Abstract
Objective
To analyse whether gender-specific health behaviour can be an explanation for why women outlive men, while having worse morbidity outcomes, known as the morbidity-mortality or gender paradox.
Setting
The working population in Sweden.
Participants
Thirty per cent random sample of Swedish women and men aged 40–59 with a hospital admission in the 1993–2004 period were included. The sample for analysis consists of 233 274 individuals (115 430 men and 117 844 women) and in total 1 867 013 observations on sickness absence.
Intervention
Hospital admission across 18 disease categories.
Main outcome measures
The main outcome measures were sickness absence (morbidity) and mortality. Longitudinal data at the individual level allow us to study how sickness absence changed after a hospital admission in men and women using a difference-in-differences regression analysis. Cox regression models are used to study differences in mortality after the admission.
Results
Women increased their sickness absence after a hospital admission by around five more days per year than men (95% CI 5.25 to 6.22). At the same time, men had higher mortality in the 18 diagnosis categories analysed. The pattern of more sickness absence in women was the same across 17 different diagnosis categories. For neoplasm, with a 57% higher risk of death for men (54.18%–59.89%), the results depended on the imputation method of sickness for those deceased. By using the premortality means of sickness absence, men had an additional 14.47 (-16.30– -12.64) days of absence, but with zero imputation women had an additional 1.6 days of absence (0.05–3.20). Analyses with or without covariates revealed a coherent picture.
Conclusions
The pattern of increased sickness absence (morbidity) and lower mortality in women provides evidence on the more proactive and preventive behaviour of women than of men, which could thus explain the morbidity-mortality paradox.
Keywords: population register data; sick leave; mortality; health, sex differences; difference-in-difference design
Strengths and limitations of this study.
The empirical analysis is based on a difference-in-differences design commonly used in social science and increasingly applied in medical science.
The longitudinal characteristics of our data allow us to condition on group differences in health, working conditions and other time-invariant factors (eg, differences in household duties), which might confound the relation between absenteeism and gender-specific health behaviour.
The conclusion of a larger increase in sickness absence in women than in men after a hospital admission does not depend on covariate adjustment.
Introduction
In many countries, women are relatively more absent for health reasons than men.1 Furthermore, similar sex differences exist in other common measures of morbidity, such as medical care utilisation and self-reported health.2 Yet, while most commonly used observed health measures show an over-representation of women, there is one major exception to this rule—the remaining life expectancy. One much-quoted fact of sex differences is that women outlive men. In fact, the remaining life expectancy is higher in women than in men in all ages and in nearly all parts of the world. The global average sex difference in life expectancy was about 4 years in 2010 and has been persistently so for a long time.3 This has led some scholars to label this relationship as the morbidity-mortality or gender paradox.4
One suggested explanation for this apparently inconsistent pattern has been the existence of sex differences in health behaviour. Differences in behaviour could be with regard to smoking, drinking, diet and so on, but can also be manifested in common measures of morbidity. Women may, for example, proactively make more use of healthcare and may take more sickness absence from work to keep themselves healthier, which would then prolong their lives relative to men (cf refs 4–7). This particular explanation for the so-called morbidity-mortality paradox has been discussed in the 17th century; English demographer John Graunt8 observed that both birth and death rates of men were higher than women, while at the same time ‘[Physicians] have two women patients to one man’.
This conjecture of behavioural differences has support in experimental studies in social science (cf ref 9). In particular, it has often been noted that women, in general, act more proactively in matters regarding their own and other family members’ health and that they tend to be more risk-averse than men. The implication is that if women pay more attention to potential future illnesses, by more frequent use of medical services or health insurance, poor health can be detected at an earlier stage, remediated, and consequently increase their relative life expectancy in relation to men. The large cross-country variation in life expectancy (see, eg, ref 10) also suggests that the general picture of women outliving men to some extent stems from gender-specific health behaviour based on differences in cultural norms.
This article empirically tests for sex differences in behaviour as a factor for understanding the morbidity-mortality paradox by using the evolution of morbidity (sickness absence) and mortality of men and women after a hospital admission. If women act more proactively than men do, we should find that women take more sickness absence after a comparable health change compared with men, while at the same time women do not experience higher mortality rates. Thus, if we find such a pattern in our data, this supports the conjecture that the morbidity-mortality conundrum is driven by a more proactive health behaviour among women. On the other hand, if we find an increase in sickness absence and that women’s mortality rate is higher after hospital admission, we would conclude that it is likely that actual health differentials between men and women are causing the increase in sickness absence.
Since measures of morbidity are almost exclusively discussed from an adverse standpoint, it is an important question for health policy whether and to which extent sex differences in outcomes reflect differences in behaviour rather than differences in health. Therefore, our aim was to study the morbidity-mortality paradox and analyse whether gender-specific health behaviour can be an explanation for why women outlive men, while having worse morbidity outcomes.
