Abstract
Introduction
Supraventricular arrhythmias contribute to haemodynamic compromise in septic shock. A retrospective study generated the hypothesis that propafenone could be more effective than amiodarone in achieving and maintaining sinus rhythm (SR). Certain echocardiographic parameters may predict a successful cardioversion and help in the decision on rhythm or rate control strategy.
Methods and analysis
The trial includes septic shock patients with new-onset arrhythmia, but without severe impairment of the left ventricular ejection fraction. After baseline echocardiography, the patient is randomised to receive a bolus and maintenance dose of either amiodarone or propafenone. The primary outcome is the proportion of patients that have achieved rhythm control at 24 hours after the start of the infusion. The secondary outcomes are the percentages of patients that needed rescue treatments (DC cardioversion or unblinding and crossover of the antiarrhythmics), the recurrence of arrhythmias, intensive care unit mortality, 28-day and 1-year mortality. In the posthoc analysis, we separately assess subgroups of patients with pulmonary hypertension and right ventricular dysfunction. In the exploratory part of the study, we assess whether the presence of a transmitral diastolic A wave and its higher velocity-time integral is predictive for the sustainability of mechanical SR and whether the indexed left atrial endsystolic volume is predictive of recurrent arrhythmia. Considering that the restoration of SR within 24 hours occurred in 74% of the amiodarone-treated patients and in 89% of the patients treated with propafenone, we plan to include 200 patients to have an 80% chance to demonstrate the superiority of propafenone at p=0.05.
Ethics and dissemination
The trial is recruiting patients according to its second protocol version approved by the University Hospital Ethical Board on the 6 October 2017 (No. 1691/16S-IV). The results will be disseminated through peer reviewed publications and conference presentations.
Trial registration number
Keywords: supraventricular arrhythmia, septic shock, propafenone, amiodarone, intensive care
Strengths and limitations of this study.
Randomised controlled trial comparing propafenone versus amiodarone in septic shock patients with normal to moderately reduced ejection fraction of left ventricle (EF_LV) should eliminate the bias of previous trials where patients with all levels of LV systolic function and various illness severities were compared.
The trial should answer the issue of safety of the 1C class agent propafenone given within the summary of product characteristics in the critically ill—in contrast to the older trials on less severely ill patients.
The outcomes of cardioverted patients with improved diastolic function will be compared with matched patients who remain in persisting arrhythmias.
The analysis of applied complex echocardiography protocol may propose simple echo parameters which may help in the decision on rhythm versus rate control approach.
Due to the scarcity of data in the current literature, the hypotheses are based on a single large retrospective study on septic shock patients with SV arrhythmias.
Introduction
The incidence of supraventricular (SV) arrhythmias varies between 8% and 25% in the critically ill depending on the illness severity.1–5 New onset SV arrhythmias are a contributor to diastolic and systolic heart failure.6 Loss of atrial systole associates with two to five times increased mortality among critically ill patients1–3 which is in contrast to lacking evidence that reverting back to sinus rhythm (SR) improves outcome.7 8 The uncertainty whether to aim for rate control rather than for rhythm control therapy also originates from the observed recurrence of arrhythmias and the side effects of the antiarrhythmics.
Besides improving oxygenation, preload and electrolyte corrections, the electric cardioversion is indicated in unstable patients with no contraindications and is more feasible in combination with an antiarrhythmic agent due to high rates of an early relapse of atrial fibrillation (AF).9
The data on various antiarrhythmic medications in the current literature show some important limitations, particularly the absence of an echocardiographic protocol before deciding on treatment.6 Some of the available studies lack an attempt to avoid potentially unfeasible medication in an unstable, critically ill patient. For example, a large pool (36%) of patients in sepsis was medicated with calcium channel blockers which can help with rate control at the cost of reducing ventricular contractility and promotion of vasodilatation. These side effects may impact on haemodynamic stability in a patient with left ventricular compromise and/or septic vasoplegia.10 In the studies suggesting beneficial effects of betablockers,11–14 haemodynamic monitoring did not include echocardiography and the comparisons to control patients were fraught with high mortality of the control group.12 Particularly, the severe left ventricular (LV) systolic dysfunction and conduction disorders should be excluded prior to beta-blocker administration in the septic shock patients.13 15
