Abstract
Objectives
Timely recognition of medication misuse and dependence is crucial to avoid both adverse drug events and increasing health expenditure. Yet the detection of these disorders in older people remains challenging due to the paucity of evidence on characteristics of patients at risk. This study investigates sociodemographic, pharmacological and clinical characteristics and factors associated with prolonged medication use, misuse and dependence in hospitalised older patients, focusing on three commonly prescribed central nervous system depressants (CNSDs): opioid analgesics, benzodiazepines and z-hypnotics.
Design
A prospective, cross-sectional study complying with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Setting
Somatic departments of the Akershus University Hospital, Norway.
Participants
246 patients aged 65–90 were included.
Outcome measures
Prolonged use was defined as using CNSDs for ≥4 weeks. Misuse and dependence were assessed with the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for substance abuse and dependence. We used descriptive statistics to report patients’ characteristics and logistic regression to demonstrate factors associated with prolonged use, and misuse or dependence.
Results
Forty per cent of participants reported using CNSDs for ≥4 weeks. The odds of prolonged use were higher for patients aged 75–84 (OR=2.32, 95% CI 1.16 to 4.65) and ≥85 (OR=3.33, 95% CI 1.25 to 8.87) vs <75 years, for pain intensity (OR=1.02, 95% CI 1.01 to 1.04), and polypharmacy versus no polypharmacy (OR=5.16, 95% CI 2.13 to 12.55). The odds were lower for patients who completed secondary education (OR=0.33, 95% CI 0.13 to 0.83) compared with those with only basic education. Factors associated with misuse or dependence were pain intensity (OR=1.02, 95% CI 1.01 to 1.04) and concurrent use of ≥2 CNSDs (OR=3.99, 95% CI 1.34 to 11.88).
Conclusion
CNSD overuse is prevalent among hospitalised older patients, despite clear guidelines and recommendations. Our findings underline a need for stronger focus on responsible prescribing, timely detection and prevention of this issue, with special attention towards older patients, those with enhanced pain, polypharmacy and/or concurrent use of several CNSDs.
Trial registration number
Keywords: characteristics, geriatric patients, risk factors, addiction, prescription drug abuse
Strengths and limitations of this study.
This is the first and comprehensive study of the characteristics and factors associated with commonly prescribed central nervous system depressants (CNSDs) prolonged use, misuse and dependence in older hospitalised patients.
Characteristics of at-risk patients and significant associations revealed in this study can be used to inform ways for implementing future research initiatives and interventions aiming at early detection, prevention and treatment for CNSD overuse among older patients.
We used validated and generally accepted diagnostic criteria to assess medication misuse and dependence in older patients (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for substance abuse and dependence).
The use of cross-sectional data and a hospital-based sample precludes us from inferring causal relationships and generalising the study findings to the general population.
Introduction
Central nervous system depressants (CNSDs) such as opioid analgesics, benzodiazepines and z-hypnotics are commonly prescribed among older patients for the treatment of chronic pain, anxiety and insomnia. While these medications are essential for moderate-severe cases, long-term use is not recommended owing to the risk of adverse events, including hyperalgesia, fractures, falls, cognitive impairment and dependence.1–3 This underlines the importance of rational use and prescription of CNSDs for older patients. To achieve this, one of the practical steps is for clinicians to be able to timely recognise older patients at risk or suffering from medication misuse and dependence.
According to the Norwegian Prescription Database, the consumption of potentially addictive drugs such as opioid analgesics (ie, oxycodone and tramadol), benzodiazepines (ie, diazepam, oxazepam and nitrazepam) and z-hypnotics (ie, zopiclone and zolpidem) is high among individuals aged 65 and older. These drugs were among the 30 most commonly prescribed drugs to older patients in Norway in 2017.4
A Norwegian study showed that patients’ regular general practitioners (GPs) prescribed the largest proportion of addictive drugs to their older patients (77%) compared with other groups of physicians.5 In line with this, another study found that CNSDs were frequently prescribed by GPs in large quantities and through indirect contacts without consultation.6 Both inappropriate prescribing and the high consumption of such addictive medications may put older patients at risk of medication misuse and dependence—a condition characterised by persistent and compulsive use of a medication despite impairment in physical, social and psychological health.7
Given the vulnerability to serious adverse effects and interactions as a result of age-related changes in pharmacokinetics and pharmacodynamics, timely recognition and treatment of medication misuse and dependence in older patients are crucial to ensure medication safety and to avoid increasing health expenditure.8 Yet the detection of these disorders in older people is challenging and requires both valid screening tools and evidence-based knowledge on long-term use of CNSDs, including patients’ characteristics related to misuse and dependence.7 9 Addressing these knowledge gaps forms the basis for developing evidence-based intervention.
This study intends to provide such information by investigating sociodemographic, pharmacological and clinical characteristics and factors associated with prolonged medication use, misuse and dependence in hospitalised older patients, focusing on three commonly prescribed CNSDs: opioid analgesics, benzodiazepines and z-hypnotics.
Methods
Study design
The study was a prospective, cross-sectional, in-hospital study and complied with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Participants and setting
The recruitment process took place between May 2017 and September 2018, at three somatic departments of the Akershus University Hospital: geriatrics, general internal medicine and neurology. The catchment area of the hospital covers roughly 10% of the total population of Norway. Participants were recruited at the first few days of admission based on predefined inclusion and exclusion criteria, and not on their vulnerability, reasons for admission, diagnosis or severity of disease. As Norway has an all-covering national health insurance, all patients enter the hospital on the same conditions and with the same inpatient threshold. The study inclusion criteria were hospitalised patients aged between 65 and 90 years old. The exclusion criteria included Mini-Mental State Examination score ≤21 (incapacity to give informed consent)10 11; pre-existing diagnosis of severe depression, stroke, dementia and psychotic disorders; serious visual or hearing impairment; and insufficient Norwegian language, all generally assumed to bias participants’ responses on self-rated health questions. We precluded participants who were in a too serious medical condition or palliative treatment, defined by physicians at the study setting.
