Abstract
Background
Poly(amidoamine) (PAMAM) dendrimers are of considerable interest when used as a carrier for topical drugs for the skin, although little is known about their possible side effects. Therefore, our study was about the impact of 2nd and 3rd generation PAMAM dendrimers on human keratinocytes and fibroblasts cells.
Methods
The effect of the tested compounds on collagen biosynthesis was determined using 5[3H]-proline incorporation bioassay. Morphological changes accompanying cell growth inhibition were observed using a confocal microscope. To evaluate the percentage of apoptotic/necrotic cells and the cell growth dynamic of apoptotic features, we performed Annexin V/PI double staining assay, assessed caspase activity, and performed cell cycle analysis by flow cytometry. The flow cytometry method was also used to determine the effect of dendrimers on pro-inflammatory cytokines (IL-6, IL-8 IL-1β).
Results
The obtained results showed that as the concentration and the generation of dendrimers increased, collagen biosynthesis decreased. We also observed abnormalities in cell differentiation, which may have caused disturbed secretion of pro-inflammatory cytokines. We found that dendrimers cause chronic inflammation which may cause adverse changes in the skin, ultimately– leading to apoptosis in the case of dendrimers in lower concentrations or necrosis at higher concentrations (especially 3rd generation dendrimers). In addition, the inflammatory path induced by the tested compounds was caused by damage in the mitochondria, which we observed as a significant decrease in the mitochondrial membrane potential.
Conclusion
The results of our study showed that PAMAM dendrimers can cause disorders of cell proliferation and differentiation and may be the cause of cell cycle deregulation and chronic adverse inflammation.
Keywords: nanocarriers, drug delivery, carrier for topical drugs, apoptosis, cell cycle
Introduction
The use of nanotechnology for various therapeutic purposes, such as diagnostics and treatment, is possible due to the design of suitable nanoparticles being molecules whose size does not exceed 100 nm.1 In medicine, one of the most commonly used groups of nanoparticles as drug carriers are dendrimers.2 Dendrimers are created by gradually adding polymer layers around the central core, thus creating subsequent generations. A unique feature of these structures is their polyvalence, which is directly related to the occurrence of many different functional groups on their surface. Dendrimers also enable precise control of the size, shape, and distribution of functional groups on the surface of the carrier, which is desirable in drug design.3
Dendrimers most commonly used in medical applications belong to the group of polyamidoamines (PAMAM). PAMAM dendrimers are called “artificial proteins” due to the similarity of sizes and shapes to proteins and other bioorganic molecules. For example, insulin (≈30Å), cytochrome (≈40Å) and hemoglobin (≈55Å) have a similar shape and size to generations 3, 4, and 5 of PAMAM dendrimers, in which the ammonia molecule is the core of these macromolecules.4 The chemically compatible, flexible surface of nanoparticles is able to attach to different types of ligands that enable the distribution of the drug in a precisely defined manner.3
One of the first examples of using dendrimers was as a carrier for cisplatin - such a complex exhibited slower drug release, higher accumulation in tumor tissue, and lower toxicity in comparison with free cisplatin.5 In combination with dendrimers, methotrexate,6 doxorubicin7 or ibuprofen8 are used in the studies. Numerous studies are underway on the use of PAMAM dendrimers in anticancer therapy.9–12 In addition, appropriately modified dendrimers capable of activating each other to form a hydrogel matrix may be used as sealing substances for wound corneas.13 Moreover, dendrimers can improve the percutaneous availability of drugs.14 They also block the herpes simplex virus connected to the cells.15
PAMAM dendrimers are able to improve dermal therapy efficiency due to their capability to enhance drug molecule permeability through the membranes, to improve drug solubility and pharmacological activity. In addition, thanks to the hydrophilic outer shells and the hydrophobic interiors, they can act as effective penetration enhancers.16–18 For example, a study using ketoprofen and dilfunisal (in vitro permeation studies on excised rat skins) showed that PAMAM dendrimers significantly enhanced the steady-state flux of both drugs compared with the drug suspensions without PAMAM dendrimers.19 Similarly, a study using indaminacin and PAMAM dendrimers showed that PAMAM dendrimers significantly improved the degree of drug absorption compared with the drug suspension without PAMAM dendrimers.20
Dosing the drug to the skin with microparticles and nanocarriers could significantly improve the treatment of many skin diseases. In any case, toxicological and environmental safety of micro- and nanoparticles should be considered with great caution. For this reason, it is important to determine their toxicity on skin-derived keratinocytes and fibroblasts. They are two of the main cell types that react to the inflammatory phase in the skin repair/regeneration process. Inflammatory signals initiate the proliferation and maturation of these two cell types, which is necessary for wound healing.21,22 These two cell lineages have been used in well-defined experimental models in several pharmacology studies, which were designed to investigate intracellular signaling pathways and responses to different types of stimulation.23 The aim of the study was to investigate the influence of 2nd and 3rd generation PAMAM dendrimers on keratinocyte and fibroblast skin cells.
Materials and methods
Materials
Poly(amidoamine) (PAMAM) dendrimers with ethylenediamine core (2nd and 3rd generation) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Human cell lines: keratinocytes and fibroblasts, were obtained from American Type Culture Collection (Manassas, VA, USA). Keratinocyte Serum-Free Medium, Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and PBS used in a cell culture were products of Gibco (San Diego, CA, USA). Glutamine, penicillin, and streptomycin were obtained from Quality Biologicals Inc. (Gaithersburg, MD, USA). 5[3H]-proline and scintillation cocktail “Ultima GoldXR” were from PerkinElmer (Waltham, MA, USA). Annexin V Apoptosis Detection Kit II, JC-1 MitoScreen Kit, Propodium iodide, Cytometric Bead Array (CBA) Human Inflammatory Cytokines Kit were from BD Pharmingen (San Diego, CA, USA). Additionally, FLICA Caspase 3 Kit, FLICA Caspase 8 Kit (ImmunoChemistry Technologies, Bloomington, MN, USA), and RNase A Solution (Promega, Madison, WI, USA) were used.
Biological activity
Cell lines and cell culture
Keratinocytes were cultured in Keratinocyte Serum-Free Medium, and fibroblasts were cultured in Dulbecco’s Modified Eagle Medium supplemented with fetal bovine serum (10%) and 50 U/mL penicillin and 50 μg/mL streptomycin. The cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C. When the cells (passage 6–8) reached 70% confluence, they were washed with heated PBS (37 °C) and were exposed to 2nd and 3rd generation PAMAM dendrimers in concentrations: 0.3 mg/mL, 1.5 mg/mL, 3.0 mg/mL. The cells were incubated for 24 h under standard conditions.