Methods
Study design and participants
Our empirical analysis exploited microdata originating from administrative population registers on sickness absence, hospitalisations, mortality and socioeconomic variables. Data on socioeconomic variables covering the entire Swedish population in the 16–65 age interval for the years 1993–2004 were obtained from Statistics Sweden. These data were linked to data on sickness absence and inpatient care over the same time period using registers at the Swedish Social Insurance Agency and the Swedish National Board of Health and Welfare, respectively. Data on sickness absence cover all individual spells of paid sick leave from the statutory sickness insurance in Sweden. The National Patient Register covers all inpatient medical contacts in public hospitals. The diagnoses are made at discharge by the responsible senior consultant and classified according to the WHO’s International Statistical Classification of Diseases and Related Health Problems (ICD-10).
Analyses were performed using a 30% random sample of the population of employed individuals 40–59 years of age in 1993 who were hospitalised at some point between the years 1994 and 2004. The sample consists of 233 274 individuals in total, of whom 49.5% are men. The fraction of individual in the age strata 40–44, 45–49, 50–54 and 55–59 is 20%, 25%, 28% and 27%, respectively. This sample constitutes around 37% of the employed individual in this age span. In comparison with those not hospitalised during the same period, the age distribution is comparable, but they have somewhat lower income. Descriptive statistics for the 30% sample of both population (hospitalised and non-hospitalised) are provided in online supplementary appendix tables 1 and 2. We made use of the first hospital admission only. For sampled individuals with their first hospital admission in 1999, we hence observed their sickness absence 5 years before and 5 years after the admission. For other years, we did not observe the complete number of leads and lags, leading to an unbalanced panel. To account for potential sample composition effects, factors (or fixed effects) for years and age were included in our empirical specification.
bmjopen-2018-024098supp001.pdf (77.3KB, pdf)
The reason for age and employment restrictions prior to hospital admission was that sickness absence is only a valid morbidity measure if individuals are eligible for sickness benefits, that is, have employment (or searching for a job but with previous employment). Eligibility is tied to belonging to the labour force and being below the mandatory retirement age of 65. Thus, as individuals in general leave the labour force before the age of 65, we restricted the analysis to individuals younger than 60.
Statistical analyses
In the analyses we made use of regression analysis and adjusted for age in years, level of education (three levels: less than secondary, secondary and postsecondary), own and spousal earnings, and a factor for whether the individual or the spouse had earnings above the sickness insurance cap, and factors for year of admission, occupational sector and disease category.
The regression analysis can be denoted by a difference-in-differences design. The idea has been proposed in 1855 by John Snow,11 who used the fact that Lambeth Company in London moved its waterwork upriver, relatively free from sewage, as a means to empirically test the theory of water quality affecting cholera. He compared the change in the occurrence of cholera in people served by Lambeth Company before and after the move of the waterwork against the change in the occurrence of cholera during the same time period in people served by another company that did not change their location. By making use of the two differences over time (ie, difference-in-differences), he controlled for the fact that the change of the water quality was not randomly assigned. For an easily assessable discussion on this idea for the analysis of healthcare policies, see ref 12.
The difference-in-differences design allowed us to adjust for unobserved confounders of importance for sickness absence that may differ between men and women before admission to the hospital. Adjusting for preadmission sex differences, we then estimated the relative effect from the admission of women compared with men using an ordinary least squares estimator. We imputed the sickness absence for the deceased the year before the death for each year after their death. If men have a higher mortality rate than women, this strategy is conservative as a means to test for more proactive behaviour of women compared with men. On the other hand, if men and women have similar mortality rates, imputing zero days of absence for each year after their death provides a conservative test for more proactive behaviour of women. Both imputation methods were used in the analysis. However, the first results take use of the mean imputation strategy. Furthermore, the sickness and disability insurance are integrated parts of the social insurance system and therefore interrelated. An individual on full-time disability benefits cannot receive sickness benefits, but part-time disabled persons can. In the analysis, we therefore defined days on sickness absence as the number of days on sickness benefits and/or days on disability benefits in a given year.
In the mortality analyses, we made use of daily data and estimated discrete time Cox proportional hazard regression models using maximum likelihood.
Patient and public involvement
Patients were not involved in the design or conduct of this large observational, register-based study. It will not be possible to disseminate the results directly to the individuals involved since all analyses were done on depersonalised data. Hence, the results will be disseminated to the public through publication in scientific and popular scientific journals.
