Abstract
Purpose
To investigate and evaluate the role of nucleated red blood cells (NRBCs) and other markers in predicting remission failure in chronic myeloid leukemia (CML) patients treated with imatinib.
Methods
Seventy-one CML patients with BCR-ABL(+) in bone marrow cells were selected for this study. Molecular response evaluations were done every three months according to the recommendations of European LeukemiaNet (ELN). Patients were defined as remission failure if BCR-ABL transcripts >10% after 6 months (T6), >1% after 12 months (T12), and >0.1% after 18 (T18) months of treatment. The logistic regression was used to determine the optimal cut-off point of each marker and test the association of marker level with remission failure.
Results
The median NRBC, white blood cells, blast cells, basophils, and platelets were declined parallel with the decreases of BCR-ABL transcripts in bone marrow cells after 6 months of treatment (P<0.001). In addition, NRBC was almost not found in the blood of patients who archived good response at T6, T12, and T18 time-points. Interestingly, patients with a high level of NRBC (cut-off: 0.003×109/L) have higher BCR-ABL transcripts compared to others. The elevated NRBC at T6 (OR=6.49, P=0.042), T12 (OR=6.73, P=0.007), and T18 (OR=5.96, P=0.009) time-points was identified as an independent factor for the remission failure.
Conclusion
The results of this study showed that a high number of NRBC in peripheral blood of CML patients is associated with higher BCR-ABL transcripts in bone marrow cells. The elevated NRBC might serve as an independent marker for molecular remission failure in CML.
Keywords: NRBC, BCR-ABL, CML
Introduction
Chronic myeloid leukemia (CML) is a hematological disease which has been characterized by the malignant proliferation of hematopoietic stem cells carried the translocation t(9;22)(q34;q11).1 This translocation leads to the formation of the BCR-ABL fusion gene which encodes for the similar name protein with hyper tyrosine kinase activity in cancer cells. The BCR-ABL rearrangement occurs in ≈95% CML patients with two major (b3a2 and b2a2; or p210 variant), one minor (e1a2 or p190), and some rare variants.1 Before the imatinib era, CML patients were treated with chemotherapy agents in classical regimens such as hydroxyurea, cytarabine, and interferon-alpha. Imatinib therapy (a tyrosine kinase inhibitor, TKI) is the breakthrough in treatment for CML patients which help to improve the response rate, survival, and quality of life for patients compared to the classical chemotherapy.2 The drug acts as the competitor with adenosine triphosphate to block the BCR-ABL fusion protein, hence inhibits the transductions of intracellular signaling pathways, and inhibits the uncontrolled proliferation of CML cells. After the success of imatinib, dasatinib and nilotinib were approved as the first-line agents in CML treatment recently.1,3,4
On TKI treatment monitoring, the BCR-ABL transcripts were recommended to be measured every three months to determine whether patients reach the molecular remission or not.1 This diagnostic test was suggested to perform on the real-time quantitative polymerase chain reaction which is the current standard technique. Although the peripheral blood cells were approved recently as a sample source for BCR-ABL quantification, the diagnostic test performed on bone marrow cells still remains as the gold standard method.1 Besides the BCR-ABL transcripts, the restore of hematopoiesis to normal, and some related immune cells such as neutrophils, blast cells, and basophils have been used in response evaluation for CML.1,5–7
One of the well-known cells of the hematopoietic system, the nucleated red blood cell (NRBC) is a promising marker for clinical application. The presence of NRBC in peripheral blood has been shown to be associated with a variety of serious conditions including solid cancer, hematological diseases, cardiovascular diseases, hemorrhage, infections, and others.8,9 Besides, this cell plays an important role as a powerfully predictive factor of survival for both hospitalized adult patients and infants.8,10–25 However, a little information of NRBC in CML has been shown, especially in patients treated with TKI.9 This study aims to investigate and evaluate the role of NRBC and other routine tests in predicting remission failure with imatinib in CML, as the simple, cost-effective markers.