Data collection
Data collection (by SC, TGS and CL) involved three steps. After consent had been given, all eligible participants were asked to complete a questionnaire on sociodemographic background, pain, anxiety and depression. At this stage, the investigators were blinded to the use of medications as this was registered in the electronic patient record (EPR) which could only be accessed once a written informed consent had been obtained. Having fulfilled this requirement, the EPRs were reviewed to document the use of medications (type, duration, frequency and polypharmacy). Finally, the presence of medication misuse or dependence among participants identified as prolonged users of CNSDs was assessed through a structured interview. Prior to the start of the study, the three data collectors had gone through training sessions in order to optimise congruent use of the interview. More details on definition, data sources and measurements for variables under investigation are given in the section below. The questionnaire and interview guide used to collect data from participants can be found in online supplementary additional file 1.
bmjopen-2019-031483supp001.pdf (53.6KB, pdf)
Sociodemographic and clinical variables
Sociodemographic variables included age (65–74, 75–84 and ≥85), sex (male, female), education (basic, secondary and higher education), annual income (<200 000, 200 000–349 000 and ≥350 000 Norwegian krone per year) and living alone (yes, no). Clinical variables consisted of pain intensity (continuous, measured using Visual Analogue Scale (VAS)); anxiety and depressive symptoms were assessed with the Hospital Anxiety and Depression Scale (HADS). The Cronbach’s alpha coefficient for the HADS anxiety and depression subscales, reported by Helvik et al 12, was 0.78 and 0.71, respectively. Optimal cut-off values for diagnosing anxiety and depression in older hospitalised patients using HADS remain to be established.12 13 To avoid underestimation and misclassification bias of individuals with anxiety and depression, we used anxiety and depression scores as continuous variables. Higher scores indicate higher levels of anxiety and depressive symptoms.14 Data for all of these variables were collected through a self-completed questionnaire.
Pharmacological variables
We defined prolonged use of CNSDs as the use of opioid analgesics, benzodiazepines and/or z-hypnotics for 4 weeks or longer continuously up to the point of recruitment,15 16 while non-prolonged use was non-use or use of these medications for less than 4 weeks. Medication misuse and dependence were defined based on the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for substance abuse and dependence, through structured interviews, using the Norwegian version of the Mini-International Neuropsychiatric Interview Guide.17 Dependence was defined to be present if patients met three or more of the dependence criteria. Misuse was confirmed if the dependence criteria were not met and the respondents satisfied one or more of the abuse criteria.18 For the purpose of the present study, and as few patients fulfilled the misuse criteria (n=6), misuse and dependence were grouped together as medication misuse or dependence (n=39). Other pharmacological variables entailed types of CNSD medications used (categorised as exclusive or concurrent use), duration of use (continuous, in week), frequency of use (categorised as sporadic use if the medication was taken intermittently <5 days per week and as daily use if the medication was taken ≥5 days per week) and polypharmacy19 (defined as use of ≥5 medications daily, coded as yes or no). The main source of pharmacological data was the EPR. We also sought to verify this against information from patients and GP referral documents. To ensure the accuracy of data on CNSD use patterns, we checked for evidence of use and consistency across prescriptions and relevant documents reported from both primary care and hospital settings.
Statistical analysis
We analysed the characteristics of older patients with and without prolonged use, and that of those with and without misuse or dependence using descriptive statistics. We assessed the associations between patient characteristics and the presence of prolonged CNSD use, and misuse or dependence using bivariate and multiple logistic regression analyses. The analyses included adjustment for sociodemographic subgroups (age groups, sex, education, annual income, living alone), clinical (pain intensity, anxiety and depression scores) and pharmacological variables (polypharmacy, duration and concurrent use of CNSDs). Multiple imputations, under the missing at random assumption, using chained equations (with 20 imputed data sets) were performed to handle missing data. Sensitivity analyses were conducted for the same models using complete case analysis (individuals with missing data are excluded). No multicollinearity was detected. Stata-SE V.15 software was used for all statistical analyses.20
Patient and public involvement
A user advisory board established at the Akershus University Hospital (the study setting), which included both representatives of older patients and health service officials, supported this study. The board met on a regular basis throughout the study period. They provided project-specific inputs on ethics, design and methodology, as well as highlighted research focus based on patient and public interests. They will also be involved in the dissemination of the findings.