Results
Sickness absence in relation to gender
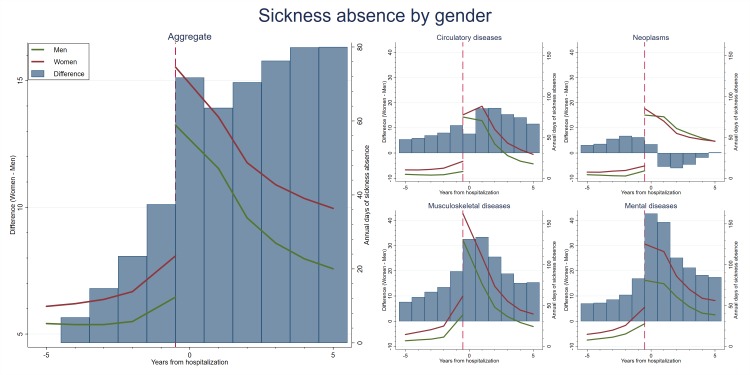
Figure 1 shows the average number of days of sickness absence of men and women before and after hospitalisation. The left panel shows the overall difference, while the right panel displays the average for four large disease categories: neoplasms (ICD-10=C00-D48), circulatory diseases (ICD-10=I00-I99), musculoskeletal diseases (ICD-10=M00–M99), and mental and behavioural disorders (ICD-10=F00–F99).
Figure 1.
Number of days of absence for men and women before and after a (first) hospital admission for the population of employed (prior to the hospital admission) individuals 40–59 years of age in 1993–2004. The left panel shows the average, while the right panel is conditional on cancer, myocardial infarction, musculoskeletal and mental diseases.
From the left panel it can be seen that the sickness absence for both men and women increased in the years prior to the hospital admission, but also that this increase is greater for women. In the period after the hospital admission, a sharp increase in sick leave for both men and women was seen, but the increase was much greater for women. The right panel of figure 1 shows the same pattern before the hospital admission for the four large disease categories. After the hospital admission, however, there are some differences across these categories. For neoplasms, sickness absence was higher for men 1–4 years after the admission. For the other diseases, women had higher sickness absence than men for the whole follow-up period. For circulatory diseases, this difference was small during the admission year, while for the two other the sex differences were initially large but then tapered off.
Mortality in relation to gender
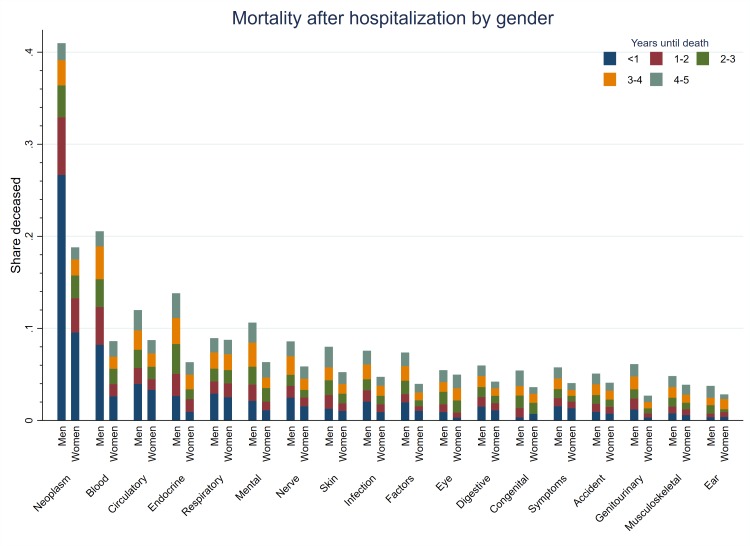
Figure 2 reports the disease-specific share of men and women who died within 5 years after the hospitalisation, separated into mortality within yearly follow-up categories for 18 different disease categories in total. A remarkable pattern was shown; for all disease categories, men had a higher probability of dying (also within follow-up categories) after hospitalisation.
Figure 2.
Five-year mortality risk for men and women after a hospital admission by diagnosis category for the population of employed (before the hospital admission) individuals 40–59 years of age in 1993–2004.
For neoplasms, the risk of dying in the 5-year follow-up period was 22 percentage points higher in men than in women (42% compared with 20%). For circulatory diseases, mental and behavioural (mental in the following) disorders and musculoskeletal diseases, there was a corresponding 4 (14%–10%), 4 (12%–8%) and 1.5 (6%–4.5%) percentage points increased risk in men, respectively.
For the sickness absence data, we imputed the sickness absence the year before the death for the deceased. The sex differences in mortality could thus possibly explain some of the posthospital admission pattern with regard to sickness absence. This explanation is most likely to be the most important for neoplasms.
