Abstract
A 20-year-old male patient presented to our emergency surgery department with blunt trauma to the abdomen and in a state of shock. The patient was resuscitated and a Contrast-Enhanced Computed Tomography (CECT) was done which showed a grade 2 liver injury involving segment VIII. The patient was managed conservatively and discharged after 8 days. The patient again presented after 3 weeks with severe anaemia, fever and melena. An upper gastrointestinal endoscopy revealed bile mixed with blood at the ampulla of Vater, consistent with haemobilia. CT angiography showed grade 2 injury of the liver with large haematoma in segment VIII. A large right subcapsular collection, a saccular area consistent with pseudoaneurysm of the replaced right hepatic artery arising from the superior mesenteric artery, was seen. A replaced left hepatic artery arising from the left gastric artery was also observed. The patient underwent right hepatic artery coil embolisation, with postprocedure digital subtraction scan showing no extravasation of contrast. The patient recovered well in the follow-up.
Keywords: radiology (diagnostics), surgical diagnostic tests, trauma, interventional radiology, vascular surgery
Background
Pseudoaneurysm of the visceral arteries is very rare (0.01%–0.2%).1 2 Blunt trauma to the abdomen is a very uncommon cause of pseudoaneurysm of the visceral arteries. Hepatic artery pseudoaneurysm is an uncommon entity and pseudoaneurysm from a replaced hepatic artery is even rarer. Pseudoaneurysms of the hepatic artery can present from asymptomatic to intermittent or life-threatening haemorrhage.3–5 Surgical resection has been the mainstream management of pseudoaneurysms for a long time. With new interventions such as percutaneous and minimally invasive angiographic techniques, these patients can be treated without an incision, significantly reducing overall morbidity and mortality.6
In our case, the patient presented with recurrent episodes of melena after sustaining a blunt trauma to the abdomen which was diagnosed to be pseudoaneurysm of the right replaced hepatic artery and managed by percutaneous angiographic coil embolisation.
Case presentation
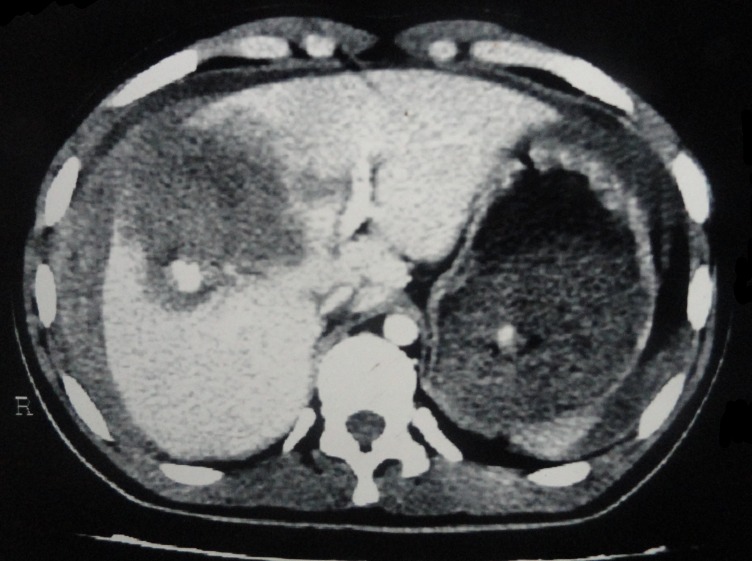
A 20-year-old male patient presented to surgery emergency after 8 hours of a road traffic accident with blunt trauma to the abdomen. The patient was dehydrated, with a pulse rate of 110 per minute, blood pressure of 90/70 mm Hg and pallor. The patient’s haemoglobin was 8.1 g/L. The patient was resuscitated and haemodynamically stabilised. A Contrast-Enhanced Computed Tomography (CECT) showed 2 cm liver laceration and a haematoma 8.1×5.4×6.8 cm in size in segment VIII extending to the subhepatic space and the right paracolic gutter with a moderate amount of haemoperitoneum (figure 1). No other visceral injuries were noted. The patient was managed conservatively, received 2 units of packed cells and no further fall in haemoglobin was noticed. The patient improved and was discharged after 8 days.
Figure 1.
Pre-embolisation Contrast-Enhanced Computed Tomography of the abdomen showing liver laceration and haematoma.
The patient again presented after 3 weeks with high-grade fever and black tarry stool. On examination, the patient was febrile with severe pallor and haemoglobin of 6.5g/L. The patient was again transfused 2 units of packed cells and stabilised. The cause of fever was speculated to be infected pseudocyst or infected haematoma; however, the blood cultures (two, at 24-hour interval) came to be ‘no growth’.
