv.2017.09.021. [DOI] [PubMed] [Google Scholar]
- Barson, Fleming & Allan (1992).Barson G, Fleming DA, Allan E. Laboratory assessment of the behavioural responses of residual populations of Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae) to the contact insecticide pirimiphos-methyl by linear logistic modelling. Journal of Stored Products Research. 1992;28(3):161–170. doi: 10.1016/0022-474X(92)90036-P. [DOI] [Google Scholar]
- Boyer et al. (2018).Boyer S, Lopes S, Prasetyo D, Hustedt J, Sarady AS, Doum D, Yean S, Peng B, Bunleng S, Leang R, Fontenille D, Hii J. Resistance of Aedes aegypti (Diptera: Culicidae) populations to Deltamethrin, Permethrin, and Temephos in Cambodia. Asia Pacific Journal of Public Health. 2018;30(2):158–166. doi: 10.1177/1010539517753876. [DOI] [PubMed] [Google Scholar]
- Brasaemle (2007).Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. Journal of Lipid Research. 2007;48(12):2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- Buchon et al. (2009).Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host & Microbe. 2009;5(2):200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Burt et al. (2012).Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012;379(9816):662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- Cao et al. (2014).Cao Z, Xu F, Covaci A, Wu M, Wang H, Yu G, Wang B, Deng S, Huang J, Wang X. Distribution patterns of brominated, chlorinated, and phosphorus flame retardants with particle size in indoor and outdoor dust and implications for human exposure. Environmental Science & Technology. 2014;48(15):8839–8846. doi: 10.1021/es501224b. [DOI] [PubMed] [Google Scholar]
- Carlson, Dougherty & Getz (2016).Carlson CJ, Dougherty ER, Getz W. An ecological assessment of the pandemic threat of Zika virus. PLOS Neglected Tropical Diseases. 2016;10(8):e0004968. doi: 10.1371/journal.pntd.0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho et al. (2017).Carvalho MS, Honorio NA, Garcia LMT, De Sá Carvalho LC. Aedes aegypti control in urban areas: a systemic approach to a complex dynamic. PLOS Neglected Tropical Diseases. 2017;11(7):e0005632. doi: 10.1371/journal.pntd.0005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catae et al. (2018).Catae AF, Roat TC, Pratavieira M, Da Silva Menegasso AR, Palma MS, Malaspina O. Exposure to a sublethal concentration of imidacloprid and the side effects on target and nontarget organs of Apis mellifera (Hymenoptera, Apidae) Ecotoxicology. 2018;27(2):109–121. doi: 10.1007/s10646-017-1874-4. [DOI] [PubMed] [Google Scholar]
- Chediak et al. (2016).Chediak M, Pimenta FG, Jr, Coelho GE, Braga IA, Lima JBP, Cavalcante KRL, De Sousa LC, De Melo-Santos MAV, Macoris MLG, De Araújo AP, Ayres CFJ, Andrighetti MTM, Gomes RGA, Campos KB, Guedes RNC. Spatial and temporal country-wide survey of temephos resistance in Brazilian populations of Aedes aegypti. Memórias do Instituto Oswaldo Cruz. 2016;111(5):311–321. doi: 10.1590/0074-02760150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon et al. (2006).Cheon H-M, Shin SW, Bian G, Park J-H, Raikhel AS. Regulation of lipid metabolism genes, lipid carrier protein lipophorin, and its receptor during immune challenge in the mosquito Aedes aegypti. Journal of Biological Chemistry. 2006;281(13):8426–8435. doi: 10.1074/jbc.M510957200. [DOI] [PubMed] [Google Scholar]
