Abstract
Objective
This study investigated payments made by pharmaceutical companies to oncology specialists in Japan, what the payments were for and whether the receipt of such payments contravened any conflict of interest (COI) regulations.
Design, setting and participants
Payment data to physicians, as reported by all pharmaceutical companies belonging to the Japan Pharmaceutical Manufacturers Association, were retrospectively extracted for 2016. Of the named individual recipients of payments, all certified oncologists were identified, using certification data from the Japanese Society of Medical Oncology (JSMO). The individual specialisations of each of the oncologists was also identified.
Outcome
Payments to individual cancer specialists and what they were for were identified. Factors associated with receipt of higher value payments and payment flows to specialties were determined. Companies selling oncology drugs with annual sales of ≥5 billion yen (£33.9 million, €40.2 million and $46.0 million) (high revenue-generating drugs) were identified.
Results
In total, 59 companies made at least one payment to oncologists. Of the 1080 oncology specialists identified, 763 (70.6%) received at least one payment, while 317 received no payment. Of the 763, some 142 (13.1%) receiving at least 1 million yen (£6,800, €8,000 and $9200) accounted for 71.5% of the total. After adjustment of covariates, working for university hospitals and cancer hospitals and male gender were key factors associated with larger monetary payments. Payments preferentially targeted on cancer specialties using high revenue-generating drugs. The JSMO has its own COI policy for its members, but the policy did not mention any specific guidelines for certified oncology specialists.
Conclusion
Financial relationships were identified and quantified between pharmaceutical companies and oncology specialists, but the extent and worth varied significantly. Given the frequency and amounts of money involved in such linkages, it would be beneficial for specific COI regulations to be developed and policed for oncologists.
Keywords: conflict of interest, oncology specialist, Japan, industry payment, Japanese Society of Medical Oncology
Strengths and limitations of this study.
We considered oncology specialists certified by the Japan Society of Medical Oncology, one of the largest professional medical associations in Japan’s clinical oncology field.
The authors independently organised payment data for speaking, writing and consulting work, as published by the major pharmaceutical companies, and created a single uniform payment database.
Accuracy of the affiliations and subspecialties of some oncology specialist in the study year (2016) were estimated using the data on relevant websites and other data sources on the internet, possibly causing some measurement errors in these variables.
This study only covered limited types of payment data in the single year (2016), which hampered a comprehensive and/or longitudinal analysis of the type and value of the payments among the oncology specialists.
Introduction
Increasing global attention is being paid with respect to how pharmaceutical companies (pharma) operate and their relationships with regard to payments they make to doctors working in national health systems. There is growing concern that specialised physicians receiving financial payments from pharma commercially connected with their field of expertise may be inadvertently or unethically being influenced and that their impartiality and ability to act in the best interests of their patients is being compromised. The approval earlier in 2019 by the US Food and Drug Administration(FDA) of onasemnogene abeparvovec-xioi (Zolgensma), a gene therapy for children less than 2 years old with spinal muscular atrophy, which is now the most expensive drug on the market, illustrates the amounts of money that are involved. If a physician prescribes Zolgensma treatment, and a single administration is all that is required, it costs $2.1 million (£1.6 million, €1.9 million and 231.2 million yen) per patient. In Japan, a new treatment for leukaemia and other haematological cancers was approved in May 2019, which will cost 33.5 million yen (£226 800, €269 000 and $307 800). The drug, Tisagenlecleucel (Kymriah), manufactured and marketed by Novartis Pharma KK, is the most expensive drug on the Japanese market and is covered under Japan’s national health insurance. In view of the sums of money involved and the possibility of corruption creeping into the system, there is an increasing need for transparency with respect to all forms of payment, or gifts of any kind, being dispensed by pharma to physicians. According to the World Medical Association, ‘although the cooperation between physicians and commercial enterprises may lead to significant advances in medicine, including the development of new drugs and treatments, it may also result in a conflict of interest (COI) between commercial enterprises and physicians that may have adverse effects on patients’ care and the reputation of physicians’. Consequently, medical and governmental facilities worldwide are considering steps to help create transparency in the relationship between physicians and the pharmaceutical industry, as exemplified by the USA’s Physician Payments Sunshine Act, enacted in 2010, and the US government’s Open Payments Data (https://openpaymentsdata.cms.gov).1 2 In Japan, members of the Japan Pharmaceutical Manufacturers Association (JPMA) are attempting to improve the transparency and acceptability of the relationship between corporate activities of pharma and medical institutions and individual physicians and, in 2015, the JPMA introduced a self-regulatory guideline for all its members to promote clarity and deeper understanding of the beneficial contribution that pharma makes to medicine and pharmacy and so that pharma activities are conducted with high ethical standards and for maximum benefit to patients.
Cancer has been the leading cause of mortality in Japan since 1981. In 2016, there were 372 986 cancer deaths in Japan, with malignant neoplasms costing the nation an estimated 3.6 trillion yen (£24.4 billion, €28.9 billion and $33.1 billion) in medical expenditure. In 2016, lung cancer was the leading cause of cancerous deaths (52 430) in men, followed by gastric cancer (29 854) and colorectal cancer (27 026), while colorectal cancer was the leading cause of cancerous death in women (23 073), followed by lung cancer (21 408) and pancreatic cancer (16 415).3 The risk factors for cancer are diverse, including tobacco use, infection, obesity, radiation exposure, reproductive and hormonal factors, and other environmental and occupational pollutants and carcinogens.4 In Japan, principally because of its ageing population, cancer rates are forecast to continue to rise for the foreseeable future.3
For the pharmaceutical industry, medical and therapeutic practice generates substantial income, allowing it exploit various opportunities to accomplish the goal of the maximisation of profits.5–7 From the 1950s, the main business model of the pharma was the production of low-price drugs to treat diseases and conditions that were primarily chronic and prevalent (eg, hypertension and diabetes).8–10 Following advances in drug development against infectious and chronic diseases, cancer became an ever-increasing and major problem, with 17.2 million incidents and 213.2 million cancer-associated disability-adjusted life-years lost during 1990–2016 worldwide.11 Pharma therefore adopted a new business model, the discovery and development of anticancer agents that could be sold at extremely high price but usually for short treatment durations.5–7 This guaranteed a hefty profit in a short timeframe—provided that the drugs would be prescribed and used—while imposing an extraordinarily high cost on patients and health systems.5–7 12
Physicians remain paramount decision makers on the demand side of the pharmaceutical market. It is known that even subtle financial interactions between physicians and a pharmaceutical company can affect their prescribing behaviour13–18 and so could encourage irrational or preferential use of a company’s drug. Perhaps unsurprisingly, given the cost of anticancer drugs, oncologists have latterly become primary targets for approaches from companies with high-cost anticancer products to sell. Indeed, significant financial relationships between such companies and the authors of the oncology Clinical Practice Guidelines (CPGs) have been reported both in the USA and Japan.19 20 Given these far from ideal circumstances, there has been a growing need for intervention, in the form of policy implementation and education about the implications of these interactions, to help protect physicians, patients, institutions and the companies themselves.15–18
Although Japan has the world’s third largest pharmaceutical market, with annual sales of $76 billion (£56 billion, €66 billion, 8.3 trillion yen) in 2017,21 its overall scale has been declining at approximately 2% annually.22 To maintain sales in these competitive and tightening markets, forceful advertisement of high-price products, namely novel oncology drugs, has become increasingly important for pharmaceutical companies. Indeed, sales of oncology drugs have recently been rising in Japan, exceeding 1 trillion yen (£6.8 billion, €8.0 billion and $9.2 billion) for the first time in 2016.23 Furthermore, sales are predicted to increase 1.5-fold in the next decade with the increasing application of immunotherapy in clinical practice.23 It would therefore be reasonable to assume that pharmaceutical companies will increasingly deploy marketing measures and incentives targeting oncology specialists for the immediate and foreseeable future.