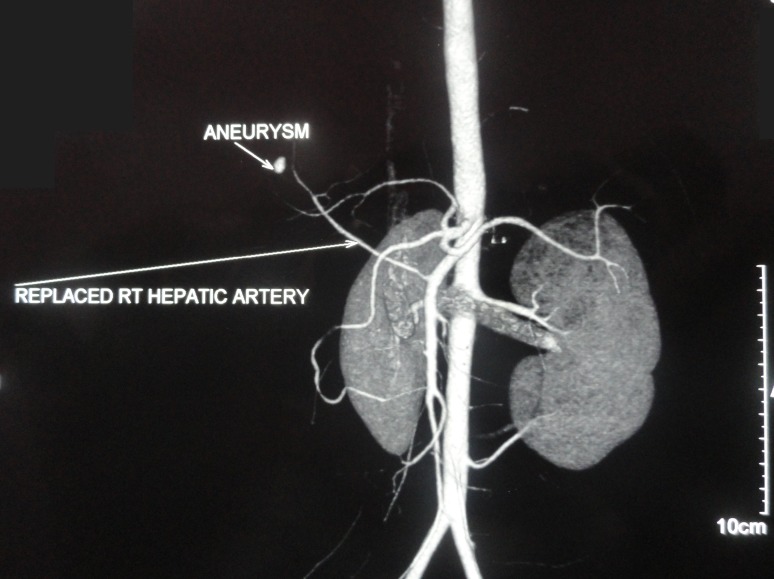
An upper gastrointestinal (GI) endoscopy showed bile mixed with blood at the ampulla, suggesting haemobilia. CT angiography showed large haematoma of size 9×7×8.2 cm in segment VIII of the right lobe, with 2.4×1.5 cm laceration in segment VIII extending up to the liver surface, along with a subcapsular collection along segments VI, VII and VIII of the right lobe extending inferiorly along the hepatorenal space. A replaced right hepatic artery arising from the superior mesenteric artery was observed along with replaced left hepatic artery arising from the left gastric artery. An 11×7 mm-sized saccular area of contrast pooling within the haematoma was seen in proximity to the anterior branch of the right replaced hepatic artery, consistent with pseudoaneurysm (figure 2).
Figure 2.
Pre-embolisation CT angiography showing replaced right (RT) hepatic artery from the superior mesenteric artery.
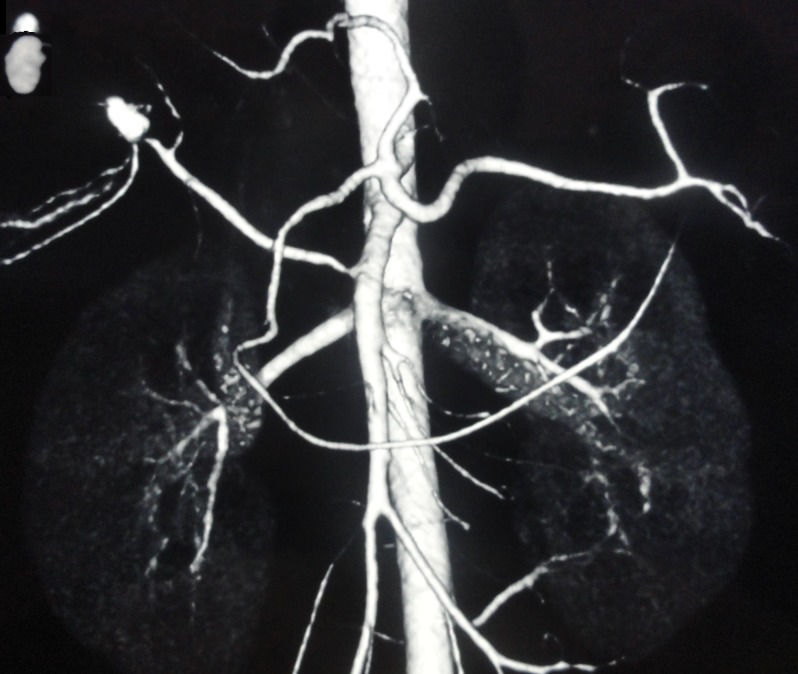
Digital Subtraction Angiography (DSA) showed extravasation of contrast at a distal end of the right hepatic artery. The patient underwent hepatic artery coil embolisation with two coils of 0.15–2 mm and one coil of 0.35–3 mm placed in the right hepatic artery proximal to the extravasation. Repeat DSA was done which showed no extravasation (figure 3).
Figure 3.
Postembolisation status of the pseudoaneurysm.
Investigations
CECT (at first presentation) showed 2 cm liver laceration and a haematoma of size 8.1×5.4×6.8 cm in segment VIII extending to the subhepatic space and the right paracolic gutter with a moderate amount of haemoperitoneum. No free air or other visceral injury was seen.
Upper GI endoscopy (at later presentation) showed bile mixed with blood at the ampulla, suggesting haemobilia.