- Chung & Ding (2009).Chung H-W, Ding W-H. Determination of organophosphate flame retardants in sediments by microwave-assisted extraction and gas chromatography–mass spectrometry with electron impact and chemical ionization. Analytical and Bioanalytical Chemistry. 2009;395(7):2325–2334. doi: 10.1007/s00216-009-3139-4. [DOI] [PubMed] [Google Scholar]
- Costa et al. (2014).Costa MS, Cossolin JF, Pereira MJ, Sant’Ana AE, Lima MD, Zanuncio JC, Serrão JE. Larvicidal and cytotoxic potential of squamocin on the midgut of Aedes aegypti (Diptera: Culicidae) Toxins. 2014;6(4):1169–1176. doi: 10.3390/toxins6041169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristale et al. (2013).Cristale J, Katsoyiannis A, Sweetman AJ, Jones KC, Lacorte S. Occurrence and risk assessment of organophosphorus and brominated flame retardants in the River Aire (UK) Environmental Pollution. 2013;179:194–200. doi: 10.1016/j.envpol.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Dettloff et al. (2001).Dettloff M, Wittwer D, Weise C, Wiesner A. Lipophorin of lower density is formed during immune responses in the lepidopteran insect Galleria mellonella. Cell and Tissue Research. 2001;306(3):449–458. doi: 10.1007/s00441-001-0468-9. [DOI] [PubMed] [Google Scholar]
- Ding et al. (2015).Ding J, Shen X, Liu W, Covaci A, Yang F. Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China. Science of the Total Environment. 2015;538:959–965. doi: 10.1016/j.scitotenv.2015.08.101. [DOI] [PubMed] [Google Scholar]
- Engström-Öst & Lehtiniemi (2004).Engström-Öst J, Lehtiniemi M. Threat-sensitive predator avoidance by pike larvae. Journal of Fish Biology. 2004;65(1):251–261. doi: 10.1111/j.0022-1112.2004.00448.x. [DOI] [Google Scholar]
- Eulaers et al. (2014).Eulaers I, Jaspers VLB, Halley DJ, Lepoint G, Nygård T, Pinxten R, Covaci A, Eens M. Brominated and phosphorus flame retardants in White-tailed Eagle Haliaeetus albicilla nestlings: bioaccumulation and associations with dietary proxies (δ13C, δ15N and δ34S) Science of the Total Environment. 2014;478:48–57. doi: 10.1016/j.scitotenv.2014.01.051. [DOI] [PubMed] [Google Scholar]
- Fang et al. (2013).Fang M, Webster TF, Gooden D, Cooper EM, McClean MD, Carignan C, Makey C, Stapleton HM. Investigating a novel flame retardant known as V6: measurements in baby products, house dust, and car dust. Environmental Science & Technology. 2013;47(9):4449–4454. doi: 10.1021/es400032v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes et al. (2015).Fernandes KM, Gonzaga WG, Pascini TV, Miranda FR, Tome HVV, Serrão JE, Martins GF. Imidacloprid impairs the post-embryonic development of the midgut in the yellow fever mosquito Stegomyia aegypti (Aedes aegypti) Medical and Veterinary Entomology. 2015;29(3):245–254. doi: 10.1111/mve.12122. [DOI] [PubMed] [Google Scholar]
- Fernandes et al. (2014).Fernandes KM, Neves CA, Serrão JE, Martins GF. Aedes aegypti midgut remodeling during metamorphosis. Parasitology International. 2014;63(3):506–512. doi: 10.1016/j.parint.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Fialho et al. (2009).Fialho MDCQ, Zanuncio JC, Neves CA, Ramalho FS, Serrão JE. Ultrastructure of the digestive cells in the midgut of the predator Brontocoris tabidus (Heteroptera: Pentatomidae) after different feeding periods on prey and plants. Annals of the Entomological Society of America. 2009;102(1):119–127. doi: 10.1603/008.102.0113. [DOI] [Google Scholar]
- Fiaz et al. (2018a).Fiaz M, Martínez LC, Da Silva Costa M, Cossolin JFS, Plata-Rueda A, Gonçalves WG, Sant’Ana AEG, Zanuncio JC, Serrão JE. Squamocin induce histological and ultrastructural changes in the midgut cells of Anticarsia gemmatalis (Lepidoptera: Noctuidae) Ecotoxicology and Environmental Safety. 2018a;156:1–8. doi: 10.1016/j.ecoenv.2018.02.080. [DOI] [PubMed] [Google Scholar]