The mainstay of antiarrhythmic therapy6 is represented by amiodarone which is preferred for its lower cardiodepressant side effect compared with other agents and electric cardioversion.1 16–18 Extensive use of amiodarone contrasts with its multiorgan side effects and its application even in patients with normal LV systolic function19 20 demonstrates poor compliance with current guidelines.21 Hypotension may occur due to amiodarone’s vasodilatatory effects and QTc prolongation associates with the occurrence of torsades-des-pointes type of ventricular tachycardia. In the long-term administration the adverse effects involve particularly thyroid function,22 hepatic dysfunction,23 interstitial pneumonia and pulmonary fibrosis.24–26
The use of 1C agents has been discouraged by studies describing poor outcome during long-term administration in the cardiology population.27 Few available case reports demonstrate serious adverse effects apparently related to the dose-related cardiotoxicity.27–29 Consequently, 1C class agents like propafenone and flecainide30 are scarcely used in the critically ill. In contrast to flecainide and encainide, propafenone is derived from propandiolamine, which is a chemical compound of betablockers and acts on the rapid depolarizing phase (phase 0) and also, to a minimal extent, on beta-adrenergic receptors.31–33 Compared with flecainide, propafenone also lacks any evidence of its relationship to mortality.34
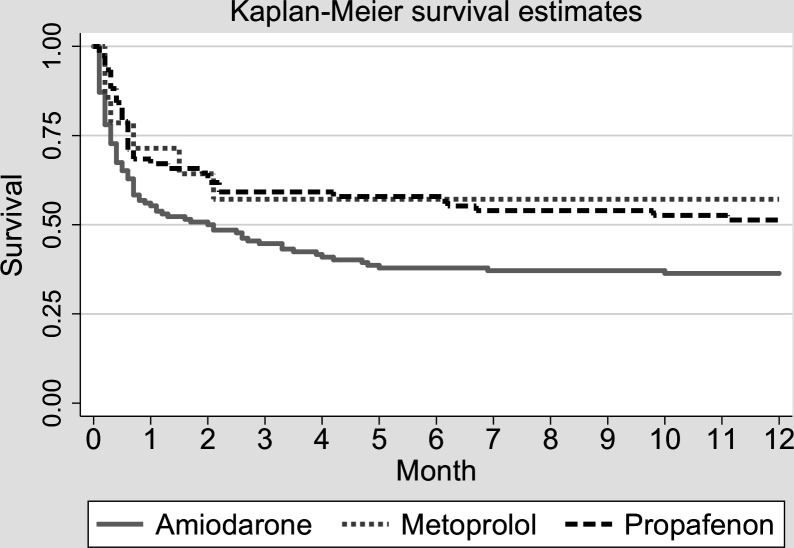
Our retrospective study4 5 suggests that propafenone might be feasible to restore SR without an adverse effect on haemodynamics and with a possible benefit on the outcome of the septic shock patients (figure 1).4 5 A chance to cardiovert seemed to be significantly higher under propafenone than in amiodarone and was close to the cardioversion rates of the betablocker metoprolol. No secondary arrhythmias or conduction disorders requiring treatment other than adjustment of the rate of infusion were observed.4 5 Another recent retrospective study found faster and more successful cardioversion with propafenone as compared with amiodarone for new onset atrial fibrillation in an emergency department. The safety profiles of the two agents were not different.35
Figure 1.
Univariate analysis showing long-term survival of the propafenone patients similar to the metoprolol group and higher than in the amiodarone medicated patients in septic shock (HR1.76 (1.06 to 2.3), p=0.024). Copied from the author’s pilot retrospective study.4
The current trial is intended to prospectively verify the efficacy and safety of propafenone administered under echocardiography control in the critically ill with septic shock. The trial also challenges the concept of amiodarone applied as a relatively toxic universal antiarrhythmic agent with a similar short-term safety profile as propafenone, while being slower and less efficient in cardioverting a SV arrhythmia in a septic shock patient. The authors also hypothesise that actively pursuing SR and cardioverting patients may contribute to the therapy of diastolic dysfunction with a positive impact on mortality.7
Methods/Design
We designed a prospective double-blinded randomised trial comparing propafenone to amiodarone administered for a SV arrhythmia in critically ill patients with septic shock.
Primary aims
The trial should prove that propafenone is more efficient than amiodarone in cardioverting a SV arrhythmia in patients with normal to moderately reduced ejection fraction of left ventricle (EF_LV) at 24 hours from the onset. The rationale stems from the retrospective data set where the primary cardioversion rate of SV arrhythmia under propafenone was 88.9% vs 73.5% under amiodarone.4 5 The authors also expect faster cardioversion under propafenone and lower rates of arrhythmia recurrence in the propafenone group. Despite prejudices arising particularly from the CAST trial and case reports on dose dependent toxicity, research should prove the safety of the 1C class agent propafenone given within the summary of product characteristics.28 30 The retrospective study4 5 has shown that the intensive care unit (ICU) and 28-day mortalities of patients treated with propafenone were better than the parameters of the amiodarone patients. In other words, propafenone administration did not increase mortality as suggested by the older trials on non-ICU patients.27–29 Moreover, patients with a SV arrhythmia treated with propafenone had a significantly better adjusted 12-month survival than the critically ill treated with amiodarone (figure 1). If proven, physicians could avoid a widespread use of amiodarone in the critically ill.
