Abstract
Chronic pain of uncertain etiology often presents a challenge to both patients and their health care providers. It is a complex condition influenced by structural and physiological changes in the peripheral and central nervous systems, and it directly influences, and is modulated by, psychological well-being and personality style, mood, sleep, activity level and social circumstances. Consequently, in order to effectively treat the pain, all of these need to be evaluated and addressed. An effective management strategy takes a multidisciplinary biopsychosocial approach, with review of all current medications and identification and careful withdrawal of those that may actually be contributing to ongoing pain. The management approach is primarily nonpharmacological, with carefully considered addition of medication, beginning with pain-modulating treatments, if necessary. In this article, we present a primary care approach to the assessment and management of a patient with chronic pain where the cause cannot be identified.
Keywords: etiology, biopsychosocial, central sensitization, chronic, pain
Introduction
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage.1 Acute pain is usually self-limiting and serves a protective function, influencing behavior to avoid further tissue damage and limiting movement to support healing. In contrast, in much the same way that a small flame from a match can cause a large forest fire, inadequately treated acute pain causes changes in the peripheral and central nervous systems that maintain persistent pain independent of the initial inciting painful stimulus.2–5
Chronic pain is defined as pain that persists beyond the normal time expected for tissue healing (usually accepted as 3 months) and without apparent benefit.6 Treatment can be complex and difficult.3 While there are guidelines to direct management of chronic pain associated with specific disorders such as cancer,7 osteoarthritis,8,9 fibromyalgia10–12 or neuropathic pain,13–15 often there is no obvious cause for pain that persists despite treatment.2 Under these circumstances, uncertainty and frustration on the part of both practitioner and patient can lead to inappropriate polypharmacy and escalating doses of medications, exposing patients to unnecessary treatments and associated side effects.
For most patients with chronic pain, the general practitioner remains the most appropriate health care professional to treat and, where necessary, coordinate multidisciplinary management. However, to do so requires an understanding of why pain becomes chronic, the multitude of factors that may complicate ongoing pain, and management of chronic pain needs to be differentiated from management of acute exacerbations. It requires careful establishment of realistic expectations and formulation of an individualized, tiered, multimodal plan that can successfully bring pain relief and improve function.
How common is chronic pain?
Limited data, differing definitions of chronic pain, data collection and reporting methods and differences in the prevalence of contributing factors (eg, psychological trauma, socioeconomic status and interpersonal violence) between countries pose significant challenges to estimating the global prevalence of chronic pain. However, depending on the definition, it is estimated that 25–30% of the world’s adult population will suffer from chronic pain during their lifetime.6,16 In general, the prevalence is higher among women, older individuals and those with mental stresses, depression and anxiety.17,18 In a considerable proportion of those with chronic pain, the etiology is uncertain (Box 1).19,20 Although the prevalence of neuropathic pain is usually reported to be lower (approximately 5–10%), pathologic changes in the peripheral and central nervous systems play a major role in chronic pain, either alone or in combination with other pain states (mixed pain), and, where the etiology is uncertain, central sensitization is an important focus of treatment.4,7,15,21–23
Box 1.
Common types of chronic pain with uncertain etiology
| Low back pain |
| Chronic headache |
| Musculoskeletal/joint pain |
| Chronic pelvic pain |
| Temporomandibular disorder |
| Abdominal pain/irritable bowel syndrome |
| Fibromyalgia |
| Chronic widespread pain |
Is chronic pain a disease in its own right?
Chronic pain is a cause of considerable long-term morbidity and disability, associated with both physical and psychosocial changes that arise consequent to, but also which contribute to persistent pain (Box 2).7,24,25
Box 2.
Associated and contributory behavioral and psychological factors in chronic pain
|
In order to increase awareness of the magnitude of suffering associated with the disorder and to facilitate changes in social policy, training and research, there have been calls to consider chronic pain a disease in its own right.24,26,27 Although this proposal has been hotly debated, and the causal relationship between some of the brain structural and functional changes and pain are uncertain, it does emphasize that chronic pain is not merely a symptom, but rather a complex and multifactorial disorder.27–30 Patients’ experiences of pain are profoundly influenced by their emotional and psychological well-being, social circumstances and cultural and spiritual beliefs. Pain is isolating, emotionally exhausting and adversely impacts on social relationships, daily functions, sleep and self-worth. It is impossible to directly measure pain, and it may be difficult for the practitioner to fully appreciate or understand the suffering experienced by the individual who is experiencing it.7,31 Consequently, management of chronic pain must extend beyond solely providing pain-relieving medication. Holistic treatment requires a careful and compassionate assessment with consideration of all of the underlying pathologies and conditions associated with and contributing to ongoing pain. Effective management requires both a multimodal (pharmacological and nonpharmacological) and multidisciplinary approach tailored specifically to each individual patient.
Pathophysiology of chronic pain
The pathophysiology of chronic pain is complex and distinct depending on its origin, being different for nociceptive, neuropathic, visceral and mixed (eg, cancer) pain.