Results
Participants
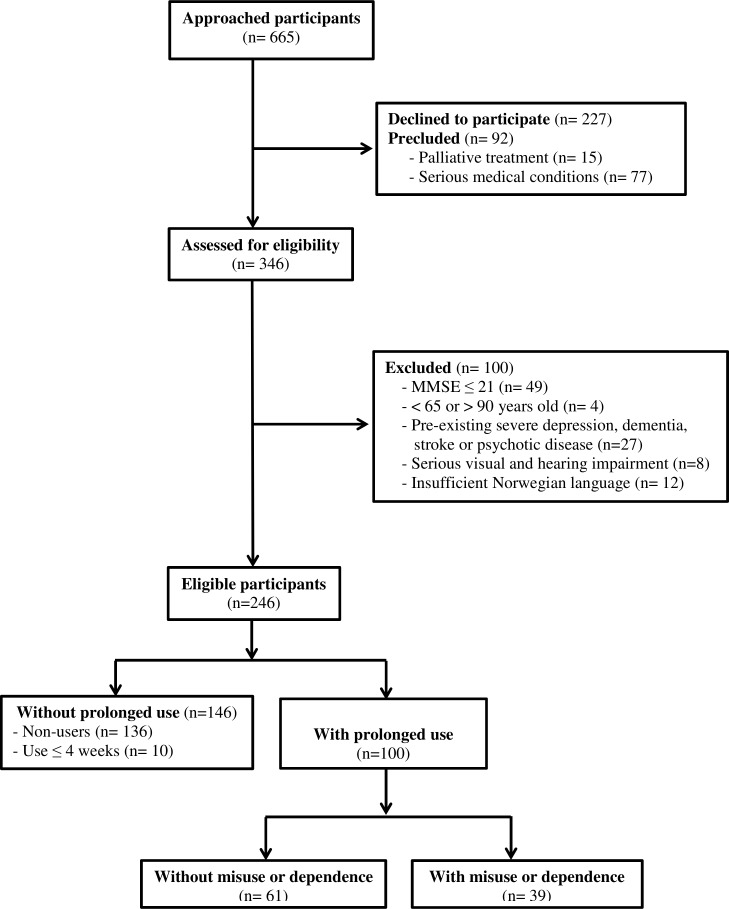
In total, we consecutively approached 665 adults aged 65–90. Of these, 227 participants declined to participate, while 92 others were precluded due to either being in a too serious medical condition or palliative treatment. Of the remaining patients, 100 were excluded based on predefined exclusion criteria. This resulted in 246 older patients eligible for the study, of whom 100 were thereafter identified as prolonged users of CNSDs (≥4 weeks). Figure 1 provides more details on the flow of participants through the study.
Figure 1.
Flow of participants through the study. MMSE, Mini-Mental State Examination.
There were no missing data in the variables: prolonged use, misuse or dependence, concurrent use, duration of CNSD use, age groups, sex, living alone, and polypharmacy. The following variables had missing data: pain intensity (7%, 18/246), anxiety scores (7%, 17/246), depression scores (7%, 17/246), education (6%, 14/246) and income (16%, 39/246).
Descriptive data
Overall, in descriptive analyses, older patients with prolonged use of CNSDs were more often female, aged 75–85, living alone, with lower socioeconomic status (completed ≤secondary education and earned <350 000 Norwegian krone per year), polypharmacy, and accompanied by higher pain intensity and depression scores (table 1). Patients screening positive on misuse or dependence were mainly those living alone (69%) and those on concurrent use of several CNSDs (46% vs 20%). More details on patient characteristics are provided in table 1.
Table 1.
Patient characteristics
| Patient characteristics | Prolonged use of CNSDs | CNSD misuse or dependence | ||
| No (n=146) | Yes (n=100) | No (n=61) | Yes (n=39) | |
| Sex | ||||
| Female | 71 (49%) | 66 (66%) | 38 (62%) | 28 (72%) |
| Male | 75 (51%) | 34 (34%) | 23 (38%) | 11 (28%) |
| Age groups, years | ||||
| 65–74 | 73 (50%) | 28 (28%) | 18 (29%) | 10 (25%) |
| 75–84 | 59 (40%) | 51 (51%) | 34 (56%) | 17 (44%) |
| ≥85 | 14 (10%) | 21 (21%) | 9 (15%) | 12 (31%) |
| Education, years | ||||
| Basic education (≤10) | 16 (12%) | 30 (32%) | 17 (29%) | 13 (36%) |
| Secondary education (11–13) | 64 (46%) | 31 (33%) | 21 (36%) | 10 (28%) |
| Higher education (≥14) | 58 (42%) | 33 (35%) | 20 (35%) | 13 (36%) |
| Income (Norwegian krone/year) | ||||
| <200 000 | 8 (7%) | 13 (15%) | 7 (14%) | 6 (18%) |
| 200 000–349 000 | 42 (34%) | 43 (51%) | 24 (46%) | 19 (58%) |
| ≥350 000 | 72 (59%) | 29 (34%) | 21 (40%) | 8 (24%) |
| Living alone | ||||
| No | 87 (60%) | 45 (45%) | 33 (54%) | 12 (31%) |
| Yes | 59 (40%) | 55 (55%) | 28 (46%) | 27 (69%) |
| Polypharmacy (≥5 drugs/day) | ||||
| No | 55 (38%) | 8 (8%) | 6 (10%) | 2 (5%) |
| Yes | 91 (62%) | 92 (92%) | 55 (90%) | 37 (95%) |
| Anxiety scores (HADS-A) | ||||
| Mean (SD) | 4.13 (3.28) | 4.97 (3.91) | 4.47 (3.54) | 5.68 (4.34) |
| Median (range) | 4 (0–14) | 4 (0–16) | 4 (0–16) | 5 (0–15) |
| Depression scores (HADS-D) | ||||
| Mean (SD) | 3.60 (2.98) | 5.13 (3.49) | 4.89 (3.26) | 5.49 (3.81) |
| Median (range) | 3 (0–13) | 4 (0–15) | 4 (0–12) | 5 (0–15) |
| Pain intensity (mm on VAS) | ||||
| Mean (SD) | 18.07 (24.21) | 35.20 (30.35) | 30.56 (28.30) | 42.08 (32.34) |
| Median (range) | 7 (0–91) | 29.50 (0–97) | 27 (0–97) | 48 (0–93) |
| Duration of CNSD use (weeks) | ||||
| Mean (SD) | – | 71.47 (113.44) | 72.10 (138.38) | 70.48 (57.36) |
| Median (range) | – | 50.50 (4-988) | 33 (4–988) | 52 (4–232) |
| Concurrent use (of >1 CNSDs) | ||||
| No (exclusive use) | – | 70 (70%) | 49 (80%) | 21 (54%) |
| Yes | – | 30 (30%) | 12 (20%) | 18 (46%) |
CNSD, central nervous system depressant drugs; HADS-D/A, Hospital Anxiety and Depression Scale, depression or anxiety subscale; VAS, Visual Analogue Scale.