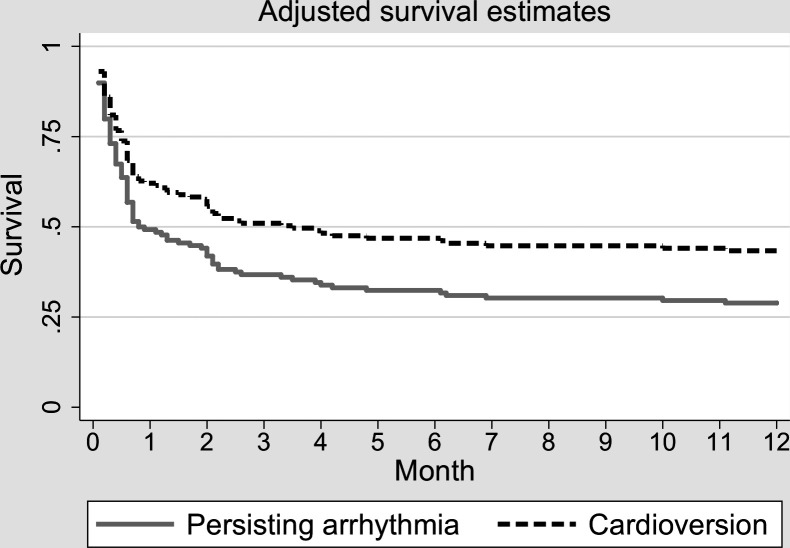
The cardioverted patients (rhythm control) may showcase better outcome parameters (ICU mortality, 28-day mortality, 1-year mortality) than those remaining in an acute onset arrhythmia (rate control). A rationale beyond this hypothesis is in the pilot study,4 5 which also included patients with severe LV dysfunction and associated higher rates of recurrent SV arrhythmias. Focusing on only normal to moderate LV systolic dysfunction may minimise bias associated with arrhythmia treatment of patients with severe LV systolic dysfunction. Likewise, patients with severe LV dysfunction were also included in the published trials dealing with either 1C class antiarrhythmics (eg, CAST trial),27 or in the trials studying rhythm versus rate control (eg, AFFIRM, RACE or AF-CHF Trial).36–39 Due to high success of rhythm control therapy (74.4% and 87% excluding chronic AF) in the retrospective study on 234 patients,4 5 the group with persisting acute onset SV arrhythmia was significantly smaller in number causing an asymmetry in statistic evaluation. This may also account for not significantly better outcome of the cardioverted versus those remaining in the SV arrhythmias (figure 2).
Figure 2.
Multivariate analysis showing insignificant 12-month benefit in cardioverting septic shock patients to sinus rhythm (HR 0.67, p=0.113). Copied from the author’s pilot retrospective study.4
Secondary aims
The presence of a transmitral diastolic A wave and its higher velocity-time integral at 4-hour postcardioversion would indicate a presence of mechanical SR. A small or negligible A wave may represent only the electric sinus in the absence of its mechanical correlate. This finding could be related to the increased indexed left atrial end-systolic volume (LAVi) and to a recurrence of a SV arrhythmia.40 41 The LAVi in all patients and altered filling pressures estimated by echocardiography could be predictive of arrhythmia recurrence.42 43
Propafenone could be more efficient than amiodarone in patients with pulmonary hypertension and right ventricular (RV) dysfunction without left ventricular systolic dysfunction.
A left ventricular relaxation disorder and a pseudonormal LV filling are more dependent on the atrial kick compared with the restrictive LV filling which is often accompanied by a dilated poorly contracting left atrium. The classic stratification of diastolic dysfunction relates to the patient’s prognosis in septic shock.44 Hence, a complex echo assessment may contribute to the decision whether to aim for rhythm or for rate control only. Evaluation of the Doppler parameters will depend on rhythm, heart rate, regularity of arrhythmia and peripheral pulse deficit.40 42
Flowchart and study setting
Patients are randomised by the unblinded team lead by a research nurse. The planned number of included patients is 100 in each arm of the study with a total of 220 randomised patients. A dropout of 10% is anticipated. The estimated duration of the study is 4 years including follow-up. The patients have been recruited since November 2017 in three university hospital ICUs. The department of Anaesthesia and Intensive Care of the General University Hospital has been performing for years as a teaching centre for critical care echocardiography and ultrasound. Together with the Coronary Care Unit of the General University Hospital, both departments are integrated as a Complex Cardiovascular Centre. The department of Anaesthesia and Intensive Care of the University Hospital Vinohrady is a mainstay of the Complex Prague Trauma Centre.