Results from regression estimation
Table 1 presents the results from regression analyses of sex differences in sick leave and mortality for the 5-year follow-up period after the hospital admission. The results on both sickness absence and mortality were in line with the previous results reported in figures 1 and 2. From column (3) in panel A of table 1, it can be seen that women used a statistically significant 5.73 additional days of sickness absence than men per year over the 5-year posthospitalisation sampling window (95% CI 5.25 to 6.22). For a hospital admission for neoplasm, circulatory disease, musculoskeletal disease and mental disorder, the corresponding sex differences were −14.47, 7.44, 5.77 and 5.30 days, respectively (−16.30 to −12.64, 5.91–8.96, 3.63–7.91 and 1.96–8.64). Finally, from column (3) in panel B, it can be seen that women had around 27% () lower posthospitalisation mortality risk than men (24.18%–29.62%). For the neoplasm, circulatory, musculoskeletal and mental diseases, the corresponding figures were 57%, 38%, 27% and 45% lower mortality risks (54.18%–59.89%, 30.73%–43.94%, 13.02%–38.40% and 33.89%–54.98%).
Table 1.
Regression (linear and Cox) slope parameter (SE within parentheses) of sex differences in sickness absence and mortality 5 years after a hospital admission, by disease type
| (1) | (2) | (3) | |
| ( A ) Linear regressions (difference -in - difference s design on sex difference s in the effect of an admission on days of sickness absence ) | |||
| All | 5.728*** | 4.963*** | 5.738*** |
| n=1 867 013† | (5.25–6.22) | (4.47–5.45) | (5.26–6.22) |
| Circulatory (ICD-10=I00–I99) | 7.102*** | 6.621*** | 7.436*** |
| n=255 687 | (5.55–8.65) | (5.09–8.15) | (5.91–8.96) |
| Neoplasms (ICD-10=C00–D48) | −9.36*** | −15.082*** | −14.471*** |
| n=223 875 | (−11.12 to −7.53) | (−16.93 to −13.24) | (−16.30 to −12.64) |
| Musculoskeletal (ICD-10=M00–M99) | 3.149*** | 4.165*** | 5.772*** |
| n=149 846 | (0.96–5.33) | (2.00–6.33) | (3.63–7.91) |
| Mental (ICD-10=F00–F99) | 4.109* | 3.584* | 5.305*** |
| n=63 065 | (0.74–7.48) | (0.24–6.93) | (1.96–8.64) |
| (B) Cox proportional hazard regressions on sex differences in postadmission mortality | |||
| All | −0.279*** | −0.226*** | −0.314*** |
| n=233 274 | (−0.31 to −0.24) | (−0.26 to 0.19) | (−0.35 to −0.28) |
| Circulatory (ICD-10=I00–I99) | −0.449*** | −0.400*** | −0.473*** |
| n=31 838 | (−0.55 to −0.34) | (−0.50 to −0.30) | (−0.58 to −0.37) |
| Neoplasms (ICD-10=C00–D48) | −0.918*** | −0.752*** | −0.847*** |
| n=27 781 | (−0.98 to −0.86) | (0.82 to −0.69) | (−0.91 to −0.78) |
| Musculoskeletal (ICD-10=M00–M99) | −0.197* | −0.253*** | −0.312*** |
| n=18 875 | (−0.37 to −0.03) | (−0.42 to −0.08) | (−0.484 to −0.140) |
| Mental (ICD-10=F00–F99) | −0.578*** | −0.559*** | −0.606*** |
| n=8236 | (−0.764 to −0.39) | (−0.74 to −0.37) | (−0.80 to −0.41) |
| Covariates‡ | √ | √ | |
| Factors§ | √ | ||
For the deceased, we impute the sickness absence the year before the death for all years after the death.
Column (1) makes no covariate adjustments. Column (2) adjusts for covariates observed before the admission (see notes in the table). Column (3) adjusts for factors (see notes in the table).
*p<0.05, ***p<0.001.
†n is the sample size. In the sickness absence analysis, this is the number of individuals multiplied by the number of time periods they are included in the analysis, while in the mortality analysis it is the number of individuals.
‡Age in years, level of education (three levels: less than secondary, secondary and postsecondary), own and spousal earnings, and dummies for whether the individual or the spouse has earnings above the sickness insurance cap.
§Indicators for calendar year, occupational sector and disease category (where feasible).
ICD-10, International Statistical Classification of Diseases and Related Health Problems.
The results from the analyses on sickness absence for the 18 disease categories are provided in table 2. The general conclusion from these analyses is similar as from the overall sex difference analysis: women increased their absence more for all categories (statistically significant for 12 of these) except for neoplasm 5 years after the hospital admission than men.
Table 2.