Medication use patterns
Forty per cent (100 out of 246 participants) of the older patients enrolled in this study were identified as prolonged users (≥4 weeks) of opioid analgesics, benzodiazepines and/or z-hypnotics. The dominant group of medications was z-hypnotics, accounting for 42% (42/100) as exclusive use and 26% (26/100) as concurrent use of z-hypnotics plus other CNSDs, followed by opioid analgesics, which comprised 21% (21/100) as exclusive use and 23% (23/100) as concurrent use. Benzodiazepines were less commonly used and constituted 7% (7/100) as exclusive use and 13% (13/100) as concurrent use. Of the prolonged users in our sample, 30% (30/100) concurrently consumed two or more different types of CNSDs. The most prevalent pattern of concurrent use was the combination of z-hypnotics and opioid analgesics, amounting to 57% (17/30) of all forms of current use.
The majority of older patients using CNSDs did so on long-term and daily basis. The medians for duration of use for opioid analgesics, benzodiazepines and z-hypnotics were 42 (4–988), 51 (4–208) and 52 (4–232) weeks, respectively. More than half of the prolonged users reported using these medications daily (5–7 days per week): opioid analgesics (26/44), benzodiazepines (15/20) and/or z-hypnotics (51/68).
Among all the prolonged users of CNSDs, 39% (39/100) met the DSM-IV criteria for substance abuse or dependence. The non-mutually exclusive proportions of misuse or dependence for opioid analgesics, benzodiazepines and z-hypnotics were 30% (13/44), 40% (8/20) and 41% (28/68), respectively.
Factors associated with prolonged use of CNSDs
In the multivariate model, factors associated with increasing odds for prolonged use of CNSDs included being aged 75–84 (OR=2.32, 95% CI 1.16 to 4.65) and ≥85 years old (OR=3.33, 95% CI 1.25 to 8.87), compared with age <75, having more intense pain according to the VAS (OR=1.02, 95% CI 1.01 to 1.04) and polypharmacy (OR=5.16, 95% CI 2.13 to 12.55). On the contrary, the odds were lower among patients who had completed at least secondary education (OR=0.33, 95% CI 0.13 to 0.83) compared with only basic education (table 2). In the sensitivity analysis using complete case analysis, the associations between these factors and the prolonged use of CNSDs remained significant (online supplementary additional file 2). Sex, income, anxiety and depression scores were not significantly associated with prolonged use of CNSDs.
Table 2.
Logistic regression models for factors associated with prolonged use of CNSDs, estimated using multiple imputations
| Independent variable | Bivariate model | Multivariate model | ||
| OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 2.05 (1.21 to 3.47) | 0.007 | 1.56 (0.80 to 3.02) | 0.19 |
| Age groups, years | ||||
| 65–74 | 1 | 1 | ||
| 75–84 | 2.25 (1.27 to 4.00) | 0.006 | 2.32 (1.16 to 4.65) | 0.02 |
| ≥85 | 3.91 (1.75 to 8.74) | 0.001 | 3.33 (1.25 to 8.87) | 0.02 |
| Education, years | ||||
| Basic education (≤10) | 1 | 1 | ||
| Secondary education (11–13) | 0.29 (0.14 to 0.60) | 0.001 | 0.33 (0.13 to 0.83) | 0.02 |
| Higher education (≥14) | 0.34 (0.16 to 0.71) | 0.004 | 0.45 (0.18 to 1.12) | 0.09 |
| Income (Norwegian krone/year) | ||||
| <200 000 | 1 | 1 | ||
| 200 000–349 000 | 0.73 (0.27 to 1.93) | 0.52 | 0.67 (0.20 to 2.24) | 0.52 |
| ≥350 000 | 0.33 (0.12 to 0.88) | 0.03 | 0.35 (0.10 to 1.23) | 0.10 |
| Living alone | ||||
| No | 1 | 1 | ||
| Yes | 1.80 (1.08 to 3.01) | 0.03 | 0.94 (0.47 to 1.88) | 0.86 |
| Polypharmacy (≥5 drugs/day) | ||||
| No | 1 | 1 | ||
| Yes | 6.95 (3.13 to 15.41) | < 0.001 | 5.16 (2.13 to 12.55) | < 0.001 |
| Anxiety scores (HADS-A) | 1.06 (0.98 to 1.14) | 0.14 | 1.01 (0.91 to 1.13) | 0.80 |
| Depression scores (HADS-D) | 1.14 (1.05 to 1.24) | 0.002 | 1.08 (0.95 to 1.22) | 0.21 |
| Pain intensity (mm on VAS) | 1.02 (1.01 to 1.03) | < 0.001 | 1.02 (1.01 to 1.04) | < 0.001 |
Bold, P value <0.05.
CNSD, central nervous system depressant drugs; HADS-D/A, Hospital Anxiety and Depression Scale, depression or anxiety subscale; VAS, Visual Analogue Scale.
bmjopen-2019-031483supp002.pdf (24.9KB, pdf)
Factors associated with CNSD misuse or dependence
Table 3 shows the results of bivariate and multiple logistic regression analyses of the factors associated with CNSD misuse or dependence, estimated using multiple imputations. We found that concurrent use of CNSDs and pain intensity had a significant association with misuse or dependence. In the multivariate model, we found that compared with exclusive use, concurrent use of two or more CNSDs increased the odds for misuse or dependence by four times (OR=3.99, 95% CI 1.34 to 11.88).