Inclusion criteria
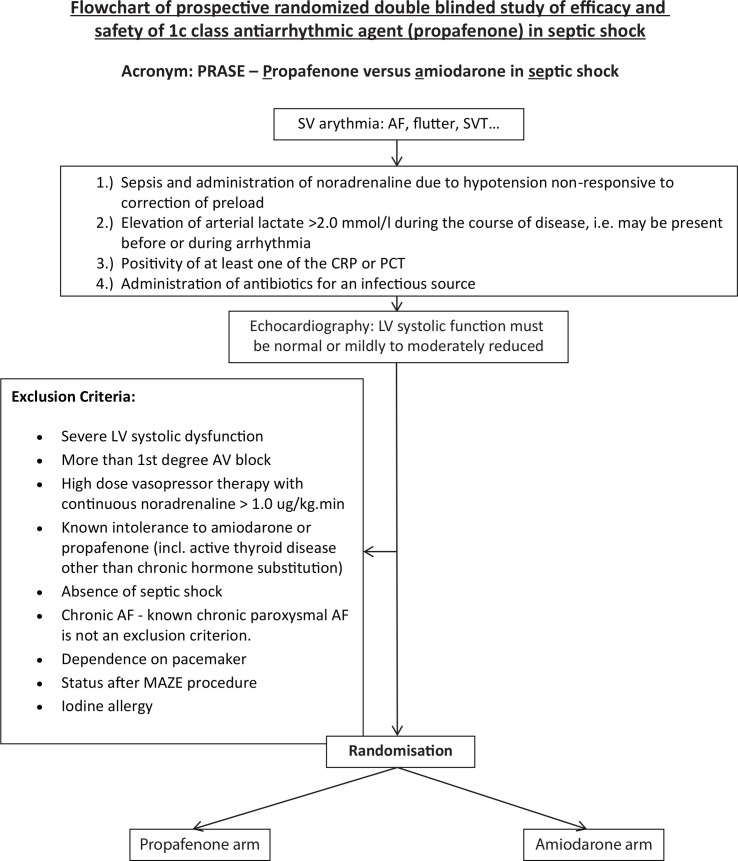
The study targets adult patients (16–85 years) in septic shock with a new onset SV arrhythmia or known paroxysmal SV arrhythmia who show normal or mildly to moderately reduced LV systolic function according to the echocardiography examination (ie, EF_LV ≥35%)(figure 3). A diagnosis of septic shock is made according to the 2016 definition45 as sepsis with a vasopressor requirement to maintain a mean arterial pressure of 65 mm Hg or greater. The arterial lactate level should be greater than 2 mmol/L in the absence of hypovolaemia or low cardiac output. The highest arterial lactate level is recorded, that is, lactate <2.0 mmol/L at the time of randomisation does not exclude a patient from the study. This might also be justified by the reported incidence of sepsis-related cardiac dysfunction which is highest 72–96 hours after the onset of septic shock.46 The presence of a suspected infection is for the purpose of this study defined as a positivity of at least one inflammatory marker of the monitored C reactive protein and procalcitonin and a clinical decision to administer antibiotic treatment for a specified infection source.
Figure 3.
Flowchart of the study.
Exclusion criteria
The study respects all exclusion criteria for a blinded administration of propafenone or amiodarone. These are severe LV systolic dysfunction (ie, EF <35%), a history indicating more than the first degree atrioventricular (AV) block and high dose vasopressor therapy represented by continuous norepinephrine administration of more than 1.0 ug/kg.min. Contraindications to randomisation are known intolerance to amiodarone or propafenone, iodine allergy and an active thyroid disease other than chronic hormone substitution for benign goitre. An interstitial pneumonia is not considered a contraindication to randomisation with regard to delayed effects of amiodarone on the lung parenchyma26 and expected short period of its administration. Similarly, liver dysfunction is not a contraindication for amiodarone assuming a titrated short duration of the medication. Chronic persistent AF represents an exclusion while known chronic paroxysmal AF is not an exclusion criterion. Patients dependent on a pacemaker or after a Maze procedure are also excluded.
Interventions and research protocol
Screened patients will have a haemodynamic examination provided according to the study protocol. With the onset of arrhythmia, the usual treatment is expected including preload correction, reduction of unnecessary vasopressors, ion supplementation (aiming particularly for K+>4.0 mM and Mg2+>1.0 mM) and maintenance of tissue oxygen delivery. Echocardiography should also guide optimisation of preload.
The complex protocol is formatted in an electronic case report form (CRF). After checking up the inclusion and exclusion criteria the CRF allocates the patients randomly using built in software (http://www.randomization.com) into the propafenone or amiodarone arm (figure 3).
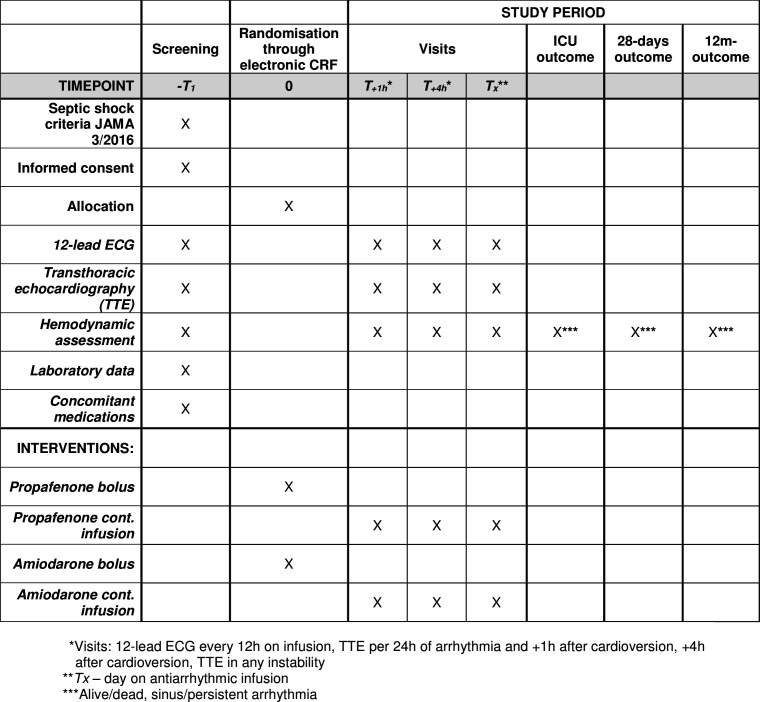
The patient’s characteristics include the illness severity scores, source of septic shock, data on mechanical ventilation and homeostasis, baseline haemodynamic data, baseline laboratory data, patient’s medications, haemodynamic data at proposed steps plus follow-up data including outcome (figure 4).