Collagen biosynthesis
The measurement of collagen biosynthesis was made according to the method described by Peterkofsky et al.24 Keratinocyte and fibroblast cells were used for the study in the state of 70% confluence. Cells with test compounds and addition of 5[3H]-proline (5 μCi/mL) were incubated 24 hrs. The amount of collagen formed is expressed in dpm of 5[3H]-proline embedded in proteins susceptible to bacterial collagenase, per 106 cells. The value obtained in the control samples was taken as 100%, while the values from the samples were expressed as a percentage of the control value.
Flow cytometric analysis of inflammatory cytokines
IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF were determined using a Cytometric Bead Array (CBA) Human Inflammatory Cytokines Kit (BD Biosciences, San Jose, CA, USA). The tests were performed according to the manufacturer’s protocols. From the Human Inflammatory Cytokine Kit, 50 µL of assay beads, 50 µL of the studied sample, or standard and 50 µL of PE-labeled antibodies (Detection Reagent) were added consecutively to each sample tube. The samples were incubated at room temperature in the dark for 3 h. Next, the samples were washed with 1 mL of Wash Buffer, centrifuged and the resulting pellet was re-suspended in 300 µL of Wash Buffer. Samples prepared in this way were analyzed using a BD FACSCanto II flow cytometer and FCAP Array v3 software (both from BD Biosciences Systems, San Jose, CA, USA).
Cell morphological analysis
To visualize the fibroblast and keratinocyte morphological specificity, cells were exposed to 2nd and 3rd generation PAMAM dendrimers. The cells, at a density of 2.5×105, were seeded into six-well plates and incubated with the tested complexes. After 24 h of incubation, the cells were washed with PBS two times. The cells were visualized using a phase contrast microscope (Nikon Eclipse Ti, Japan) at a 100× magnification.
Analysis of mitochondrial membrane potential
Mitochondrial membrane potential changes (ΔMMP) were assessed using the JC-1 MitoScreen Kit (BD Biosciences Systems, San Jose, CA, USA) as described previously.25 Sample of cells at 106 cells/mL was suspended in a buffer mixture and JC-1 dye provided by the kit manufacturer. Then the cells were incubated for 15 mins at 37 °C, washed, and re-suspended in buffer. Samples prepared in this way were subjected to cytometric analysis using a cytometer BD FACSCanto II and FACSDiva software (both from BD Biosciences Systems, San Jose, CA, USA).
Flow cytometry assessment of Annexin V binding
Apoptosis studies were performed using the FITC Annexin V Apoptosis Detection Kit II kit (BD Biosciences, USA) using flow cytometry. After 24 hrs of incubation of keratinocyte and fibroblast cells with test compounds, they were washed twice with cold PBS and suspended in buffer (provided by the kit manufacturer) at 1×106 cells/mL. Then, 100 μL of the solution was transferred to the tubes and 5 μL of V-FITC Annexin and 5 μL of propidium iodide were added. The contents of the tubes were mixed and incubated in the dark at room temperature for 15 mins. After the required time had elapsed, 400 μL of buffer was added and the test was carried out using BD FACSCanto II flow cytometer (BD Biosciences Systems, San Jose, CA, USA). Results were analyzed with FACSDiva software (BD Biosciences Systems, San Jose, CA, USA).
Caspase-3 enzymatic activity assay
Caspase-3 activity was measured using the FLICA Caspase 3 Assay Kit (ImmunoChemistry Technologies, Bloomington, MN, USA). After 24 hrs of incubation of keratinocytes and fibroblasts with the test compounds, the medium was removed, the cells were washed twice with the cold PBS solution and the buffer (provided by the kit manufacturer) was added. Then 5 μL FLICA reagent and 2 µL of Hoechst 33342 was added to sample of cells (1×106 cells/mL) and incubated at 37 °C for 30 mins in a dark place. After incubation, the cells were subjected to a 3-fold rinsing procedure using a wash buffer. Cells were suspended in 500 μL of buffer and analyzed using a flow cytometer (BD FACSCanto II flow cytometer) and FACSDiva software (both from BD Biosciences Systems, San Jose, CA, USA).
Caspase-8 enzymatic activity assay
Caspase-8 activity was measured using the FLICA Caspase 8 Assay Kit (ImmunoChemistry Technologies, Bloomington, MN, USA). After 24 hrs of incubation of keratinocytes and fibroblasts with the test compounds, the medium was removed, the cells were washed twice with the cold PBS solution and the buffer (provided by the kit manufacturer) was added. Then 5 μL FLICA reagent and 2 µl of Hoechst 33342 was added to sample of cells (1×106 cells/mL) and incubated at 37 °C for 30 mins in a dark place. After incubation, the cells were subjected to a 3-fold rinsing procedure using a wash buffer. Cells were suspended in 500 μL of buffer and analyzed using a flow cytometer (BD FACSCanto II flow cytometer) and FACSDiva software (both from BD Biosciences Systems, San Jose, CA, USA).