CT angiography (at later presentation) showed large haematoma of 9×7×8.2 cm in size, with 2.4×1.5 cm laceration in segment VIII extending up to the liver surface. A subcapsular collection along segments VI, VII and VIII of the right lobe was seen extending inferiorly along the hepatorenal space. An 11×7 mm-sized saccular area of contrast pooling within the haematoma in proximity to the anterior branch of the right hepatic artery, consistent with pseudoaneurysm of the replaced right hepatic artery arising from superior mesenteric artery (SMA), was observed along with replaced left hepatic artery arising from the left gastric artery.
Treatment
The patient underwent replaced right hepatic artery coil embolisation. DSA showed extravasation of contrast at the distal end of the right hepatic artery. Two coils of 0.15–2 mm and one coil of 0.35–3 mm were placed in the right hepatic artery proximal to extravasation. Repeat DSA was done after 2 weeks which showed no extravasation.
Outcome and follow-up
The patient recovered well thereafter and is healthy up to 8 months of follow-up.
Discussion
Hepatic artery pseudoaneurysm is an uncommon entity and pseudoaneurysm from the replaced hepatic artery is even rarer.1 Iatrogenic causes are the most common causes, but it is also associated with trauma (4%), infection (0.3%) and abdominal inflammation.3
Most of the pseudoaneurysms are formed as a result of damage to arterial wall continuity due to inflammation, trauma, or iatrogenic causes such as surgical procedure, percutaneous biopsy or drainage.7 Under the impact of persistent blood pressure, blood percolates through the intima of the damaged artery into the tissues and forms a perfused sac that connects with the arterial lumen. This sac is enclosed by the media or adventitia or simply by the constructions of soft tissues that surround the wounded vessel.8
A replaced hepatic artery is a vessel that does not originate from the common hepatic artery. The most common site for the origin of an aberrant or replaced artery is the superior mesenteric artery (11%).1 It is classified as type 3 variant of the hepatic anomaly by Michel et al,9 which is seen in our case. A right replaced or aberrant hepatic artery has an incidence of 5%–25% in the general population.10 However, in recent years, the incidence of replaced right hepatic artery arising from the superior mesenteric artery is 3.7% of all cases.11
Tessier et al 6 reported haemobilia (64%) as the most common presenting symptom, and others being haematemesis (30%), haematochezia (14%), abdominal pain (20%) and some asymptomatic patients (10%). Unusual presentations depend on the size and location of the pseudoaneurysm, including compressive symptoms such as jaundice and pruritus.8 Catastrophic outcomes include haemobilia and delayed haemorrhage with free rupture up to 2 weeks. The reported rate of rupture in pseudoaneurysm ranges from 21% to 80%, with a mortality of 69%–100%.6
Modern imaging modalities enable early detection and therapeutic treatment before the catastrophic results of pseudoaneurysm manifest clinically. These include endoscopic procedures which may show bleeding from the ampulla of Vater and help rule out other causes of bleeding. Angiography can accurately diagnose pseudoaneurysm and is also useful in identifying additional aneurysms in the vascular anatomy (20% were multiple).12–14 It also provides therapeutic options for embolisation or stenting. Pseudoaneurysm of the hepatic arterial vasculature has a tendency for continuous enlargement and eventual rupture.
Symptomatic pseudoaneurysm must be treated. The treatment for asymptomatic patients is unclear. It varies according to the different anatomical sites of pseudoaneurysm and patient comorbidities. Some can be spontaneously thrombosed, and others are asymptomatic. However, currently, it is very difficult to predict spontaneous thrombosis in pseudoaneurysm.12–14
There is a paradigm change in therapeutic options. Less invasive approaches have replaced surgical management, and have dramatically decreased the rates of morbidity and mortality.15–17 Endovascular treatment, particularly coil embolisation, has become a therapeutic intervention of choice. When embolising the right and left hepatic arteries, flow is established immediately in the intrahepatic translobar collaterals and no infarction occurs.11 In our case, the patient was treated with coil embolisation of the replaced right hepatic artery. The bleeding was arrested in a single procedure.
Surgery is considered in patients with failure of embolisation or have coexisting complications requiring surgical intervention, and surgical management includes ligation or excision of the pseudoaneurysm. Embolisation is associated with a 25% lower mortality and 67% less morbidity in comparison with surgical intervention.8
Learning Points.
Pseudoaneurysms arise from a disruption in arterial wall continuity resulting from inflammation, trauma, or iatrogenic causes such as surgical procedures, percutaneous biopsy or drainage.
Symptomatic pseudoaneurysms (intermittent or continuous bleeding) should be treated.
There is an increasing paradigm shift towards minimally invasive management of pseudoaneurysms as a consequence of technological developments in ultrasound-guided and endoluminal management of pseudoaneurysms.