Acute nociceptive pain arises from activation of nociceptors in the periphery by noxious stimuli (eg, mechanical pressure, heat, cold or chemicals) that damage or threaten to damage tissue. Afferent nociceptive signals can be altered by a descending or modulatory system originating from several regions of the central nervous system, including the somatosensory cortex, hypothalamus, periaqueductal gray (PAG), pons, lateral tegmental area and nucleus raphe magnus. Activation of these descending pathways promotes an analgesic effect (descending inhibition) effected and modulated by various neurotransmitters, including noradrenaline and serotonin.2–5
Persistent acute pain may lead to neuronal remodeling both in the periphery and centrally (in the spinal cord and brain). These changes in structure and function are associated with reduced modulation of painful nerve impulses, increased excitability and sensitivity of nerve cells that transmit pain signals, and increased connection between cells in the periphery and those in the central nervous system. Peripheral and central sensitization result in an exaggerated response to painful stimuli (hyperalgesia) and pain in response to normally nonpainful stimuli (allodynia), leading to persistent chronic pain that is independent of the initial painful insult. Dysfunction of descending serotonergic and noradrenergic modulatory pathways results in an imbalance between inhibitory and excitatory pain signaling pathways within the central nervous system. Over time, pain hypersensitivity also produces structural changes in the brain that perpetuate chronic pain.2–5
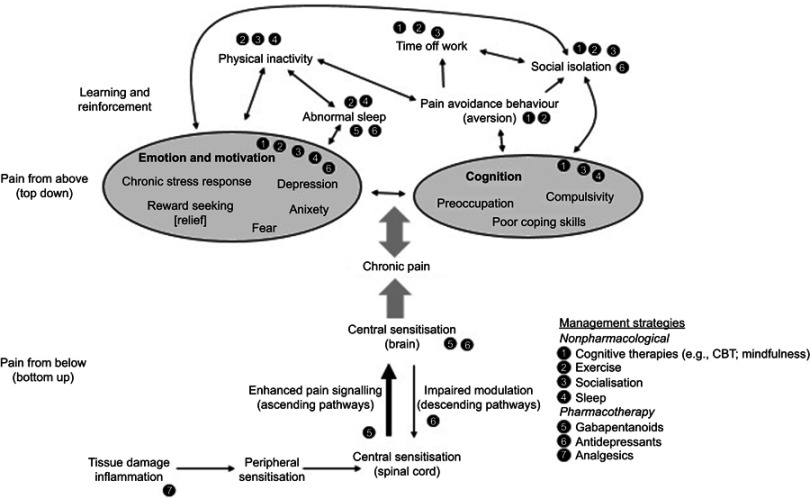
Genetics, personality type, social influences and psychological factors have a significant effect on the vulnerability of the individual to progress from acute to chronic pain. Psychological factors (Box 2) exacerbate pain perception in a top-down fashion through their effect on the inhibitory descending pathways (Figure 1).5,18
Figure 1.
Multiple mechanisms of chronic pain and potential effects of management strategies.
Due to this complex nature of chronic pain, it does not respond to analgesic or anti-inflammatory drugs on their own. However, it may respond, at least partially, to specific modulators of pain signaling in the central nervous system (Table 1).5
Table 1.
Effect of pain modulators on general pain mechanisms
| Mechanism | Pain modulator | ||
|---|---|---|---|
| Anti-inflammatory; immunosuppressant | Gabapentanoid | Antidepressant | |
| Peripheral sensitization | ✓ | Possibly | |
| Ectopic activity | ✓ | ||
| Central sensitization | ✓ | ✓ | |
| Central disinhibition | ✓ | ✓ | |
Note: Data from Vardeh et al.4
Pain, depression and sleep
Chronic pain is bidirectionally associated with anxiety, depression and insomnia.35,36
The association between these pathologies extends beyond cause and effect. The complex neural pathways in the brain involved in processing pain expand painful sensations to a subjective consciousness of internal state, external circumstance, memory and mood. These are the same regions of the brain responsible for mood and sleep regulation.37–39 Overlapping neurophysiology explains why these conditions are so frequently comorbid and why addressing one, without addressing the others, is unlikely to lead to effective or lasting relief of any of them (Figure 2).
Figure 2.
Bidirectional relationships between pain, mood and sleep.
Notes: Adapted with permission from Jain R, Webb DA. Chronic pain: addressing the triad of pain, sleep and depression/anxiety. SA J Diabetes. 2016;9(3):7–11. © Homestead Publishing (Pty) Ltd.39
Long-term use of opioids may worsen chronic pain
While opioids may be useful for short-term (less than 3–7 days) treatment of acute pain when inflammation and/or nociception are present and for palliative care at end of life, most patients with chronic noncancer pain will not benefit from long-term opioids.7,40–43
Long-term use of opioids for chronic non-cancer pain is associated with variable and unpredictable efficacy, tolerance to the analgesic effect and potential for serious side effects. Among others, these include suppression of endogenous opioids (endorphins) and down-regulation of opioid receptors, thereby lowering the pain threshold; physical dependence, abuse and risk of addiction.44–50 Common comorbidities, such as depression, anxiety, sleep-disordered breathing and alcohol dependence, increase the risk of serious harm associated with long-term use of opioids.41,50,51
Opioid analgesia may also induce central sensitization, which promotes persistent pain, and hyperalgesia, a paradoxical state of increased pain sensitivity that extends to areas of the body beyond that of the original pain (opioid-induced hyperalgesia [OIH]). Consequently, patients experience sustained pain that is apparently unresponsive to treatment, causing a vicious cycle of ongoing suffering, unnecessary treatments and escalating drug doses with resultant exacerbation of diffuse hyperalgesic symptoms.46 Unfortunately, because OIH occurs consequent to neuroplastic changes in the central nervous system, it may be prolonged even after cessation of opioid drugs, and it is difficult to manage.48
Practical approach to pain management in chronic pain of uncertain etiology
The general principles of management for chronic pain of uncertain etiology are listed in Box 3.
Box 3.
General principles for biopsychosocial management of chronic pain of uncertain etiology
|
This biopsychosocial approach requires an interdisciplinary team, with early referral as necessary. Communication between health professionals is paramount, not only to ensure a co-ordinated approach to an individualized management plan, but also to ensure that information and guidance provided to the patient is consistent, clear and coherent.
Perform a biopsychosocial assessment and address expectations
Where there is an identifiable cause of chronic pain (eg, diabetes, fibromyalgia), management should proceed as per the current available treatment guidelines with appropriate monitoring and follow-up. However, after appropriate investigations, in patients in whom the cause of chronic pain cannot be determined, repeated radiological and other special investigations are rarely helpful and should be avoided.