Figure 4.
SPIRIT table for the schedule of enrolment, interventions and assessments.
Haemodynamic evaluation includes ICU standard plus echocardiography (figure 4). The study team involves eight intensivists with a European Accreditation in Echocardiography (either ESC or EACTA backed) and three qualified cardiologists-intensivists.
By no means is an antiarrhythmic given out of the summary of product characteristics. Both arms will have standard treatment, and there are no limits to electric cardioversion as part of the treatment which is indicated at anytime in haemodynamic compromise and in signs of low cardiac output or insufficient perfusion pressures due to arrhythmia.
The propafenone arm constitutes administering a bolus of 35–70 mg of intravenous propafenone followed by a continuous infusion of 400–840 mg/24 hours in a black syringe. The amiodarone arm constitutes administering a bolus of 150–300 mg of intravenous amiodarone followed by a continuous infusion of 600–1800 mg/24 hours in a black syringe.
A 12-lead ECG is taken every 12 hours while on the antiarrhythmic infusion. Besides echocardiography prerandomisation the control echocardiography is performed 1-hour postcardioversion and 4-hour postcardioversion. Echocardiography is also performed every day until cardioversion, it is also mandatory in any kind of haemodynamic instability. All the Doppler measurements are recorded at end-expiration and three cardiac cycles when in SR and 5–10 during arrhythmia are analysed and averaged. All recordings should be acquired with an ECG (lead II), and ideally, at the speed of 100 mm/s.
If electrically cardioverted in addition to administered pharmacotherapy, then echocardiography is performed 1-hour postcardioversion and 4-hour postcardioversion.
If cardioverted later than within 24 hours after randomisation, then echocardiography is performed at 1 hour and 4 hours after cardioversion. The times of cardioversion and arrhythmia relapses are always recorded.
If a patient spontaneously cardioverts before the drug is administered, that is, between randomisation and drip initiation, the patient is monitored accordingly and included in the intention-to-treat analysis.
Primary outcome measures
The efficacy in restoration of SR assessed as the proportion of patients who are in SR 24 hours after the beginning of the infusion of the study drug and remain in SR until discharge from ICU. The primary outcome will be assessed in all randomised patients (ie, intention-to-treat analysis).
A priori defined subgroup analysis: Primary outcome will be analysed in the following subgroups of patients: (1) with and without indexed LAVi higher than >40 mL/m2. (2) with and without pulmonary hypertension (defined as pulmonary artery systolic pressures (PAPs)>40 mm Hg) associated with moderate to severe RV dysfunction (dilated RV with tricuspidannular plane excursion <15 mm).
Secondary outcome measures
The cumulative proportion of patients receiving rescue treatment for arrhythmia defined as direct current cardioversion or administration of an alternative antiarrhythmic drug during the first 24 hours (cross-over from one arm to the other resulting in unblinding of the study, eg, from amiodarone to propafenone due to a persisting arrhythmia or from propafenone to amiodarone due to a decrease in LV systolic function).
The cumulative proportion of patients receiving rescue treatment for arrhythmia defined as direct current cardioversion, cross-over to the alternative study drug or another antiarrhythmic drug during ICU stay.
Mortality at discharge from ICU, at 28 days and at 1 year.
Vasopressor-free days at day 28.
Safety issues and patient’s monitoring
Besides cardioversion monitoring, transthoracic echocardiography is also acquired in any kind of haemodynamic instability (ie, change in vasopressor support). This is important to avoid administering a potentially cardiodepressant propafenone in a patient developing septic cardiomyopathy. Twelve-hourly 12-lead ECG for the monitoring of conduction times (PQ, QRS, QTc) is performed while the patient is on the antiarrhythmic infusion. In case of an AV block of the first degree or extension of the conduction times (QRS or QTc), the slowing or temporary ceasing of the medication in relation to heart rate is mandatory. Adjustment of the infusion rate or eventual termination of an antiarrhythmic medication does not exclude the patient from the study. Cessation of medication after reaching SR equally does not exclude the patient. If an infusion is interrupted and restarted, then the number of infusion hours are counted up as a sum of infusion hours.
In case of progression of septic cardiomyopathy and a decrease of contractility (decrease of EFLV to <35%) or a progression of mitral regurgitation with a risk of low cardiac output the study drug is unblinded and propafenone discontinued. Further treatment is decided by the clinician. If the study is unblinded due to haemodynamic instability, the second drug after study arm cross-over is administered without an initial bolus.
Anytime the patient becomes haemodynamically unstable or has another reason (as per discretion of the treating clinician) to benefit from electric cardioversion (DCC), then DCC is delivered without delay.
Should there be a concern at any point in time about the safety of the drug, the treating clinicians are encouraged to unblind the treatment drug without delay and alter the treatment accordingly. The course of the trial is regularly reported to the hospital Ethical Board which acts as the research supervising body. The minimum frequency of the report is once per year throughout the duration of the trial which is proposed from 2018 to 2021.