- Fiaz et al. (2018b).Fiaz M, Martínez LC, Plata-Rueda A, Gonçalves WG, Shareef M, Zanuncio JC, Serrão JE. Toxicological and morphological effects of tebufenozide on Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae. Chemosphere. 2018b;212:337–345. doi: 10.1016/j.chemosphere.2018.08.088. [DOI] [PubMed] [Google Scholar]
- Finney (1964).Finney DJ. Probit Analysis. Cambridge: Cambridge University Press; 1964. [Google Scholar]
- Forkpah et al. (2014).Forkpah C, Dixon LR, Fahrbach SE, Rueppell O. Xenobiotic effects on the intestinal stem cell proliferation in adult honey bee (Apis melliera L) workers. PLOS ONE. 2014;9(3):e91180. doi: 10.1371/journal.pone.0091180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster & Walker (2002).Foster WA, Walker ED. Mosquitoes (Culicidae) In: Mullen G, Durden L, editors. Medical and Veterinary Entomology. San Diego: Academic press; 2002. pp. 203–262. 597. [Google Scholar]
- Gaban et al. (2015).Gaban CRG, Dourado DM, Da Silva LMGE, Paulo CÃ, Cabrini I. Morphological changes in the digestive system of Aedes aegypti L. induced by [Cu (EDTA)]2−complex ions. Journal of Mosquito Research. 2015;5(21):1–9. doi: 10.5376/jmr.2015.05.0021. [DOI] [Google Scholar]
- Gardner, Chen & Sarkar (2016).Gardner LM, Chen N, Sarkar S. Global risk of Zika virus depends critically on vector status of Aedes albopictus. Lancet Infectious Diseases. 2016;16(5):522–523. doi: 10.1016/S1473-3099(16)00176-6. [DOI] [PubMed] [Google Scholar]
- Goellner et al. (2018).Goellner E, Schmitt AT, Couto JL, Müller ND, Pilz-Junior HL, Schrekker HS, Silva CE, Da Silva OS. Larvicidal and residual activity of imidazolium salts against Aedes aegypti (Diptera: Culicidae) Pest Management Science. 2018;74(4):1013–1019. doi: 10.1002/ps.4803. [DOI] [PubMed] [Google Scholar]
- Goindin et al. (2017).Goindin D, Delannay C, Gelasse A, Ramdini C, Gaude T, Faucon F, David J, Gustave J, Vega-Rua A, Fouque F. Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies) Infectious Diseases of Poverty. 2017;6(1):38. doi: 10.1186/s40249-017-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross et al. (2017).Gross AD, Norris EJ, Kimber MJ, Bartholomay LC, Coats JR. Essential oils enhance the toxicity of permethrin against Aedes aegypti and Anopheles gambiae. Medical and Veterinary Entomology. 2017;31(1):55–62. doi: 10.1111/mve.12197. [DOI] [PubMed] [Google Scholar]
- Gutiérrez et al. (2016).Gutiérrez Y, Santos HP, Serrão JE, Oliveira EE. Deltamethrin-mediated toxicity and cytomorphological changes in the midgut and nervous system of the mayfly Callibaetis radiatus. PLOS ONE. 2016;11(3):e0152383. doi: 10.1371/journal.pone.0152383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazarika et al. (2018).Hazarika H, Tyagi V, Krishnatreyya H, Kishor S, Karmakar S, Bhattacharyya DR, Zaman K, Chattopadhyay P. Toxicity of essential oils on Aedes aegypti: a vector of chikungunya and dengue fever. International Journal of Mosquito Research. 2018;5(3):51–57. [Google Scholar]
- Henne, Reese & Goodman (2018).Henne WM, Reese ML, Goodman JM. The assembly of lipid droplets and their roles in challenged cells. EMBO Journal. 2018;37(12):e98947. doi: 10.15252/embj.201898947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2014).Hu M, Li J, Zhang B, Cui Q, Wei S, Yu H. Regional distribution of halogenated organophosphate flame retardants in seawater samples from three coastal cities in China. Marine Pollution Bulletin. 2014;86(1–2):569–574. doi: 10.1016/j.marpolbul.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Hustedt et al. (2017).Hustedt J, Doum D, Keo V, Ly S, Sam B, Chan V, Alexander N, Bradley J, Prasetyo DB, Rechmat A, Muhammad S, Lopes S, Leang R, Hii J. Determining the efficacy of guppies and pyriproxyfen (Sumilarv® 2MR) combined with community engagement on dengue vectors in Cambodia: study protocol for a randomized controlled trial. Trials. 2017;18(1):367. doi: 10.1186/s13063-017-2105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invest & Lucas (2008).Invest JF, Lucas JR. Pyriproxyfen as a mosquito larvicide. Proceedings of the Sixth International Conference on Urban Pests; Budapest. 2008. pp. 239–245. [Google Scholar]
- Jansen & Beebe (2010).Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next. Microbes and Infection. 2010;12(4):272–279. doi: 10.1016/j.micinf.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Janssens & Stoks (2012).Janssens L, Stoks R. How does a pesticide pulse increase vulnerability to predation? Combined effects on behavioral antipredator traits and escape swimming. Aquatic Toxicology. 2012;110–111:91–98. doi: 10.1016/j.aquatox.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Jaspers et al. (2011).Jaspers VLB, Rodriguez FS, Boertmann D, Sonne C, Dietz R, Rasmussen LM, Eens M, Covaci A. Body feathers as a potential new biomonitoring tool in raptors: a study on organohalogenated contaminants in different feather types and preen oil of West Greenland white-tailed eagles (Haliaeetus albicilla) Environment International. 2011;37(8):1349–1356. doi: 10.1016/j.envint.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Kroemer, Mariño & Levine (2010).Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Molecular Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leta et al. (2018).Leta S, Beyene TJ, De Clercq EM, Amenu K, Kraemer MUG, Revie CW. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. International Journal of Infectious Diseases. 2018;67:25–35. doi: 10.1016/j.ijid.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2018).Liu J, Zhang X, Wu H, Gao Y, Silver K, Ma E, Zhang J, Zhu KY. Comparisons of microsomal cytochrome P450 content and enzymatic activity towards selected model substrates and insecticides in different tissues from the migratory locust (Locusta migratoria) Chemosphere. 2018;208:366–373. doi: 10.1016/j.chemosphere.2018.05.179. [DOI] [PubMed] [Google Scholar]
- Maharajan et al. (2018).Maharajan K, Muthulakshmi S, Nataraj B, Ramesh M, Kadirvelu K. Toxicity assessment of pyriproxyfen in vertebrate model zebrafish embryos (Danio rerio): a multi biomarker study. Aquatic Toxicology. 2018;196:132–145. doi: 10.1016/j.aquatox.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Mäkinen et al. (2009).Mäkinen MSE, Mäkinen MRA, Koistinen JTB, Pasanen A-L, Pasanen PO, Kalliokoski PJ, Korpi AM. Respiratory and dermal exposure to organophosphorus flame retardants and tetrabromobisphenol A at five work environments. Environmental Science & Technology. 2009;43(3):941–947. doi: 10.1021/es802593t. [DOI] [PubMed] [Google Scholar]
- Mangia et al. (2018).Mangia C, Vismarra A, Genchi M, Epis S, Bandi C, Grandi G, Bell-Sakyi L, Otranto D, Passeri B, Kramer L. Exposure to amitraz, fipronil and permethrin affects cell viability and ABC transporter gene expression in an Ixodes ricinus cell line. Parasites & Vectors. 2018;11(1):437. doi: 10.1186/s13071-018-3020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes & Ximenes (2016).Marcondes CB, Ximenes MDFFD. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Revista da Sociedade Brasileira de Medicina Tropical. 2016;49(1):4–10. doi: 10.1590/0037-8682-0220-2015. [DOI] [PubMed] [Google Scholar]
- Maricopa County Environmental Services (2006).Maricopa County Environmental Services . Lifecycle and information on Aedes aegypti mosquitoes. Phoenix: Vector Control Office & Lab.; 2006. [27 February 2017]. [Google Scholar]
- Marriel et al. (2016).Marriel NB, Tomé HVV, Guedes RCN, Martins GF. Deltamethrin-mediated survival, behavior, and oenocyte morphology of insecticide-susceptible and resistant yellow fever mosquitos (Aedes aegypti) Acta Tropica. 2016;158:88–96. doi: 10.1016/j.actatropica.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Martínez et al. (2018b).Martínez LC, Plata-Rueda A, Da Silva Neves G, Cossolin JF, Dos Santos MH, Zanuncio JC, Serrão JE. Morphology, ultrastructure, and chemical compounds of the osmeterium of Heraclides thoas (Lepidoptera: Papilionidae) Protoplasma. 2018b;255(6):1693–1702. doi: 10.1007/s00709-018-1261-x. [DOI] [PubMed] [Google Scholar]
- Martínez et al. (2018a).Martínez LC, Plata-Rueda A, Da Silva Neves G, Gonçalves WG, Zanuncio JC, Bozdoğan H, Serrão JE. Permethrin induces histological and cytological changes in the midgut of the predatory bug, Podisus nigrispinus. Chemosphere. 2018a;212:629–637. doi: 10.1016/j.chemosphere.2018.08.134. [DOI] [PubMed] [Google Scholar]
- Martins et al. (2006).Martins GF, Neves CA, Campos LAO, Serrão JE. The regenerative cells during the metamorphosis in the midgut of bess. Micron. 2006;37(2):161–168. doi: 10.1016/j.micron.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2018).Meng Q-W, Xu Q-Y, Deng P, Fu K-Y, Guo W-C, Li G-Q. Transcriptional response of Methoprene-tolerant (Met) gene to three insect growth disruptors in Leptinotarsa decemlineata (Say) Journal of Asia-Pacific Entomology. 2018;21(2):466–473. doi: 10.1016/j.aspen.2018.02.011. [DOI] [Google Scholar]
- Messina et al. (2016).Messina JP, Kraemer MU, Brady OJ, Pigott DM, Shearer FM, Weiss DJ, Golding N, Ruktanonchai CW, Gething PW, Cohn E, Brownstein JS, Khan K, Tatem AJ, Jaenisch T, Murray CJL, Marinho F, Scott TW, Hay SI. Mapping global environmental suitability for Zika virus. Elife. 2016;5:e15272. doi: 10.7554/eLife.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizouchi et al. (2015).Mizouchi S, Ichiba M, Takigami H, Kajiwara N, Takamuku T, Miyajima T, Kodama H, Someya T, Ueno D. Exposure assessment of organophosphorus and organobromine flame retardants via indoor dust from elementary schools and domestic houses. Chemosphere. 2015;123:17–25. doi: 10.1016/j.chemosphere.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Mullen & Goldsworthy (2003).Mullen L, Goldsworthy G. Changes in lipophorins are related to the activation of phenoloxidase in the haemolymph of Locusta migratoria in response to injection of immunogens. Insect Biochemistry and Molecular Biology. 2003;33(7):661–670. doi: 10.1016/S0965-1748(03)00045-6. [DOI] [PubMed] [Google Scholar]
- Nagata (2018).Nagata S. Apoptosis and clearance of apoptotic cells. Annual Review of Immunology. 2018;36(1):489–517. doi: 10.1146/annurev-immunol-042617-053010. [DOI] [PubMed] [Google Scholar]
- Nardi & Bee (2012).Nardi JB, Bee CM. Regenerative cells and the architecture of beetle midgut epithelia. Journal of Morphology. 2012;273(9):1010–1020. doi: 10.1002/jmor.20038. [DOI] [PubMed] [Google Scholar]
- Nardi, Bee & Miller (2010).Nardi JB, Bee CM, Miller LA. Stem cells of the beetle midgut epithelium. Journal of Insect Physiology. 2010;56(3):296–303. doi: 10.1016/j.jinsphys.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Nisbet (2000).Nisbet AJ. Azadirachtin from the neem tree Azadirachta indica: its action against insects. Anais da Sociedade Entomológica do Brasil. 2000;29(4):615–632. doi: 10.1590/S0301-80592000000400001. [DOI] [Google Scholar]
- Oo et al. (2018).Oo SZM, Thaung S, Maung YNM, Aye KM, Aung ZZ, Thu HM, Thant KZ, Minakawa N. Effectiveness of a novel long-lasting pyriproxyfen larvicide (SumiLarv® 2MR) against Aedes mosquitoes in schools in Yangon. Myanmar Parasites & Vectors. 2018;11(1):16. doi: 10.1186/s13071-017-2603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupy et al. (2010).Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, Hervé J-P, Leroy E, Simard F. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector-Borne and Zoonotic Diseases. 2010;10(3):259–266. doi: 10.1089/vbz.2009.0005. [DOI] [PubMed] [Google Scholar]
- Prophiro et al. (2011).Prophiro JS, Silva OS, Luna JED, Piccoli CF, Kanis LA, Silva MAND. Aedes aegypti and Aedes albopictus (Diptera: Culicidae): coexistence and susceptibility to temephos, in municipalities with occurrence of dengue and differentiated characteristics of urbanization. Revista da Sociedade Brasileira de Medicina Tropical. 2011;44(3):300–305. doi: 10.1590/S0037-86822011005000025. [DOI] [PubMed] [Google Scholar]
- Reiter (2010).Reiter P. Yellow fever and dengue: a threat to Europe? Eurosurveillance. 2010;15(10):19509. doi: 10.2807/ese.15.10.19509-en. [DOI] [PubMed] [Google Scholar]
- Reynolds (1963).Reynolds ES. Use of lead citrate at high ph as an electron-opaque stain in electron microscopy. Journal of Cell Biology. 1963;17(1):208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha et al. (2014).Rocha LLV, Neves CA, Zanuncio JC, Serrão JE. Endocrine and regenerative cells in the midgut of Chagas’ disease vector Triatoma vitticeps during different starvation periods. Folia Biologica. 2014;62(3):259–267. doi: 10.3409/fb62_3.259. [DOI] [PubMed] [Google Scholar]
- Rosen et al. (1983).Rosen L, Shroyer DA, Tesh RB, Freier JE, Lien JC. Transovarial transmission of dengue viruses by mosquitoes: Aedes albopictus and Aedes aegypti. American Journal of Tropical Medicine and Hygiene. 1983;32(5):1108–1119. doi: 10.4269/ajtmh.1983.32.1108. [DOI] [PubMed] [Google Scholar]
- Rost-Roszkowska et al. (2010).Rost-Roszkowska MM, Poprawa I, Klag J, Migula P, Mesjasz-Przybylowicz J, Przybylowicz W. Differentiation of regenerative cell in the midgut epithelium of Epilachna cf. nylanderi (Mulsant 1850) (Insecta, Coleoptera, Coccinellidae) Folia Biologica. 2010;58(3):209–216. doi: 10.3409/fb58_3-4.209-216. [DOI] [PubMed] [Google Scholar]
- Sachs & Malaney (2002).Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415(6872):680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- Salehzadeh et al. (2002).Salehzadeh A, Jabbar A, Jennens L, Ley SV, Annadurai RS, Adams R, Strang RHC. The effects of phytochemical pesticides on the growth of cultured invertebrate and vertebrate cells. Pest Management Science: formerly Pesticide Science. 2002;58(3):268–276. doi: 10.1002/ps.449. [DOI] [PubMed] [Google Scholar]
- Schaefer & Mulligan (1991).Schaefer CH, Mulligan FS., III Potential for resistance to pyriproxyfen: a promising new mosquito larvicide. Journal of the American Mosquito Control Association. 1991;7(3):409–411. [PubMed] [Google Scholar]
- Shepard et al. (2011).Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. American Journal of Tropical Medicine and Hygiene. 2011;84(2):200–207. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva et al. (2018).Silva IM, Martins GF, Melo CR, Santana AS, Faro RR, Blank AF, Alves PB, Picanço MC, Cristaldo PF, Araújo APA, Bacci L. Alternative control of Aedes aegypti resistant to pyrethroids: lethal and sublethal effects of monoterpene bioinsecticides. Pest Management Science. 2018;74(4):1001–1012. doi: 10.1002/ps.4801. [DOI] [PubMed] [Google Scholar]
- Smagghe et al. (2003).Smagghe G, Braeckman BP, Huys N, Raes H. Cultured mosquito cells Aedes albopictus C6/36 (Dip., Culicidae) responsive to 20-hydroxyecdysone and non-steroidal ecdysone agonist. Journal of Applied Entomology. 2003;127(3):167–173. doi: 10.1046/j.1439-0418.2003.00727.x. [DOI] [Google Scholar]
- Steele (1982).Steele JE. Glycogen phosphorylase in insects. Insect Biochemistry. 1982;12(2):131–147. doi: 10.1016/0020-1790(82)90001-4. [DOI] [Google Scholar]
- Stefanini, Martino & Zamboni (1967).Stefanini M, Martino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967;216(5111):173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Suman et al. (2018).Suman DS, Wang Y, Faraji A, Williams GM, Williges E, Gaugler R. Seasonal field efficacy of pyriproxyfen autodissemination stations against container-inhabiting mosquito Aedes albopictus under different habitat conditions. Pest Management Science. 2018;74(4):885–895. doi: 10.1002/ps.4780. [DOI] [PubMed] [Google Scholar]
- Suresh Kumar et al. (2013).Suresh Kumar RS, Shiny PJ, Anjali CH, Jerobin J, Goshen KM, Magdassi S, Mukherjee A, Chandrasekaran N. Distinctive effects of nano-sized permethrin in the environment. Environmental Science and Pollution Research. 2013;20(4):2593–2602. doi: 10.1007/s11356-012-1161-0. [DOI] [PubMed] [Google Scholar]
- Tan et al. (2016).Tan X-X, Luo X-J, Zheng X-B, Li Z-R, Sun R-X, Mai B-X. Distribution of organophosphorus flame retardants in sediments from the Pearl River Delta in South China. Science of the Total Environment. 2016;544:77–84. doi: 10.1016/j.scitotenv.2015.11.089. [DOI] [PubMed] [Google Scholar]
- Thangamani et al. (2016).Thangamani S, Huang J, Hart CE, Guzman H, Tesh RB. Vertical transmission of Zika virus in Aedes aegypti mosquitoes. American Journal of Tropical Medicine and Hygiene. 2016;95(5):1169–1173. doi: 10.4269/ajtmh.16-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuten et al. (2016).Tuten HC, Moosmann P, Mathis A, Schaffner F. Effects of pyriproxifen on Aedes japonicus development and its auto-dissemination by gravid females in laboratory trials. Journal of the American Mosquito Control Association. 2016;32(1):55–58. doi: 10.2987/moco-32-01-55-58.1. [DOI] [PubMed] [Google Scholar]
- Valzania et al. (2018).Valzania L, Martinson VG, Harrison RE, Boyd BM, Coon KL, Brown MR, Strand MR. Both living bacteria and eukaryotes in the mosquito gut promote growth of larvae. PLOS Neglected Tropical Diseases. 2018;12(7):e0006638. doi: 10.1371/journal.pntd.0006638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha-Srinivasan et al. (2018).Vasantha-Srinivasan P, Thanigaivel A, Edwin ES, Ponsankar A, Senthil-Nathan S, Selin-Rania S, Kalaivani K, Hunter WB, Duraipandiyan V, Al-Dhabi NA. Toxicological effects of chemical constituents from Piper against the environmental burden Aedes aegypti Liston and their impact on non-target toxicity evaluation against biomonitoring aquatic insects. Environmental Science and Pollution Research. 2018;25(11):10434–10446. doi: 10.1007/s11356-017-9714-x. [DOI] [PubMed] [Google Scholar]