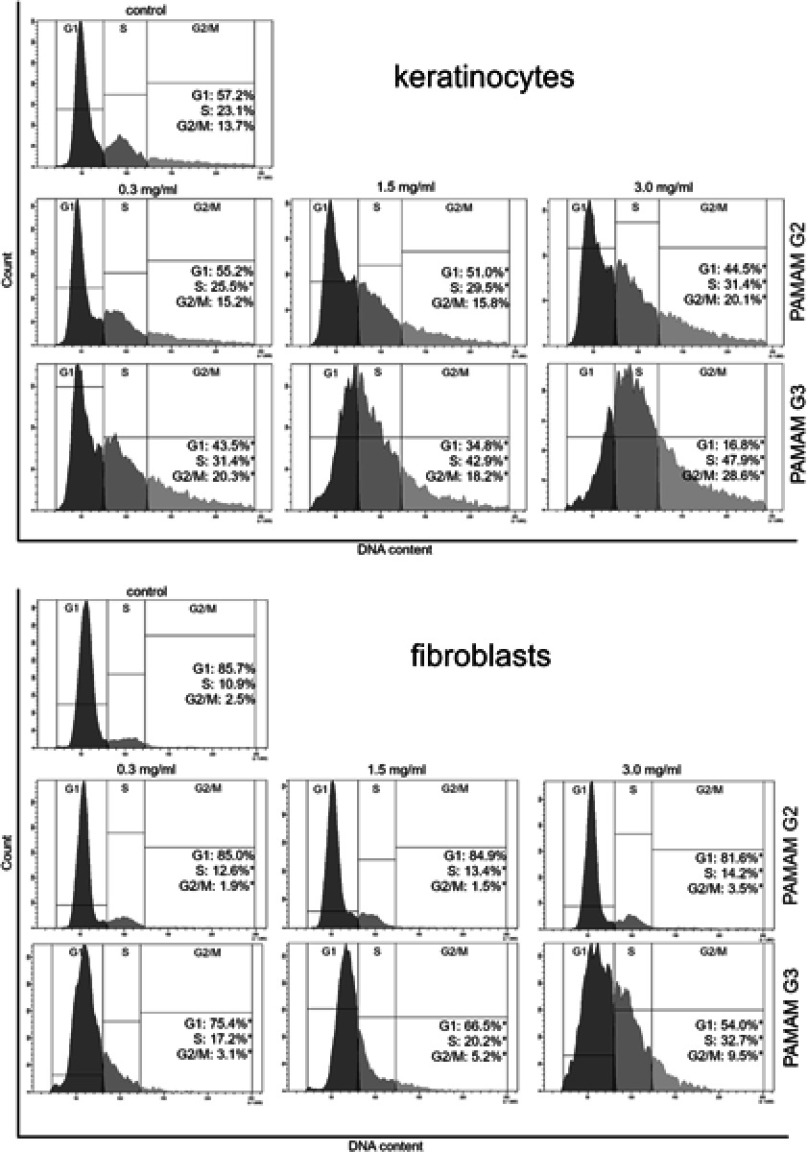
Cell cycle analysis
The distribution of cell cycle phases was analyzed by flow cytometry. After 24 h of incubation of keratinocyte and fibroblast cells with test compounds, the cells were harvested and then fixed with 1 mL of 70% ethanol and kept overnight at −20 °C. Before analysis, the cells were re-suspended in PBS, treated with 50 μg/mL of DNase-free RNase A Solution (Promega, Madison, WI, USA) and stained with 100 μg/mL of propidium iodide (PI). The FACSCanto II flow cytometer (BD Biosciences Systems, San Jose, CA, USA) was used to read the fluorescence.
Statistical analysis
All numerical data are presented as mean ± standard deviation (SD) from at least three independent experiments. Statistical analysis was conducted using the Origin 7.5 software (OriginLab, Northampton, MA, USA). Statistical differences in multiple groups were determined by one-way ANOVA followed by Tukey’s test. P < 0.05 was considered statistically significant.
Results
Effect of 2nd and 3rd generation PAMAM dendrimers on collagen biosynthesis
One of the characteristic changes accompanying the aging process is the impairment of collagen biosynthesis and its content in tissues.26 To check whether 2nd and 3rd generation PAMAM dendrimers affect this process, we conducted a study on collagen biosynthesis using 5[3H]-proline incorporation. As shown in Figure 1, the inhibitory effect was dose dependent; with an increasing concentration of the test compounds there was a significant decrease in collagen biosynthesis. In addition, the inhibition of collagen biosynthesis was dependent on dendrimer generation. A larger decrease in biosynthesis was observed in the case of 3rd generation PAMAM dendrimers. This situation may result from different amounts of −NH2 groups in both dendrimers. Cheng et al have shown that primary amine groups on the dendrimer surface interact with phospholipids in the cell membrane, followed by disturbance of these amphiphilic molecules and formation of holes in the cell membrane, resulting disrupting the cell structure.27 The removal of surface amine groups by acetylation or PEGylation can effectively decrease the cytotoxicity of PAMAM dendrimers indicating that surface charge plays an important role in the cytotoxicity of both dendrimers.28,29 In addition on the example of many cell lines, it was found that toxicity of dendrimers also depends on their size.29 Shao et al have proved that, 4th and 5th generation PAMAM dendrimers show much higher cytotoxicity to MCF-7 cells than 3rd generation PAMAM dendrimer at an equivalent concentration of surface charge.30 In connection with the above, we can state that both the number of −NH2 groups and the size of the dendrimer is responsible for the decrease in collagen biosynthesis. However, we can’t rule out other factors responsible for this process.
Figure 1.

Effect of dendrimers (PAMAM) on collagen biosynthesis in keratinocytes and fibroblasts. The cells were subjected to various concentrations of dendrimers (0.3 mg/mL, 1.5 mg/mL and 3.0 mg/mL) for 24 h. Values represent the mean (% of the control) ± SD of six experiments. The asterisk (*) indicates statistically significant differences compared with the untreated control. ***p<0.001 vs the control group.
Effect of 2nd and 3rd generation PAMAM on cytokine secretion
To evaluate the inflammatory responses elicited by various concentrations of PAMAM dendrimers, we treated cultured keratinocytes and fibroblasts with dendrimers for 24 h and then assessed the secreted levels of representative pro-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF) using a flow cytometer. The PAMAM dendrimers showed complex activity on cytokine secretion, which depends on the cytokine considered, as well as dendrimer concentration and generation. As shown in Figures 2–4, the expression levels of IL-1β, IL-6 and IL-8 gradually increased. We clearly noticed that the highest increase in the secretion of the determined cytokines occurred in the case of the highest dendrimer concentrations (3.0 mg/mL). Whereby, the increase was higher in the case of the third generation PANAM dendrimer. Only at the lowest concentration (0.3 mg/mL) in the case of the two tested cell lines, a decrease in IL-6 and IL-8 levels was observed below the control. No rational explanation is available for this phenomenon and additional studies are required to explain this complex effect.
Figure 3.
Flow cytometric analysis of population keratinocyte and fibroblast cells treated for 24 h with 2nd and 3rd generation PAMAM dendrimers (0.3 mg/mL, 1.5 mg/mL and 3.0 mg/mL) for IL-6. Mean percentage values from three independent experiments (n=6) done in duplicate are presented. *p<0.05 vs the control group, **p<0.01 vs the control group, ***p<0.001 vs the control group.
Figure 2.

Flow cytometric analysis of population keratinocyte and fibroblast cells treated for 24 h with 2nd and 3rd generation PAMAM dendrimers (0.3 mg/mL, 1.5 mg/mL and 3.0 mg/mL) for IL-1β. Mean percentage values from three independent experiments (n=6) done in duplicate are presented. *p<0.05 vs the control group, **p<0.01 vs the control group, ***p<0.001 vs the control group.
Figure 4.

Flow cytometric analysis of populations keratinocyte and fibroblast cells treated for 24 h with 2nd and 3rd generation PAMAM dendrimers (0.3 mg/mL, 1.5 mg/mL and 3.0 mg/mL) for IL-8. Mean percentage values from three independent experiments (n=6) done in duplicate are presented. *p<0.05 vs the control group, **p<0.01 vs the control group, ***p<0.001 vs the control group.
Cytokine values lower than the detection limit (IL-10, IL-12p70 and TNF) were considered as not detectable, and are not shown. It is obvious that the most marked effects were observed on the most expressed cytokines.
Effect of 2nd and 3rd generation PAMAM dendrimers on cell morphology
Confocal microscopy revealed that cells treated with the tested compounds were different in shape, and that their density decreased (Figure 5). Moreover, we observed a decrease in cell adhesion. Cell proliferation inhibition was dose-dependent and the most significant decrease in survival was observed with the 3rd generation PAMAM dendrimers. At high dendrimer concentrations, we observed the disappearance of connections between cells. The cells were shortened and destabilized.
Figure 5.
Morphological changes in keratinocyte and fibroblast cells incubated with different concentrations (0.3 mg/mL, 1.5 mg/mL and 3.0 mg/mL) of 2nd and 3rd generation PAMAM dendrimers for 24 h exposure. Representative photographs are shown. Morphological effects evaluated by phase contrast microscopy (magnification × 100).
2nd and 3rd generation PAMAM dendrimers decrease mitochondrial membrane potential
JC-1 fluorescence dye staining was used to assess mitochondrial membrane potential changes of fraction−treated fibroblasts and keratinocytes. Determination of the ratio of aggregate to monomer (red/green) fluorescence, which corresponded to active mitochondria, showed that after 24 h of treatment with dendrimers we observed a decrease in this ratio compared with the control (Figure 6). This is particularly evident in the case of the 3rd generation dendrimers, where in the case of keratinocytes at 1.5 mg/mL, more than 90% of the cells showed a decrease in this parameter. In the case of fibroblasts, the value of over a 90% decrease in mitochondrial potential was observed only at a concentration of 3.0 mg/mL.
Figure 6.

Fluorescence of keratinocyte and fibroblast cells treated for 24 h with 2nd and 3rd generation PAMAM dendrimers (0.3 mg/mL, 1.5 mg/mL and 3.0 mg/mL) incubated with mitochondrial membrane potential probe JC-1. The x- and y-axes are green and red fluorescence, respectively. Mean percentage values from three independent experiments (n=3) done in duplicate are presented. *p<0.05 versus the control group, **p<0.01 vs the control group.
2nd and 3rd generation PAMAM dendrimers induce apoptosis/necrosis
We sought to determine whether the growth inhibitory effect of dendrimers was due to the induction of apoptosis/necrosis. For this purpose, we used flow cytometry and Annexin V- FITC staining. The results of the determination together with percentages are shown in Figure 7. Treatment of 2nd generation PAMAM dendrimer cells in increasing doses showed an increase in the number of apoptotic cells. We observed this increase mainly in the case of keratinocytes. Fibroblasts were less sensitive to 2nd generation PAMAM dendrimers. However, in the case of the 3rd generation PAMAM dendrimers, with increased dosage, a significant increase in the number of necrotic cells was observed, which may indicate the high toxicity of this nanoparticle.
Figure 7.

Flow cytometric analysis of keratinocyte and fibroblast cancer cells after incubation with 2nd and 3rd generation PAMAM dendrimers (0.3 mg/mL, 1.5 mg/mL and 3.0 mg/mL) for 24 h and subsequent staining with Annexin V and propidium iodide (PI). Dots with Annexin V−/PI− (left lower square), Annexin V+/PI− (right bottom square), Annexin V+/PI+ (right upper square), and Annexin V−/PI+ (left upper square) feature represent intact, early apoptotic, late apoptotic, and necrotic cells, respectively. Mean percentage values from three independent experiments (n=3) done in duplicate are presented. *p<0.05 versus the control group.
Activation of caspase-3 and caspase-8
We conducted flow cytometer analysis to determine the effect of the dendrimers on the expression of caspases. Figure 8 shows 24 h treatment of cells with different concentrations of active caspase-3, while Figure 9 of active caspase-8. With the increasing concentrations of dendrimers used for the study, we observed an increase in the amount of active caspase-3 and caspase-8. The increase in active forms of caspases was directly proportional to increasing compound concentrations. However, in the case of keratinocytes, the amount of caspase-3 and caspase-8 released was greater than in the case of fibroblasts. This may indicate a higher sensitivity of keratinocytes to the tested compounds compared with fibroblasts. These results indicated that 2nd and 3rd generation PAMAM dendrimers triggered the caspase cascade and active caspase-3 may cause DNA damage.
Figure 8.

Flow cytometric analysis of population keratinocyte and fibroblast cells treated for 24 h with 2nd and 3rd generation PAMAM dendrimers (0.3 mg/mL, 1.5 mg/mL and 3.0 mg/mL) for active caspase-3. Mean percentage values from three independent experiments (n=3) done in duplicate are presented. *p<0.05 versus the control group.
Figure 9.

Flow cytometric analysis of population keratinocyte and fibroblast cells treated for 24 h with 2nd and 3rd generation PAMAM dendrimers (0.3 mg/mL, 1.5 mg/mL and 3.0 mg/mL) for active caspase-8. Mean percentage values from three independent experiments (n=3) done in duplicate are presented. *p<0.05 versus the control group.
Alteration of cell cycle progression by 2nd and 3rd generation PAMAM dendrimers
The cell cycle distribution was determined using flow cytometric analysis with propidium iodide staining. After 24 hr treatment of fibroblasts and keratinocytes with different concentrations of 2nd and 3rd generation PAMAM dendrimers, we observed a decrease in the proportion of cells in the G1 phase, however the number in the S phase increased (Figure 10). The increase in the S phase was directly proportional to the increase in dendrimer concentrations and generation. The biggest difference between the decrease in the number of cells in the G1 phase and their increase in the S phase was observed in the case of the 3rd generation dendrimers at a concentration of 3 mg/mL.
Figure 10.
Flow cytometric analysis of cell cycle of keratinocyte and fibroblast cells after 24 h of incubation with different concentrations (0.3 mg/mL, 1.5 mg/mL and 3.0 mg/mL) of 2nd and 3rd generation PAMAM dendrimers using propidium iodide staining. Mean percentage values from three independent experiments (n=3) done in duplicate are presented. *p<0.05 versus the control group.
Discussion
There has been a very large increase in the interest of research teams in dendrimers as carriers of drugs in skin diseases.31,32 A good in vitro model for testing skin diseases are keratinocytes and fibroblasts. Keratinocytes occupy an important place in the immune response of the skin by the release of pro-inflammatory chemokines, cytokines, proteases, growth factors, and matrix metalloproteinases. These cells also recruit, stimulate and coordinate the activities of many types of cells involved in healing and recapitulate the epidermal barrier layer of the skin.20 Fibroblasts are cells necessary for tissue repair. They are one of the first cells that appear in damaged places. They secrete paracrine factors in damaged sites, such as basic fibroblast growth factor (bFGF/FGF-2),20,33 keratinocyte growth factor (KGF/FGF-7),34 vascular endothelial growth factor A (VEGF-A)20 and insulin-like growth factor-1 (IGF-1),35 which signals neighboring keratinocytes. Furthermore, keratinocytes and fibroblasts communicate with each other via double paracrine signaling loops, known as cross talk or dynamic reciprocity, which coordinates their actions to restore normal tissue homeostasis after wounding.33 Fibroblasts are responsible for the production and repair of damage in collagen. The test of collagen biosynthesis performed in our paper indicates that under the influence of 2nd and 3rd generation of PAMAM dendrimers, its biosynthesis is impaired in keratinocytes and fibroblasts.
The observed decrease of collagen biosynthesis is directly proportional to dendrimer concentration and generation. The higher the concentration and the higher the nanoparticle generation, the greater the decrease. Disturbed biosynthesis of this protein, which increases with the diameter of the nanoparticles, although it may appear to contradict commonly accepted views of increased toxicity with a reduced size of nanoparticles, but can be explained in terms of linear correlation with the number of surface amino groups of PAMAM dendrimers.
Collagen is the main structural component of connective tissue, that maintains the stability of organs and supports their structural integrity. It is synthesized mainly by fibroblasts and the intensity of this biosynthesis can be regulated by various chemical factors. Since in the skin, it is responsible for its elasticity, firmness and flexibility,36 exposure of the skin to dendrimers may intensify adverse changes in its architecture and be the cause of inflammation. One of the markers of inflammatory changes is cytokines. These soluble mediators are responsible for the inflammatory response by attracting neutrophils. Neutrophils are designed to integrate a network of cellular interactions to maintain immune homeostasis. Thus, they provide a gradual transition to successive phases of wound healing and restoration of tissue homeostasis.37
At the same time, dysregulated excessive or persistent production of these inflammatory mediators causes a sustained pro-inflammatory state that causes tissue damage. Inflammation is not just a pathological process. This physiological action is primarily a defense mechanism that hinders the spread of tissue infections, because wounds are often contaminated with pathogens. Inflammation becomes harmful when it is not properly terminated and goes into a chronic condition that is accompanied by the permanent production of pro-inflammatory mediators.38
Inflammatory response induced by nanoparticles may have a toxic mechanism,39 and in this paper we highlighted the inflammatory mediators (IL-6, IL-8 IL-1β) induced by PAMAM dendrimers. We noticed that the secretion level of all three inflammatory mediators, following the exposure of fibroblasts and keratinocytes to PAMAM dendrimers, is dependent on dendrimer concentration and generation. An increase in IL-1β secretion occurred from the lowest concentration of PAMAM dendrimers used in the study (0.3 mg/mL). In turn, in the case of IL-6 and IL-8, this increase began at a concentration of 1.5 mg/mL. Interestingly, in the case of both of these cytokines at 0.3 mg/mL, we observed a decrease in the interleukin concentration below the control.
It is well known that in the inflammatory process the primary inflammatory cytokine interleukin-1β (IL-1β) induces IL-6 expression.40 We also observed this trend in our work. IL-1β is the major pro-inflammatory cytokine responsible for mediating several physiological responses, such as fever, activation of lymphocytes, and induction of acute phase protein synthesis.40
Recent findings suggest that cellular responses to IL-1β are mediated by cascades of intracellular events, including the activation of mitogen-activated protein kinases (MAPKs) involved in the activation of AP-1 and IκB kinases (IKKs) involved in the activation of NF-κB, which ultimately activate IL-6.41 Catherine M. Cahill showed that there is a cross-talk phenomenon between IL-1β and IL-6, the excessive secretion of which can lead to tumor growth and development.41 Also, the observed excessive secretion of IL-8 under the influence of PAMAM dendrimers may be a bad prognostic. The excessive secretion of IL-8 may result in fibroplasia, the characteristic feature of psoriasis.
Moreover, it has been proven that excessive secretion IL-6 and IL-8 was found to locally contribute to neoangiogenesis.42 IL-8 has also been shown to play an important role in tumor growth and metastasis.43 In response to epidermal injuries or certain pathologic conditions, epidermal keratinocytes not only produce pro-inflammatory cytokines, but also migrate to sites of injury and enable weakening of intercellular adhesions such as desmosomes. Some preparations cause a disruptor in the metabolism of keratinocytes and their abnormal keratosis, consisting of the separation of individual cells in the spinous layer, with formation of horn seeds and the formation of so-called round cells44 that we also observed in our study. This may confirm the results of Winnicka’s research, in which she observed that the morphological changes of epidermal cells of rats treated with PAMAM dendrimers included cytoplasmic vacuolization of keratinocytes in the basal and spinous layers.17
The inflammatory pathway induced by PAMAM dendrimers may be one of localizing them in the mitochondria leading to a disruption of the mitochondrial electron transduction chain and additional O2 production resulting in oxidative stress.45 The cationic PAMAM dendrimers with mitochondrial markers were observed by other researchers.46,47 The explanation of the interaction between cationic dendrimers and lipid bilayers of cells revealed the presence of electrochemical as well as hydrophobic interactions that favor the breakdown of the mitochondrial membrane and disrupt the mechanisms of transport.48 The formation of pores in the outer mitochondrial membrane promotes the cell’s entry into the apoptotic pathway.49
Lee et al demonstrated in human lung cells (WI-26 VA4) exposed to G4 PAMAM dendrimers co-localized with mitochondria changed the potential of the mitochondrial membrane and released cytochrome c, inducing apoptosis.50 It should be noted that a strong interaction between cationic dendrimers and lipid bilayers has produced increased pore formation. In contrast to cationic linear polymers contacting only a single bilayer leaflet and do not perforate membranes, relatively rigid dendrimers penetrate the bilayer, because they can achieve a similar level of contact between the charged groups, interacting with both leaflets.51 Perforation by dendrimer may be one of the mechanisms responsible for mitochondrial damage that we observed in our study as a result of mitochondrial potential reduction.
Additionally, as a result of decreased mitochondrial membrane potential, intermembrane space proteins, such as cytochrome c and Bcl-2 family proteins including Bax and Bak, are released and initiate caspase cleavage and activation.49 It is clear from the observations in this study that PAMAM dendrimers induced mitochondrial membrane permeability. In addition to the Bcl-2 family proteins, mitochondrial membrane permeability affects other components, including mitochondrial lipids. Pro-apoptotic signals may come from their intermediate metabolites, redox processes, sphingolipids, ion gradients, transcription factors, kinases and phosphatases.52 It seems that one of these factors may be responsible for permeabilization by dendrimers.
The process of apoptosis is closely related to proteins called caspases.25 In our study, we observed an increase in the active forms of caspase-3 and −8. Differences in caspase-3 activity are consistent with a trend similar to caspase-8. In both cases, the activity profile and percentage activity compared to the control depend on the generation and concentrations of the dendrimer. To explain the possible pathways of response to apoptosis, it should be noted that apoptosis can be mediated through two major pathways, the death-receptor (extrinsic) pathway and the mitochondrial (intrinsic) pathway.53 As a result of the initiation, the extrinsic pathway proceeds an activation of caspase-8, whereas the initiation of the intrinsic pathway occurs as a result of the mitochondrial potential reduction.54
Activation of caspase-8 and −3 occurs almost immediately after permeabilization of the mitochondrial membrane, in the early and late stages of apoptosis. Activation of caspase-8 is involved as a preliminary process for caspase-3 activation through activating pro-caspase-3.54 Our results suggest that apoptosis of fibroblasts and keratinocytes in the presence of PAMAM dendrimers follows the mitochondrial pathway, with a decrease in mitochondrial membrane potential, as well as the extrinsic pathway with a significant increase in expression and caspase-8.
As assessed by means of a flow cytometer, there was an increase in the number of apoptotic cells in each of the tested concentrations of PAMAM dendrimers. Apoptosis of keratinocytes is an important element in maintaining the normal physiological function of the skin and is associated to the development of various skin diseases.55 Clinical and histological studies have shown that excessive apoptosis of keratinocytes is a fundamental element in the development of atopic dermatitis, allergic contact dermatitis and eczematous dermatitis.56 With increasing concentrations, not only was there an increase in the number of apoptotic cells, but at the highest concentration of 3.0 mg/mL of third generation dendrimers a significant increase in the number of necrotic cells was observed. This was particularly noticeable in the case of fibroblasts.
Significantly higher cell death as a result of necrosis after treatment with higher concentrations of third generation dendrimers may suggest a disorganization of cell proliferation. In addition, along with the increase in apoptosis/necrosis of fibroblasts and keratinocytes, we noticed cell cycle abnormalities. The cell cycle was stopped mainly in phase S. The observed phenomenon of S-phase arrest can lead to numerous mutations, and ultimately cell death.57 Furthermore, abnormal cell proliferation, which results from cell cycle deregulation, might even lead to neoplastic changes.
The results of the experiment obtained by us clearly show that the mechanisms of dendrimer cellular uptake vary considerably with their generation and concentration, although the exact roles of these parameters – individually and collectively – remain unclear. Cellular uptake and intracellular transport is known to be dependent on the dendrimer surface charge and the cell type. The intracellular fate of dendrimers depends on the mechanisms of their cellular uptake, where the size of vesicles, the type of proteins involved and the cell type in which they are found can vary considerably.15 Albertazzi et al studied the impact of dendrimer surface chemistry (cationic, neutral and hydrophobic/lipidated) and size (PAMAM G2, PAMAM G4 and PAMAM G6) on the uptake mechanisms by cervical cancer (HeLa) cells.58 He showed that the membrane affinity of the dendrimers to depend upon their generation or the amount of positive charges on their periphery (G6>G4>G2). It turned out that their internalization properties correlated with the molecular structure. We observed similar relation in our study. With the increase in concentration and generation of dendrimers, and hence with more cationic groups –NH2, we observed an increase in pro-inflammatory activity. Therefore, it can be concluded that the increase in pro-inflammatory activity may be correlated with the amount of –NH2 groups present in dendrimers. It is also interesting how the uptake of dendrimers by cells occurs. The main mechanism of transporting dendrimers into the cell is endocytosis and subsequent oxidative stress.59 On the example of dermal cells lines (HaCaT), Mukherjee et al showed that PAMAM dendrimers to localize in mitochondria and produce reactive oxygen species (ROS) resulting in DNA damage and cell death. The cationic PAMAM dendrimers enter the cell through endocytosis and locate in mitochondria. The dendrimers increase the internal pH of the mitochondria because of an acid-base equilibrium reaction between secondary amines and their conjugate base, resulting in the production of ROS.60,61 Finally, DNA damage occurs which increases with dendrimer generation, possibly due to the corresponding increase in charge density which promotes dendrimer binding to negatively charged DNA.