Table 3.
Logistic regression models for factors associated with CNSD misuse or dependence, estimated using multiple imputations
| Independent variables | Bivariate model | Multivariate model | ||
| OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 1.54 (0.65 to 3.67) | 0.33 | 1.13 (0.39 to 3.21) | 0.83 |
| Age groups, years | ||||
| 65–74 | 1 | 1 | ||
| 75–84 | 0.90 (0.34 to 2.37) | 0.83 | 1.37 (0.42 to 4.50) | 0.60 |
| ≥85 | 2.40 (0.75 to 7.65) | 0.14 | 2.30 (0.56 to 9.45) | 0.25 |
| Education, years | ||||
| Basic education (≤10) | 1 | 1 | ||
| Secondary education (11–13) | 0.61 (0.22 to 1.72) | 0.35 | 0.84 (0.23 to 3.03) | 0.78 |
| Higher education (≥14) | 0.86 (0.32 to 2.34) | 0.77 | 1.09 (0.31 to 3.81) | 0.89 |
| Income (Norwegian krone/year) | ||||
| <200 000 | 1 | 1 | ||
| 200 000–349 000 | 0.85 (0.24 to 2.98) | 0.80 | 1.26 (0.26 to 6.26) | 0.77 |
| ≥350 000 | 0.49 (0.13 to 1.88) | 0.30 | 0.73 (0.11 to 4.83) | 0.75 |
| Living alone | ||||
| No | 1 | 1 | ||
| Yes | 2.65 (1.14 to 6.18) | 0.02 | 2.06 (0.65 to 6.48) | 0.22 |
| Polypharmacy (≥5 drugs/day) | ||||
| No | 1 | 1 | ||
| Yes | 2.02 (0.39 to 10.55) | 0.41 | 1.88 (0.25 to 14.21) | 0.54 |
| Concurrent use (of >1 CNSDs) | ||||
| No (exclusive use) | 1 | 1 | ||
| Yes | 3.5 (1.44 to 8.54) | 0.006 | 3.99 (1.34 to 11.88) | 0.01 |
| Duration of CNSD use (weeks) | 1.00 (0.99 to 1.00) | 0.94 | 1.00 (0.99 to 1.00) | 0.33 |
| Anxiety scores (HADS-A) | 1.08 (0.97 to 1.21) | 0.14 | 1.06 (0.88 to 1.26) | 0.55 |
| Depression scores (HADS-D) | 1.05 (0.93 to 1.19) | 0.41 | 0.99 (0.81 to 1.20) | 0.90 |
| Pain intensity (mm, VAS) | 1.01 (0.99 to 1.03) | 0.08 | 1.02 (1.01 to 1.04) | 0.04 |
Bold, P value <0.05.
CNSD, central nervous system depressant drugs; HADS-D/A, Hospital Anxiety and Depression Scale, depression or anxiety subscale; VAS, Visual Analogue Scale.
Moreover, as pain intensity increased by 1 mm on the 100 mm VAS, the odds for misuse or dependence increased by 1.02 units (OR=1.02, 95% CI 1.01 to 1.04). Sensitivity analysis using complete case analysis yields consistent results (online supplementary additional file 2).
Discussion
Our study shows that prolonged, concurrent and daily use of CNSDs is prevalent among hospitalised older patients. The z-hypnotics were the most commonly used drugs, both in exclusive and concurrent use patterns (with other CNSDs). In this particular group of patients, the presence of prolonged CNSD use was more common among patients with the following characteristics: being female, aged 75–84 years old, living alone, with lower socioeconomic status, polypharmacy, higher pain intensity and depression scores. Patients with misuse or dependence, according to the DSM-IV criteria, were mainly those living alone and those on concurrent use of several CNSDs. The odds for prolonged CNSD use were higher among patients aged ≥75 years old and those with higher pain intensity and polypharmacy. Having completed secondary education was protective against the prolonged use. In older patients, enhanced pain and concurrent use of ≥2 different types of CNSDs rather than the duration of use increased the likelihood of misuse or dependence.
One of the strengths of this study is that it provides evidence on the characteristics of older patients with prolonged CNSD use, misuse and dependence, from many different aspects (sociodemographic, pharmacological and clinical profiles). We adhered strictly to the STROBE reporting guidelines and ethical standards, and outcome measures were predefined. Moreover, we used validated and generally accepted criteria (DSM-IV criteria) to assess medication misuse and dependence in older patients. Nonetheless, the study has some limitations. We acknowledge that the use of a consecutive hospital-based sample represents a limitation regarding generalisability of the study findings to the general population. However, our study should be reasonably representative for somatic hospital populations of older people. We suggest that hospitals may be good settings for conducting research on medication-related problems as they represent settings where older patients often get their medication regimens changed and also where they may therefore be at risk for adverse drug events and, consequently, where this focus is important. Furthermore, it has recently been pointed out that long-term use of benzodiazepine/z-hypnotics often started in hospitals and the prescription is continued by GPs.21 Another issue, which suggests some care in the interpretation of our results, is the relatively high number of patients who declined participation. It may be that those who declined represent either those with the most serious medical conditions or those who were not interested in being queried regarding their medication use, thus suggesting that our sample may be somewhat biased towards milder cases. In addition, the use of cross-sectional data precludes us from inferring causality of the observed associations.