Conventional angiography remains the reference standard for diagnosis, but being invasive new non-invasive diagnostic methods should be included in the initial work-up.
Complete work-up is crucial in selecting the therapeutic method to determine the position of the pseudoaneurysm and to assess adjacent structures and appropriate vascular anatomy.
Footnotes
Contributors: RK: research, preparation of the manuscript and submission. LB: consultant, guidelines for management of the case and primary research work. RS: preparation of the manuscript, research and corrections. DK: preparation of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Gachabayov M, Kubachev K, Mityushin S, et al. Recurrent hemobilia due to right hepatic artery pseudoaneurysm. Clin Med Res 2017;15:96–9. 10.3121/cmr.2017.1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker KS, Tisnado J, Cho SR, et al. Splanchnic artery aneurysms and pseudoaneurysms: transcatheter embolization. Radiology 1987;163:135–9. 10.1148/radiology.163.1.3823426 [DOI] [PubMed] [Google Scholar]
- 3. Velmahos GC, Demetriades D, Chahwan S, et al. Angiographic embolization for arrest of bleeding after penetrating trauma to the abdomen. Am J Surg 1999;178:367–73. 10.1016/S0002-9610(99)00212-3 [DOI] [PubMed] [Google Scholar]
- 4. Croce MA, Fabian TC, Spiers JP, et al. Traumatic hepatic artery pseudoaneurysm with hemobilia. Am J Surg 1994;168:235–8. 10.1016/S0002-9610(05)80193-X [DOI] [PubMed] [Google Scholar]
- 5. Arata MA, Cope C. Principles used in the management of visceral aneurysms. Tech Vasc Intervent Radiol 2000;3:124–9. [Google Scholar]
- 6. Tessier DJ, Fowl RJ, Stone WM, et al. Iatrogenic hepatic artery pseudoaneurysms: an uncommon complication after hepatic, biliary, and pancreatic procedures. Ann Vasc Surg 2003;17:663–9. 10.1007/s10016-003-0075-1 [DOI] [PubMed] [Google Scholar]
- 7. Reber PU, Baer HU, Patel AG, et al. Superselective microcoil embolization: treatment of choice in high-risk patients with extrahepatic pseudoaneurysms of the hepatic arteries. J Am Coll Surg 1998;186:325–30. 10.1016/S1072-7515(98)00032-5 [DOI] [PubMed] [Google Scholar]
- 8. Lumsden AB, Mattar SG, Allen RC, et al. Hepatic artery aneurysms: the management of 22 patients. J Surg Res 1996;60:345–50. 10.1006/jsre.1996.0055 [DOI] [PubMed] [Google Scholar]
- 9. Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966;112:337–47. 10.1016/0002-9610(66)90201-7 [DOI] [PubMed] [Google Scholar]
- 10. Modi R, Patted SV, Halkati PC, et al. CHA2DS2-VASc-HSF score - New predictor of severity of coronary artery disease in 2976 patients. Int J Cardiol 2017;228:1002–6. 10.1016/j.ijcard.2016.10.093 [DOI] [PubMed] [Google Scholar]
- 11. Noussios G, Dimitriou I, Chatzis I, et al. The Main Anatomic Variations of the Hepatic Artery and Their Importance in Surgical Practice: Review of the Literature. J Clin Med Res 2017;9:248–52. 10.14740/jocmr2902w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Driscoll D, Olliff SP, Olliff JF. Hepatic artery aneurysm. Br J Radiol 1999;72:1018–25. 10.1259/bjr.72.862.10673957 [DOI] [PubMed] [Google Scholar]
- 13. Messina LM, Shanley CJ. Visceral artery aneurysms. Surg Clin North Am 1997;77:425–42. 10.1016/S0039-6109(05)70559-4 [DOI] [PubMed] [Google Scholar]
- 14. Abbas MA, Fowl RJ, Stone WM, et al. Hepatic artery aneurysm: factors that predict complications. J Vasc Surg 2003;38:41–5. 10.1016/S0741-5214(03)00090-9 [DOI] [PubMed] [Google Scholar]
- 15. Yoon W, Jeong YY, Kim JK, et al. CT in blunt liver trauma. Radiographics 2005;25:87–104. 10.1148/rg.251045079 [DOI] [PubMed] [Google Scholar]
- 16. Sun L, Guan YS, Wu H, et al. Post-traumatic hepatic artery pseudo-aneurysm combined with subphrenic liver abscess treated with embolization. World J Gastroenterol 2006;12:2798–9. 10.3748/wjg.v12.i17.2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dambrin C, Marcheix B, Birsan T, et al. Posttraumatic pseudoaneurysm of the hepatic artery: treatment with ultrasound-guided percutaneous transhepatic thrombin injection. J Trauma 2005;59:239–42. 10.1097/01.TA.0000171526.24911.B2 [DOI] [PubMed] [Google Scholar]