Linear regression slope parameter, that is, the difference-in-differences estimate of sex differences in sickness absence 5 years after a hospital admission for 18 disease categories
| (1) | (2) | (3) | |
| Accident, n=201 273† | 5.033*** | 6.541*** | 7.653*** |
| Blood, n=9973 | 7.613* | 3.717 | 3.768 |
| Congenital, n=5530 | 5.365 | 3.116 | 3.924 |
| Digestive, n=219 619 | 7.861*** | 7.628*** | 8.447*** |
| Ear, n=25 660 | 4.459* | 4.559* | 5.952*** |
| Endocrine, n=40 538 | −0.871 | −0.964 | 0.157 |
| Eye, n=22 685 | 4.086* | 4.648* | 5.248*** |
| Factors, n=55 136 | −0.147 | 2.113 | 3.633*** |
| Genitourinary, n=168 659 | 4.273*** | 0.667 | 0.860 |
| Circulatory (ICD-10=I00–I99), n=255 687 | 7.102*** | 6.621*** | 7.436†*** |
| Infection, n=40 946 | 3.555* | 3.380* | 3.660* |
| Mental (ICD-10=F00–F99), n=63 065 | 4.109* | 3.584* | 5.305*** |
| Neoplasms (ICD-10=C00–D48), n=223 875 | −9.365*** | −15.082*** | −14.471*** |
| Nerve, n=44 075 | 9.461*** | 10.397*** | 11.395*** |
| Respiratory, n=81 981 | 7.952*** | 7.819*** | 8.688*** |
| Skin, n=14 040 | −0.219 | 0.983 | 2.355 |
| Symptoms, n=244 425 | 10.072*** | 9.972*** | 10.752*** |
| Covariates‡ | √ | √ | |
| Factors§ | √ |
For the deceased, we impute the sickness absence the year before the death for all years after the death.
Column (1) makes no covariate adjustments. Column (2) adjusts for covariates observed before the admission (see notes in the table). Column (3) adjusts for factors (see notes in the table).
*p<0.05, p<0.10, ***p<0.01.
†n is the sample size. This is the number of individuals multiplied by the number of time periods included in the analysis.
‡Age in years, level of education (three levels: less than secondary, secondary and postsecondary), own and spousal earnings, and dummies for whether the individual or the spouse has earnings above the sickness insurance cap.
§Indicators for calendar year, occupational sector and disease category (where feasible).
ICD-10, International Statistical Classification of Diseases and Related Health Problems.
To find out the importance of the mean, imputation method, an analysis where we imputed zero for those deceased after their death, was conducted. The results from this sensitivity analysis are shown in table 3. The overall results were basically unaffected, but in the sensitivity analysis statistically significant increases were found in sickness absence for women in 16 disease categories, including neoplasm. For this disease women increased their absence by 1.6 days more than men after the admission over the 5-year follow-up period (0.05–3.20).
Table 3.
Linear regression slope parameter, that is, the difference-in-differences estimate of sex differences in sickness absence 5 years after a hospital admission (imputing zero days of absence for all years after a death for those deceased) for 18 disease categories
| (1) | (2) | (3) | |
| All, n=1 867 013 | 5.156*** | 4.392*** | 5.126*** |
| Accident, n=201 273† | 5.175*** | 6.693*** | 7.771*** |
| Blood, n=9973 | 16.757*** | 12.188*** | 12.320*** |
| Congenital, n=5530 | 5.940 | 3.660 | 4.458 |
| Digestive, n=219 619 | 7.569*** | 7.349*** | 8.137*** |
| Ear, n=25 660 | 4.068* | 4.190* | 5.567*** |
| Endocrine, n=40 538 | 0.240 | 0.122 | 1.212 |
| Eye, n=22 685 | 5.576*** | 6.132*** | 6.717*** |
| Factors, n=55 136 | 0.641 | 2.662** | 4.150*** |
| Genitourinary, n=168 659 | 5.230*** | 1.570* | 1.759* |
| Circulatory (ICD-10=I00–I99), n=255 687 | 7.385*** | 6.900*** | 7.779*** |
| Infection, n=40 946 | 4.349*** | 4.153*** | 4.411*** |
| Mental (ICD-10=F00–F99), n=63 065 | 5.474*** | 4.947*** | 6.713*** |
| Musculoskeletal (ICD-10=M00–M99), n=149 846 | 2.981*** | 4.009*** | 5.592*** |
| Neoplasms (ICD-10=C00–D48), n=223 875 | 6.097*** | 1.108 | 1.626* |
| Nerve, n=44 075 | 9.607*** | 10.469*** | 11.461*** |
| Respiratory, n=81 981 | 7.317*** | 7.294*** | 8.061*** |
| Skin, n=14 040 | 0.114 | 1.342 | 2.710 |
| Symptoms, n=244 425 | 9.487*** | 9.419*** | 10.173*** |
| Covariates‡ | √ | √ | |
| Factors§ | √ |
Column (1) makes no covariate adjustments. Column (2) adjusts for covariates observed before the admission (see notes in the table). Column (3) adjusts for factors (see notes in the table).
*p<0.05, **p<0.01, ***p<0.001.
†n is the sample size. This is the number of individuals multiplied by the number of time periods included in the analysis.