Management is guided by careful assessment for the presence of risk factors for chronic pain, psychosocial history, assessment of pain severity and the degree of functional impairment. The Brief Pain Inventory (BPI) is recommended as a simple tool to measure pain intensity and physical and psychosocial components of pain.
Psychosocial and behavioral factors (Box 2) may complicate diagnosis, management and the course of chronic pain, and it is necessary to conduct a careful assessment for these using simple questions (Box 4) and/or self-rating scales (eg, Beck’s Depression Inventory). Detailed assessment tools are time-consuming and usually unnecessary. It is important to point out that many of these psychosocial factors are normal psychological processes and/or responses to adversity. However, in some patients they can influence the subjective experience of pain and directly modulate pain pathways leading to maladaptive coping and persistent pain that is apparently non-responsive to treatment.32,33 Management in the primary care setting is often sufficient, but if necessary patients should be referred to an appropriate mental health professional.
Box 4.
Helpful brief screening questions to identify risk factors for chronic pain, disability and delayed return to work
|
It is important to explain to patients and their family/caregivers that chronic pain is multifactorial and does not necessarily indicate harm, and also to set realistic expectations in terms of goals of treatment. In chronic pain, complete pain relief is rarely achieved and aims of therapy are to reduce pain, improve function and return to work and physical activity. A clinically meaningful improvement is at least a 30% reduction in pain (or ≥2 points on a 0–10 numerical rating scale) and/or 30% improvement in function.13,55 Other important outcomes include improvement in sleep and mood, reduced analgesic consumption and reduced health care consultations.
Work absenteeism is associated with low self-esteem, depression, loss of skills, delayed recovery, and in some patients, personal re-identification as a “disabled person”. Because the likelihood of returning to work diminishes with increasing duration of absence, it is important to encourage patients who are still working to continue to do so. Those who are off work should be encouraged to return to work as soon as possible.52,60,61
Assess current medication
All medications should be evaluated and discussed. This includes prescribed pharmacotherapy, over-the-counter medicines, alternative treatments, supplements and illicit/recreational drugs (including cannabis).
Analgesic consumption must be carefully evaluated. Frequent use of all types of pain-relieving drugs, in particular triptans, ergotamine, opioids, caffeine, meprobamate and codeine-containing products, may be associated with rebound headache, which itself is associated with greater pain-related disability.53,54 Withdrawal of treatment often leads to improvement and evolution to episodic headache. Where chronic headache does not resolve with withdrawal of analgesics, the patient should be referred for specialist assessment.
Opioids should be tapered and discontinued
Where patients who present with ongoing pain of uncertain etiology are already on long-term opioids, the dose should be carefully tapered and the opioid should preferably be discontinued. This includes combination analgesic formulations containing codeine and/or meprobamate, which are commonly used and may be available over-the-counter.
While careful tapering and discontinuation of opioids may take time, and both patients and health care professionals may be reluctant to withdraw any medication they perceive to be pain-relieving, in patients with chronic pain it is generally an essential step if pain is to be effectively managed. Opioid withdrawal itself can result in clinically meaningful pain relief.46
The opioid dose should be reduced slowly by approximately 10% per week, or more slowly in patients who are anxious or who are suspected of being physically dependent on opioids. When one-third of the original dose is reached, reduce the rate of tapering to one half or less of the initial rate. With careful tapering, withdrawal symptoms in patients with chronic pain are less common and less severe than those which occur in people with opioid addiction and, if they do occur can usually be managed symptomatically. Where there is an increase in pain during tapering, dose reduction should be discontinued and consideration could be given to temporarily increasing the current dose before beginning the tapering process again.55 Buprenorphine substitution and clonidine may be helpful for more severe withdrawal symptoms and buprenorphine also for acute pain during opioid withdrawal.62
Care must be taken to ensure that the patient is not lost to follow-up during the time taken for opioid withdrawal. Patients should be provided with written instructions, dates for follow-up consultations and encouraged to ask questions so that their concerns are addressed. Where a reasonable attempt to withdraw the opioid is not successful, the patient should be referred to a pain clinician.
Assess sleep and pay attention to sleep hygiene
Patients should be asked about sleep and habits that may affect sleep. Advice for improving sleep is listed in Box 5.
Encourage physical activity
Physical activity helps to prevent the negative effects of immobility, including muscle weakness, joint and muscle stiffness, as well as depression, weight gain and cardiovascular deconditioning.52 Physical activities should be individualized and commensurate with personal capability and preferences. Examples include cardiovascular exercise, resistance training, yoga and pilates. Exercise in water (aquacise) is reassuring for patients with musculoskeletal pain. Group activities may increase motivation and provide an opportunity for social engagement. If costs allow, early referral to a biokineticist is desirable for an individualized and supervised exercise program. Where feasible, the use of downloadable exercise-related apps (eg, step counter) is encouraged to help motivate, set daily goals and keep track of activity.
Encourage healthy nutrition and maintenance of healthy body weight
The association between body fat mass and pain extends beyond merely excessive overloading of joints. Adipose is an active endocrine organ that secretes many cytokines and hormones that may be relevant in the development of pain. Obesity may therefore be responsible for metabolic in addition to structural and psychological mechanisms that link adiposity to pain. Excessive adiposity is associated with increased risk of incident and worsening single-site and widespread pain.64
There is a bidirectional relationship between obesity and inflammation, and weight loss is associated with reduced serum concentration of inflammatory markers and the number of macrophages and inflammatory mediators in adipose tissue, the liver and the colon.56,65
Nutrition influences many of the factors that influence chronic pain, including psychological factors, pain pathophysiology, inflammation and response to medication.56,57 Patients should be given practical dietary instructions on how to implement an anti-inflammatory/Mediterranean/DASH-type diet. These diets encourage adequate hydration and consumption of fresh fruit and vegetables, proteins and whole grains, with avoidance of refined and processed foods (Table 2).56,57,65–68 In order to be sustainable, diet should be culturally acceptable, practical, affordable and enjoyable.69
Table 2.