Conclusion
As numerous literature data indicate, dendrimers have found application as a drug delivery system in various models with good results. Controlled release of the drug into skin and targeting of hair follicle specific cell populations, transcutaneous vaccination and transdermal gene therapy are among these applications. Our research has shown that PAMAM dendrimers of the second and third generation may be the cause of abnormal cell proliferation, cell cycle disorders, and chronic inflammation in skin-derived human keratinocytes and fibroblasts. Cell proliferation inhibition was dose-dependent and the most significant decrease in survival was observed with the 3rd generation PAMAM dendrimers. Therefore, at the stage of designing nanocarriers based on dendrimers to deliver the drug into the skin, low carrier concentration should be preferred and the use of the 2nd PAMAM should be considered.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW. Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 2007;150(5):552−558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu HJ, Bugno J, Lee SR, Hong S. Dendrimer-based nanocarriers: a versatile platform for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(1). doi: 10.1002/wnan.1409 [DOI] [PubMed] [Google Scholar]
- 3.Da Silva Santos S, Igne Ferreira E, Giarolla J. Dendrimer prodrugs. Molecules. 2016;21(6):686. doi: 10.3390/molecules21060686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisal DS, Yellepeddi VK, Kumar A, et al. Permeability of surface-modified polyamidoamine (PAMAM) dendrimers across Caco-2 cell monolayers. Int J Pharm. 2008;350(1–2):113−121. doi: 10.1016/j.ijpharm.2007.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik N, Evagorou EG, Duncan R. Dendrimer-platinate: a novel approach to cancer chemotherapy. Anticancer Drugs. 1999;10(8):767−776. doi: 10.1097/00001813-199909000-00010 [DOI] [PubMed] [Google Scholar]
- 6.Gurdag S, Khandare J, Stapels S, Matherly LH, Kannan RM. Activity of dendrimer-methotrexate conjugates on methotrexate-sensitive and -resistant cell lines. Bioconjug Chem. 2006;17(2):275−283. doi: 10.1021/bc0501855 [DOI] [PubMed] [Google Scholar]
- 7.Ke W, Zhao Y, Huang R, Jiang C, Pei Y. Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J Pharm Sci. 2008;97(6):2208−2216. doi: 10.1002/jps.21155 [DOI] [PubMed] [Google Scholar]
- 8.Kurtoglu YE, Mishra MK, Kannan S, Kannan RM. Drug release characteristics of PAMAM dendrimer-drug conjugates with different linkers. Int J Pharm. 2010;384(1−2):189−194. doi: 10.1016/j.ijpharm.2009.10.017 [DOI] [PubMed] [Google Scholar]
- 9.Li J, Liang H, Liu J, Wang Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int J Pharm. 2018;546(1−2):215−225. doi: 10.1016/j.ijpharm.2018.05.045 [DOI] [PubMed] [Google Scholar]
- 10.Salimi M, Sarkar S, Fathi S, et al. Biodistribution, pharmacokinetics, and toxicity of dendrimer-coated iron oxide nanoparticles in BALB/c mice. Int J Nanomedicine. 2018;13:1483−1493. doi: 10.2147/IJN.S157293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winnicka K, Bielawski K, Bielawska A. Synthesis and cytotoxic activity of G3 PAMAM-NH(2) dendrimer-modified digoxin and proscillaridin A conjugates in breast cancer cells. Pharmacol Rep. 2010;62(2):414−423. doi: 10.1016/S1734-1140(10)70283-8 [DOI] [PubMed] [Google Scholar]
- 12.Bielawski K, Bielawska A, Muszyńska A, Popławska B, Czarnomysy R. Cytotoxic activity of G3 PAMAM-NH₂ dendrimer-chlorambucil conjugate in human breast cancer cells. Environ Toxicol Pharmacol. 2011;32(3):364−372. doi: 10.1016/j.etap.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 13.Paszko E, Ehrhardt C, Senge MO, Kelleher DP, Reynolds JV. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn Ther. 2011;8(1):14−29. doi: 10.1016/j.pdpdt.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 14.Ficker M, Theeuwen MJM, Janaszewska A, et al. Complexes of indomethacin with 4-carbomethoxy-pyrrolidone PAMAM dendrimers show improved anti-inflammatory properties and temperature-dependent binding and release profile. Mol Pharm. 2018. doi: 10.1021/acs.molpharmaceut.8b00567 [DOI] [PubMed] [Google Scholar]
- 15.Fox LJ, Richardson RM, Briscoe WH. PAMAM dendrimer - cell membrane interactions. Adv Colloid Interface Sci. 2018;257:1−18. doi: 10.1016/j.cis.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 16.Wróblewska M, Winnicka K. The effect of cationic polyamidoamine dendrimers on physicochemical characteristics of hydrogels with erythromycin. Int J Mol Sci. 2015;16(9):20277−20289. doi: 10.3390/ijms160920277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winnicka K, Wróblewska M, Sosnowska K, Car H, Kasacka I. Evaluation of cationic polyamidoamine dendrimers’ dermal toxicity in the rat skin model. Drug Des Devel Ther. 2015;9:1367−1377. doi: 10.2147/DDDT.S78336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maji S, Agarwal T, Maiti TK. PAMAM (generation 4) incorporated gelatin 3D matrix as an improved dermal substitute for skin tissue engineering. Colloids Surf B Biointerfaces. 2017;155:128−134. doi: 10.1016/j.colsurfb.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y, Man N, Xu T, et al. Transdermal delivery of nonsteroidal anti-inflammatory drugs mediated by polyamidoamine (PAMAM) dendrimers. J Pharm Sci. 2007;96(3):595−602. [DOI] [PubMed] [Google Scholar]
- 20.Chauhan AS, Sridevi S, Chalasani KB, et al. Dendrimer-mediated transdermal delivery: enhanced bioavailability of indomethacin. J Control Release. 2003;90(3):335−343. doi: 10.1016/S0168-3659(03)00200-1 [DOI] [PubMed] [Google Scholar]
- 21.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585−601. doi: 10.1111/j.1524-475X.2008.00410.x [DOI] [PubMed] [Google Scholar]
- 22.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122(18):3209−3213. doi: 10.1242/jcs.031187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira LE, Muniz BV, Burga-Sánchez J, et al. The effect of two drug delivery systems in ropivacaine cytotoxicity and cytokine release by human keratinocytes and fibroblasts. J Pharm Pharmacol. 2017;69(2):161−171. doi: 10.1111/jphp.12680 [DOI] [PubMed] [Google Scholar]
- 24.Peterkofsky B, Chojkier M, Bateman J. Immunochemistry of the extracellular matrix In: H Furthmayr, editor. Determination of collagen synthesis in tissue and cell culture system. Vol 2 CRC Press; 2018:19–47. [Google Scholar]
- 25.Czarnomysy R, Surażyński A, Muszyńska A, Gornowicz A, Bielawska A, Bielawski K. A novel series of pyrazole-platinum(II) complexes as potential anti-cancer agents that induce cell cycle arrest and apoptosis in breast cancer cells. J Enzyme Inhib Med Chem. 2018;33(1):1006–1023. doi: 10.1080/14756366.2018.1471687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaręba I, Celinska-Janowicz K, Surażynski A, Miltyk W, Pałka J. Proline oxidase silencing induces proline-dependent pro-survival pathways in MCF-7 cells. Oncotarget. 2018;9(17):13748–13757. doi: 10.18632/oncotarget.24466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y, Zhao L, Li Y, Xu T. Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem Soc Rev. 2011;40(5):2673–2703. doi: 10.1039/c0cs00097c [DOI] [PubMed] [Google Scholar]
- 28.Yang K, Weng L, Cheng Y, et al. Host-guest chemistry of dendrimer-drug complexes. 6. Fully acetylated dendrimers as biocompatible drug vehicles using dexamethasone 21-phosphate as a model drug. J Phys Chem B. 2011;115(10):2185–2195. doi: 10.1021/jp111044k [DOI] [PubMed] [Google Scholar]
- 29.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57(15):2215–2237. doi: 10.1016/j.addr.2005.09.019 [DOI] [PubMed] [Google Scholar]
- 30.Shao N, Su Y, Hu J, Zhang J, Zhang H, Cheng Y. Comparison of generation 3 polyamidoamine dendrimer and generation 4 polypropylenimine dendrimer on drug loading, complex structure, release behavior, and cytotoxicity. Int J Nanomedicine. 2011;6:3361–3372. doi: 10.2147/IJN.S27028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dave K, Krishna Venuganti VV. Dendritic polymers for dermal drug delivery. Ther Deliv. 2017;8(12):1077–1096. doi: 10.4155/tde-2017-0091 [DOI] [PubMed] [Google Scholar]
- 32.Shetty PK, Manikkath J, Tupally K, et al. Skin delivery of EGCG and silibinin: potential of peptide dendrimers for enhanced skin permeation and deposition. AAPS PharmSciTech. 2017;18(6):2346–2357. doi: 10.1208/s12249-015-0403-0 [DOI] [PubMed] [Google Scholar]
- 33.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134–148. doi: 10.1111/j.1524-475X.2010.00647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998–1008. doi: 10.1038/sj.jid.5700647 [DOI] [PubMed] [Google Scholar]
- 35.Shirakata Y. Regulation of epidermal keratinocytes by growth factors. J Dermatol Sci. 2010;59(2):73–80. doi: 10.1016/j.jdermsci.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 36.Donejko M, Przylipiak A, Rysiak E, et al. Hyaluronic acid abrogates ethanol-dependent inhibition of collagen biosynthesis in cultured human fibroblasts. Drug Des Devel Ther. 2015;9:6225–6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juranova J, Frankova J, Ulrichova J. The role of keratinocytes in inflammation. J Appl Biomed. 2017;15:169−179. doi: 10.1016/j.jab.2017.05.003 [DOI] [Google Scholar]
- 38.Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861−3885. doi: 10.1007/s00018-016-2268-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monteiller C, Tran L, MacNee W, et al. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med. 2007;64(9):609−615. doi: 10.1136/oem.2005.024802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30(10):1075−1079. doi: 10.1016/S1357-2725(98)00081-8 [DOI] [PubMed] [Google Scholar]
- 41.Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem. 2008;283(38):25900−25912. doi: 10.1074/jbc.M707692200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998;77(1):80−84. doi: 10.1006/jsre.1998.5345 [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Wang L, Zhang M, Jin M, Bai C, Wang X. Potential mechanism of interleukin-8 production from lung cancer cells: an involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J Cell Physiol. 2012;227(1):35−43. doi: 10.1002/jcp.22722 [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Zhang D, Yan T, et al. BNIP3 promotes the motility and migration of keratinocyte under hypoxia. Exp Dermatol. 2017;26(5):416−422. doi: 10.1111/exd.13248 [DOI] [PubMed] [Google Scholar]
- 45.Naha PC, Davoren M, Lyng FM, Byrne HJ. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol Appl Pharmacol. 2010;246(1–2):91−99. doi: 10.1016/j.taap.2010.04.014 [DOI] [PubMed] [Google Scholar]
- 46.Pai CL, Shieh MJ, Lou PJ, Huang FH, Wang TW, Lai PS. Characterization of the uptake and intracellular trafficking of G4 polyamidoamine dendrimers. Aust J Chem. 2011;64(3):302−308. doi: 10.1071/CH10358 [DOI] [Google Scholar]
- 47.Nyitrai G, Héja L, Jablonkai I, Pál I, Visy J, Kardos J. Polyamidoamine dendrimer impairs mitochondrial oxidation in brain tissue. J Nanobiotechnology. 2013;11:9. doi: 10.1186/1477-3155-11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith PE, Brender JR, Dürr UH, et al. Solid-state NMR reveals the hydrophobic-core location of poly(amidoamine) dendrimers in biomembranes. J Am Chem Soc. 2010;132(23):8087−8097. doi: 10.1021/ja101524z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith DJ, Ng H, Kluck RM, Nagley P. The mitochondrial gateway to cell death. IUBMB Life. 2008;60(6):383−389. doi: 10.1002/iub.44 [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Cha KE, Kim MS, et al. Nanosized polyamidoamine (PAMAM) dendrimer-induced apoptosis mediated by mitochondrial dysfunction. Toxicol Lett. 2009;190(2):202−207. doi: 10.1016/j.toxlet.2009.07.018 [DOI] [PubMed] [Google Scholar]
- 51.Lee H, Larson RG. Multiscale modeling of dendrimers and their interactions with bilayers and polyelectrolytes. Molecules. 2009;14(1):423−438. doi: 10.3390/molecules14031081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87(1):99−163. doi: 10.1152/physrev.00013.2006 [DOI] [PubMed] [Google Scholar]
- 53.Czarnomysy R, Bielawska A, Muszyńska A, Bielawski K. Effects of novel alkyl pyridine platinum complexes on apoptosis in Ishikawa endometrial cancer cells. Med Chem. 2015;11(6):540−550. doi: 10.2174/1573406411666150206163547 [DOI] [PubMed] [Google Scholar]
- 54.Barman J, Kumar R, Saha G, Tiwari K, Dubey VK. Apoptosis: mediator molecules, interplay with other cell death processes and therapeutic potentials. Curr Pharm Biotechnol. 2018;19(8):644−663. doi: 10.2174/1389201019666181022115405 [DOI] [PubMed] [Google Scholar]
- 55.Albanesi C, Scarponi C, Giustizieri ML, Girolomoni G. Keratinocytes in inflammatory skin diseases. Curr Drug Targets Inflamm Allergy. 2005;4(3):329−334. doi: 10.2174/1568010054022033 [DOI] [PubMed] [Google Scholar]
- 56.Albanesi C. Keratinocytes in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2010;10(5):452−456. doi: 10.1097/ACI.0b013e32833e08ae [DOI] [PubMed] [Google Scholar]
- 57.Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007;120(20):3700−3712. doi: 10.1242/jcs.03484 [DOI] [PubMed] [Google Scholar]
- 58.Albertazzi L, Serresi M, Albanese A, Beltram F. Dendrimer internalization and intracellular trafficking in living cells. Mol Pharm. 2010;7(3):680−688. doi: 10.1021/mp9002464 [DOI] [PubMed] [Google Scholar]
- 59.Beddoes CM, Case CP, Briscoe WH. Understanding nanoparticle cellular entry: a physicochemical perspective. Adv Colloid Interface Sci. 2015;218:48−68. doi: 10.1016/j.cis.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 60.Mukherjee SP, Davoren M, Byrne HJ. In vitro mammalian cytotoxicological study of PAMAM dendrimers - towards quantitative structure activity relationships. Toxicol In Vitro. 2010;24(1):169−177. doi: 10.1016/j.tiv.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 61.Mukherjee SP, Lyng FM, Garcia A, Davoren M, Byrne HJ. Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells. Toxicol Appl Pharmacol. 2010;248(3):259−268. doi: 10.1016/j.taap.2010.08.016 [DOI] [PubMed] [Google Scholar]