‡Age in years, level of education (three levels: less than secondary, secondary and postsecondary), own and spousal earnings, and dummies for whether the individual or the spouse has earnings above the sickness insurance cap.
§Indicators for calendar year, occupational sector and disease category (where feasible).
ICD-10, International Statistical Classification of Diseases and Related Health Problems.
Previous studies have reported on sex differences in mortality after an inpatient care visit for an acute myocardial infarction (AMI) (see, eg, refs 13 14 and 15). For this reason, additional analyses on AMI inpatient care visits were made. We re-estimated our models using the AMI sample on (1) total 5-year mortality, (2) in-hospital death (ie, where the patient dies before discharge), (3) 1-year follow-up period (conditional on discharge) and (4) a follow-up period of 1–5 years after the inpatient care visit. We estimated the total effects but also separately for the age groups 40–44, 45–49, 50–54 and 55–59.
Table 4 provides the results from the regressions where we adjusted for the same variables as in the previous analyses. From column (1) it can be seen that men in this population had higher risk of dying within 5 years and that men in the oldest stratum is primarily driving this effect. For the other outcomes, we found no statistically significant sex differences.
Table 4.
Cox regression slope parameters (SE within parentheses): the sex difference in mortality after acute myocardial infarction hospitalisation by ‘timing of death’ and age categories
| (1) Total | (2) In-hospital | (3) Postdischarge (<1 year) |
(4) Postdischarge (1–5 years) |
|
| All | −0.030* | −0.007 | −0.009 | −0.013 |
| n=3545† | (−0.057 to −0.003) | (−0.019 to 0.005) | (−0.019 to 0.001) | (−0.035 to 0.009) |
| Age cohorts | ||||
| 40–44 | −0.054 | −0.011 | −0.032 | −0.010 |
| n=211 | (−0.140 to 0.032) | (−0.046 to 0.024) | (−0.081 to 0.017) | (−0.075 to 0.055) |
| 45–49 | −0.016 | −0.004 | −0.003 | −0.009 |
| n=604 | (−0.081 to 0.049) | (−0.031 to 0.023) | (−0.028 to 0.022) | (−0.064 to 0.046) |
| 50–54 | −0.005 | −0.013 | −0.008 | 0.016 |
| n=1175 | (−0.052 to 0.042) | (−0.035 to 0.009) | (−0.026 to 0.010) | (−0.023 to 0.055) |
| 55–59 | −0.050* | −0.003 | −0.009 | −0.038* |
| n=1555 | (−0.093 to −0.007) | (−0.021 to 0.015) | (−0.027 to 0.009) | (−0.073 to −0.003) |
| Covariates and factors‡ | √ | √ | √ | √ |
*p<0.05.
†n is the number of individuals.
‡Age in years, level of education (three levels: less than secondary, secondary and postsecondary), own and spousal earnings, and dummies for whether the individual or the spouse has earnings above the sickness insurance cap, and indicators for calendar year, occupational sector and disease category (where feasible).
Discussion
Measures of morbidity are often used as measures of health in the population, as well as inputs to adjust for the remuneration when healthcare is paid by capitation. Ideally, these measures should not be affected by patients’ preferences for healthcare. If these morbidity measures do not reflect real health, remuneration by capitation will be misleading and inefficient. For instance, a recently published study shows that among fee-for-service Medicare beneficiaries, there is an inverse relationship between the regional frequency of diagnosis and the case-fatality rate for chronic conditions.16 The present study focuses on the differences between sexes and to what extent that sex differences in observed morbidity outcomes reflect differences in behaviour rather than differences in health. We test this hypothesis using a novel design made possible by the supply of longitudinal data on a morbidity measure (sickness absence) on a population of working men and women. We found that women extracted relatively more sickness absence and simultaneously had a lower mortality risk than men both before, but in particular after the hospitalisation. This provides strong evidence on the more proactive and preventive behaviour of women compared with men.
Case and Paxson17 and Singh-Manoux et al 18 could not confirm the hypothesis of the differences in preferences between sexes, that is, a more proactive behaviour of women than of men, or a [18, p. 2251] ‘greater stoicism among men and a greater willingness among women to use health services, report health problems and factor in less-serious ailments when assessing their own health’.18As a morbidity measure Case and Paxson17 focused on self-assessed health, while Singh-Manoux et al 18 used self-rated health, long-standing illness, respiratory illness, sickness absence, hypertension and coronary heart disease (CHD) prevalence. The lack of systematic statistically significant differences in the association between mortality and morbidity measures was taken as evidence against the theory. One should, however, note that there are patterns in both studies that support the theory. For example, 8 of 11 morbidity measures have a stronger association to mortality for men than for women and for one (sickness absence) is this difference statistically significant. Men with respiratory cancer, cardiovascular disease and bronchitis were found to have higher incidence of hospital episodes and mortality than women who suffer from the same self-reported conditions in the study by Case and Paxson.17 This suggests that this theory may be one explanation for the observed gender pattern, but that the sample size needs to be large and that one needs methods not sensitive to unmeasured confounders. The strategy used in this paper was originally suggested by Avdic and Johansson,19 who applied the method to a sample of working Swedish men and women aged 40–45. This paper extends on this study by studying a larger population and by a more elaborate analysis over diagnosis codes. However, the results from the two papers are in agreement.