In Japan, the JPMA encompasses a majority of companies that manufacture brand name drugs. Its members accounted for 80.8% of total pharmaceutical sales in Japan in 2015.24 In 2011, the JPMA published a transparency guideline requiring all member companies to disclose all payments for speaking, writing and consulting work made to all individuals, specifying their names and affiliations.25 The guideline was updated in 2015 and made more comprehensive. The aim was to improve the transparency of linkages between pharmaceutical companies and physicians, as in the Open Payments Data in the USA.1 2 The 2015 revised JPMA Guideline obliges pharma to itemise payments made for: (1) research and development; (2) academic support; (3) lecturing/writing/consultancy work; (4) expenses related to provision of information and (5) expenses for hospitality and so on. However, the disclosure format, whereby companies involved published the required data on their own individual websites, has differed among and between companies and the aggregated, standardised payment data have not been readily available.26 As a result, an easy examination of company/physicians links and payments in a meaningful way has proved almost impossible. Thus, we independently organised payment data for speaking, writing, and consulting work, and created a single uniform payment database.
The aims of the current study were: (1) to understand and evaluate the characteristics and distributions of financial payments made by pharmaceutical companies to oncology specialists: (2) to examine whether or not pharmaceutical companies may be making payments to help promote sales of their own products; and (3) to elucidate what Japanese oncology specialists are obliged to disclose with respect to any COI.
Methods
Study setting and participants
The Japan Society of Medical Oncology (JSMO), with over 9154 general members, is the primary professional medical society in the clinical oncology field in Japan. The JSMO began operating a specialty registration system for members in 2004, which required JSMO members wishing to be certified to meet specific requirements for both oncology care and academic achievement. Only after passing the requisite examination could they become board-certified oncology specialists, with renewal of certification being required every 5 years. All 1081 oncology specialists certified by the JSMO as of 1 April 2016 were included in this study.
Sources of payment data
The sources of the payment data were the websites of 78 pharmaceutical companies that were members of the JPMA in fiscal 2016. These companies were required to publish data of payments made to physicians and other researchers annually under the transparency guidelines of the JPMA. They were categorised into 71 active JPMA members, six affiliated entities of these companies and one past member. The companies included in this study, plus their payment data, are listed in online supplementary material 1.
bmjopen-2018-028805supp001.pdf (207.6KB, pdf)
We obtained each company’s data and organised them into a unified, easy-to-compare database. This was done because no data were published as a spreadsheet. Consequently, data with differing character codes were converted into a spreadsheet format, and data with no character codes were converted into text files using an optical character reader. Moreover, where data were protected against facsimile or reproduction, we used FullShot10 software (Inbit, California, USA) to scan photos of the data and converted the data into text files. The accuracy of the reorganised data was confirmed by comparing it with the original data. The database included physicians’ names, their main institutional affiliation, payments received, the form of the payments and the total amount paid. The form of payment was categorised into three types: payment for speaking, payment for writing and consulting fees. For the purposes of this study, we converted Japanese yen (¥) to pounds sterling (£), euro (€) and US dollars ($), using the average monthly exchange rate for 2016, namely 147.7 yen per £1, 124.5 yen per €1 and 108.8 yen per US$1.
Data collection
We examined payment data for all oncology specialists included in this study. We extracted their working institutions and regional locations, along with the year of their certification by the JSMO. We further confirmed the accuracy of such information, collating data from institutional websites and other sources. We determined the sex of all the oncologists, using data from Japan’s Ministry of Health, Labour and Welfare,27 institutional websites and other sources. We further estimated the primary cancer specialty (respirology, gastroenterology, haematology, breast and so on) of all oncologists included in the study. We also determined the COI policy of the JSMO prevailing at the time.
Data analysis
To examine the characteristics and distributions of payments, we performed descriptive analyses of the data on an individual oncology specialist and pharmaceutical company basis. We then summarised the characteristics of oncology specialists according to the total monetary value of the payment they received, dividing the patients into the three groups: 1 million yen (£6800, €8000 and $9200) or above (high-payment group (HPG)); 1 yen–1 million yen (low-payment group (LPG)) and 0 yen (no-payment group (NPG)). In general, 1 million yen is approximately 25% of the median annual income of a Japanese citizen.28
Using a multivariate negative binomial regression model, we subsequently examined possible factors associated with the monetary value of the payment to each individual, with sex, institutional place of work, regional working locations, year of experience after board certification and cancer specialty as covariates. The payment data were rounded down as a unit of 1 million yen. Since the payment of those receiving less than 1 million yen (LPG and NPG) was regarded as zero in the regression analysis, among this group, we further examined possible factors associated with the monetary value of any payment using the same model adopted for the overall population. For this analysis, the payment data were rounded down as a unit of 100 000 (£677, €803 and $919).
For more detailed examination, a Sankey diagram was created to illustrate the distribution of payments to each cancer specialty on an individual company basis. The Sankey diagram is a flow diagram, where band width proportionally represents the flow quantity.29 Payment values from individual companies, according to cancer specialty, are depicted in the bands in the diagram, width being proportional to the total amount of the payment. In addition, to see whether the payment was linked to any specific oncology drugs, we examined such drugs with annual Japanese domestic sales of 5 billion yen (£33.9 million, €40.2 million and $46.0 million) or above (high revenue-generating drugs) in fiscal 2016, and if each drug was covered under the Japanese National Health Insurance scheme in specific oncology subspecialty by the end of the same fiscal year (31 March 2017). We further examined newly approved drugs and drugs with a new indication added during the fiscal years of 2015 and 2016 (1 April 2015–31 March 2017).
Human subject involvement
The present study is a retrospective analysis of existing databases and public domain information. No patients or any other individuals other than oncology specialists identified in the public domain, and whose names are not identified in this report, were included in the study.
Results
The JSMO had over 9000 members at the time the study was undertaken, with 1081 physicians having been board certified as oncology specialists. We excluded one oncologist whose professional affiliation we were unable to confirm, and he did not receive any payment from the pharmaceutical companies. Thus, we included a total of 1080 specialist oncologists in our analyses.
Table 1 summarises the details of certified oncologists and payments from Japanese pharmaceutical firms. Of the 1080 individuals involved, 907 were men (84.0%) and 173 (16.0%) were women. Of the total, 442 (40.9%), 183 (16.9%) and 455 (42.1%) worked for university hospitals, cancer hospitals and other institutions, respectively. In 2016, the most common specialty was respirology (285, 26.4%), followed by gastroenterology (278, 25.7%) and haematology (250, 23.2%).
Table 1.
Characteristics of oncology specialists and pharmaceutical payments received by individual doctors
| Variable | |
| Characteristics of oncology specialists (n=1080) | |
| Sex (N, %) | |
| Men | 907 (84.0) |
| Women | 173 (16.0) |
| Working institutions (N, %) | |
| University hospitals | 442 (40.9) |
| Cancer hospitals | 183 (16.9) |
| Other types of institutions | 455 (42.1) |
| Working regions (N, %) | |
| Hokkaido | 52 (4.8) |
| Tohoku | 58 (5.4) |
| Kanto | 302 (28.0) |
| Chubu | 194 (18.0) |
| Kinki | 208 (19.3) |
| Chugoku | 88 (8.2) |
| Shikoku | 43 (4.0) |
| Kyushu | 135 (12.5) |
| Year of certification (N, %) | |
| 2006 | 45 (4.2) |
| 2007 | 77 (7.1) |
| 2008 | 71 (6.6) |
| 2009 | 98 (9.1) |
| 2010 | 133 (12.3) |
| 2011 | 130 (12.0) |
| 2012 | 124 (11.5) |
| 2013 | 143 (13.2) |
| 2014 | 98 (9.1) |
| 2015 | 85 (7.9) |
| 2016 | 76 (7.0) |
| Specialty (N, %) | |
| Respirology | 285 (26.4) |
| Gastroenterology | 278 (25.7) |
| Haematology | 250 (23.2) |
| Breast | 72 (6.7) |
| Head and neck | 12 (1.1) |
| Gynaecology | 10 (0.9) |
| Urology | 9 (0.8) |
| Dermatology | 2 (0.2) |
| Other or undetermined | 162 (15.0) |
| Characteristics of payments (n=1080) | |
| Total monetary value of payments | 585 453 314 (3 963 800) |
| Total count of payments | 7325 |
| Type of payments (¥ (£), %) | |
| Speaking | 467 802 690 (3 167 249), 79.9 |
| Consulting | 94 682 807 (641 048), 16.2 |
| Writing | 22 266 186 (150 753), 3.8 |
| Missing | 701 631 (4 750), 0.1 |
| Payment per individual specialist | |
| Median value per individual specialist (¥ (£), IQR) | 120 016 (813) (0(0)–449 378 (3043)) |
| Median count per individual specialist (IQR) | 2 (0–7) |
| Number of oncology specialists with payment (N, %) | |
| Any | 763 (70.6) |
| 1 million yen (£6800) or above | 142 (13.1) |
| 5 million yen (£33 900) or above | 19 (1.8) |
| 10 million yen (£67 700)or above | 2 (0.2) |
| Monetary value of payment according to specialties (¥ (£), %) | |
| Respirology | 216 806 522 (1 467 884), 37.0 |
| Gastroenterology | 139 690 202 (945 770), 23.9 |
| Haematology | 119 219 713 (807 175), 20.4 |
| Breast | 49 287 661 (333 701), 8.4 |
| Head and neck | 9 213 401 (62 379), 1.6 |
| Gynaecology | 570 533 (3 863), 0.1 |
| Urology | 7 862 285 (53 231), 1.3 |
| Dermatology | 562 502 (3,808), 0.1 |
| Other or undetermined | 42 240 495 (285 988), 7.2 |
We converted Japanese yen to pound sterling (£), using the average monthly exchange rate for 2016, namely 147.7 yen per £1.
A total of 7325 payments were recorded, the total monetary value being 585 453 314 yen (£3 963 800, €4 702 436 and $5 381 005). Of this total, 467 802 690 yen (£3 167 249, €3 757 451 and $4 299 657) was for speaking, 94 682 807 yen (£641 048, €760 504 and $870 246) was for consulting and 22 266 186 yen (£150 753, €178 845 and $204 652) was paid for writing. The median monetary value and count of an individual payment was 120 016 yen (£813, €964 and $1103). (IQR 0 yen–449 378 yen (£3043, €3609 and $4130) and 2 (IQR 0–7), respectively.
Of the 1080 individuals, 763 (70.6%) received at least one payment. Furthermore, 142 (13.1%) received payments totalling ≥1 million yen, while 19 (1.8%) received ≥5 million yen (£33 900, €40 200 and $46 000). Two individuals (0.2%) received ≥10 million (£67 700, €80 300 and $91 900).
Respirology was the specialty that attracted the largest payment (216 806 522 yen (£1 467 884, €1 741 418 and $1 992 707) from the pharmaceutical companies, followed by gastroenterology (139 690 202 yen (£945 770, €1 122 010 and $1 283 917) and haematology (119 219 713 yen (£807 175, €957 588 and $1 095 769).
Table 2 summarises the monetary values and counts of payments made by the 78 pharmaceutical companies. In total, 59 (75.6%) companies made at least one payment to oncology specialists. The Chugai Pharmaceutical Co Ltd, a subsidiary of F. Hoffmann-La Roche Ltd, made the largest accumulated payment of 103 830 493 yen (£702 982, €833 980 and $954 324). The median monetary value and count among the 78 companies was 645 946 yen (£4373, €5188 and $5937) (IQR 33 410 yen (£226, €268 and $307)–5 196 201 (£35 181, €41 737 and $47 759) and 10 (IQR 1–71), respectively.
Table 2.
Companies making payments to oncology specialists and monetary value and count of their payments
| Pharmaceutical company | Monetary value (¥ (£)) | Count |
| Chugai Pharmaceutical Co, Ltd | 103 830 493 (702 982) | 1248 |
| AstraZeneca plc | 51 928 785 (351 583) | 592 |
| Taiho Pharmaceutical Co, Ltd | 50 723 560 (343 423) | 688 |
| Ono Pharmaceutical Co, Ltd | 47 831 737 (323 844) | 624 |
| Eli Lilly Japan K.K. | 44 825 340 (303 489) | 502 |
| Bristol-Myers Squibb K.K. | 33 443 966 (226 432) | 405 |
| Takeda Pharmaceutical Company Ltd | 28 280 960 (191 476) | 306 |
| Novartis Pharma K.K. | 27 203 346 (184 180) | 336 |
| Nippon Boehringer Ingelheim Co, Ltd | 25 987 859 (175 950) | 325 |
| Kyowa Hakko Kirin Company, Ltd | 20 208 095 (136 819) | 267 |
| Pfizer Japan Inc | 16 509 478 (111 777) | 185 |
| Merck Serono Co, Ltd | 16 377 746 (110 885) | 229 |
| Eisai Co, Ltd | 16 309 136 (110 421) | 220 |
| Celgene Corporation | 15 207 296 (102 961) | 212 |
| Daiichi Sankyo Company, Limited | 8 772 101 (59 391) | 117 |
| Bayer Yakuhin, Ltd | 8 340 481 (56 469) | 97 |
| Yakult Honsha Company, Limited | 8 318 026 (56 317) | 121 |
| Janssen Pharmaceutical K.K. | 7 723 516 (52 292) | 84 |
| MSD K.K. | 6 317 468 (42 772) | 71 |
| Sumitomo Dainippon Pharma Co, Ltd | 5 196 201 (35 181) | 92 |
| Nippon Kayaku Co, Ltd | 3 868 780 (26 194) | 46 |
| Astellas Pharma Inc | 3 590 000 (24 306) | 53 |
| Nippon Shinyaku Co, Ltd | 3 129 497 (21 188) | 53 |
| Asahi Kasei Pharma Corporation | 3 102 452 (21 005) | 45 |
| Sanofi K.K. | 2 592 500 (17 552) | 31 |
| Otsuka Holdings Co, Ltd | 2 204 198 (14 923) | 40 |
| Mochida Pharmaceutical Co, Ltd | 2 149 441 (14 553) | 31 |
| Teijin Pharma Limited | 2 099 790 (14 217) | 27 |
| AbbVie GK | 2 082 626 (14 100) | 17 |
| Shionogi & Co, Ltd | 1 948 968 (13 195) | 28 |
| Kyorin Pharmaceutical Co, Ltd | 1 918 033 (12 986) | 34 |
| Tsumura & Co | 1 681 688 (11 386) | 21 |
| Meiji Seika Pharma Co, Ltd | 1 289 000 (8727) | 24 |
| Terumo Corporation | 1 214 840 (8225) | 16 |
| Kissei Pharmaceutical Co, Ltd | 1 124 840 (7616) | 9 |
| Zeria Pharmaceutical Co, Ltd | 946 645 (6409) | 12 |
| Mitsubishi Tanabe Pharma Corporation | 935 508 (6334) | 17 |
| EA Pharma Co, Ltd | 783 712 (5306) | 17 |
| Taisho Toyama Pharmaceutical Co, Ltd | 701 631 (4750) | 11 |
| Kowa Company, Ltd | 590 262 (3996) | 5 |
| Hisamitsu Pharmaceutical Co, Inc | 539 030 (3649) | 11 |
| Novo Nordisk Pharma Ltd | 474 360 (3212) | 8 |
| Sanwa Kagaku Kenkyusho Co, Ltd | 445 480 (3016) | 4 |
| Aska Pharmaceutical Co, Ltd | 423 206 (2865) | 6 |
| Shire Japan KK | 367 521 (2488) | 5 |
| Nihon Pharmaceutical Co, Ltd | 311 836 (2111) | 8 |
| Nippon Chemiphar Co, Ltd | 278 425 (1885) | 3 |
| Ayumi Pharmaceutical Corporation | 226 864 (1536) | 3 |
| Mylan Seiyaku Ltd | 206 240 (1396) | 4 |
| Kracie Holdings, Ltd | 134 056 (908) | 2 |
| GlaxoSmithKline K.K. | 111 370 (754) | 2 |
| Minophagen Pharmaceutical Co | 110 440 (748) | 2 |
| Maruho Co, Ltd | 103 120 (698) | 1 |
| Torii Pharmaceutical Co, Ltd | 102 260 (692) | 2 |
| EN Otsuka Pharmaceutical Co, Ltd | 89 096 (603) | 2 |
| Kaken Pharmaceutical Co, Ltd | 77 959 (528) | 1 |
| Toray Industries, Inc | 77 080 (522) | 1 |
| Santen Pharmaceutical Co, Ltd | 51 560 (349) | 1 |
| Toyama Chemical Co, Ltd | 33 410 (226) | 1 |
| Bee Brand Medico Dental Co, Ltd | 0 (0) | 0 |
| Biofermin Seiyaku Co, Ltd | 0 (0) | 0 |
| Fujimoto Pharmaceutical Corporation | 0 (0) | 0 |
| Fuso Pharmaceutical Industries, Ltd | 0 (0) | 0 |
| Japan Tobacco Inc | 0 (0) | 0 |
| Kyoto Pharmaceutical Industries, Ltd | 0 (0) | 0 |
| Maruishi Pharmaceutical Co, Ltd | 0 (0) | 0 |
| Nippon Zoki Pharmaceutical Co, Ltd | 0 (0) | 0 |
| Otsuka Pharmaceutical Co, Ltd | 0 (0) | 0 |
| Otsuka Pharmaceutical Factory, Inc | 0 (0) | 0 |
| POLA-Pharma | 0 (0) | 0 |
| Research Institute for Microbial Diseases | 0 (0) | 0 |
| Seikagaku Corporation | 0 (0) | 0 |
| Senju Pharmaceutical Co, Ltd | 0 (0) | 0 |
| Taisho Pharmaceutical Co, Ltd | 0 (0) | 0 |
| Teikoku Seiyaku Co, Ltd | 0 (0) | 0 |
| Toa Eiyo Ltd | 0 (0) | 0 |
| UCB Japan Co, Ltd | 0 (0) | 0 |
| Wakamoto Pharmaceutical Co, Ltd | 0 (0) | 0 |
We converted Japanese yen to pound sterling (£), using the average monthly exchange rate for 2016, namely 147.7 yen per £1.
Table 3 ranks the oncology specialists according to the monetary value of the payments they received. In the HPG (n=142), women accounted for only 6.3% (9) of the total, while in the LPG (n=621), women accounted for 10.6% (66) of the total. However, women accounted for 30.9% (98) in the NPG (n=317). With respect to male recipients, 75.9% (688/907) received at least one payment, compared with only 43.4% of women (75/173). Of the oncologists in the HPG, 52.8% (75) worked for university hospitals and 28.2% (40) worked for cancer hospitals: these figures were higher than those seen in the other two groups. Furthermore, while only 19.7% (28) of the specialists in the HPG were certified during the previous 5 years (2012–2016), 49.4% (307) and 60.3% (191) of individuals in the LPG and NPG, respectively, were certified during these 5 years. The proportion of specialist respirology oncologists was larger in the HPG (55, 38.7%) than in either the LPG (165, 26.6%) or NPG (65, 20.5%).
Table 3.
Characteristics of oncology specialists and pharmaceutical company payments received in 2016, according to the monetary value of the payments
| Variable | High-payment group (1 million yen (£6800) or more) (n=142) | Low-payment group (1 yen–1 million yen (£6800)) (n=621) | No-payment group (0 yen (n=317) |
| Characteristics of oncology specialists | |||
| Sex (N, %) | |||
| Men | 133 (93.7) | 555 (89.4) | 219 (69.1) |
| Women | 9 (6.3) | 66 (10.6) | 98 (30.9) |
| Working institutions (N, %) | |||
| University hospitals | 75 (52.8) | 248 (39.9) | 119 (37.5) |
| Cancer hospitals | 40 (28.2) | 98 (15.8) | 45 (14.2) |
| Other types of institutions | 27 (19.0) | 275 (44.3) | 153 (48.3) |
| Working regions (N, %) | |||
| Hokkaido | 4 (2.8) | 37 (6.0) | 11 (3.5) |
| Tohoku | 11 (7.8) | 30 (4.8) | 17 (5.4) |
| Kanto | 45 (31.7) | 162 (26.1) | 95 (30.0) |
| Chubu | 23 (16.2) | 113 (18.2) | 58 (18.3) |
| Kinki | 29 (20.4) | 108 (17.4) | 71 (22.4) |
| Chugoku | 9 (6.3) | 60 (9.7) | 19 (6.0) |
| Shikoku | 5 (3.5) | 31 (5.0) | 7 (2.2) |
| Kyushu | 16 (11.3) | 80 (12.9) | 39 (12.3) |
| Year of certification (N, %) | |||
| 2006 | 22 (15.5) | 21 (3.4) | 2 (0.6) |
| 2007 | 15 (10.6) | 46 (7.4) | 16 (5.1) |
| 2008 | 19 (13.4) | 38 (6.1) | 14 (4.4) |
| 2009 | 19 (13.4) | 56 (9.0) | 23 (7.3) |
| 2010 | 23 (16.2) | 80 (12.9) | 30 (9.5) |
| 2011 | 16 (11.3) | 73 (11.8) | 41 (12.9) |
| 2012 | 9 (6.3) | 72 (11.6) | 43 (13.6) |
| 2013 | 9 (6.3) | 79 (12.7) | 55 (17.4) |
| 2014 | 5 (3.5) | 65 (10.5) | 28 (8.8) |
| 2015 | 2 (1.4) | 51 (8.2) | 32 (10.1) |
| 2016 | 3 (2.1) | 40 (6.4) | 33 (10.4) |
| Specialty (N, %) | |||
| Respirology | 55 (38.7) | 165 (26.6) | 65 (20.5) |
| Gastroenterology | 33 (23.2) | 178 (28.7) | 67 (21.1) |
| Haematology | 28 (19.7) | 139 (22.4) | 83 (26.2) |
| Breast | 16 (11.3) | 34 (5.5) | 22 (6.9) |
| Head and neck | 3 (2.1) | 4 (0.6) | 5 (1.6) |
| Gynaecology | 0 (0.0) | 5 (0.8) | 5 (1.6) |
| Urology | 1 (0.7) | 6 (1.0) | 2 (0.6) |
| Dermatology | 0 (0.0) | 1 (0.2) | 1 (0.3) |
| Other or undetermined | 6 (4.2) | 89 (14.3) | 67 (21.1) |
| Characteristics of pharmaceutical payments | |||
| Total payments | |||
| Total value of payments (¥ (£)) | 418 345 258 (2 832 398) | 167 108 056 (1 131 402) | |
| Total count of payment | 4466 | 2859 | |
| Type of payments (¥ (£), %) | |||
| Speaking | 327 075 925 (2 214 461), 78.2 | 140 726 765 (952 788), 84.2 | |
| Consulting | 73 870 218 (500 137), 17.7 | 20 812 589 (140 911), 12.5 | |
| Writing | 17 053 868 (115 463), 4.1 | 5 212 318 (35 290), 3.1 | |
| Missing | 345 247 (2337), 0.1 | 356 384 (2413), 0.2 | |
| Payments per individual specialist | |||
| Median monetary value (¥ (£), IQR) | 2 269 622 (15 366) (1 439 448 (9746)–3 681 775 (24 927)) | 171 086 (1158) (89 096 (603)–380 886 (2579)) | |
| Median count (IQR) | 24 (19–38) | 3 (2–6) | |
| Monetary value of payments according to specialties (¥ (£), %) | |||
| Respirology | 169 761 707 (1 149 368), 40.6 | 47 044 815 (318 516), 28.2 | |
| Gastroenterology | 92 334 612 (625 150), 22.1 | 47 335 590 (320 485), 28.3 | |
| Haematology | 81 963 421 (554 932), 19.6 | 37 256 292 (252 243), 22.3 | |
| Breast | 42 090 455 (284 973), 10.1 | 7 197 206 (48 729), 4.3 | |
| Head and neck | 8 689 962 (58 835), 2.1 | 523 439 (3544), 0.3 | |
| Gynaecology | 0 (0), 0 | 570 533 (3 863), 0.3 | |
| Urology | 5 527 458 (37 424), 1.3 | 2 334 827 (15 808), 1.4 | |
| Dermatology | 0 (0), 0 | 562 502 (3 808), 0.3 | |
| Other or undetermined | 17 977 643 (121 717), 4.3 | 24 262 852 (164 and 271), 14.5 |
We converted Japanese yen to pound sterling (£), using the average monthly exchange rate for 2016, namely 147.7 yen per £1.
In the HPG, the total monetary value paid and number of payments were 418 345 258 yen (£2 832 398, €3 360 203 and $3 845 085) and 4466, respectively, accounting for 71.5% and 61.0% of the totals.
Table 4 displays findings of the multivariate regression analyses for the monetary value of payments. Female oncologists tended to receive a smaller value of payments than their male counterparts (relative monetary value (RMV) 0.40, 95% CI 0.20 to 0.79). Oncologists working for university hospitals (RMV 5.78, 95% CI 3.34 to 10.02) and those working for cancer hospitals (RMV 5.47, 95% CI 3.30 to 9.06) also tended to receive higher payments. Oncologists with longer experience after board certification were significantly more likely to receive larger payments compared with those with shorter experience (RMV 1.40, 95% CI 1.30 to 1.50). Those working in haematology were likely to receive smaller payments than those working in respirology (RMV 0.49, 95% CI 0.30 to 0.83). In the LPG and NPG, there were no significant differences in the monetary value of the payments with respect to the type of affiliation of recipients.
Table 4.
Multivariate negative binomial model for the monetary value of payments on an individual basis
| Variable | All (n=1080) Relative monetary value per year (95% CI) |
Low-payment and no-payment groups (0–1 million yen (£6800)) (n=938) Relative monetary value per year (95% CI) |
| Sex | ||
| Men | Ref. | Ref. |
| Women | 0.40 (0.20 to 0.79)** | 0.40 (0.28 to 0.58)*** |
| Types of affiliations | ||
| Other type of institutions | Ref. | Ref. |
| University hospitals | 5.78 (3.34 to 10.02)*** | 1.08 (0.80 to 1.47) |
| Cancer hospitals | 5.47 (3.30 to 9.06)*** | 1.11 (0.90 to 1.37) |
| Working region | ||
| Kanto | Ref. | Ref. |
| Hokkaido | 0.45 (0.16 to 1.26) | 0.82 (0.54 to 1.23) |
| Tohoku | 1.41 (0.62 to 3.20) | 1.07 (0.69 to 1.67) |
| Chubu | 0.86 (0.41 to 1.81) | 0.96 (0.72 to 1.29) |
| Kinki | 1.14 (0.66 to 1.96) | 0.74 (0.54 to 1.03) |
| Chugoku | 1.47 (0.50 to 4.32) | 0.80 (0.53 to 1.22) |
| Shikoku | 0.69 (0.32 to 1.53) | 1.22 (0.78 to 1.89) |
| Kyushu | 1.18 (0.55 to 2.51) | 1.04 (0.74 to 1.45) |
| Year of experience after the board certification | 1.40 (1.30 to 1.50)*** | 1.13 (1.09 to 1.17)*** |
| Subspecialty | ||
| Respirology | Ref. | Ref. |
| Gastroenterology | 0.57 (0.32 to 1.01) | 0.94 (0.72 to 1.22) |
| Haematology | 0.49 (0.30 to 0.83)** | 0.76 (0.57 to 1.00)* |
| Breast | 1.50 (0.68 to 3.33) | 0.73 (0.45 to 1.19) |
| Other or undetermined* | 0.28 (0.12 to 0.64)** | 0.69 (0.51 to 0.93)* |
*Other or undetermined specialties included head and neck cancer, gynaecology, urology and dermatology. Due to the small number of physicians in these four specialties, they were included in the ‘other or undetermined’ category; *<0.05, **<0.01, ***<0.001.
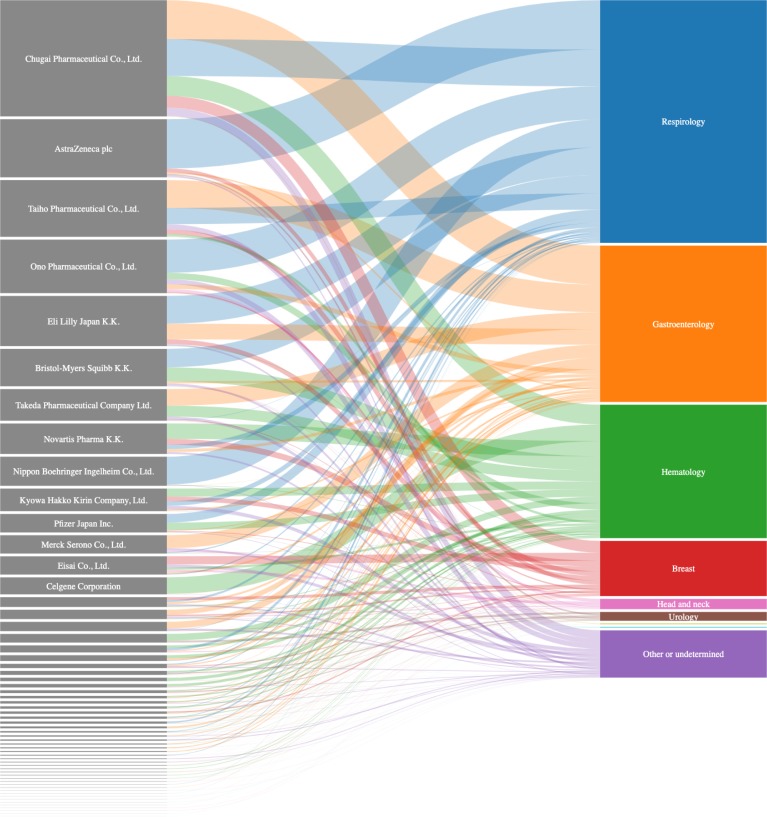
Figure 1 displays payment distributions to each cancer specialty on an individual company basis. Details of the payments are provided in online supplementary material 2. Furthermore, in online supplementary material 3, we summarise the list of high revenue-generating oncology drugs. In the Chugai Pharmaceutical Co, Ltd, which made the largest specialty payment, the top four specialties were gastroenterology (34 760 717 yen (£235 347, €279 203 and $319 492), 33.5%), respirology (32 937 605 yen (£223 003, €264 559 and $302 735), 31.7%), haematology (17 702 450 yen (£119 854, €142 188 and $162 706), 17.0%) and breast cancer (10 548 519 yen (£71 419, €84 727, $96 953), 12.0%). The Chugai company manufactured eight high revenue-generating oncology drugs (online supplementary material 3), and four, three, one and five drugs were respectively covered under the National Health Insurance scheme for the field of gastroenterology, respirology, haematology and breast cancer. Nivolumab (Opdivo), manufactured by the Ono Pharmaceutical Co, Ltd, mainly used in lung cancer and melanoma, had the largest domestic sales in 2016 (103.9 billion yen (£703.5 million, €834.5 million and $955.0 million)). The total monetary value of the company’s payments was 47 831 737 yen (£323 844, €384 191 and $439 630), (representing fourth place in the payment table), of which 29 657 836 yen ((£200 798, €238 216 and $272 590), 62.0%) was specifically distributed to respirology specialists. All of the top eight companies with regard to the monetary value of the payments (online supplementary material 2) had at least one drug which was newly approved or that had an added anticancer indication under the National Health Insurance scheme in the fiscal years of 2015 and 2016 (online supplementary material 4). While AstraZeneca Plc had no high revenue-generating oncology drugs (online supplementary material 3), vandetanib (Caprelsa) and osimertinib (Tagrisso) were newly approved for thyroid cancer in September 2015 and non-small cell lung cancer in March 2016, respectively (online supplementary material 4). The total monetary value of the company’s payments was second, accounting for 51 928 785 yen (£351 583, €417 099 and $477 287). Of the total, 84.8% (44 013 864 yen (£297 995, €353 525 and $404 539)) was specifically allocated to oncologists with a specialism in respirology.
Figure 1.
Distribution of payments to each subspecialty on an individual company basis. The companies and specialties are sorted in descending order with regard to payment value (proportionally expressed in the box height and band width in figure 1). Band colour represents the payment destination specialties. Due to space limitations, names of companies with payment values of less than 10 million yen (£67 700, €80 300 and $91 900) have been omitted.
The JSMO has established a guideline on COI disclosure for its members that requires them to disclose any COI associated with publications and other research presentations. Furthermore, executive board members, auditors and other high-level members, as well as presidents and vice-presidents of conferences and committee members operating under the JSMO are required to disclose any COI associated with their work and positions. These include, with respect to any for-profit organisation, reporting any: (1) position as an officer or advisor, (2) stock ownership, (3) patent royalties or licensing fees, (4) honoraria (eg, lecture fees), (5) fees paid for any writing or publication work, (6) receipt of research funding, (7) advisory fees or financial remuneration in exchange for testimony, (8) acceptance of researchers from any for-profit enterprise, (9) endowed chairs offered and (10) any remuneration (travel, gifts, or other in-kind payments not directly related to research). However, there are no rules specifically referring to oncology specialists.
Discussion
In 2016, approximately 600 million yen (£4.1 million, €4.8 million and $5.5 million) was paid by Japanese pharmaceutical companies to 763 (70.6%) certified oncology specialists. Payments appeared to be concentrated on specific targets, notably experienced male oncologists working for university hospitals and cancer hospitals.
The proportion of oncologists receiving payments was larger compared with general physicians in the USA (39.9%)30 and Japan (33.3%).31 However, the proportion was slightly smaller than that of National Comprehensive Cancer Network oncology CPG authors in the USA (86.4%).19 Although the mean value of payments in our study was approximately half of that of the CPG authors ($4982 (£3670, €4354 and 542 086 yen) (data not shown) vs $10 011 (£7374, €8479 and 1 089 197 yen)), a simple comparison is not valid, as our analysis only covered data for speaking, writing and consultancy work. It did not include payments related to meals, transportation and accommodation, stock ownership, investment interest or payments from medical device companies, as is complied in the US’s Open Payments Data.30 The CPG authors strongly influence oncology practice, both in the USA and internationally32 by recommending treatment algorithms. They may well be identified as prime targets for representatives of pharma attempting to promote the sale of their anticancer products. It is thus reasonable to assume that Japanese pharma with similar anticancer interests may well be trying to target oncology specialists in an attempt to help boost the sales and use of their specific products.
We observed a large disparity in payments to specialists. Those receiving 1 million yen or more accounted for 13.1% of all oncologists studied but received 71.5% of the total paid. Oncologists working for university hospitals and those working for cancer hospitals similarly received large value payments. In Japan, cancer centres are generally more likely to treat more patients with cancer compared with university hospitals. Indeed, cancer centres top the nationwide ratings for treatments of most of the common cancers, including lung, colon, gastric and breast cancer.33 In contrast, university hospitals are regarded as symbols of academic excellence and authority, and medical school professors traditionally have a strong influence on both physicians and medical practice in their field of expertise. They are more influential in setting treatment protocols that are usually followed without question by less senior medical staff nationwide. Thus, our findings suggest that Japanese pharmaceutical companies have placed emphasis on expertise and authority, as well as clinical experience, in the selection of targets for their promotional activities.
A particularly significant finding was that a smaller proportion of female oncologists received payments from pharma compared with their male colleagues. Furthermore, women also tended to receive smaller payment amounts than men. These findings are in line with similar studies performed in the USA.34 35 In the relatively unique, patriarchal Japanese society, there may be very specific reasons for these results. First, there are far fewer female oncologists than males, and they have considerably less spare time for industry-related work due to women needing to fulfil their socially perceived duty to be the main person responsible for raising any children in the family.36 Furthermore, pharma may tend to target men rather than women34 because in Japan’s male-dominated society, the status of women has traditionally been low, and their contribution, presence and influence in biomedicine and the higher echelons of power and influence has not been actively encouraged.37 38
We found that respirology attracted the greatest financial outlay. In Japan, lung cancer is of primary concern at present, covering a large patient volume and consequently attracting multiple novel oncology drugs, such as alectinib (Alecensa) (Chugai Pharmaceutical Co, Ltd (approved 2014)), afatinib (Gilotrif) (Nippon Boehringer lngelheim Co, Ltd (approved 2014)), nivolumab (Opdivo) (Ono Pharmaceutical Co, Ltd (approved 2015)), ceritinib (Zykadia) (Novartis Pharma K.K. (approved 2016)), osimertinib (Tagrisso) (AstraZeneca plc (approved 2016)), pembrolizumab (Keytruda) (MSD K.K. (approved 2016)), ramucirumab (Cyramza) (Eli Lilly Japan K.K. (approved 2016)), all for non-small cell lung cancer (online supplementary materials 3 and 4). As such, for the pharmaceutical companies, this field is a critical yet highly competitive target in any strategy to maximise the cost-effectiveness of their promotional endeavours.
The examples of Chugai Pharmaceutical and Ono Pharmaceutical chiefly support the belief that there is an association between the value and destination of payments dependent on the products the companies in question manufacture. In contrast, the example of AstraZeneca adds credence to the notion that that funds were mainly allocated to promote their novel product: osimertinib (Tagrisso) was approved for non-small cell lung cancer in March 2016. Indeed, 84.8% of the company’s total payment was allocated to respirology specialists.
As we have demonstrated, there are extensive financial relationships between pharmaceutical companies and oncologists in Japan. It is true that the receipt of payments by physicians in Japan is not illegal, especially as they are supposedly given as remuneration for work undertaken or services rendered. However, we believe that there is an ethical problem inherent in such relationships, given that this practice can be seen by the public and neutral observers as being instigated and developed to possibly end up expanding the profit of pharmaceutical companies, rather than promoting the health and well-being of patients. Indeed, even a subtle but reputable financial relationship with the industry, such as collaborating in a field trial, could bias a physician’s prescription patterns in a manner that benefits the companies.13–18 Oncologists handle extraordinary and very potent life-saving drugs and have a degree of autonomy in their prescribing actions. Their decisions substantially influence the treatment and outcome for their patients, as well as having significant economic impact due to the high cost of anticancer medications.5–7 It would therefore appear sensible to have regulations in place that necessitate the open and accessible reporting of any financial dealings between physicians and pharma, so as to avoid any potential nefarious or underhand behaviour or undue pressure on physicians to alter their usual treatment practices. Indeed, it is possible that these arrangements may have contributed to the multiple cases of scientific misconduct that have recently been reported in Japan. The most infamous case was when employee misconduct was discovered in a series of clinical trials for Valsartan, an antihypertensive medication manufactured by Novartis Pharma K.K., leading to a retraction of the associated academic papers.39 40 Also, the company illegally obtained the information about patients participating in another clinical trial for chronic myelogenous leukaemia using nilotinib (Tasigna) from a university hospital in Japan.41 42 A breast cancer clinical trial (CREATE-X trial) with a questionable pharmaceutical payment has also been identified.43 44
Since January 2019, the new regulations in Japan have already been weakened by allowing pharma to aggregate payment data they should publish into a single amount, making matters much less transparent.45 To prevent similar cases in future, we call for the implementation of a transparent, independent mechanism that would enable a comprehensive assessment of any and all payments being made by any pharmaceutical company to any individual physician or, for that matter, medical institution where the company’s products may be used and not just with respect to oncology. Ideally, these actions should be mandatory and legally binding on the side of both the company and physician. New schemes along these lines, such as the US’s Open Payments Data, may prove successful, but it is too soon to know.46 The Disclosure UK mechanism may not prove to be so effective as it is voluntary.47 Additionally, given that such mechanisms allow for direct comparison between what is allegedly paid and what is allegedly received, any new system will probably necessitate a fair, equitable and timely mechanism for dispute settlement, probably involving the use of third parties.44
Study limitations
Several limitations in this preliminary study should be acknowledged. First, there could be measurement errors in the affiliations and subspecialties of the included specialists, as we collated these data in the study year (2016), using the websites and other data sources on the internet. Second, there might be minor measurement errors in the payment database as well. Most of the pharmaceutical companies involved did not disclose their payment data in a uniform or readily available format. As a result, we manually entered all the payment data from a variety of formats, and despite repeated and careful review, the database may include minor errors. Third, the present research analysed only limited payment types, namely speaking, writing and consultancy work. Currently, Japanese pharmaceutical companies do not disclose any payment data for stock holdings, royalties, individual data for costs of meals, transportation and accommodation and so on. As, unlike the pharmaceutical companies, the JMSO and other similar academic and learnt societies in Japan, where such data may be registered, refuse to open their databases on payments to public scrutiny, we were not able to consider these data in this study. Fourth, most of the pharmaceutical companies only publish single-year data so we could only consider payments made in fiscal 2016. To understand temporal trends and the extent and distribution of pharmaceutical company payments, a continuous assessment of the payment data is warranted in future.
Concluding remarks
Japanese certified oncologists receive financial payments directly from pharmaceutical companies, usually from companies active in the specialist field of the physician in question. In today’s prevailing climate of fake news, inaccurate scientific data, vaccine hesitancy and suspicion about many financial dealings involving pharma, this raises several queries with regard to ethical, medical and legal issues. The value and specialty targets of the payments varied substantially, which also raises yet more questions as to why. We believe that the lessons learnt from our analyses should be shared among the global medical community to help put in place safeguards to prevent any form of inducements from the pharmaceutical industry and to help protect physicians from outside influences. It is essential to establish a robust, comprehensive and legally binding system for identifying and avoiding any and all potential COIs, of any nature, involving physicians or other medical professionals, both in Japan and internationally. While it is too early to evaluate whether similar systems, such as the US-based Open Payments Data, will be truly effective, financial transparency is a fundamental component in illustrating that there is an open, honest and ethically correct relationship between pharmaceutical companies and physicians. A more comprehensive study should be planned, to include all Japanese oncologists, to try and confirm our findings and to help identify the best way forward to ensure that COIs are minimised and so that physicians and pharmaceutical companies can work harmoniously and synergistically to provide Japan with the best cancer prophylaxis, treatment and cures possible.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Masahiro Kami and Professor Andy Crump for their constructive opinions and insights and the Waseda Chronicle for extensive support.
Footnotes
Contributors: AO acquired and controlled all sources of data and oversaw all data analyses. AO, HS, YO, TS, YukS, YurS, AT and TT were involved in the study concept and design. AO, HS, YO, TS, YukS, YurS, AT and TT were involved in the analysis, interpretation of results and formation of conclusions. AO drafted the manuscript.
Funding: The Medical Governance Research Institute (MEGRI) is a non-profit enterprise, which receives donations from various organisations and individuals. Among the donors, Ain Pharmaciez Inc is part of the pharmaceutical industry. Waseda Chronicle, an independent non-profit news organisation dedicated to investigative journalism, also contributed funding to this study.
Disclaimer: The funders made no contribution whatsoever to either the design of the study, the work carried out or the interpretation of the study findings.
Competing interests: AO and TT receive personal fees from Medical Network Systems (MNES Inc); HS has received a research honorarium from TAIHO Pharmaceutical Co, Ltd.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Institutional Review Board of the MEGRI on 16 May 2018.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
- 1. Agrawal S, Brown D. The physician payments sunshine act--two years of the open payments program. N Engl J Med 2016;374:906–9. 10.1056/NEJMp1509103 [DOI] [PubMed] [Google Scholar]
- 2. Agrawal S, Brennan N, Budetti P. The sunshine act--effects on physicians. N Engl J Med 2013;368:2054–7. 10.1056/NEJMp1303523 [DOI] [PubMed] [Google Scholar]
- 3. Cancer Information Service NCC, Japan Cancer registry and statistics, 2018. Available: https://ganjoho.jp/reg_stat/statistics/stat/index.html
- 4. Jemal A, Vinela P, Bray F, et al. . The cancer atlas. 2nd Edition GA: American Cancer Society, 2014. [Google Scholar]
- 5. Experts in Chronic Myeloid Leukemia The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood 2013;121:4439–42. 10.1182/blood-2013-03-490003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Light DW, Kantarjian H. Market spiral pricing of cancer drugs. Cancer 2013;119:3900–2. 10.1002/cncr.28321 [DOI] [PubMed] [Google Scholar]
- 7. Kantarjian H, Rajkumar SV. Why are cancer drugs so expensive in the United States, and what are the solutions? Mayo Clin Proc 2015;90:500–4. 10.1016/j.mayocp.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 8. Odell TW, Gregory MC. Cost of hypertension treatment. J Gen Intern Med 1995;10:686–8. 10.1007/BF02602764 [DOI] [PubMed] [Google Scholar]
- 9. Tibi-Levy Y, de Pouvourville G, Westerloppe J, et al. . The cost of treating high blood pressure in general practice in France. Eur J Health Econ 2008;9:229–36. 10.1007/s10198-007-0065-2 [DOI] [PubMed] [Google Scholar]
- 10. Zhuo X, Zhang P, Kahn HS, et al. . Change in medical spending attributable to diabetes: national data from 1987 to 2011. Diabetes Care 2015;38:dc141687–7. 10.2337/dc14-1687 [DOI] [PubMed] [Google Scholar]
- 11. Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. . Global, regional, and National cancer incidence, mortality, years of life lost, years lived with disability, and Disability-Adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol 2018;4:1553–68. 10.1001/jamaoncol.2018.2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zafar SY, Peppercorn JM, Schrag D, et al. . The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient's experience. Oncologist 2013;18:381–90. 10.1634/theoncologist.2012-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeJong C, Aguilar T, Tseng C-W, et al. . Pharmaceutical industry-sponsored meals and physician prescribing patterns for Medicare beneficiaries. JAMA Intern Med 2016;176:1114–22. 10.1001/jamainternmed.2016.2765 [DOI] [PubMed] [Google Scholar]
- 14. De Ferrari A, Gentille C, Davalos L, et al. . Attitudes and relationship between physicians and the pharmaceutical industry in a public General Hospital in Lima, Peru. PLoS One 2014;9:e100114 10.1371/journal.pone.0100114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians' attitudes and prescribing habits: a systematic review. BMJ Open 2017;7:e016408 10.1136/bmjopen-2017-016408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riese F, Guloksuz S, Roventa C, et al. . Pharmaceutical industry interactions of psychiatric trainees from 20 European countries. Eur Psychiatry 2015;30:284–90. 10.1016/j.eurpsy.2014.09.417 [DOI] [PubMed] [Google Scholar]
- 17. Lee D, Begley CE. Physician report of industry gifts and quality of care. Health Care Manage Rev 2016;41:275–83. 10.1097/HMR.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 18. Montastruc F, Moulis G, Palmaro A, et al. . Interactions between medical residents and drug companies: a national survey after the Mediator® affair. PLoS One 2014;9:e104828 10.1371/journal.pone.0104828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell AP, Basch EM, Dusetzina SB. Financial relationships with industry among national comprehensive cancer network guideline authors. JAMA Oncol 2016;2:1628–31. 10.1001/jamaoncol.2016.2710 [DOI] [PubMed] [Google Scholar]
- 20. Saito H, Ozaki A, Sawano T, et al. . Evaluation of pharmaceutical company payments and conflict of interest disclosures among oncology clinical practice guideline authors in Japan. JAMA Netw Open 2019;2:e192834 10.1001/jamanetworkopen.2019.2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. IQVIA Top 10 pharmaceutical markets worldwide, 2017, 2017. Available: https://www.iqvia.com/-/media/iqvia/pdfs/canada-location-site/top10worldwidesales_en_17.pdf?la=en&hash=C1D59CB0CB2B060CF458F14CF5390D96F3B28238 [Accessed 12 Jul 2019].