Examples of foods appropriate to an anti-inflammatory eating plan
| Eat more of | Eat less of |
|---|---|
|
|
Encourage social connectedness
Social isolation and lack of meaningful relationships are risk factors for psychological disorders (eg, depression, stress), ongoing chronic pain and poor health in general, including cardiovascular disease and cancer.63,70–72 Participation in support groups, especially those that encourage talking with others who share the same experience (eg, chronic pain support groups), can help to improve coping skills, self-efficacy, enjoyment of life and hope.73–77 Community groups associated with activity (eg, Walk for Life) are also encouraged.78
Encourage cognitive and mind-body therapies and relaxation
Patients need to be encouraged to take responsibility for their condition and recovery. Treatment is more likely to be successful in patients who are resilient, have good coping skills, are able to problem solve, who are less likely to ruminate over their condition and who are motivated to get better.25,32,33
In patients with chronic pain, when added to usual care, cognitive and mind-body therapies are associated with reduced pain and increased function. They also have potential to reduce the burden on caregivers, increase work productivity and reduce consumption of analgesic medication.25,58 Examples include cognitive behavioral therapy (CBT), acceptance therapy, mindfulness-based stress reduction (MBSR), yoga and tai chi. In some patients, including those with catastrophizing or pain-related anxiety, engaging in distracting thoughts or activities might be beneficial to reduce pain and pain-related distress.79–81 Hobbies and pursuit of other interests should be encouraged.
Pharmacological treatment
For most patients in primary care, pain modulators are the mainstay of pharmacological management of chronic pain of uncertain etiology. The aim of treatment is to reduce central sensitization/hyperalgesia and to break the ongoing cycle of exacerbated pain and medication side effects.
Analgesics are generally reserved for intermittent use for acute pain flare-ups and to facilitate rehabilitation, starting with simple analgesics (eg, paracetamol or nonsteroidal anti-inflammatories) and following a step-wise approach appropriate to the severity of pain and the individual.7 However, many patients with chronic pain are over-treated with analgesics or use them inappropriately, and long-term use is not recommended.82–86 Attention needs to be paid to use of rational combination therapy using the least number of medications as is absolutely necessary.
Role of pain modulators
Pain modulators include the gabapentanoids (gabapentin and pregabalin), tricyclic antidepressants (TCI; eg, amitriptyline) and serotonin noradrenaline re-uptake inhibitors (SNRI; eg, duloxetine).13–15,87–89 Some indications that may be helpful to guide initial choice of therapy are listed in Table 3.
Table 3.
Relative indications for choosing a specific pain modulator
| Amitriptyline | Pregabalin/gabapentin | Duloxetine |
|---|---|---|
|
|
|
Practical approach:
Amitriptyline: Start with 10 mg at night and if necessary escalate the dose weekly by 10–25 mg/day, up to a maximum dose of 50–150 mg/day.15 The usual dose for pain relief is 25–50 mg/day. Since the analgesic effect is independent of the mood-altering effect, the dose used for pain is lower than the antidepressant dose (100–300 mg).90–93
Pregabalin: Start low and titrate slowly according to tolerability, initially 25 mg at night, increasing by 25 mg increments every 3 days. Dosing is initially nocté, followed by higher doses, to a maximum daily dose of 300 mg, administered in two divided doses.15 Some patients prefer to take only a night-time dose.
Gabapentin: Start with a low dose (100–300 mg at bedtime or 100–300 mg three times daily). Because it has nonlinear pharmacokinetics, it requires slow and careful titration, increasing the dose by 100–300 mg three times daily every 1–7 days as tolerated. The maximum dose is 3600 mg per day in divided doses (1200 mg three times daily).15
Duloxetine: Start with 30 mg and increase to 60 mg after 1 week. The dose may be increased to 120 mg/day (60 mg twice daily).15
Patients with chronic pain have often been taking one of these medications in the past. Lack of efficacy may be consequent to inappropriate dosing or use of monotherapy. Dosing should be optimized and patients generally require combination therapy, which, in comparison with monotherapy, may be more effective and permit lower doses, with improvement in tolerability.13,15 However, the potential for additive adverse effects, increased risk of drug interactions and reduced adherence due to increasing complexity of dosing are also important considerations. Once the dose of a single agent has been stabilized at a therapeutic level, if response is not adequate after 2–4 weeks (and treatment is tolerable), then combination therapy may be considered, using any of the other classes of pain modulators.15
Compliance should be assessed by asking the patient to bring all of their medications with them to each consultation.
Reduce and remove daily analgesic therapy
Regular analgesic use may be associated with increased pain sensitivity and should be withdrawn with careful weaning. For some patients, especially those who have been living with daily analgesia for sometimes months or even years, this approach can be quite distressing. Therefore, communication with both patient and their caregivers, including reasons for withdrawing medication and clear explanation of the entire pain management plan and outcome expectations, is essential, with confirmation that this information has been understood. If the patient is not fully committed to the structured pain management plan, improvement in outcomes is unlikely.
Pharmacological sleep management
Sleep is a critical component of treatment and sleep management aims to ensure sufficient quantity of good quality sleep.
Short-term use of benzodiazepines or z-drugs (zolpidem and zopiclone) may be helpful during the initial stage of pain management. However, this should be limited to the shortest time possible (ie, less than 2 weeks), with withdrawal as pain and function improve. Reasons for choosing these drugs should be discussed with the patient, including that the duration of use will be limited to a certain period of time, which must be specified.
Thereafter, if sleep remains a problem, short-term intermittent use of sedative antidepressants (eg, mirtazapine, trazodone) is preferred. In carefully selected patients, it may be appropriate to prescribe Z-drugs for intermittent use. Because of potential for adverse effects, long-term use of benzodiazepines should be avoided (Box 6). Patients who are already taking benzodiazepines should preferably be slowly weaned (over approximately a month) until the medication can be discontinued altogether.
Box 6.
Concerns associated with long-term use of benzodiazepines
|
|
Notes: Data from Ashton.94
Box 5.
Sleep health recommendations
|
Notes: Data from Webb et al.63
Refer to pain clinician
If the steps listed above are not successful, referral to a pain clinician or multidisciplinary pain clinic is recommended.
Conclusion
Chronic pain, especially where there is no obvious biological cause, may be associated with considerable suffering and despair. However, with an individualized biopsychosocial management plan, it is usually possible to relieve at least some of the pain and improve function and quality of life. Managing pain takes time and needs to be done in partnership with the patient. Careful communication is essential to manage expectations, encourage a healthy lifestyle and to explain why some medications need to be stopped or changed. Nevertheless, with a little extra effort, the systematic approach described in this article can be extremely rewarding for both health care providers and their patients.
Acknowledgments
Writing was supported by an unrestricted grant from Cipla. The content and writing was independent of the sponsor.
Author contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
SB, FM, HM, JS and EV report no conflicts of interest relevant to this article. SS reports grants from Cipla. EA reports grants from Cipla and non-financial support from Psychiatry Management Group (PsychMg) and Glynnview Multiprofessional Practice (GMPP) during the conduct of the study; grants from Aspen Pharmacare, Adcock Ingram, Pfizer, Janssens Pharmaceuticals, Sanofi-Aventis and Litha Pharma, and non-financial support from Rickett Benckiser and Sandoz outside the submitted work; and relationships with Pain SA and with several medical professionals that manage patients with chronic pain to form a virtual pain clinic. REH reports grants or personal fees from Mundipharma, Pfizer, Takeda, Sandoz, Abbvie, Aspen-GSK and Sanofi. DW is a medical writer and reports personal fees from Cipla during the conduct of the study; personal fees from Accord, Adcock Ingram, Alcon, Astra Zeneca, Eli Lilly, Fresenius Kabi, Litha, MSD, Novartis, Novo Nordisk, Pfizer, Pharma Dynamics, Reckitt Benkiser, Sandoz, Sanofi-Aventis, Takeda, MundiPharma and Abbvie outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Part III: pain terms, a current list with definitions and notes on usage In: Merskey H, Bogduk N, editors. Classification of Chronic Pain. 2nd IASP Task Force on Taxonomy Seattle: IASP Press; 1994:209–214. Most recent update: December 14, 2017. Available from: http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698. Accessed October 2, 2018. [Google Scholar]
- 2.Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth. 2010;105(S1):i69–i85. doi: 10.1093/bja/aeq323 [DOI] [PubMed] [Google Scholar]
- 3.Scholz J. Mechanisms of chronic pain. Mol Pain. 2014;10(Suppl 1):O15. doi: 10.1186/1744-8069-10-S1-O15 [DOI] [Google Scholar]
- 4.Vardeh D, Mannion RJ, Woolf CJ. Toward a mechanism-based approach to pain diagnosis. J Pain. 2016;9(Suppl 2):T50–T69. doi: 10.1016/j.jpain.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90(4):532–545. doi: 10.1016/j.mayocp.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 6.International Association for the Study of Pain (IASP). How prevalent is chronic pain? Pain Clinical Updates. 2003;XI(2). Available from: http://www.iasp-pain.org/PublicationsNews/NewsletterIssue.aspx?ItemNumber=2136. Accessed 6 August 2018. [Google Scholar]
- 7.Blanchard C, Chetty S, Ganca L, et al. Guide to the Treatment of Cancer Pain in South Africa. Pretoria (SA): Medspec Publishing; 2015. doi: 10.6084/m9.figshare.1612170 [DOI] [Google Scholar]
- 8.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465–474. [DOI] [PubMed] [Google Scholar]
- 9.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–388. doi: 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 10.Kia S, Choy E. Update on treatment guideline in fibromyalgia syndrome with focus on pharmacology. Biomedicines. 2017:5(4):20 Published online. doi: 10.3390/biomedicines5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzcharles M-A, Ste-Marie PA, Goldenberg DL, et al. 2012 Canadian guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res Manag. 2013;18(3):119–126. doi: 10.1155/2013/918216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macfarlane G, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76:318–328. doi: 10.1136/annrheumdis-2016-209724 [DOI] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence (NICE). Neuropathic pain – pharmacological management. The pharmacological management of neuropathic pain in adults in non-specialist settings. NICE clinical guideline 173; 2017. Available from: http://guidance.nice.org.uk/CG173. Accessed July26, 2019.
- 14.Cruccu G, Truini A. A review of neuropathic pain: from guidelines to clinical practice. Pain Ther. 2017;6(Suppl 1):S35–S42. doi: 10.1007/s40122-017-0087-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chetty S, Baalbergen E, Bhigjee AI, et al. Clinical practice guidelines for the management of neuropathic pain: expert panel recommendations for South Africa. S Afr Med J. 2012;102(5):312–325. [DOI] [PubMed] [Google Scholar]
- 16.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being. A world health organization study in primary care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147 [DOI] [PubMed] [Google Scholar]
- 17.Jackson T, Thomas S, Stabile V, et al. Chronic pain without clear etiology in low and middle-income countries: a narrative review. Anesth Analg. 2016;122:2028–2039. doi: 10.1213/ANE.0000000000001287 [DOI] [PubMed] [Google Scholar]
- 18.van Hecke TN, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth. 2013;111(1):13–18. doi: 10.1093/bja/aet123 [DOI] [PubMed] [Google Scholar]
- 19.Jackson T, Thomas S, Stabile V, et al. A systematic review and meta-analysis of the global burden of chronic pain without clear etiology in low and middle-income countries: trends in heterogeneous data and a proposal for new assessment methods. Anesth Analg. 2016;123:739–748. doi: 10.1213/ANE.0000000000001389 [DOI] [PubMed] [Google Scholar]
- 20.Treede R-D, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–1007. doi: 10.1097/j.pain.0000000000000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elzahaf R, Anabela S, Maynard M, Tashani OAA. Meta-analytic review of the prevalence of neuropathic pain in the general population of the global south compared to the global north (2017) Presented at the British Pain Society's 50th Anniversary Annual Scientific Meeting; Birmingham, UK; May 3–5, 2017. [Google Scholar]
- 22.DiBonaventura MD, Sadosky A, Concialdi K, et al. The prevalence of probable neuropathic pain in the US: results from a multinational general-population survey. J Pain Res. 2017;10:2525–2538. doi: 10.2147/JPR.S127014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Hecke O, Austin SK, Khan RA, et al. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662. doi: 10.1016/j.pain.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 24.International Association of Study of Pain (IASP), European Federation of IASP Chapters (EFIC). IASP now recommends the global adoption of EFIC’s declaration on chronic pain as a major healthcare problem, a disease in its own right; October 2004. Available from: https://s3.amazonaws.com/rdcms-iasp/files/production/public/Content/ContentFolders/GlobalYearAgainstPain2/20042005RighttoPainRelief/painasadisease.pdf. Accessed 6August 2018.
- 25.Sullivan MJL, Scott W, Trost Z. Perceived injustice. A risk factor for problematic pain outcomes. Clin J Pain. 2012;28:484–488. doi: 10.1097/AJP.0b013e3182527d13 [DOI] [PubMed] [Google Scholar]
- 26.Siddall PJ, Cousins MJ. Persistent pain as a disease entity: implications for clinical management. Anesth Analg. 2004;99:510–520. doi: 10.1213/01.ANE.0000133383.17666.3A [DOI] [PubMed] [Google Scholar]
- 27.Raffaeli W, Arnaudo E. Pain as a disease: an overview. J Pain Res. 2017;10:2003–2008. doi: 10.2147/JPR.S138864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen M, Quintner J, Buchanan D. Is chronic pain a disease? Pain Med. 2013;14:1284–1288. doi: 10.1111/pme.12025 [DOI] [PubMed] [Google Scholar]
- 29.Siddall P. Is chronic pain a disease? Pain Med. 2013;14:1289–1290. doi: 10.1111/pme.12162 [DOI] [PubMed] [Google Scholar]
- 30.Smith RC Chronic pain is not a disease. Psychology Today May 27, 2018. Available from: https://www.psychologytoday.com/intl/blog/patient-zero/201805/chronic-pain-is-not-disease. Accessed August6, 2018.
- 31.Teno JM, Okun SN, Casey V, et al. Toolkit of instruments to measure end of life care (TIME). Resource Guide: achieving Quality of Care at Life’s End. Center for Gerontology and Health Care Research, Brown University; 2001. Available from: http://as800.chcr.brown.edu/pcoc/resourceguide/resourceguide.pdf. Accessed February10, 2014.
- 32.Borsook D, Youssef AM, Simons L, et al. When pain gets stuck: the evolution of pain chronification and treatment resistance. Pain. 2018;159(12):2421–2436. doi: 10.1097/j.pain.0000000000001401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholas MK. Why do some people develop chronic, treatment resistance pain and not others? Pain. 2018;159(12):2419–2420. doi: 10.1097/j.pain.0000000000001404 [DOI] [PubMed] [Google Scholar]
- 34.Kendall NAS, Linton SJ, Main CJ Guide to assessing psycho-social yellow flags in acute low back pain: risk factors for long-term disability and work loss. Accident compensation corporation and the New Zealand guidelines group. Wellington, New Zealand; October, 2004. Edition. Available from: https://www.healthnavigator.org.nz/media/1006/nz-acute-low-back-pain-guide-acc.pdf. Accessed August8, 2018. [Google Scholar]
- 35.Gupta A, Silman AJ, Ray D, et al. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology. 2007;46(4):666–671. doi: 10.1093/rheumatology/kel363 [DOI] [PubMed] [Google Scholar]
- 36.Gore M, Brandenburg NA, Dukes E, et al. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005;30(4):374–385. doi: 10.1016/j.jpainsymman.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 37.Borsook D, Moulton EA, Schmidt KF, Becerra LR. Neuroimaging revolutionizes therapeutic approaches to chronic pain. Mol Pain. 2007;11(3):25. doi: 10.1186/1744-8069-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 39.Jain R, Webb DA. Chronic pain: addressing the triad of pain, sleep and depression/anxiety. SA J Diabetes. 2016;9(3):7–11. [Google Scholar]
- 40.Mitra S, Carlyle D, Kodumudi G, et al. New advances in acute postoperative pain management. Curr Pain Headache Rep. 2018;22(5):35. doi: 10.1007/s11916-018-0690-8 [DOI] [PubMed] [Google Scholar]
- 41.Ashburn MA, Fleisher LA. Increasing evidence for the limited role of opioids to treat chronic noncancer pain. JAMA. 2018;320(23):2427–2428. doi: 10.1001/jama.2018.19327 [DOI] [PubMed] [Google Scholar]
- 42.Busse JW, Wang L, Kamaleldin M, et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320(23):2448–2460. doi: 10.1001/jama.2018.18472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meske DS, Lawal OD, Elder H, et al. Efficacy of opioids versus placebo in chronic pain: a systematic review and meta-analysis of enriched enrolment randomized withdrawal trials. J Pain Res. 2018;11:923–934. doi: 10.2147/JPR.S160255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrone LA, Scuteri D, Rombolà L, et al. Opioids resistance in chronic pain management. Curr Neuropharmacol. 2017;15:444–456. doi: 10.2174/1570159X14666161101092822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volkow ND, McLellan T. Opioid abuse in chronic pain – misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–1263. doi: 10.1056/NEJMra1507771 [DOI] [PubMed] [Google Scholar]
- 46.Arout CA, Edens E, Petrakis IL, Sofuoglu M. Targeting opioid-induced hyperalgesia in clinical treatment: neurobiological considerations. CNS Drugs. 2015;29:465–486. Published online. doi: 10.1007/s40263-015-0255-x [DOI] [PubMed] [Google Scholar]
- 47.International Association for the Study of Pain (IASP). IASP statement on opioids February 2018. Available from: http://www.iasp-pain.org/Advocacy/Content.aspx?ItemNumber=7194. Accessed August8, 2018.
- 48.Rivat C, Ballantyne J. The dark side of opioids in pain management: basic science explains clinical observation. Pain Rep. 2016;1:e570 Published online. doi: 10.1097/PR9.0000000000000570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sprouse-Blum A, Smith G, Sugai D, Parsa FD. Understanding endorphins and their importance in pain management. Hawaii Med J. 2010;69:70–71. [PMC free article] [PubMed] [Google Scholar]
- 50.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med. 2015;162(4):276–286. doi: 10.7326/M14-2559 [DOI] [PubMed] [Google Scholar]
- 51.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36(10):2179–2192. doi: 10.1016/j.neubiorev.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jurisic M, Bean M, Harbaugh J, et al. ACOAM position statement. The personal physician’s role in helping patients with medical conditions stay at work or return to work. J Occup Environ Med. 2017;59(6):e125–e131. doi: 10.1097/JOM.0000000000001055 [DOI] [PubMed] [Google Scholar]
- 53.Peck KR, Roland MM, Smitherman TA. Factors associated with medication-overuse headache in patients seeking treatment for primary headache. Headache. 2018;58:648–660. doi: 10.1111/head.13294 [DOI] [PubMed] [Google Scholar]
- 54.Alstadhaug KB, Ofte HK, Kristoffersen ES. Preventing and treating medication overuse headache. Pain Rep. 2017;2(4):e612. doi: 10.1097/PR9.0000000000000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raff M, Crosier J, Eppel S, et al. South African guideline for the use of chronic opioid therapy for chronic noncancer pain. S Afr Med J. 2014;104(1 Suppl 1):78–89. doi: 10.7196/SAMJ.7316 [DOI] [PubMed] [Google Scholar]
- 56.Sears B, Ricordi C. Anti-inflammatory nutrition as a pharmacological approach to treat obesity. J Obesity. 2011;2011:1–14. Published online. doi: 10.1155/2011/431985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Gregori M, Muscoli C, Schatman ME, et al. Combining pain therapy with lifestyle: the role of personalized nutrition and nutritional supplements according to the SIMPAR feed your destiny approach. J Pain Res. 2016;9:1179–1189. doi: 10.2147/JPR.S115068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cherkin DC, Herman PM. Cognitive and mind-body therapies for chronic low back pain and neck pain. Effectiveness and value. JAMA. 2018;178(4):556–557. doi: 10.1001/jamainternmed.2018.0113 [DOI] [PubMed] [Google Scholar]
- 59.Goertz M, Thorson D, Bonsell J, et al. Institute for Clinical Systems Improvement. Adult Acute and Subacute Low Back Pain; [Updated November 2012]. Available from:https://www.icsi.org/_asset/bjvqrj/LBP.pdf. Accessed July26, 2019.
- 60.The Royal Australasian College of Physicians, Australasian Faculty of Occupational and Environmental Medicine. Realising the health benefits of work. A position statement; October 2011. Available from: https://www.racp.edu.au/docs/default-source/default-document-library/read-realising-the-health-benefits-of-work-position-statement-october-2011-(pdf-654kb).pdf?sfvrsn=032. Accessed 8August 2018.
- 61.Christian J, Martin D, Brown D, et al. Preventing needless work disability by helping people stay employed. A report from the stay-at-work & return-to-work committee of the American College of Occupational & Environmental Medicine (ACOEM). J Occup Environ Med. 2006;48(9):972–987. doi: 10.1097/01.jom.0000235915.61746.0d [DOI] [PubMed] [Google Scholar]
- 62.Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9:1–10. doi: 10.1097/ADM.0000000000000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webb D, Jain R, Jain S. Treating patients for better outcomes in depression: incorporating a patient-centered wellness program into everyday practice. SA Psychiatry. 2017;11:35–43. [Google Scholar]
- 64.Walsh TP, Arnold JB, Evans AM, et al. The association between body fat and musculoskeletal pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2018;19:233. doi: 10.1186/s12891-018-2137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Todoric J, Antonucci L, Karin M. Targeting inflammation in cancer prevention and therapy. Cancer Prev Res. 2016;9(12):895–905. doi: 10.1158/1940-6207.CAPR-16-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soltani S, Chitsazi MJ, Salehi-Abarqouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr. 2018;37(2):542–550. doi: 10.1016/j.clnu.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 67.Sureda A, Del Mar Bibiloni M, Julibert A, et al. Adherence to the Mediterranean diet and inflammatory markers. Clin Nutr. 2018;10(1):pii:E62. doi: 10.3390/nu10010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rondanelli M, Faliva MA, Miccono A, et al. Food pyramid for subjects with chronic pain: foods and dietary constituents as anti-inflammatory and antioxidant agents. Nutr Res Rev. 2018;31(1):131–151. doi: 10.1017/S0954422417000270 [DOI] [PubMed] [Google Scholar]
- 69.Vorster HH, Badham JB, Venter CS. An introduction to the revised food-based dietary guidelines for South Africa. S Afr J Clin Nutr. 2013;26(3):S1–S164. [Google Scholar]
- 70.Cohen S. Social relationships and health. Am Psychol. 2004;59(8):676–684. doi: 10.1037/0003-066X.59.8.676 [DOI] [PubMed] [Google Scholar]
- 71.Dueñas M, Ojeda B, Salazar A, et al. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457–467. doi: 10.2147/JPR.S105892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu E, Graham DP. Association of chronic pain and community integration of returning veterans with and without traumatic brain injury. J Head Trauma Rehabil. 2016;31(1):E1–E12. doi: 10.1097/HTR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 73.Subramaniam V, Stewart MW, Smith JF. The development and impact of a chronic pain support group: a qualitative and quantitative study. J Pain Sympt Manage. 1999;17(5):376–383. doi: 10.1016/S0885-3924(99)00012-3 [DOI] [PubMed] [Google Scholar]
- 74.Carnes D, Homer K, Underwood M, et al. Pain management for chronic musculoskeletal conditions: the development of an evidence-based and theory informed pain self-management course. BMJ Open. 2013;3:e003534. doi: 10.1136/bmjopen-2013-003534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dargan PJ, Simm R, Murray C. New approaches towards chronic pain: patient experiences of a solution-focused pain management programme. Br J Pain. 2014;8(1):34–42. doi: 10.1177/2049463713516755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simm R, Iddon J, Barker C. A community pain service solution-focused pain management programme: delivery and preliminary outcome data. Br J Pain. 2014;8(1):49–56. doi: 10.1177/2049463713507910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simm R, Barker C. Five years of a community pain service solution-focused pain management programme: extended data and reflections. Br J Pain. 2018;12(2):113–121. doi: 10.1177/2049463717744358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geneen LJ, Moore RA, Clarke C, et al. Physical activity and exercise for chronic pain in adults: an overview of cochrane reviews. Cochrane Database Syst Rev. 2017;4:Art. No.: CD011279. doi: 10.1002/14651858.CD011279.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Ryckeghem DM, Van Damme S, Eccleston C, Crombez G. The efficacy of attentional distraction and sensory monitoring in chronic pain patients: a meta-analysis. Clin Psychol Rev. 2018;59:16–29. doi: 10.1016/j.cpr.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 80.Schreiber KL, Campbell C, Martel MO, et al. Distraction analgesia in chronic pain patients. The impact of catastrophizing. Anesthesiol. 2014;121:1292–1301. doi: 10.1097/ALN.0000000000000465 [DOI] [PubMed] [Google Scholar]
- 81.Verhoeven K, Van Damme S, Eccleston C, et al. Distraction from pain and executive functioning: an experimental investigation of the role of inhibition, task switching and working memory. Eur J Pain. 2011;15(8):866–873. doi: 10.1016/j.ejpain.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 82.Tompkins DA, Hobelmann JG, Compton P. Providing chronic pain management in the “Fifth Vital Sign” Era: historical and treatment perspectives on a modern-day medical dilemma. Drug Alcohol Depend. 2017;173(Suppl 1):S11–S21. doi: 10.1016/j.drugalcdep.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.British Medical Association (BMA). Chronic pain: supporting safer prescribing of analgesics. London: BMA; March 2017. Available from: https://www.bma.org.uk/-/media/files/pdfs/collective%20voice/policy%20research/public%20and%20population%20health/analgesics-chronic-pain.pdf?la=en. Accessed 31May 2019. [Google Scholar]
- 84.Wehling M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse events. Eur J Clin Pharmacol. 2014;70(10):1159–1172. doi: 10.1007/s00228-014-1734-6 [DOI] [PubMed] [Google Scholar]
- 85.Cryer B, Barnett MA, Wagner J, Wilcox CM. Overuse and misperceptions of nonsteroidal anti-inflammatory drugs in the United States. Am J Med Sci. 2016;352(5):472–480. doi: 10.1016/j.amjms.2016.08.028 [DOI] [PubMed] [Google Scholar]
- 86.Cavagna L, Caporali R, Trifiro G, et al. Overuse of prescription and OTC non-steroidal anti-inflammatory drugs in patients with rheumatoid arthritis and osteoarthritis. Int J Immunopathol Pharmacol. 2013;26(1):279–281. doi: 10.1177/039463201302600132 [DOI] [PubMed] [Google Scholar]
- 87.Kirkpatrick DR, McEntire DM, Hambsch BS, et al. Therapeutic basis of clinical pain modulation. Clin Trans Sci. 2015;8:848–856. doi: 10.1111/cts.12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schliessbach J, Maurer K. Pharmacology of pain transmission and modulation In: Yong RJ, Nguyen M, Nelson E, ED U, editors. Pain Medicine. An Essential Review. Cham: Springer; 2017:7–9. [Google Scholar]
- 89.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verdu B, Decosterd I, Buclin T, et al. Antidepressants for the treatment of chronic pain. Drugs. 2008;68(18):2611–2632. doi: 10.2165/0003495-200868180-00007 [DOI] [PubMed] [Google Scholar]
- 91.Kalita J, Kohat AK, Misra UK, Bhoi SK. An open label randomized controlled trial of pregabalin versus amitriptyline in chronic low backache. J Neurol Sci. 2014;342:127–132. doi: 10.1016/j.jns.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 92.Urquhart DM, Wluka AE, Sim MR, et al. Is low-dose amitriptyline effective in the management of chronic low back pain? Study protocol for a randomised controlled trial. Trials. 2016;17:514. doi: 10.1186/s13063-016-1637-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med. 2018;178(11):1474–1481. doi: 10.1001/jamainternmed.2018.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ashton CH. Benzodiazepines: how they work and how to withdraw. Institute of Neuroscience, Newcastle University; August 2002. Available from: http://www.benzo.org.uk/manual/index.htm. Accessed 9June 201606.10 39.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- International Association of Study of Pain (IASP), European Federation of IASP Chapters (EFIC). IASP now recommends the global adoption of EFIC’s declaration on chronic pain as a major healthcare problem, a disease in its own right; October 2004. Available from: https://s3.amazonaws.com/rdcms-iasp/files/production/public/Content/ContentFolders/GlobalYearAgainstPain2/20042005RighttoPainRelief/painasadisease.pdf. Accessed 6August 2018.