Materials and methods
Patients and sample
A total of 71 CML patients with BCR-ABL(+) in bone marrow cells who were treated with imatinib from Jan-2016 to May-2019 at Cho Ray Hospital were selected for this study (approval number 602-2017-CN-HDDD) (Figure 1). The molecular response evaluations were done every three months according to the recommendations of ELN.1 Patients reach response milestones if the quantitative BCR-ABL transcripts ≤10% after 6 months, ≤1% after 12 months, and ≤0.1% after 18 months of treatment. Conversely, patients were defined as remission failure if BCR-ABL transcripts above these values. About 2mL bone marrow sample was used for BCR-ABL transcripts quantification at diagnosis time-point and serial treatments. The NRBC with other immune cells and biochemical markers were recalled from the laboratory database. These blood tests were performed on the Alinity-hq hematology analyzer and Accelerator a3600 automation system (Abbott Laboratories, Illinois, USA). Other diagnostic results of bone marrow cytology and t(9;22) translocation were collected from the medical records. Authors were permitted to collect the data with the responsibilities of personal information security. Because of the retrospective study, patients were not requested to write consent form.
Figure 1.
Patient selection.
Total RNA extraction
The total RNA was extracted from 107 leukocytes from bone marrow sample using kit QIAamp RNA Blood Mini (Cat No./ID: 52304) according to the instructions of the manufacturer (Qiagen, Hilden, Germany). Briefly, samples were mixed with 10 mL EL buffer and incubated in 4 °C/15 mins to lysis red blood cells, then centrifuged at 400×g/4 °C/10 mins to remove supernatant. The collected leukocytes were lysed by 600 µL RLT buffer added 10 µL β2-mercapthoethanol 100%. After that, the entire lysate was transferred to the QIAshredder column and centrifuged for 2 mins at 14,000×g to homogenize the samples. The RNA in the homogenized lysate was precipitated by 600 µL ethanol 70%, then captured on the silica membrane of QIAamp spin column in the centrifuge step (10,000×g/4 °C/1 min). Approx of 700 µL RW1 and 700 µL RPE buffers were added to wash silica membrane, respectively. The total RNA was eluted in 30 µL RNase-free water and checked the concentration with purity by the BioDrop µLITE machine (BioDrop Ltd, UK) before storing in −80 °C until uses.
BCR-ABL transcripts quantification
The BCR-ABL p210 b2a2 or b3a2, and p190 e1a2 transcripts in patient’s sample were quantified by using kit Ipsogen BCR-ABL1 Mbcr IS-MMR DX (Cat No./ID: 670823) and Ipsogen BCR-ABL1 mbcr (Cat No./ID: 670023), performed on the RotorGene Q 5Plex HRM machine according to the instructions of the manufacturer (Qiagen, Hilden, Germany). In the first step, reverse transcription reaction was prepared by mixing 1 µg heated RNA (10 µL) with 15 µL of RT buffer, then cycled at 25 °C for 15 mins, 50 °C for 60 mins, and 85 °C for 5 mins. In the second step, the quantitative polymerase chain reactions (qPCR) were prepared by mixing 12.5 µL qPCR master mix with 6.5 µL nuclease-free water, 1 µL primers and probe mix, and 5 µL PCR product from step one. PCR temperatures were set up as follows: 95 °C/10 seconds; 50 cycles of 95 °C/5 seconds and 60 °C/1 min; and then at 36 °C for 1 min. The standard curve was constructed using a standard series of 101–106 copies BCR-ABL/5 µL which were run within one-batch with the patient’s sample. PCR reactions were analyzed by the Rotor-Gene Q Series Software V.2.3.1. BCR-ABL transcripts were reported as normalized copy number on the international scale.
Statistical analysis
The Chi-square or Fisher’s exact tests were used to compare the relative frequencies while the Kruskal-Wallis rank was used to test the difference of median diagnostic values between groups. To determine the optimal cut-off point of each marker for the treatment failure (failure to reach response milestones), the logistic regression was used to construct the receiver operating characteristic (ROC) curve and calculate the Youden’s index. Association of marker levels with treatment failure were assessed by the odds ratio (OR) with 95% confident interval (95% CI). All data of the study were analyzed by R statistical software v.3.5.1 (R foundation, 1020 Vienna, Austria). P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 71 CML (39 males and 32 female) patients with BCR-ABL (+) in bone marrow cells were selected for this study (Figure 1). Patients were categorized into three groups based on the leukemic phenotype and NRBC cut-off (0.003×109/L): 7 cases of accelerated phase with NRBC≥0.003×109/L; 64 cases of chronic phase including 31 cases with NRBC≥0.003×109/L and 33 cases with NRBC<0.003×109/L (Table 1). The median age of all patients was 39 (from 31 to 47) years old.
Table 1.
Baseline characteristics of the patients
| Variable | Total (n=71) | Accelerated phase, n=7 | Chronic phase, n=64 | P-value | |
|---|---|---|---|---|---|
| High-NRBC, n=31 | Low-NRBC, n=33 | ||||
| Age, years (95% CI) | 39 (31–47) | 45 (26–59) | 39 (28–46) | 39 (29–49) | - |
| Gender | 0.832 | ||||
| Female | 32 | 4 | 14 | 14 | |
| Male | 39 | 3 | 17 | 19 | |
| Bone marrow cellularity | 0.443 | ||||
| Normal | 15 | 0 | 8 | 7 | |
| Hyper | 56 | 7 | 23 | 26 | |
| Myeloid:Erythroid ratio | 0.952 | ||||
| ≤5:1 | 13 | 1 | 6 | 6 | |
| >5:1 | 58 | 6 | 25 | 27 | |
| Peripheral white blood cells | 0.192 | ||||
| ≤50×109/L | 8 | 0 | 6 | 2 | |
| >50×109/L | 63 | 7 | 25 | 31 | |
| Blast cells | <0.001 | ||||
| <10% | 64 | 0 | 31 | 33 | |
| ≥10% | 7 | 7 | 0 | 0 | |
| Basophils | 0.099 | ||||
| ≤20% | 70 | 6 | 31 | 33 | |
| >20% | 1 | 1 | 0 | 0 | |
| Platelets | 0.545 | ||||
| ≤1000×109/L | 63 | 7 | 26 | 30 | |
| >1000×109/L | 8 | 0 | 5 | 3 | |
| FISH: t (9;22) translocation | 0.996 | ||||
| ≤35% | 10 | 1 | 4 | 5 | |
| >35% | 61 | 6 | 27 | 28 | |
| BCR-ABL transcripts | 0.805 | ||||
| ≤35% | 7 | 0 | 4 | 3 | |
| >35% | 64 | 7 | 27 | 30 | |
| Treatment | 0.046 | ||||
| Imatinib | 67 | 5 | 30 | 32 | |
| Imatinib+Nilotinib | 4 | 2 | 1 | 1 | |
Notes: High-NRBC: NRBC≥0.003×109/L; Low-NRBC: NRBC<0.003×109/L.
Abbreviations: 95% CI, 95% confident interval; FISH, fluorescent in situ hybridization; NRBC, nucleated red blood cell.
Most of the patients had been diagnosed with the hypercellular bone marrow (56 cases, 78.9%), myeloid-to-erythroid ratio >5:1 (58 cases, 81.7%) and peripheral white blood cells >50×109/L (63 cases, 88.7%) (Table 1). The increased blast cells (≥10%; in the bone marrow and peripheral blood) was observed in 7 cases (accelerated phase), of which one patient had basophils number >20%. In the cytogenetic assessment, the t(9;22) translocation was found in bone marrow cells with a median value of 56.5% (95% CI: 49.2–62.7%). Sixty-one cases (85.9%) have the translocation value >35%. The PCR analyses found the BCR-ABL p210 transcripts in bone marrow cells with a median value of 63.81% (95% CI: 53.44–74.37%). Almost patients (64 cases, 90.1%) have the quantitative BCR-ABL p210 transcripts >35% at the diagnosis. BCR-ABL p190 transcript was not found in the study subjects.
Changes of BCR-ABL transcripts and other diagnostic values during treatment
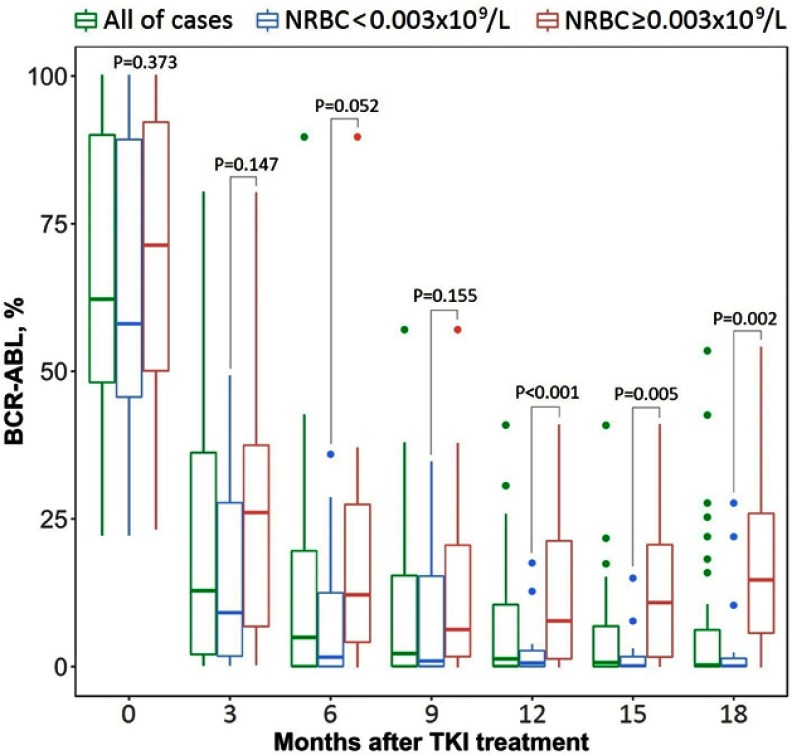
All of 71 patients were treated with a standard dose of imatinib (400 mg/day), in which 4 cases were switched to the treatment with Nilotinib (300 mg/day) (Novartis, Basel, Switzerland). The median time of the treatment follow-up was 39 months. We noted that median values of BCR-ABL transcripts, white blood cells, blast cells, basophils, platelets, and NRBC were declined after 6 months of treatment compared to the initial values (at T0 time-point) (P<0.001) (Table 2). After 12 and 18 months of treatment, the BCR-ABL transcripts were continued to be decreased considerably compared to the value at T6 time-point (P=0.008) while white blood cells (P=0.582) and basophils (P=0.070) were not changed. We also noted that median blast cells and circulating NRBC dropped down to zero from the 6th months to beyond (Table 2). Interestingly, we found that patients with a high level of circulating NRBC (≥0.003×109/L) have higher BCR-ABL transcripts compared to others (Figure 2).
Table 2.
Laboratory data at serial treatment time-points
| Variable | T0 (n=71) | T6 (n=71) | T12 (n=59) | T18 (n=58) | P-value |
|---|---|---|---|---|---|
| BCR-ABL, % (All patients) | 63.81 (53.44–74.37) | 4.83 (1.73–10.82) | 1.07 (0.28–2.98) | 0.51 (0.09–1.81) | <0.001a 0.008b |
| BCR-ABL, % (Low-NRBC) | 58.04 (48.61–74.54) | 1.59 (0.06–9.92) | 0.60 (0.04–2.66) | 0.09 (0.04–0.67) | <0.001a 0.069b |
| BCR-ABL, % (High-NRBC) | 71.35 (52.47–80.93) | 12.15 (4.09–26.17) | 7.73 (3.47–25.07) | 17.04 (2.10–38.86) | <0.001a 0.724b |
| RBC, ×1012/L | 3.26 (3.08–3.41) | 3.73 (3.57–4.02) | 3.71 (3.46–3.94) | 3.70 (3.51–3.86) | <0.001a 0.754b |
| NRBC, ×109/L | 0.043 (0.000–0.253) | 0.000 (0.000–0.001) | 0.000 (0.000–0.002) | 0.000 (0.000–0.000) | <0.001a 0.766b |
| WBC, ×109/L | 160.7 (138.4–185.7) | 5.5 (4.8–6.2) | 5.5 (4.3–6.1) | 5.5 (4.8–6.3) | <0.001a 0.582b |
| Blast cells, % | 1.6 (1.3–1.9) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | <0.001a 0.998b |
| BASO, % | 7.5 (6.7–8.9) | 0.5 (0.4–0.5) | 0.5 (0.4–0.7) | 0.6 (0.5–0.6) | <0.001a 0.070b |
| PLT, ×109/L | 402 (352–487) | 157 (126–162) | 157 (135–176) | 183 (163–204) | <0.001a 0.004b |
| Uric acid, mg/dL | 5.52 (4.92–6.32) | 4.74 (4.12–5.80) | 4.95 (4.71–5.59) | 5.11 (4.69–5.46) | 0.776a 0.968b |
| Bilirubin, mg/dL | 0.59 (0.53–0.67) | 0.60 (0.55–0.70) | 0.60 (0.51–0.71) | 0.60 (0.52–0.67) | 0.802a 0.618b |
| Creatinine, mg/dL | 0.75 (0.70–0.81) | 0.71 (0.70–0.81) | 0.75 (0.70–0.80) | 0.78 (0.70–0.80) | 0.284a 0.104b |
| Sodium, mmol/L | 138.0 (138.0–139.0) | 139.5 (138.9–140.1) | 139.2 (138.6–139.7) | 139.2 (138.7–139.7) | 0.091a 0.480b |
| Potassium, mmol/L | 3.89 (3.70–3.99) | 3.62 (3.57–3.70) | 3.64 (3.60–3.75) | 3.67 (3.61–3.83) | 0.001a 0.252b |
| Chloride, mmol/L | 104.8 (104.0–105.4) | 107.1 (106.2–108.0) | 107.2 (106.6–107.9) | 107.0 (106.4–107.6) | 0.003a 0.962b |
| Calcium, mmol/L | 2.2 (2.1–2.2) | 2.2 (2.1–2.2) | 2.2 (2.2–2.2) | 2.2 (2.2–2.2) | 0.673a 0.577b |
Notes: Results were presented as median with 95% confident interval (95% CI); High-NRBC: NRBC≥0.003×109/L; Low-NRBC: NRBC<0.003×109/L; aT6, T12, T18 versus T0. bT6 versus T12 versus T18.
Abbreviations: RBC, red blood cells; WBC, white blood cells; BASO, basophils; NRBC, nucleated red blood cells; PLT, platelets; T0, at diagnosis; T6, after 6 months of treatment; T12, after 12 months of treatment; T18, after 18 months of treatment.
Figure 2.
Changes of quantitative BCR-ABL transcripts during tyrosine kinase inhibitor (TKI) therapy.
The median red blood cells, electrolyte sodium, and chloride values were increased after 6 months of treatment (P-values were <0.001, 0.091, and 0.003, respectively), then stable for the following months (P=0.754, 0.480, and 0.962, respectively) (Table 2). Conversely, the potassium level was decreased after 6 months (P=0.001), and unchanging for the later (P=0.252). Other biochemical values were not changed during treatment (P>0.05).
Associated factors for the response failure
By the end of 6 months of treatment, forty-four (62.0%) patients reached good response (BCR-ABL≤10%), in which optimal response (BCR-ABL<1%) was noted in 27 (38.0%) cases. Twenty-seven (38.0%) cases failed to reach response milestone at T6 time-point (Table 3). At T12 time-point, the molecular data of 59 cases were available for response evaluation. Of which, thirty patients (50.8%) attained while 29 (49.2%) cases failed to reach the response target (BCR-ABL≤1%). These numbers at T18 time-point were 24 (41.4%) and 34 (58.6%) cases, respectively.
Table 3.
Molecular response of the study subjects
| Response targets | Yes, n (%) | No, n (%) | ||
|---|---|---|---|---|
| High-NRBC | Low-NRBC | High-NRBC | Low-NRBC | |
| BCR-ABL≤10% at T6 (n=71) | 2 (2.8) | 42 (59.2) | 8 (11.3) | 19 (26.7) |
| BCR-ABL≤1% at T12 (n=59) | 3 (5.0) | 27 (45.8) | 10 (17.0) | 19 (32.2) |
| BCR-ABL≤0.1% at T18 (n=58) | 2 (3.5) | 22 (37.9) | 12 (20.7) | 22 (37.9) |
Notes: High-NRBC: NRBC≥0.003×109/L; Low-NRBC: NRBC<0.003×109/L.
Abbreviations: T6, after 6 months of treatment; T12, after 12 months of treatment; T18, after 18 months of treatment.
The optimal cut-off point and the area under the ROC curve (AUC) value of each marker for the treatment failure at T6, T12, and T18 time-points were shown in Table 4 (only markers with AUC≥0.55 have been shown). At the first milestone (T6), patients with high levels of circulating NRBC (≥0.003×109/L, P=0.009), basophils (≥0.6%, P=0.029), and low levels of peripheral red blood cells (≤4.29×1012/L, P=0.057), electrolyte sodium (≤138.9 mmol/L, P=0.051), and chloride (≤108.1 mmol/L, P=0.018) have the higher incidence of treatment failure (BCR-ABL>10%) compared to others. The multivariable analysis has shown that elevated NRBC is an independent factor for the treatment failure at T6 (adjusted OR=6.49, 95% CI: 1.09–39.33, P=0.042).
Table 4.
Associated factors for the treatment failure
| Variable | Cut-off | AUC | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| BCR-ABL>10% after 6 months of treatment | ||||||
| RBC, ×1012/L | ≤4.29 | 0.6166 | 3.73 (0.96–14.51) | 0.057 | – | – |
| NRBC, ×109/L | ≥0.003 | 0.6006 | 8.84 (1.71–45.65) | 0.009 | 6.49 (1.09–39.33) | 0.042 |
| BASO, % | ≥0.6 | 0.5816 | 3.16 (1.12–8.87) | 0.029 | – | – |
| Sodium, mmol/L | ≤138.9 | 0.5913 | 2.42 (0.98–6.45) | 0.051 | – | – |
| Chloride, mmol/L | ≤108.1 | 0.6481 | 4.37 (1.29–14.77) | 0.018 | – | – |
| BCR-ABL>1% after 12 months of treatment | ||||||
| RBC, ×1012/L | ≤4.01 | 0.6517 | 5.78 (1.42–23.44) | 0.014 | 7.09 (1.29–38.84) | 0.024 |
| NRBC, ×109/L | ≥0.003 | 0.6977 | 4.92 (1.55–15.64) | 0.007 | 6.73 (1.69–26.76) | 0.007 |
| Potassium, mmol/L | ≥3.62 | 0.5989 | 2.54 (0.87–7.36) | 0.086 | – | – |
| Chloride, mmol/L | ≤104.7 | 0.5672 | 3.43 (0.81–14.53) | 0.095 | – | – |
| BCR-ABL>0.1% after 18 months of treatment | ||||||
| RBC, ×1012/L | ≤4.01 | 0.6294 | 3.10 (1.17–8.24) | 0.023 | 4.11 (1.28–13.20) | 0.018 |
| NRBC, ×109/L | ≥0.003 | 0.6690 | 6.00 (1.59–18.10) | 0.007 | 5.96 (1.54–21.71) | 0.009 |
| BASO, % | ≥0.8 | 0.6183 | 3.08 (0.99–9.66) | 0.051 | – | – |
| Sodium, mmol/L | ≤139.8 | 0.6218 | 3.67 (1.31–10.24) | 0.013 | – | – |
Abbreviations: RBC, red blood cells; WBC, white blood cells; BASO, basophils; NRBC, nucleated red blood cells; PLT, platelets; AUC, area under the ROC curve; OR, odds ratio.
At the second and third milestones (T12 and T18), we noted that red blood cells and circulating NRBC are independent factors for the failure of response targets (adjusted OR: 7.09 and 4.11 for RBC; 6.73 and 5.96 for NRBC, respectively). Remarkably, at all three evaluation time-points, the elevated NRBC is the worse indicator in treatment with imatinib (P=0.042, 0.007, and 0.009, respectively).
Discussion
NRBC is a part of the hematopoietic system which appears in peripheral blood of neonates for several days after birth, but not in the blood of healthy adults.10,26 The mechanism in which NRBC is released into the blood of critical illness such as CML is uncleared to date. It might because of the hypoxia and inflammation in the tumor microenvironment which causes hematopoietic stress, or the increased level of erythropoietin, and cytokines such as interleukin 3 and interleukin 6.10,19,27–30 Previous studies have shown that the presence of NRBC in peripheral blood is associated with high mortality in many severe diseases including leukemia.8,10–25 Some studies even showed the role of NRBC as a supplementing factor which helps to improve the prognostic power of the current used clinical standard.14,16 In spite of that, a few studies described the role of this cell in CML, especially in patients treated with targeted therapy.9
We investigated and showed that the number of NRBC and other immune cells such as white blood cells, blast cells, and basophils dropped quickly parallel with the decreases of BCR-ABL transcripts in CML bone marrow cells after 6 months of treatment with imatinib (P<0.001). Besides, the NRBC has not been found in peripheral blood of almost patients who archived good response (Table 3). This is consistent with results of Danise et al, in which 100% of patients at diagnosis decreased to 0% of cases at remission time-point have the presence of NRBC in peripheral blood.9 Notably, we found that the maintenance of high NRBC number in the blood is associated with high BCR-ABL transcripts. This might be explained by the existence of a high number of leukemic cells with BCR-ABL arrangement which causes the extramedullary hematopoiesis leads to the penetration of NRBC to the bloodstream.10
Importantly, patients with elevated NRBC have a higher likelihood of remission failure at different milestones (T6, T12, and T18) according to the ELN definitions (P=0.042, 0.007, 0.009, respectively). To our knowledge, this is the first time that NRBC is identified as an independent factor for the treatment failure in CML. In clinical practice, the appearance with a high number of NRBC suggests the clinicians about the loss of remission, thereby lead to the next decision makings such as drug interaction evaluation and mutational analysis.1 Besides, NRBC is a simple marker integrated with the complete blood count, therefore the uses of this parameter in treatment evaluation do not require additional costs.
In this study, the BCR-ABL transcripts in bone marrow cells (gold standard sample) were quantified and reported according to the international scale which was highly recommended in targeted treatment monitoring for CML.1 However, this study has limitations of a single-center and retrospective study. In addition, the sample size in the study is limited. Further research with a large cohort should be conducted to confirm our findings.
In conclusion, the results of this study showed that patients with a high number of NRBC in peripheral blood have higher BCR-ABL transcripts in bone marrow cells compared to others. The elevated NRBC might serve as an independent marker for remission failure in CML treated with imatinib.
Ethics approval and informed consent
This study was considered and approved by the Ethics Committees of Cho Ray Hospital (approval number: 602-2017-CN-HDDD). Patient consent was not required.
Data availability
The data of this study are available from the corresponding author with a reasonable request.
Author contributions
Son Truong Nguyen is the senior author who contributed to study design; Thang Thanh Phan, Ha The Vy, and Toan Trong Ho collected the clinical data; Tuyen Thi Bich Pham, Thao Thi Le, Suong Phuoc Pho, Vinh Thanh Tran, and Tung Thanh Tran collected the laboratory data; Thang Thanh Phan and Son Truong Nguyen performed the data analysis and wrote the manuscript. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hochhaus A, Saussele S, Rosti G, et al. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl.4):iv41–iv51. doi: 10.1093/annonc/mdx075 [DOI] [PubMed] [Google Scholar]
- 2.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Eng J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457 [DOI] [PubMed] [Google Scholar]
- 3.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333–2340. doi: 10.1200/JCO.2015.64.8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–1054. doi: 10.1038/leu.2016.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brück O, Blom S, Dufva O, et al. Immune cell contexture in the bone marrow tumor microenvironment impacts therapy response in CML. Leukemia. 2018;32:1643–1656. doi: 10.1038/s41375-018-0175-0 [DOI] [PubMed] [Google Scholar]
- 6.Lekovic D, Gotic M, Milic N, et al. Predictive parameters for imatinib failure in patients with chronic myeloid leukemia. Hematology. 2017;22(8):460–466. doi: 10.1080/10245332.2017.1302179 [DOI] [PubMed] [Google Scholar]
- 7.Chikkodi SV, Malhotra P, Naseem S, et al. Factors affecting early molecular response in chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2015;15(Suppl):S114–S119. doi: 10.1016/j.clml.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 8.Schwartz SO, Stansbury F. Significance of nucleated red blood cells in peripheral blood: analysis of 1,496 cases. JAMA. 1954;154(16):1339–1340. doi: 10.1001/jama.1954.02940500019007 [DOI] [PubMed] [Google Scholar]
- 9.Danise P, Maconi M, Barrella F, et al. Evaluation of nucleated red blood cells in the peripheral blood of hematological diseases. Clin Chem Lab Med. 2012;50(2):357–360. doi: 10.1515/cclm.2011.766 [DOI] [PubMed] [Google Scholar]
- 10.Otsubo H, Kaito K, Asai O, et al. Persistent nucleated red blood cells in peripheral blood is a poor prognostic factor in patients undergoing stem cell transplantation. Clin Lab Haem. 2005;27:242–246. doi: 10.1111/j.1365-2257.2005.00687.x [DOI] [PubMed] [Google Scholar]
- 11.Kho AN, Hui S, Kesterson JG, McDonald CJ. Which observations from the complete blood cell count predict mortality for hospitalized patients? J Hosp Med. 2007;2(1):5–12. doi: 10.1002/(ISSN)1553-5606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stachon A, Holland-Letz T, Krieg M. High in-hospital mortality of intensive care patients with nucleated red blood cells in blood. Clin Chem Lab Med. 2004;42(8):933–938. doi: 10.1515/CCLM.2004.151 [DOI] [PubMed] [Google Scholar]
- 13.Stachon A, Segbers E, Holland-Letz T, et al. Nucleated red blood cells in the blood of medical intensive care patients indicate increased mortality risk: a prospective cohort study. Crit Care. 2007;11:R62. doi: 10.1186/cc5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stachon A, Becker A, Kempf R, et al. Re-evaluation of established risk scores by measurement of nucleated red blood cells in blood of surgical intensive care patients. J Trauma. 2008;65:666–673. doi: 10.1097/TA.0b013e318181e524 [DOI] [PubMed] [Google Scholar]
- 15.Desai S, Jones SL, Turner KL, et al. Nucleated red blood cells are associated with a higher mortality rate in patients with surgical sepsis. Surg Infect. 2012;13(6):360–365. doi: 10.1089/sur.2011.089 [DOI] [PubMed] [Google Scholar]
- 16.Monteiro Junior JG, Torres Dde O, Da Silva MC, et al. Nucleated red blood cells as predictors of all cause mortality in cardiac intensive care unit patients: a prospective cohort study. PloS One. 2015;10(12):e0144259. doi: 10.1371/journal.pone.0144259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menk M, Giebelhäuser L, Vorderwülbecke G, et al. Nucleated red blood cells as predictors of mortality in patients with acute respiratory distress syndrome (ARDS): an observational study. Ann. Intensive Care. 2018;8:42. doi: 10.1186/s13613-018-0387-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purtle SW, Horkan CM, Moromizato T, et al. Nucleated red blood cells, critical illness survivors and postdischarge outcomes: a cohort study. Crit Care. 2017;21(1):154. doi: 10.1186/s13054-017-1724-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuert S, Holland-Letz T, Friese J, Stachon A. Association of nucleated red blood cells in blood and arterial oxygen partial tension. Clin Chem Lab Med. 2011;49(2):257–263. doi: 10.1515/CCLM.2011.041 [DOI] [PubMed] [Google Scholar]
- 20.Shah R, Reddy S, Horst HM, et al. Getting back to zero with nucleated red blood cells: following trends is not necessarily a bad thing. Am J Surg. 2012;203(3):343–345. doi: 10.1016/j.amjsurg.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Boskabadi H, Maamouri G, Sadeghian MH, et al. Early diagnosis of perinatal asphyxia by nucleated red blood cell count: a case-control study. Arch Iran Med. 2010;13(4):275–281. doi:010134/AIM.005 [PubMed] [Google Scholar]
- 22.Cremer M, Roll S, Gräf C, et al. Nucleated red blood cells as marker for an increased risk of unfavorable outcome and mortality in very low birth weight infants. Early Hum Dev. 2015;91(10):559–563. doi: 10.1016/j.earlhumdev.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 23.Kil TH, Han JY, Kim JB, et al. A study on the measurement of the nucleated red blood cell (nRBC) count based on birth weight and its correlation with perinatal prognosis in infants with very low birth weights. Korean J Pediatr. 2011;54(2):69–78. doi: 10.3345/kjp.2011.54.2.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Kobata K, Kamei Y, et al. Nucleated red blood cell counts: an early predictor of brain injury and 2-year outcome in neonates with hypoxic-ischemic encephalopathy in the era of cooling-based treatment. Brain Dev. 2014;36(6):472–478. doi: 10.1016/j.braindev.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 25.Walsh BH, Boylan GB, Murray DM. Nucleated red blood cells and early EEG: predicting Sarnat stage and two year outcome. Early Hum Dev. 2011;87(5):335–339. doi: 10.1016/j.earlhumdev.2011.01.041 [DOI] [PubMed] [Google Scholar]
- 26.Christensen RD, Henry E, Andres RL, Bennett ST. Reference ranges for blood concentrations of nucleated red blood cells in neonates. Neonatology. 2011;99:289–294. doi: 10.1159/000320148 [DOI] [PubMed] [Google Scholar]
- 27.Stachon A, Bolulu O, Holland-Letz T, Krieg M. Association between nucleated red blood cells in blood and the levels of erythropoietin, interleukin 3, interleukin 6, and interleukin 12p70. Shock. 2005;24(1):34–39. [DOI] [PubMed] [Google Scholar]
- 28.Dulay AT, Buhimschi IA, Zhao G, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol. 2008;198:426.e1–426.e9. doi: 10.1016/j.ajog.2008.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero R, Savasan ZA, Chaiworapongsa T, et al. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med. 2011;40(1):19–32. doi: 10.1515/JPM.2011.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danise P, Amendola G, Concilio RD, et al. Nucleated red blood cells and soluble transferrin receptor in thalassemia syndromes: relationship with global and ineffective erythropoiesis. Clin Chem Lab Med. 2009;47(12):1539–1542. doi: 10.1515/CCLM.2009.340 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available from the corresponding author with a reasonable request.