- 22. QuintilesIMS Top 10 pharmaceutical markets worldwide, 2016, 2016. Available: https://www.iqvia.com/-/media/iqvia/pdfs/canada-location-site/top-10-worldwide-sales-en-2016.pdf [Accessed 12 Jul 2019].
- 23. Mix Online Analysis of markets for oncology drugs in Japan. Available: https://www.mixonline.jp/Article/tabid/55/artid/59063/Default.aspx [Accessed 16 Oct 2018].
- 24. Japan Pharmaceutical Manufactures Association Data book 2018 [in Japanese], 2018. Available: http://www.jpma.or.jp/about/issue/gratis/databook/2018/ [Accessed 12 Jul 2019].
- 25. Japan Pharmaceutical Manufactures Association Regarding the transparency guideline for the relation between corporate activities and medical institutions, 2018. Available: http://www.jpma.or.jp/english/policies_guidelines/pdf/transparency_gl_intro_2018.pdf [Accessed 12 Jul 2019].
- 26. Saito H, Ozaki A, Kobayashi Y, et al. . Pharmaceutical company payments to executive board members of professional medical associations in Japan. JAMA Intern Med 2019;179:578 10.1001/jamainternmed.2018.7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ministry of Health, Labour and Welfare Physician Search Site [in Japanese], 2019. Available: https://licenseif.mhlw.go.jp/search_isei/jsp/top.jsp [Accessed 12 Jul 2019].
- 28. National Tax Agency of Japan Survey results of wages in 2013 (Japanese only). Available: https://www.nta.go.jp/information/release/kokuzeicho/2013/minkan/index.htm [Accessed 8 Jul 2018].
- 29. Riehmann P, Hanfler M, Froehlich B. Interactive Sankey diagrams. information visualization. 2005 IEEE symposium, INFOVIS, 2005:233–40. [Google Scholar]
- 30. Marshall DC, Jackson ME, Hattangadi-Gluth JA. Disclosure of industry payments to physicians: an epidemiologic analysis of early data from the open payments program. Mayo Clin Proc 2016;91:84–96. 10.1016/j.mayocp.2015.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Waseda Chronicle Pharmaceutical payment and physicians, 2018. Available: http://www.wasedachronicle.org/articles/docyens/e2/ [Accessed 16 Oct 2018].
- 32. Ismaila N, Salako O, Mutiu J, et al. . Oncology guidelines usage in a low- and middle-income country. J Glob Oncol 2018;4:1–6. 10.1200/JGO.17.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sony Life Insurance Co. L Operation counts of cancer in Japanese hospitals [in Japanese], 2018. Available: https://cs.sonylife.co.jp/lpv/pcms/sca/ct/medical/ranking-cancer/02.html?lpk= [Accessed 16 Oct 2018].
- 34. Tringale KR, Hattangadi-Gluth JA. Types and distributions of biomedical industry payments to men and women physicians by specialty, 2015. JAMA Intern Med 2018;178:421–3. 10.1001/jamainternmed.2017.7445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tringale KR, Marshall D, Mackey TK, et al. . Types and distribution of payments from industry to physicians in 2015. JAMA 2017;317:1774–84. 10.1001/jama.2017.3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ly DP, Seabury SA, Jena AB. Hours worked among US dual physician couples with children, 2000 to 2015. JAMA Intern Med 2017;177:1524–5. 10.1001/jamainternmed.2017.3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shibayama S, Geuna A. Gender gap in science in Japan, 2016. Available: http://www.pp.u-tokyo.ac.jp/wp-content/uploads/2016/02/GraSPP-DP-E-16-001.pdf [Accessed 8 Oct 2018].
- 38. Oshima K, Ozaki A, Mori J, et al. . Entrance examination misogyny in Japanese medical schools. Lancet 2019;393:1416 10.1016/S0140-6736(18)33180-5 [DOI] [PubMed] [Google Scholar]
- 39. Lancet Editors Retraction--Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet 2013;382:843 10.1016/S0140-6736(13)61847-4 [DOI] [PubMed] [Google Scholar]
- 40. Narumi H, Takano H, Shindo S, et al. . Retraction: effects of valsartan and amlodipine on cardiorenal protection in Japanese hypertensive patients: the valsartan amlodipine randomized trial. Hypertens Res 2017;40 10.1038/hr.2016.144 [DOI] [PubMed] [Google Scholar]
- 41. Tanimoto T, Kami M, Shibuya K. Misconduct: Japan to learn from biomedical cases. Nature 2014;512:371 10.1038/512371d [DOI] [PubMed] [Google Scholar]
- 42. McCurry J. Former Novartis employee arrested over valsartan data. Lancet 2014;383:2111 10.1016/S0140-6736(14)61015-1 [DOI] [PubMed] [Google Scholar]
- 43. Ozaki A. Conflict of interest and the CREATE-X trial in the New England Journal of medicine. Sci Eng Ethics 2018;24:1809–11. 10.1007/s11948-017-9966-3 [DOI] [PubMed] [Google Scholar]
- 44. Ozaki A, Takita M, Tanimoto T. A call for improved transparency in financial aspects of clinical trials: a case study of the CREATE-X trial in the New England Journal of medicine. Invest New Drugs 2018;36:517–22. 10.1007/s10637-018-0577-x [DOI] [PubMed] [Google Scholar]
- 45. Kobashi Y, Watanabe M, Kimura H, et al. . Are pharmaceutical company payments incentivising malpractice in Japanese physicians? Int J Health Policy Manag 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. OpenPaymentData.CMS.gov Centers for Medicare & Medicaid, 2014. Available: https://openpaymentsdata.cms.gov/ [Accessed 17 Sep 2018].
- 47. Mulinari S, Ozieranski P. Disclosure of payments by pharmaceutical companies to healthcare professionals in the UK: analysis of the association of the British pharmaceutical industry’s disclosure UK database, 2015 and 2016 cohorts. BMJ Open 2018;8:e023094 10.1136/bmjopen-2018-023094 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-028805supp001.pdf (207.6KB, pdf)