Our study delivers a number of new and important insights pertaining to medication misuse and dependence in older patients. First, our study showed that concurrent use of CNSDs is still common among older patients. This is despite recommendations specified in the national treatment guidelines and evidence on the risk of fatal overdose.22–26 Second, we comprehensively explored patient characteristics associated with the presence of CNSD prolonged use among hospitalised older patients, which may be useful for raising the awareness of patient groups that may be at increased risk.
Among geriatric patients, age-related pharmacokinetic and pharmacodynamics changes, chronic diseases, polypharmacy, and cognitive impairment can all interact and lead to greater vulnerability to harmful effects of medication overuse. According to both the Norwegian General Practice criteria (NORGEP) and international (Beers and the screening tool of older people's prescriptions (STOPP)) criteria27–29 and treatment guidelines,22 23 opioid analgesics, benzodiazepines and z-hypnotics are all classified as inappropriate drugs for older patients and should not be used on a long-term basis. Our findings are consistent with three studies previously conducted in Norway,6 16 30 and suggest that today’s prescribing behaviour is suboptimal and that more indepth research and educational interventions are needed.31 32
Problematic patterns of CNSD use may derive from different underlying factors. Overprescribing is often unintentional, due to lenient prescribing or unawareness among prescribers about the uncertainty of long-term efficacy of CNSDs.33 34 Another factor may be the failure to recognise at-risk patients, which may be a consequence of lacking validated instruments. Apart from this, doctor–patient communication may also play a role. Messages on the importance of adherence in order to avoid harmful effects of medication overuse, for instance, may not be conveyed effectively to older patients. For the older patient counterpart, potential barriers to understanding and adhering to medical advice may include cognitive impairment, low health literacy and lack of family/social support.35 36 Furthermore, older patients may hold opposing attitudes regarding the discontinuation of CNSD use,21 if adverse effects are underemphasised and hopes of attaining freedom from unnecessary pain, anxiety and sleep disturbances in old age based only on medications are overstated. Physicians may also experience other challenges in managing CNSD use.37 Both the magnitude and impacts of such underlying factors on the prolonged use of CNSDs in older patients remain poorly understood and should therefore be elaborated in future research.
Also of note, we found that the concurrent use of CNSDs significantly increased the odds for misuse or dependence in older patients, even adjusted for patients’ sociodemographic background and clinically important covariates such as duration of CNSD use and intensity of pain, anxiety and depressive symptoms (table 3). This finding suggests that coprescribing of CNSDs for older patients should be done with great care. Moreover, the present study points out a significant association between polypharmacy and CNSD prolonged use in older patients. This, to our knowledge, has not been explored by previous research. Such associations can be explained by several factors. Studies suggest that polypharmacy is associated with the co-occurrence of anxiety, sleep difficulties and discomforts,38–40 and might through unknown side effects aggravate these conditions, in turn leading to more CNSDs being prescribed for symptom relief.41
Pertaining to our finding that living alone in old age is not associated with medication prolonged use and misuse or dependence, previous research yielded inconsistent results. Some reported that older adults who lived alone had significantly poorer sleep quality and tended to use more hypnotic drugs,42 43 whereas others claimed that living alone is not associated with or even reduced the risk of long-term use of benzodiazepine and z-hypnotics.44–46 The issue clearly deserves further focus.
Finally, we found that pain intensity has a significant relationship with both prolonged use and misuse or dependence. This may suggest that pain intensity drives problematic use of CNSDs. However, another possibility is that prolonged opioid use does not contribute to improving pain. That opioid-induced hyperalgesia may worsen pain is well known among younger chronic opioid users.47 48 However, whether pain may indeed be worsened also by prolonged CNSD use among older patients remains to be studied. Notably, anxiety and depression, known to be associated with pain intensity, were not, in our study, associated with prolonged CNSD use and misuse or dependence, even though they were reported to be common among chronic z-hypnotics users (the major medication used in our sample).49 50 The inter-relationship between pain, anxiety and depression is complex.51 52 Future prospective studies over time are therefore needed to explain the interplay between these entities and their influences on CNSD dependence.
In conclusions, CNSD overuse (prolonged use, misuse and dependence) is still prevalent among hospitalised older patients, despite clear guidelines and recommendations. Our findings underline a need for stronger focus on responsible prescribing, timely detection and prevention of medication misuse and dependence, with special attention towards older patients, those with enhanced pain, polypharmacy and/or concurrent use of several CNSDs.
Supplementary Material
Acknowledgments
We express our appreciation to all participants for spending their time and effort on this study. We are thankful to all medical staff at the three departments of Akershus University Hospital (geriatrics, neurology and endocrinology) for facilitating the data collection process. We also gratefully acknowledge the statistical support of Professor Jurate Š Benth.
Footnotes
Contributors: CL conceptualised, designed and led the study. SC, ESK and CL drafted the study protocol. SC customised the protocol, recruited participants, collected and analysed the data, and drafted this manuscript. TGS was involved in the recruitment and data collection process. MG contributed to the study conception and design. All authors took part in project planning and were involved in the refinement and approval of the final version of this manuscript.
Funding: This study was funded by a grant from the Norwegian Research Council (project number 256431), the University of Oslo and the Health Services Research Unit of the Akershus University Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: CL reports having received research grants from the South-East Regional Health Authority, during the conduct of the study, as well as grants and personal fees from AbbVie Pharma and Roche Norway, outside this submitted work.