Statistics and power analysis
All analysis will be conducted in R Core Team (2019) and will be available together with the raw data. Exploratory data analysis will be performed for both baseline and outcome parameters. Continuous parameters will be described as means and SD and as medians and the IQRs if not normally distributed. Log-normally distributed parameters will be logarithmically transformed if needed. Binary data will be described as counts and frequencies. Statistical significances of differences between groups will be described as OR, HR or mean difference according to the type of analysis with 95% CI. Both intention-to-treat and per protocol analysis will be performed.
The primary outcome (proportion of patients that have achieved rhythm control at 24 hours after the start of the infusion) will be analysed using logistic regression and time to event analysis (Cox regression). The secondary outcomes (proportion of patients that needed rescue treatments), recurrence of arrhythmias, ICU mortality, 28-day and 1-year mortality will be analysed using logistic regression. If significant differences in baseline characteristics are found between analysed groups, then multivariate regression for adjustments to these variables will be performed.
The required number of patients is based on the power analysis and data from the pilot retrospective study.4 5 The entry parameters for sample size analysis were estimated by the probabilities of cardioversion of 75% for the amiodarone group and 90% for the propafenone group within 24 hours from the onset of arrhythmia, randomisation ratio 1:1, p=0.05 and power 0.8. To achieve a statistically significant difference under these conditions, 100 patients need to be included into each group, altogether 200 patients into the trial. Assuming 10% drop out the authors plan to randomise 220 patients.
Ethics approval and dissemination
The written informed consent is sought from the patient’s next of kin. The results will be disseminated through peer reviewed publications and conference presentations. The study repository will be created with the dataset available after study completion. The recruitment has begun through the electronic CRF on the 23 October 2017 and is expected to be completed in December 2021.
Patient and public involvement
Patients and public are not involved in the design and conduct of the study. The results of the trial will be disseminated to the involved patients and their next of kin on their requests, which is offered during collection of the informed consents.
Limitations and conclusions
The available literature on SV arrhythmias in septic shock shows critically ill patients with a high predicted mortality, intermittent positive pressure ventilation (IPPV) rate of 99% and high rates of continuous renal replacement therapy (27%–31%).4 5 Up to now, all the authors adhered to the septic shock criteria based on volume non-responsive systemic inflammatory response syndrome with a need for a vasopressor and antibiotic therapy administered for an infectious source.47 Applying the novel septic shock criteria of 201645 may increase specificity at the cost of lacking sensitivity to include even those who could potentially benefit from septic shock therapy.48 If applying the results of the current trial to less severe patients, for example, those classified according to the older criteria, the SOFA score and a median arterial lactate level may serve as controls adjusting the studied population in context of the novel septic shock criteria published in 2016.45
The hypothesis that propafenone might be superior to amiodarone in cardioverting newly appearing SV arrhythmia with an impact on the long-term outcome may not be proved due to the confounding factors of the retrospective study.4 5 Although being statistically insignificant, LV systolic function was mildly higher in the propafenone and betablocker patients compared with those on amiodarone. The severe LV systolic dysfunctions were medicated with amiodarone, the same being applied to patients on a higher dosage of norepinephrine compared with the patients with moderate to mild LV systolic dysfunction and those with a lower dosage of norepinephrine in the propafenone and betablocker groups.4 5
The retrospective study also included patients with a cross-over from an unsuccessful antiarrhythmic therapy to another group during 24 hours as part of the rhythm control strategy. This increased the pool of the propafenone patients after administering the agent in patients who were not able to cardiovert and maintain SR on amiodarone.4 5 This, so far, might represent an unreported synergistic effect of the two antiarrhythmic agents on achieving a high cardioversion rate, yet with a very acceptable safety profile.4 5 The current prospective trial allows a cross-over between the arms however, only in a haemodynamic instability and with immediate unblinding.
The observed median age in an adult ICU varies around 55–65 years. The age-related prevalence of hypertension and ischaemic heart disease suggests a large proportion of patients with a benefit of atrial systole and thus an indication for the rhythm control approach.7 The prevalence of newly occurring SV arrhythmias and the broad spectrum of potentially reversible triggers in the critically ill offer an opportunity for cardioversion in closely monitored patients rather than in ambulatory patients in cardiology. Moreover, septic shock is often fraught with diastolic dysfunction and to restore SR might be of paramount importance for the therapy of diastolic heart failure.
Supplementary Material
Footnotes
Contributors: MB: study coordinator, concept and design, drafting, revisions and approval of articles, provision of funding. PW and FD: concept and design, electronic case report form, statistics, article revisions, data collection. MP, JR, MO, VM, MM, TB, RS, JP, PB, ES, MF, ZS and MS: data collection, article revisions. OS: article revisions, data collection, unblinded team coordination.
Funding: The protocol has received a four year (2018-2022) grant support from the Czech Health Research Council, AZV No. NV18-06-00417, commencing on 1 May 2018.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The local ethical approvals have been received from the Ethics Committee of the 1st Medical Faculty and General University Hospital (No. 1691/16 S-IV) and from the Ethics Committee of the 3rd Medical Faculty and University Hospital Kralovske Vinohrady.