- Verheggen et al. (2007).Verheggen F, Ryne C, Olsson P-OC, Arnaud L, Lognay G, Högberg H-E, Persson D, Haubruge E, Löfstedt C. Electrophysiological and behavioral activity of secondary metabolites in the confused flour beetle, Tribolium confusum. Journal of Chemical Ecology. 2007;33(3):525–539. doi: 10.1007/s10886-006-9236-3. [DOI] [PubMed] [Google Scholar]
- Vincent et al. (2016).Vincent AE, Ng YS, White K, Davey T, Mannella C, Falkous G, Feeney C, Schaefer AM, McFarland R, Gorman GS, Taylor RW, Turnbull DM, Picard M. The spectrum of mitochondrial ultrastructural defects in mitochondrial myopathy. Scientific Reports. 2016;6(1):30610. doi: 10.1038/srep30610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson & Barson (1996).Watson E, Barson G. A laboratory assessment of the behavioural responses of three strains of Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae) to three insecticides and the insect repellent N,N-diethyl-m-toluamide. Journal of Stored Products Research. 1996;32(1):59–67. doi: 10.1016/0022-474X(95)00033-4. [DOI] [Google Scholar]
- WHO Pesticide Evaluation Scheme (WHOPES) (2000).WHO Pesticide Evaluation Scheme (WHOPES) Report of the fourth WHOPES working group meeting. Review of IR3535, KBR3023, (RS)-methoprene 20% EC and pyriproxyfen 0.5% GR. Geneva: World Health Organization; 2000. [20 August 2017]. [Google Scholar]
- World Health Organization (2015).World Health Organization Emergencies preparedness response. Zika virus infection — Brazil and Colombia. 2015. http://www.who.int/csr/don/21-october-2015-zika/en/ [23 October 2015]. http://www.who.int/csr/don/21-october-2015-zika/en/ Epidemiological Week/EW 41 (Update as of 21 of October 2015)
- World Health Organization (2016).World Health Organization . World health statistics 2016: monitoring health for the SDGs sustainable development goals. Geneva: World Health Organization; 2016. [Google Scholar]
- World Health Organization (2017).World Health Organization Number of Reported Cases of Chikungunya Fever in the Americas. by Country or Territory 2013–2015. 2017. http://www.paho.org/chikungunya. http://www.paho.org/chikungunya Epidemiological Week/EW 4 (Updated as of 30 January 2015) (accessed 5 February 2015)
- Yang et al. (2014).Yang F, Ding J, Huang W, Xie W, Liu W. Particle size-specific distributions and preliminary exposure assessments of organophosphate flame retardants in office air particulate matter. Environmental Science & Technology. 2014;48(1):63–70. doi: 10.1021/es403186z. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang Z, Statler BM, Calkins TL, Alfaro E, Esquivel CJ, Rouhier MF, Denton JS, Piermarini PM. Dynamic expression of genes encoding subunits of inward rectifier potassium (Kir) channels in the yellow fever mosquito Aedes aegypti. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2017;204:35–44. doi: 10.1016/j.cbpb.2016.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available at Figshare:
Fiaz, Muhammad (2019): Fig 1 S1.xlsx. figshare. Dataset. https://doi.org/10.6084/m9.figshare.7462082.v1.
Fiaz, Muhammad (2019): Fig 3 S2.xlsx. figshare. Dataset. https://doi.org/10.6084/m9.figshare.7462094.v1.