Our results on mortality after a hospital admission are somewhat in contrast to studies on sex differences in AMI mortality after a hospital admission. For example, some previous studies13–15 have found a higher risk of mortality after an inpatient care visit for an AMI in younger (65 or younger, or 75 or younger) women, compared with men. However, these analyses are based on hospital discharge data, implying that mortality is conditional on patient admission and that death occurred before leaving the hospital. Furthermore, other studies show that female patients with AMI have on average longer hospital stays than men.20 21 The implication is that if women have longer length of hospital stays (eg, due to differences in preferences) given a certain health condition, then this could explain women’s higher mortality. An advantage of our analysis is that it is not restricted to death in the hospital. To shed light on this potential issue, we re-estimated our analyses on the subsample of patients with AMI. This subanalysis could not confirm the results of the previous studies.13–15
Limitations
Results based on observational data can always suffer from confounding bias. We empirically analyse changes in sickness absence after a hospital admission for men and women in a difference-in-differences design commonly used in social science and increasingly applied in medical science.12 The longitudinal characteristics of our data allow us to condition on group differences in health, working conditions and other time-invariant factors (eg, differences in household duties), which might confound the relation between absenteeism and gender-specific health behaviour. In this respect, we need to stress that all displayed results are not sensitive to whether observed covariates are included or not. This result is to be expected from the design of the study. If anything, the adjustment for covariates increased, rather than decreased, the magnitude of the effects (compare column (1) with no adjustment with column (3) in tables 1–3). Hence, given that the inclusion of these covariates to some extent captures health before the hospital admission, this empirical pattern indicates that women have, on average, better preadmission health than men do. The implication would then be that the observed sex differences in sickness absence after a hospital admission are a lower bound of the more proactive and preventive behaviour of women in contrast to that of men.
Another limitation is that our results reflect the findings from a representative sample of employed Swedish individuals aged 40–59 with a hospital visit in 1991. It is not clear whether these results would apply to other populations.
Implications
Using remuneration by capitation based on morbidity measurescan be misleading and inefficient. The reason is that morbidity measures notnecessarily reflect real health. A more efficient strategy may instead be of affecting the attitudes and norms among groups with a high mortality risk. One such strategy would be to inform men to use medical services more proactively.
The processing of personal data that takes place in Sweden must still comply with the rules of the Personal Data Act. This means that data may only be transferred if the data controller in Sweden has complied with the other requirements of the Personal Data Act, for instance the fundamental requirements regarding processing of personal data and the rules about when such processing is permitted on the whole.
*In the Personal Data Act (and in the EC Directive on data protection) there are guidelines on what you have to consider when assessing the level of protection for personal data. All circumstances surrounding the transfer shall be considered. Particular consideration shall be given to the nature of data, the purpose of the processing, the duration of the processing, the country of origin, the country of final destination and the rules that exist for the processing in the third country.
The EU Commission has analysed the data protection rules of a few countries and decided that the level of protection in these countries is adequate. The decisions concern Argentina, Bailiwick of Guernsey, Faroe Islands, Isle of Man Jersey and Switzerland.
Furthermore the EU Commission has assessed that the level of protection is adequate within certain sectors or under certain conditions in the following countries:
Canada (if their legislation on protection of personal data in the private sector is applicable on the recipient’s processing of personal data).
USA (if the recipient has adhered to the so-called Safe Harbor principles).
The decisions of the EU Commission are enumerated in an annex to the Personal Data Ordinance. In the ordinance it is explicitly stated that transfers are permitted in these cases.
The Safe Harbor principle is a set of voluntary rules on privacy and data protection elaborated and decided by the US Department of Commerce (DoC). Organisations in the USA can notify the DoC that they adhere to these rules. The EU Commission has assessed that the rules (including accompanying questions and answers) constitute an adequate level of protection. Thus it is permitted to transfer personal data from EU/EEA to organisations in the USA that have adhered to the rules. On the website of the US DoC, there is a list of companies and organisations that have adhered to the Safe Harbor principles. For further information see http://www.datainspektionen.se/in-english/in-focus-transfer-of-personal-data/.
Supplementary Material
Footnotes
Contributors: DA made all analyses and interpreted the data together with the coauthors. He had approved the version of the manuscript to be published. PH interpreted the data together with the coauthors and drafted the manuscript. He had approved the version of the manuscript to be published. BL interpreted the data together with the coauthors, drafted parts of the manuscript and revised the manuscript for important intellectual content. He had approved the version of the manuscript to be published. PJ designed the study and interpreted the data together with the coauthors and drafted the manuscript of data. He had approved the version of the manuscript to be published.
Funding: PJ acknowledges funding from the Swedish Research Council for Health, Working Life and Welfare (FORTE).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Regional Ethical Review Board in Uppsala (approval number 2005:126).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available.
References
- 1. Mastekaasa A, Olsen KG. And job characteristics: a fixed effects approach. Work and Occupations 1998;25:195–2228. [Google Scholar]
- 2. Sindelar JL. Differential use of medical care by sex. Journal of Political Economy 1982;90:1003–19. 10.1086/261105 [DOI] [PubMed] [Google Scholar]
- 3. Lee C. , Health and Health Behaviors : Chrisler J, McCreary DR, Handbook of gender research in psychology. 20 Springer Science, 2010: 471–93. [Google Scholar]
- 4. Nathanson CA. Illness and the feminine role: a theoretical review. Social Science & Medicine 1975;9:57–62. 10.1016/0037-7856(75)90094-3 10.1016/0037-7856(75)90094-3 [DOI] [PubMed] [Google Scholar]
- 5. Verbrugge LM. Sex differentials in health. Public Health Report 1982;97:417–37. [PMC free article] [PubMed] [Google Scholar]
- 6. Stronegger WJ, Freidl W, Rásky E. Health behaviour and risk behaviour: socioeconomic differences in an Austrian rural County. Soc Sci Med 1997;44:423–6. 10.1016/S0277-9536(96)00181-5 [DOI] [PubMed] [Google Scholar]
- 7. Uitenbroek DG, Kerekovska A, Festchieva N. Health lifestyle behaviour and socio-demographic characteristics. A study of VARNA, Glasgow and Edinburgh. Soc Sci Med 1996;43:367–77. 10.1016/0277-9536(95)00399-1 [DOI] [PubMed] [Google Scholar]
- 8. Graunt J. Natural and political observations mentioned in a following index and made upon the bills of mortality. London: Tho: Roycroft, for John Martin, James Allestry, and Tho: Dicas, London: Martin, Allestry and Dicas, 1662. [Google Scholar]
- 9. Bertrand M. New perspectives on gender In: Ashenfelter O, Card D, eds Handbook of labor economics 4B, Elsevier LTD, 2010: 1545–92. [Google Scholar]
- 10. Cia factbook, 2011. Available: https://www.cia.gov/library/publications/the-world-factbook/index.html
- 11. Snow J. On the mode of communication of cholera. 2nd edn London: John Churchill, 1855. [Google Scholar]
- 12. Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA 2014;312:2401–2. 10.1001/jama.2014.16153 [DOI] [PubMed] [Google Scholar]
- 13. Vaccarino V, Parsons L, Every NR, et al. National Registry of myocardial infarction 2 participants. Sex-based differences in early mortality after myocardial infarction. N Engl J Med 1999;341:217–25. [DOI] [PubMed] [Google Scholar]
- 14. Vaccarino V, Krumholz HM, Yarzebski J, et al. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med 2001;134:173–81. 10.7326/0003-4819-134-3-200102060-00007 [DOI] [PubMed] [Google Scholar]
- 15. Canto JG, Rogers WJ, Goldberg RJ, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012;307:813–22. 10.1001/jama.2012.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Welch HG, Sharp SM, Gottlieb DJ. Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA 2011;305:1113–8. 10.1001/jama.2011.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Case A, Paxson CH. Sex differences in morbidity and mortality. Demography 2005;42:189–214. 10.1353/dem.2005.0011 [DOI] [PubMed] [Google Scholar]
- 18. Singh-Manoux A, Guéguen A, Ferrie J, et al. Gender differences in the association between morbidity and mortality among middle-aged men and women. Am J Public Health 2008;98:2251–7. 10.2105/AJPH.2006.107912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Avdic D, Johansson P, Absenteeism JP. Absenteeism, gender and the Morbidity–Mortality paradox. J Appl Econ 2017;32:440–62. 10.1002/jae.2516 [DOI] [Google Scholar]
- 20. Every NR, Spertus J, Fihn SD, et al. Length of hospital stay after acute myocardial infarction in the myocardial infarction triage and intervention (MITI) project registry. J Am Coll Cardiol 1996;28:287–93. 10.1016/0735-1097(96)00168-4 [DOI] [PubMed] [Google Scholar]
- 21. Kinjo K, Sato H, Nakatani D, et al. Predictors of length of hospital stay after acute myocardial infarction in Japan. Circ J 2004;68:809–15. 10.1253/circj.68.809 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-024098supp001.pdf (77.3KB, pdf)