References
- 1.Arrigo M, Bettex D, Rudiger A. Management of atrial fibrillation in critically ill patients. Crit Care Res Pract 2014;2014:1–10. 10.1155/2014/840615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuipers S, Klein Klouwenberg PMC, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care 2014;18. 10.1186/s13054-014-0688-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein Klouwenberg PMC, Frencken JF, Kuipers S, et al. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis. A cohort study. Am J Respir Crit Care Med 2017;195:205–11. 10.1164/rccm.201603-0618OC [DOI] [PubMed] [Google Scholar]
- 4.Balik M, Kolnikova I, Maly M, et al. Propafenone for supraventricular arrhythmias in septic shock—Comparison to amiodarone and metoprolol. J Crit Care 2017;41:16–23. 10.1016/j.jcrc.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 5.Balik M, Maly M, Brozek T, et al. Propafenone for supraventricular arrhythmias in septic shock-Comparison to amiodarone and metoprolol. The authors reply. J Crit Care 2018;45:247–8. 10.1016/j.jcrc.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 6.Balik M, Matousek V, Maly M, et al. Management of arrhythmia in sepsis and septic shock. Anaesthesiol Intensive Ther 2017;49:419–29. 10.5603/AIT.a2017.0061 [DOI] [PubMed] [Google Scholar]
- 7.Balik M. New-onset atrial fibrillation in critically ill patients - Implications for rhythm rather than rate control therapy? Int J Cardiol 2018;266:147–8. 10.1016/j.ijcard.2018.04.078 [DOI] [PubMed] [Google Scholar]
- 8.Liu WC, Lin WY, Lin CS, et al. Prognostic impact of restored sinus rhythm in patients with sepsis and new-onset atrial fibrillation. Crit Care 2016;20. 10.1186/s13054-016-1548-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrigo M, Jaeger N, Seifert B, et al. Disappointing success of electrical cardioversion for new-onset atrial fibrillation in Cardiosurgical ICU patients. Crit Care Med 2015;43:2354–9. 10.1097/CCM.0000000000001257 [DOI] [PubMed] [Google Scholar]
- 10.Walkey AJ, Evans SR, Winter MR, et al. Practice patterns and outcomes of treatments for atrial fibrillation during sepsis: a Propensity-Matched cohort study. Chest 2016;149:74–83. 10.1378/chest.15-0959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morelli A, Donati A, Ertmer C, Rehberg S, et al. Microvascular effects of heart rate control with esmolol in patients with septic shock: a pilot study. Crit Care Med 2013;41:2162–8. 10.1097/CCM.0b013e31828a678d [DOI] [PubMed] [Google Scholar]
- 12.Morelli A, Ertmer C, Westphal M, Rehberg S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA 2013;310:1683–91. 10.1001/jama.2013.278477 [DOI] [PubMed] [Google Scholar]
- 13.Balik M, Rulisek J, Leden P, Zakharchenko M, et al. Concomitant use of beta-1 adrenoreceptor blocker and norepinephrine in patients with septic shock. Wien Klin Wochenschr 2012;124:552–6. 10.1007/s00508-012-0209-y [DOI] [PubMed] [Google Scholar]
- 14.Balik M, Rulisek J, Leden P, Zakharchenko M, et al. Concomitant use of beta-1 adrenoreceptor blocker and norepinephrine in patients with septic shock. reply to a letter to the authors. Wien Klin Wochenschr 2014;126:246–7. 10.1007/s00508-013-0487-z [DOI] [PubMed] [Google Scholar]
- 15.McLean AS, Taccone FS, Vieillard-Baron A. Beta-Blockers in septic shock to optimize hemodynamics? no. Intensive Care Med 2016;42:1610–2. 10.1007/s00134-016-4407-3 [DOI] [PubMed] [Google Scholar]
- 16.Kirchhof P, Ammentorp B, Darius H, et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events--European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014;16:6–14. 10.1093/europace/eut263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sleeswijk ME, Van Noord T, Tulleken JE, Ligtenberg JJ, et al. Clinical review: treatment of new-onset atrial fibrillation in medical intensive care patients: a clinical framework. Crit Care 2007;11. 10.1186/cc6136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrigo M, Jaeger N, Seifert B, Spahn DR, et al. Disappointing success of electrical cardioversion for new-onset atrial fibrillation in Cardiosurgical ICU patients. Crit Care Med 2015;43:2354–9. 10.1097/CCM.0000000000001257 [DOI] [PubMed] [Google Scholar]
- 19.Allen LaPointe NM, Dai D, Thomas L, et al. Antiarrhythmic drug use in patients <65 years with atrial fibrillation and without structural heart disease. Am J Cardiol 2015;115:316–22. 10.1016/j.amjcard.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwag HB, Chun KJ, Hwang JK, et al. Which antiarrhythmic drug to choose after electrical cardioversion: a study on non-valvular atrial fibrillation patients. PLoS One 2018;13:e0197352. 10.1371/journal.pone.0197352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 22.Hofmann A, Nawara C, Ofluoglu S, Holzmannhofer J, et al. Incidence and predictability of amiodarone-induced thyrotoxicosis and hypothyroidism. Wien Klin Wochenschr 2008;120:493–8. 10.1007/s00508-008-1017-2 [DOI] [PubMed] [Google Scholar]
