Abstract
Tularaemia is a rare infectious disease endemic in most European countries caused by the bacterium Francisella tularensis. 1 Patients often show acute non-specific symptoms, which causes a delay in diagnosis and proper treatment, potentially resulting in significant morbidities such as deep neck abscess, meningitis, endocarditis and septic shock. The authors present a case of a 5-year old boy with a 4-day history of fever, sore throat and painful cervical lymphadenopathy, whose clinical progression worsened despite being treated with recommended antibiotics as per WHO guidelines once the diagnosis of Tularaemia was confirmed by serologic tests. He developed a parapharyngeal abscess and a persistent left necrotic cervical lymph node, which both were surgically drained and excised, respectively, and an extended course of antibiotic was given. Subsequently, the patient fully recovered from the illness and the follow-up was negative for relapse.
Keywords: otolaryngology/ent, infectious diseases, paediatrics
Background
Tularaemia, also known as rabbit fever, is an infectious disease caused by the bacterium Francisella tularensis. The European Centre For Disease Preventation And Control reported in their latest surveillance report that the number of cases in 2015 have more than doubled compared with 2014. In 2015, the reported rate was 0.25 cases/100 000 population among the participating European countries.2 Data from Switzerland indicate constant increase since 2008 and within 2 years (2015–2017), incidence for tularaemia rose from 0.59/100 000 to 1.55/100 000, decreasing only slightly to 1.33/100 000 in 2018.3
Diagnosis of tularaemia is challenging due to its unspecific influenza-like symptoms with fever, lymphadenopathy or a sore throat.4 However, its diagnosis is essential in order to initiate appropriate treatment. Untreated patients with tularaemia can have significant complications and may need surgical treatment in the follow-up. Given the rising incidence of tularaemia, it is essential to raise awareness among physicians for this disease. Therefore, we report about a 5-year-old boy with tularaemia initially exhibiting with tonsillitis and lymphadenopathy. In this case report, we discuss the aetiology, diagnosis and differential diagnosis of tularaemia.
Case presentation
A previously healthy 5-year-old boy presented himself to our otorhinolaryngology clinic with a 4-day history of persistent fever, sore throat, cough and headache. He was previously treated by his general practitioner for viral infection on day 2 of illness. Physical examination revealed tender bilateral cervical lymphadenopathy, inflamed tonsils and pharyngeal mucosa. Laboratory investigations revealed normal full blood count and normal streptococcal smear from a throat swab. However, his C reactive protein was elevated at 78 mg/L. He was treated for acute bacterial pharyngotonsillitis with intravenous amoxicillin–clavulanate (100 mg/kg/day) and discharged after 4 days when his sore throat resolved.
On follow-up, 2 days after discharge and on day 8 of illness, he complained of persistent fever and worsening of left cervical lymphadenopathy. He was readmitted, commenced on intravenous amoxicillin–clavulanate (100 mg/kg/day) and investigated thoroughly with blood cultures, Mantoux test, influenza test, serology tests for Epstein-Barr virus, cytomegalovirus, toxoplasmosis, Bartonella henselae, brucellosis and tularaemia. On day 15 of the disease, all serology tests were negative except for tularaemia. Immunoglobulin M titre was highly elevated at 168.6 U/mL. MRI revealed a left level II cervical lymph node with a necrotic centre of 22×20 mm size. He was then commenced on intravenous gentamicin (6 mg/kg/day) for 10 days as per WHO guidelines.5
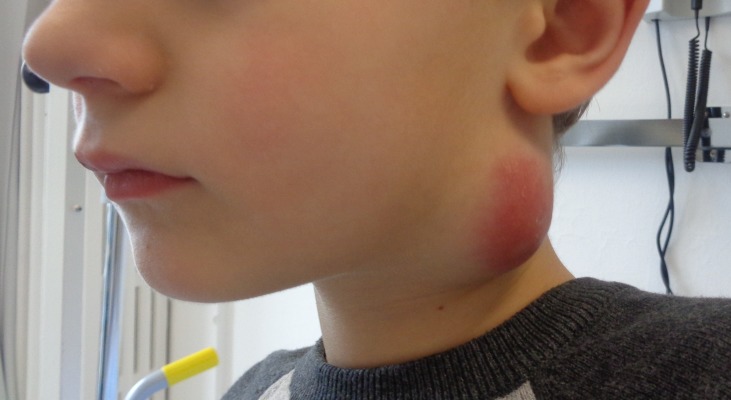
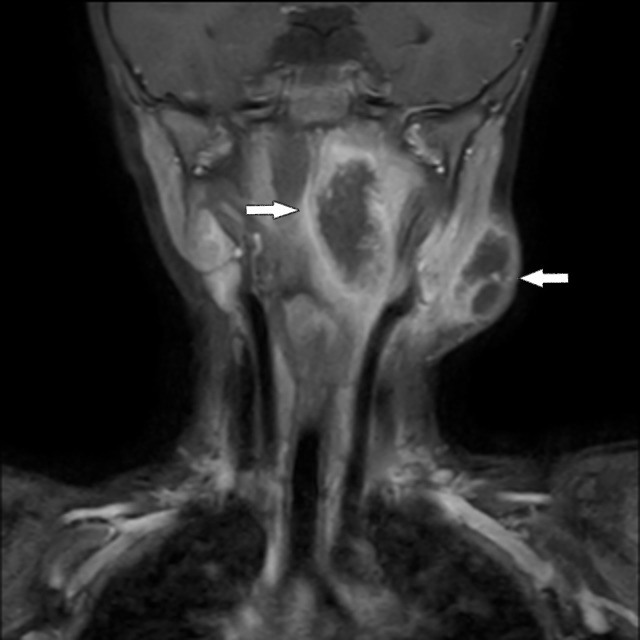
He was discharged after completing the antibiotic course. However, he was readmitted on day 28 of illness with persistent fever, dysphagia and worsening left-sided neck swelling (figure 1). A repeated cervical MRI showed a left parapharyngeal abscess with the similar necrotic lymph node with an increased size of 24×34 mm (figure 2, see the arrows). The abscess was drained and the left cervical lymph node was excised. He was put on a course of intravenous gentamicin (6 mg/kg/day) for another 2 weeks and discharged with oral doxycycline for a total of 2 weeks. He was well and free of illness on follow-up 4 weeks after discharge.
Figure 1.
Clinical picture of the boy with the suppurative, necrotic lymph node.
Figure 2.
Contrast-enhanced coronal T1-weighted MRI scan of the head and neck. It demonstrates the necrotic lymph node and parapharyngeal abscess.
Investigations
Diagnostic gold standard are serological tests. However, they can be negative during the first 2 weeks following the onset of the disease, as antibody titres cannot be detected in the early stage of the disease.4
Differential diagnosis
There is a long list of differential diagnoses. Reasons for unilateral lymphadenopathy are: atypical mycobacterial infection, carcinoma of unknown primary, lymph node metastasis, B. henselae (also known as Cat-scratch disease), Kawasaki disease, Kikuchi disease, lymphoma, toxoplasmosis, tuberculosis and tularaemia.
Reasons for bilateral lymphadenopathy are: adenovirus, cytomegalovirus or human herpes virus infection, herpes simplex infection, HIV and Lyme disease.6
Treatment
As F. tularensis is resistant to beta-lactam antibiotics, the current approach against the disease based on WHO guidelines includes for adults a treatment with parenteral aminoglycosides for severe cases. In less severe cases, oral ciprofloxacin can be given. The treatment should last for at least 10 days.
For children, the WHO suggest in severe cases parenteral aminoglycosides, for example, gentamicin. For less severe cases, they suggest ciprofloxacin. Streptomycin used to be the first choice but is used less frequently nowadays because of its ototoxicity and kidney toxicity.5
Outcome and follow-up
As soon as the serologic results were available an appropriate treatment for tularaemia was started.
Despite antibiotic treatment, the patient developed a parapharyngeal abscess which had to be drained operatively. He then got an intravenous treatment with gentamicin for 2 weeks. As the necrotic lymph node on the left cervical side persisted despite the antibiotic treatment, it was decided to remove it surgically.
In the most recent follow-up, 6 months after the initial diagnosis, he showed no signs of disease any more and a sonographic examination of the neck was blunt.
Discussion
Tularaemia is a rare zoonotic bacterial infection which is potentially fatal and can cause outbreaks and also sporadic cases.7 It is seen more commonly in adult men and less reported in children.8 Among the cases reported in children, the age range would be from 5 to 9 years and the common form of Tularaemia would be oropharyngeal disease with the suspected mode of transmission being from contaminated food or water.8 9 In this case, the patient had contact with a dead mouse.
Oropharyngeal tularaemia causes symptoms such as fever, sore throat, myalgia and signs such as inflamed pharynx and tonsils and cervical lymphadenopathy.10 Due to low clinical suspicion, these non-specific symptoms and signs often lead to a delay in diagnosis. Our patient presented with the exact same symptoms and signs and was initially treated as a viral disease and then bacterial pharyngotonsillitis.
Earlier detection of disease and earlier appropriate treatment may be achieved if there is increased awareness of tularaemia among healthcare workers in endemic areas.11
The diagnosis of tularaemia requires serologic tests by microagglutination where antibody titres of >1:160 is deemed consistent with infection.5 However, antibody levels may only start to increase in the second week of illness and testing may be negative if taken in the early stage of the disease.12 Other laboratory methods to diagnose tularaemia include PCR and culture, but the latter is difficult as it requires a biosafety level 3 facility.5
A few studies have reported that the average elapse time from the onset of symptoms to the diagnosis of tularaemia ranges from 1 to 2 months.8 13–15 In our case, the diagnosis was made on day 15 of illness, corresponding to day 8 after coming the first time to the hospital.
Compared with the above studies, our patient was diagnosed relatively early and the treatment commenced and completed as per WHO guidelines.5 However, his clinical condition worsened with the development of a parapharyngeal abscess.
According to the WHO, the mainstay treatment of tularaemia in children is gentamicin 5–6 mg/kg/day for at least 10 days with streptomycin and ciprofloxacin as alternatives.5 Treatment failure in tularaemia ranges from 20% to 40% and is described as having at least one of the following: increase in size or appearance of new enlarged lymph nodes, persistent or recurrent fever, constantly high blood inflammatory markers and presence of suppurative lymph node despite being on medical treatment for 10–14 days.14 16 17 Tezer et al mentioned that possible factors of treatment failure include being of female gender, treatment delay of >16 days and doxycycline use.18 Oz et al however did not find any association between the different medical treatments and treatment failure, but did find that treatment delay leads to the formation of neck abscesses.9 Karli et al postulated that major causes of treatment failure include treatment delay and development of suppurative lymph node.19 The presence of a persistent fluctuant lymph node and the presence of an abscess despite after adequate medical treatment are indications for surgical intervention.13 Gozel et al mentioned that treatment failure rate was only 7.7% when combined antibiotic therapy with early surgical intervention in suspected cases.15
This was true in our case where the patient responded well after extended antibiotic therapy and after the drainage of the parapharyngeal abscess and excision of the necrotic lymph node.
Learning points.
Tularaemia is a re-emerging zoonosis. It is most common in the northern hemisphere. Endemic areas are North America and the Nordic countries. Outbreaks occur in Russia, Turkey, Eastern Europe and Japan.20
It often causes unspecific influenza-like symptoms which explains why a diagnostic delay can be long. This may have consequences on therapy and outcome. To avoid abscess formation, immediate therapy initiation is crucial.
In cases of lymphadenopathy and fever not responding to beta-lactam-antibiotic treatment, further investigations should be considered and tularaemia is an important potentially lethal differential diagnosis.
Footnotes
Contributors: AN and AB: data acquisition and analysis, drafting and design of article and final approval of article. CAF: critical revision of article and final approval of article. YB: data interpretation, drafting and design of article, critical revision and final approval of article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Parental/guardian consent obtained.
References
- 1. Richard FJ, Gordon ES. Tularemia : Dennis LK, Anthony SF, Harrison’s Infectious Diseases. New York: The Mc-Graw-Hill, 2010:552–7. [Google Scholar]
- 2. European Centre for Disease Prevention and Control. Annual epidemiological report for 2015 – Tularemia. https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-tularaemia.pdf [PubMed]
- 3. Swiss Federal Office of Public Health. Overview of Tularemia. https://www.bag.admin.ch/bag/de/home/krankheiten/krankheiten-im-ueberblick/tularaemie.html
- 4. Penn RL. Francisella tularensis (Tularemia) : Bennet JE, Dolin R, Blaser MJ, Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed Philadelphia: Elsevier Saunders, 2015:2590. [Google Scholar]
- 5. World Health Organization. WHO guidelines on tularemia. http://whqlibdoc.who.int/publications/2007/9789241547376_eng.pdf (22 Jul 2013).
- 6. Turhan V, Berber U, Haholu A, et al. . Differential diagnosis of cervical lymphadenitis mimicking malignancy due to tularemia: our experiences. Indian J Pathol Microbiol 2013;56:252–7. 10.4103/0377-4929.120381 [DOI] [PubMed] [Google Scholar]
- 7. Sjostedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci 2007;1105:1–29. 10.1196/annals.1409.009 [DOI] [PubMed] [Google Scholar]
- 8. Celebi S, Hacimustafaoglu M, Gedikoglu S. Tularemia in children. Indian J Pediatr 2008;75:1129–32. 10.1007/s12098-008-0180-9 [DOI] [PubMed] [Google Scholar]
- 9. Oz F, Eksioglu A, Tanır G, et al. . Evaluation of Clinical and Sonographic Features in 55 Children with Tularemia. Vector-Borne and Zoonotic Diseases 2014;14:571–5. 10.1089/vbz.2013.1517 [DOI] [PubMed] [Google Scholar]
- 10. Ohara Y, Sato T, Fujita H, et al. . Clinical manifestations of tularemia in Japan — analysis of 1,355 cases observed between 1924 and 1987. Infection 1991;19:14–17. 10.1007/BF01643750 [DOI] [PubMed] [Google Scholar]
- 11. Chitadze N, Kuchuloria T, Clark DV, et al. . Water-borne outbreak of oropharyngeal and glandular tularemia in georgia: investigation and follow-up. Infection 2009;37:514–21. 10.1007/s15010-009-8193-5 [DOI] [PubMed] [Google Scholar]
- 12. Kılıç S, Çelebi B, Yeşilyurt M. Evaluation of a commercial immunochromatographic assay for the serologic diagnosis of tularemia. Diagn Microbiol Infect Dis 2012;74:1–5. 10.1016/j.diagmicrobio.2012.05.030 [DOI] [PubMed] [Google Scholar]
- 13. Cağlı S, Vural A, Sönmez O, et al. . Tularemia: a rare cause of neck mass, evaluation of 33 patients. Eur Arch Otorhinolaryngol 2011;268:1699–704. 10.1007/s00405-011-1722-8 [DOI] [PubMed] [Google Scholar]
- 14. Sencan I, Sahin I, Kaya D, et al. . An outbreak of oropharyngeal tularemia with cervical adenopathy predominantly in the left side. Yonsei Med J 2009;50:50–4. 10.3349/ymj.2009.50.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gozel MG, Engin A, Altuntas EE, et al. . Evaluation of clinical and laboratory findings of pediatric and adult patients with oropharyngeal tularemia in turkey: a combination of surgical drainage and antibiotic therapy increases treatment success. Jpn J Infect Dis 2014;67:295–9. 10.7883/yoken.67.295 [DOI] [PubMed] [Google Scholar]
- 16. Meric M, Willke A, Finke EJ, et al. . Evaluation of clinical, laboratory, and therapeutic features of 145 tularemia cases: the role of quinolones in oropharyngeal tularemia. APMIS 2008;116:66–73. 10.1111/j.1600-0463.2008.00901.x [DOI] [PubMed] [Google Scholar]
- 17. Ulu-Kilic A, Gulen G, Sezen F, et al. . Tularemia in central anatolia. Infection 2013;41:391–9. 10.1007/s15010-012-0355-1 [DOI] [PubMed] [Google Scholar]
- 18. Tezer H, Ozkaya-Parlakay A, Aykan H, et al. . Tularemia in children, Turkey, September 2009–November 2012. Emerg Infect Dis 2015;21:1–7. 10.3201/eid2101.131127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karlı A, Şensoy G, Paksu Ş, et al. . Treatment-failure tularemia in children. Korean J Pediatr 2018;61:49–52. 10.3345/kjp.2018.61.2.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hestvik G, Warns-Petit E, Smith LA, et al. . The status of tularemia in Europe in a one-health context: a review. Epidemiol Infect 2015;143:2137–60. 10.1017/S0950268814002398 [DOI] [PMC free article] [PubMed] [Google Scholar]