Ethics approval: Ethical approval was obtained from the Regional Committee for Medical Research Ethics – South East Norway (reference number: 2016/2289/REK sør-øst) and the Data Protection Office at Akershus University Hospital (reference number: 17-054). All participants provided written informed consents. Data handling was done accordingly.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. World Health Organization Lexicon of alcohol and drug terms. Geneva: World Health Organization, 1994. [Google Scholar]
- 2. Del Carmen Panini A, Teves MR, Giraudo E. Psychotropic Medication Use in the Elderly : PÁ G, Mesones-Arroyo HL, Psychiatry and Neuroscience Update - Vol II: A Translational Approach. Cham: Springer International Publishing, 2017: 293–306. [Google Scholar]
- 3. Chu LF, Angst MS, Clark D. Opioid-Induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 2008;24:479–96. 10.1097/AJP.0b013e31816b2f43 [DOI] [PubMed] [Google Scholar]
- 4. Berg C, Blix HS, Fenne O, Furu K, Hjellvik V, Husabø KJ. Drug use in the elderly. Norwegian prescription database 2013–2017. Oslo: Norwegian Institute of Public Health; 2018. [Google Scholar]
- 5. Kann IC, Lundqvist C, Lurås H. Prescription of addictive and non-addictive drugs to home-dwelling elderly. Drugs Aging 2014;31:453–9. 10.1007/s40266-014-0169-1 [DOI] [PubMed] [Google Scholar]
- 6. Sundseth AC, Gjelstad S, Straand J, et al. General practitioners' prescriptions of benzodiazepines, Z-hypnotics and opioid analgesics for elderly patients during direct and indirect contacts. A cross-sectional, observational study. Scand J Prim Health Care 2018;36:115–22. 10.1080/02813432.2018.1459164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng S, Siddiqui TG, Gossop M, et al. The severity of dependence scale detects medication misuse and dependence among hospitalized older patients. BMC Geriatr 2019;19:174 10.1186/s12877-019-1182-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heider D, Matschinger H, Meid AD, et al. Health service use, costs, and adverse events associated with potentially inappropriate medication in old age in Germany: retrospective matched cohort study. Drugs Aging 2017;34:289–301. 10.1007/s40266-017-0441-2 [DOI] [PubMed] [Google Scholar]
- 9. Levesque A, Nunes EV. Addiction in the older patient. Recognizing Addiction In Older Patients 2016. [Google Scholar]
- 10. Karlawish J. Measuring decision-making capacity in cognitively impaired individuals. Neurosignals 2008;16:91–8. 10.1159/000109763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Folstein MF, Folstein SE, McHugh PR, et al. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 12. Helvik A-S, Engedal K, Skancke RH, et al. A psychometric evaluation of the hospital anxiety and depression scale for the medically hospitalized elderly. Nord J Psychiatry 2011;65:338–44. 10.3109/08039488.2011.560684 [DOI] [PubMed] [Google Scholar]
- 13. Djukanovic I, Carlsson J, Årestedt K. Is the Hospital Anxiety and Depression Scale (HADS) a valid measure in a general population 65-80 years old? A psychometric evaluation study. Health Qual Life Outcomes 2017;15:193 10.1186/s12955-017-0759-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bell ML, Fairclough DL, Fiero MH, et al. Handling missing items in the hospital anxiety and depression scale (HADS): a simulation study. BMC Res Notes 2016;9:479–79. 10.1186/s13104-016-2284-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ashton H. Guidelines for the rational use of benzodiazepines. when and what to use. Drugs 1994;48:25–40. 10.2165/00003495-199448010-00004 [DOI] [PubMed] [Google Scholar]
- 16. Sakshaug S, Handal M, Hjellvik V, et al. Long-Term use of Z-Hypnotics and co-medication with benzodiazepines and opioids. Basic Clin Pharmacol Toxicol 2017;120:292–8. 10.1111/bcpt.12684 [DOI] [PubMed] [Google Scholar]
- 17. Mordal J, Gundersen Ø., Bramness JG. Norwegian version of the Mini-International neuropsychiatric interview: feasibility, acceptability and test-retest reliability in an acute psychiatric ward. Eur Psychiatry 2010;25:172–7. 10.1016/j.eurpsy.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 18. American Psychiatric Association Diagnostic and statistical manual of mental disorders. 4th edition Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
- 19. Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65:989–95. 10.1016/j.jclinepi.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 20. StataCorp Stata statistical software: release 15. College Station, TX: StataCorp LLC, 2017. [Google Scholar]
- 21. Mokhar A, Kuhn S, Topp J, et al. Long-term use of benzodiazepines and Z drugs: a qualitative study of patients’ and healthcare professionals’ perceptions and possible levers for change. BJGP Open 2019;28 10.3399/bjgpopen18X101626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Helsedirektoratet Nasjonal faglig veileder vanedannende legemidler - rekvirering og forsvarlighet. [National guidelines - addictive drugs] The Norwegian Directorate of Health; 2015. [Google Scholar]
- 23. Helsedirektoratet Nasjonal faglig veileder for bruk av opioider - ved langvarige ikke-kreftrelaterte smerter. [National guidelines for the use of opioids] The Norwegian Directorate of Health; 2016. [Google Scholar]
- 24. Hernandez I, He M, Brooks MM, et al. Exposure-Response association between concurrent opioid and benzodiazepine use and risk of Opioid-Related overdose in Medicare Part D beneficiaries. JAMA Netw Open 2018;1:e180919–e19. 10.1001/jamanetworkopen.2018.0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun EC, Dixit A, Humphreys K, et al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017;356 10.1136/bmj.j760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garg RK, Fulton-Kehoe D, Franklin GM. Patterns of opioid use and risk of opioid overdose death among Medicaid patients. Med Care 2017;55:661–8. 10.1097/MLR.0000000000000738 [DOI] [PubMed] [Google Scholar]
- 27. Nyborg G, Straand J, Klovning A, et al. The Norwegian general practice – nursing home criteria (NORGEP-NH) for potentially inappropriate medication use: a web-based Delphi study. Scand J Prim Health Care 2015;33:134–41. 10.3109/02813432.2015.1041833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panel A, Fick DM, Semla TP, et al. American geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63:2227–46. 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 29. Hamilton H, Gallagher P, Ryan C, et al. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized PatientsPIMs defined by STOPP criteria and risk of ADEs. JAMA Internal Medicine 2011;171:1013–9. [DOI] [PubMed] [Google Scholar]
- 30. Neutel CI, Skurtveit S, Berg C. Benzodiazepine and z-hypnotic use in Norwegian elderly, aged 65-79. Nor Epidemiol 2012;22 10.5324/nje.v22i2.1567 [DOI] [Google Scholar]
- 31. Voepel-Lewis T, Tait AR, Becher A, et al. An interactive web-based educational program improves prescription opioid risk knowledge and perceptions among parents. Pain Manag 2019;9:369–77. 10.2217/pmt-2019-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kline TV, Savage RL, Greenslade JH, et al. Affecting emergency department oxycodone discharge prescribing: an educational intervention. Emergency Medicine Australasia 2019;31:580–6. 10.1111/1742-6723.13261 [DOI] [PubMed] [Google Scholar]
- 33. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health 2015;36:559–74. 10.1146/annurev-publhealth-031914-122957 [DOI] [PubMed] [Google Scholar]
- 34. Weiß V, Nau R, Glaeske G, et al. The interplay of context factors in hypnotic and sedative prescription in primary and secondary care-a qualitative study. Eur J Clin Pharmacol 2019;75:87–97. 10.1007/s00228-018-2555-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kvarnström K, Airaksinen M, Liira H. Barriers and facilitators to medication adherence: a qualitative study with general practitioners. BMJ Open 2018;8:e015332 10.1136/bmjopen-2016-015332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. JWY K, Khoo HS, Lim I, et al. Communication skills in Patient-Doctor interactions: learning from patient complaints. Health Professions Education 2018;4:97–106. [Google Scholar]
- 37. Sirdifield C, Anthierens S, Creupelandt H, et al. General practitioners' experiences and perceptions of benzodiazepine prescribing: systematic review and meta-synthesis. BMC Fam Pract 2013;14:191 10.1186/1471-2296-14-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wongpakaran N, Wongpakaran T, Sirirak T, et al. Predictors of polypharmacy among elderly Thais with depressive and anxiety disorders: findings from the DAS study. BMC Geriatr 2018;18:309 10.1186/s12877-018-1001-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ancoli-Israel S, Shochat T. Chapter 135 - Insomnia in Older Adults A2 - Kryger, Meir H : Roth T, Dement WC, Principles and practice of sleep medicine. Fifth Edition Philadelphia: W.B. Saunders, 2011: 1544–50. [Google Scholar]
- 40. Rambhade S, Shrivastava A, Rambhade A, et al. A survey on polypharmacy and use of inappropriate medications. Toxicol Int 2012;19:68 10.4103/0971-6580.94506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. YWF L. Polypharmacy in elderly opioid users. The Brown University Psychopharmacology Update 2019;30:2–3. [Google Scholar]
- 42. Hägg M, Houston B, Elmståhl S, et al. Sleep quality, use of hypnotics and sleeping habits in different age-groups among older people. Scand J Caring Sci 2014;28:842–51. 10.1111/scs.12119 [DOI] [PubMed] [Google Scholar]
- 43. Yu B, Steptoe A, Niu K, et al. Prospective associations of social isolation and loneliness with poor sleep quality in older adults. Qual Life Res 2018;27:683–91. 10.1007/s11136-017-1752-9 [DOI] [PubMed] [Google Scholar]
- 44. Mokhar A, Tillenburg N, Dirmaier J, et al. Potentially inappropriate use of benzodiazepines and z-drugs in the older population-analysis of associations between long-term use and patient-related factors. PeerJ 2018;6:e4614–e14. 10.7717/peerj.4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luijendijk HJ, Tiemeier H, Hofman A, et al. Determinants of chronic benzodiazepine use in the elderly: a longitudinal study. Br J Clin Pharmacol 2008;65:593–9. 10.1111/j.1365-2125.2007.03060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Proulx-Tremblay V, Allary A, Payette M-C, et al. Social support and sleep quality in older benzodiazepine users. Aging Ment Health 2019;48:1–7. 10.1080/13607863.2019.1594167 [DOI] [PubMed] [Google Scholar]
- 47. Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011;14:145–61. [PubMed] [Google Scholar]
- 48. Mitra S. Opioid-Induced hyperalgesia: pathophysiology and clinical implications. J Opioid Manag 2018;4:123–30. 10.5055/jom.2008.0017 [DOI] [PubMed] [Google Scholar]
- 49. Kripke DF. Greater incidence of depression with hypnotic use than with placebo. BMC Psychiatry 2007;7:42 10.1186/1471-244X-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abolhassani N, Haba-Rubio J, Heinzer R, et al. Ten-Year trend in sleeping pills use in Switzerland: the CoLaus study. Sleep Med 2019. 10.1016/j.sleep.2018.06.022 [DOI] [PubMed] [Google Scholar]
- 51. Means-Christensen AJ, Roy-Byrne PP, Sherbourne CD, et al. Relationships among pain, anxiety, and depression in primary care. Depress Anxiety 2008;25:593–600. 10.1002/da.20342 [DOI] [PubMed] [Google Scholar]
- 52. Sheng J, Liu S, Wang Y, et al. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast 2017;2017:1–10. 10.1155/2017/9724371 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-031483supp001.pdf (53.6KB, pdf)
bmjopen-2019-031483supp002.pdf (24.9KB, pdf)