- 23.Rätz Bravo AE, Drewe J, Schlienger RG, et al. Hepatotoxicity during rapid intravenous loading with amiodarone: description of three cases and review of the literature. Crit Care Med 2005;33:128–34. 10.1097/01.CCM.0000151048.72393.44 [DOI] [PubMed] [Google Scholar]
- 24.Singh VK, Maheshwari V. Acute Respiratory Distress Syndrome Complicated by Amiodarone Induced Pulmonary Fibrosis: Don't Let Your Guard Down. J Clin Diagn Res 2017;11:Ud01–2. 10.7860/JCDR/2017/24710.9674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes M, Binning A. Intravenous amiodarone in intensive care. time for a reappraisal? Intensive Care Med 2000;26:1730–9. [DOI] [PubMed] [Google Scholar]
- 26.Papiris SA, Triantafillidou C, Kolilekas L, et al. Amiodarone: review of pulmonary effects and toxicity. Drug Saf 2010;33:539–58. 10.2165/11532320-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 27.Echt DS, Liebson PR, Mitchell LB, Peters RW, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. the cardiac arrhythmia suppression trial. N Engl J Med 1991;324:781–8. 10.1056/NEJM199103213241201 [DOI] [PubMed] [Google Scholar]
- 28.Chevalier P, Durand-Dubief A, Burri H, Cucherat M, et al. Amiodarone versus placebo and class Ic drugs for cardioversion of recent-onset atrial fibrillation: a meta-analysis. J Am Coll Cardiol 2003;41:255–62. 10.1016/S0735-1097(02)02705-5 [DOI] [PubMed] [Google Scholar]
- 29.Courand P-YN, Sibellas F, Ranc S, Mullier A, et al. Arrhythmogenic effect of flecainide toxicity. Cardiol J 2013;20:203–5. 10.5603/CJ.2013.0035 [DOI] [PubMed] [Google Scholar]
- 30.Aliot E, Capucci A, Crijns HJ, Goette A, et al. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace 2011;13:161–73. 10.1093/europace/euq382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varon J, Marik PE, Irwin RS, Rippe JM, eds. Irwin and Rippe's intensive care medicine. 6th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008: 1855–69. [Google Scholar]
- 32.Ganetsky MBE. Antiarythmic agents. In: Irwin RS RJ, ed. Intensive care medicine. 6th ed. Philadelphia: Wolters Kluwer/Lippincott, Williams&Wilkins, 2008: 1486–98. [Google Scholar]
- 33.Stoschitzky K, Stoschitzky G, Lercher P, et al. Propafenone shows class Ic and class II antiarrhythmic effects. Europace 2016;18:568–71. 10.1093/europace/euv195 [DOI] [PubMed] [Google Scholar]
- 34.Lafuente-Lafuente C, Valembois L, Bergmann J-F, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev 2015;151. 10.1002/14651858.CD005049.pub4 [DOI] [PubMed] [Google Scholar]
- 35.Bonora A, Turcato G, Franchi E, et al. Efficacy and safety in pharmacological cardioversion of recent-onset atrial fibrillation: a propensity score matching to compare amiodarone vs class Ic antiarrhythmic drugs. Intern Emerg Med 2017;12:853–9. 10.1007/s11739-016-1497-4 [DOI] [PubMed] [Google Scholar]
- 36.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834–40. 10.1056/NEJMoa021375 [DOI] [PubMed] [Google Scholar]
- 37.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–33. 10.1056/NEJMoa021328 [DOI] [PubMed] [Google Scholar]
- 38.Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496–506. 10.1056/NEJMoa1404380 [DOI] [PubMed] [Google Scholar]
- 39.Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med 2016;374:1911–21. 10.1056/NEJMoa1602002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung CS, Kovács SJ. Consequences of increasing heart rate on deceleration time, the velocity-time integral, and E/A. Am J Cardiol 2006;97:130–6. 10.1016/j.amjcard.2005.07.116 [DOI] [PubMed] [Google Scholar]
- 41.Fornengo C, Antolini M, Frea S, Gallo C, et al. Prediction of atrial fibrillation recurrence after cardioversion in patients with left-atrial dilation. Eur Heart J Cardiovasc Imaging 2015;16:335–41. 10.1093/ehjci/jeu193 [DOI] [PubMed] [Google Scholar]
- 42.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–93. 10.1093/ejechocard/jep007 [DOI] [PubMed] [Google Scholar]
- 43.Marchese P, Bursi F, Delle Donne G, et al. Indexed left atrial volume predicts the recurrence of non-valvular atrial fibrillation after successful cardioversion. Eur J Echocardiogr 2011;12:214–21. 10.1093/ejechocard/jeq176 [DOI] [PubMed] [Google Scholar]
- 44.Poelaert J, Declerck C, Vogelaers D, et al. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med 1997;23:553–60. 10.1007/s001340050372 [DOI] [PubMed] [Google Scholar]
- 45.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, et al. The third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Repessé X, Charron C, Vieillard-Baron A. Evaluation of left ventricular systolic function revisited in septic shock. Crit Care 2013;17. 10.1186/cc12755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 2003;31:1250–6. 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- 48.Sterling SA, Puskarich MA, Glass AF, et al. The impact of the Sepsis-3 septic shock definition on previously defined septic shock patients. Crit Care Med 2017;45:1436–42. 10.1097/CCM.0000000000002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository.