Abstract
Purpose
Breast cancer is one of the rapidly increasing cancers among women and a significant cause of cancer-related morbidity and mortality worldwide. Therefore, the current study was designed to examine and compare trends of breast cancer incidence (BCI) during the observed period (1990–2015) in specific age groups and investigate age-specific, time period, and birth cohort-related effects on BCI in China, India, Pakistan, and Thailand.
Patients and method
Data related to BCI were retrieved from the Institute for Health Metrics and Evaluation. Age–period–cohort model joint with intrinsic estimator algorithm was used to estimate the effect of age, period, and birth cohort on BCI. BCI rates were analyzed among different age groups ranging from 20 to 84 years in specified periods.
Result
Overall, results showed an increasing trend of BCI among four Asian countries during the study period especially in age groups 50 to 84 years. Higher incidence rates were observed in 2015 in the age group 70–74, 65–69, 50–54, and 60–64 in Pakistan, China, India, and Thailand, respectively. Age period cohort analysis revealed significantly raised effect of age and period and declined effect of the cohort on incidence rates.
Conclusion
The current study reported increased BCI with time in selected four Asian countries. Overall, BCI remained high in Pakistan as compared to China, India, and Thailand. Although proper registries are not available in most of the developing Asian countries, the current study highlighted the increased incidence and may play an essential role in registries development or spreading awareness against this disease. Therefore, maintaining proper records to build registries at the national level along with advancements in breast cancer screening and treatment are highly recommended to deal with the increasing burden of this disease.
Keywords: breast cancer, incidence, age–period–cohort model, APC, trend, Asia
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Breast cancer ranks as the most common malignancy and the leading cause of cancer-related morbidity and mortality all over the world. Breast cancer has become a severe health issue and alone accounts for 30% of all new cancer diagnoses among females.1 With the advancement of technology related to disease diagnosis and management, breast cancer transition has been observed from developed countries toward Asia and Africa.2 Incidence of breast cancer varies remarkably among different ethnicities. According to an estimate in 2020, the greatest cancer-related mortality will be observed in developing countries whereas disease burden will reduce in western countries due to the healthy lifestyle, tobacco resistance, awareness programs, and better health facilities.3,4 Decline in breast cancer incidence (BCI) has been reported among most of the US populations (different races/ethnicities) in the last 15 years, but increasing trend of 1.1% was observed among Asian residents of America from 2003 to 2012.5,6
Being a heavily populated country, China accounts for one-fourth of total cancer-related deaths, and an increasing trend was observed in cancer incidence, mortality rate, and onset among younger individuals.4 According to different breast cancer-related surveys, China showed 36.1% increased mortality from 1970 to 2005,7 seeking researcher’s attention toward in-depth studies. India, another heavily populated country in Asia, also has the highest breast cancer mortality rates, ie, 12.7 per 100,000 cases. The incidence of breast cancer varies significantly across the country due to demographic, lifestyle, anthropometric, and reproductive reasons. Highest rates were observed in New Delhi, Mumbai, and many Northeast cities.8,9 Temporal trends showed rising BCI rates in South Indian postmenopausal women.10
Pakistan is the seventh most populated country in the world and faces an increased burden of breast cancer due to some demographic, economic, lifestyle, reproductive, and social issues. It is the most commonly diagnosed malignancy in Pakistan, and one-ninth women are facing this disease.11,12 Trend analysis suggests 98.6% increased cancer incidence among females,11 and younger women belonging to rural areas are more to develop hereditary breast cancer due to consanguinity resulting in poor disease prognosis.13 Reproductive cancers are responsible for increased cancer-related burden in Thailand.14 Increased BCI was found in women with age of 40 years and above. Various social, environmental, economic, and reproductive factors are responsible for the devastating health effects of this cruel disease in Thailand.15
Despite advancement in the technology, BCI is still rising in Asia due to various social, demographic, environmental, reproductive, and genetic factors. Disease diagnosis at advanced stages, limited access to proper treatment, less awareness, and socioeconomic barriers contributed toward increased mortality rates among women due to breast cancer. To the best of our knowledge, no study was available on a combined comparison of incidence rate from breast cancer in China, India, Pakistan, and Thailand. Therefore, the current study aimed to examine the trends of BCI during the observed period (1990–2015) in specific age groups and compares the trends among four Asian countries having the similar sociocultural background, increased population, and limited availability of advanced treatment. Furthermore, the current study aimed to investigate age-specific, period, and birth cohort-related effects on trends of BCI using age–period–cohort (APC) model joint with intrinsic estimator (IE) algorithm in four different Asian countries due to increased BCI in them with the somewhat related sociocultural background. Examining the trends in Asian female BCI may expose new evidence about the risk factors for breast cancer, and the findings of period and cohort effect could reveal the association of social development with breast cancer burden in Asia.
Materials and methods
BCI data for four Asian representative countries like China, India, Pakistan, and Thailand were retrieved from the Institute for Health Metrics and Evaluation (IHME) 2018 (http://ghdx.healthdata.org/gbd-results-tool). The IHME is an independent global health research foundation at the University of Washington and responsible for maintaining and exchanging global registry data, surveys, censuses, and other health-related data to produce disease estimates, particularly incidence and mortality estimates. It is a source of data for researchers from all over the world and seeking their interest in scientific contributions highlighting the global burden of diseases, injuries, and associated risk factor studies. Already available literature and annual reports of IHME confirm the reliability of the source used for the current study.
Data of BCI rates from 1990 to 2015 in ages 20 to 84 for specific Asian countries were extracted from the source mentioned above. Thirteen age groups 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and 80–84 were considered for statistical analysis. Birth cohorts were calculated using the equation, cohort = period – age for four Asian countries including China, Pakistan, India, and Thailand.
Country-specific breast cancer incidence rates (BCIRs) from 1990 to 2015 for the age range 20 to 84 years were analyzed by APC analysis. APC analysis is an extensively used statistical technique to explore age, period, and cohort effect from the observed age-specific incidence rates. It is commonly used in epidemiologic, demographical, and sociological studies.16,17 IE with base Poisson log-linear model18 was used to estimate APC parameters. A linear relationship exists between age, period, and cohort, ie, period = cohort + age; it becomes difficult to estimate unique set for every age, period, and cohort effect.19 Therefore, IE was used to generate practically important and valid results.20 Fitting deviance, Akaike Information Criterion (AIC), and Bayesian Information Criterion (BIC) was applied to test the goodness of fit. Odds ratio (OR) was used to interpret the estimated parameters of the model. Additionally, exploratory and descriptive plots were produced to measure age, period, and birth cohort-related BCI trends, and area charts were used to assess the extent and variation of BCI over time in three age groups (20–49 and 50–84 and the third group included all ages) in two time periods (2000–2005 and 2010–2015). The area chart gives a quick comparison of the change in one or more quantities over the period of a year. Study data were analyzed using Stata version 12.0 (StataCrop LP, Texas, USA) and Minitab version 16.0 (Minitab LLC, State College, Pennsylvania, USA).
Results
Age, period, and birth cohort-related BCI trends
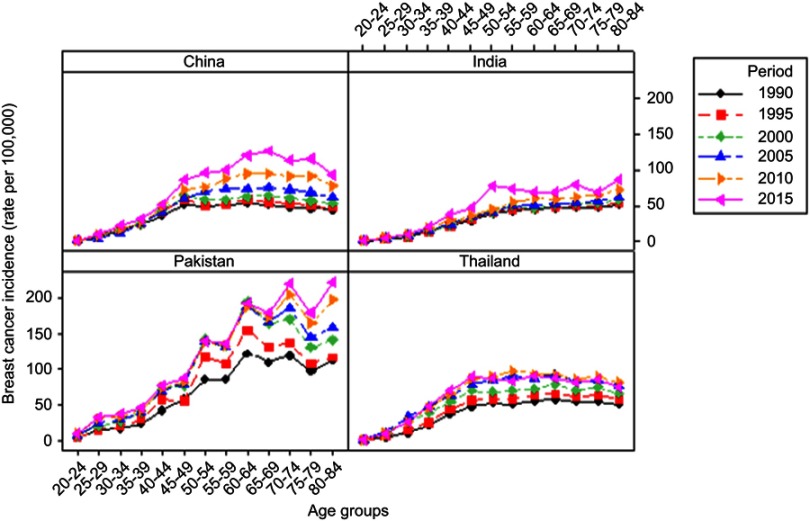
Age-related trends of BCIR from 1990 to 2015 with a 5-year interval by different age groups are illustrated in Figure 1. In general, BCIR systematically raised with time in China, Pakistan, India, and Thailand. In women above 49 years, the significantly increasing trend of BCIR was observed during all periods from 1990 to 2015. In China, BCIR in 2015 presented a sharp increase from 1.170 per 100,000 in the age group 20–24 to 127.550.30 per 100,000 in the age group 65–69. While, trends of BCIRs in India were relatively lower, peaking only in age groups 50–54 (77.279 per 100,000), 70–74 (78.149 per 100,000), and 80–84 (86.331 per 100,000) in 2015. Highest BCIRs were observed in Pakistan as compared to other countries. Specifically, age groups 50–54, 60–64, 70–74, and 80–84 showed significantly increased incidence rate in each period. Whereas, in Thailand, the increasing trend of BCIR was observed in age groups 20–24 to 45–49 and slightly decreased after that (Figure 1).
Figure 1.
Trends of female breast cancer incidence by specific age groups in different years among four Asian countries.
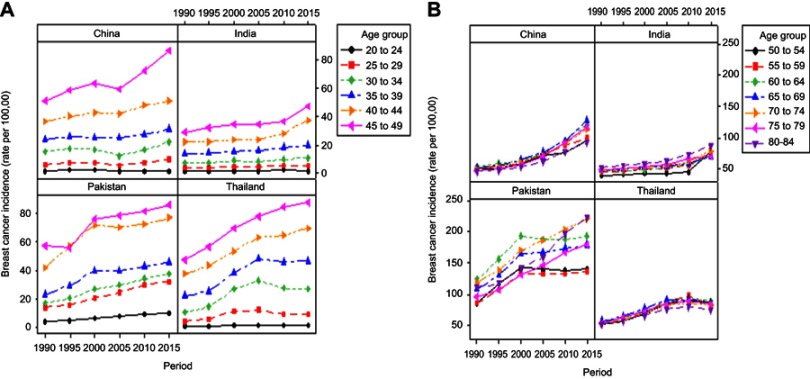
Period-based trend variation of BCIR in different age groups from 1990 to 2015 is demonstrated in Figure 2. Overall, BCIR mildly increased in lower age groups, ie, 20–49 years throughout the period in selected four Asian countries. While the upper age groups 50 to 84 years showed continuously increasing trend from 1990 to 2015 in four Asian countries. Whereas, in Pakistan, the incidence rates declined after 2000 in age group ranging from 50 to 69, but a relatively higher rate was maintained throughout the period in age groups 50–84 years compared to other countries. In China, the age group 65–69 years showed the highest incidence rate (127.550 per 100,000) in 2015. Moreover, 69.38% increased BCIR was observed in the age group 65–69 years from 75.303 to 127.550 per 100,000 in 2005 to 2015, respectively, which indicated a 6.9% annual average growth. In India, BCIR in the age group 50–54 years rapidly increased by 67.55% from 44.381 per 100,000 in 2010 to 74.364 per 100,000 in 2015, with an annual average growth of 13.51%. Slightly increasing incidence rates were observed in the age group 50–84 year over the entire observation period in Thailand (Figure 2).
Figure 2.
Trends of female breast cancer incidence by different years for age groups (A) 20 to 49 years and age groups (B) 50 to 84 years in four Asian countries.
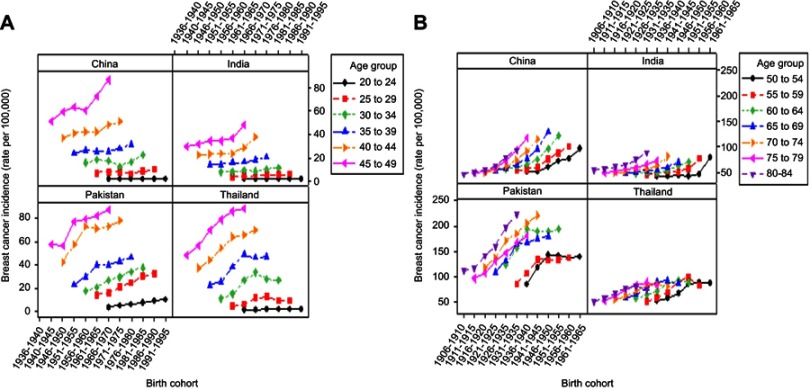
Effect of birth cohort on BCI trends was measured by age-specific incidence rates in different birth cohorts in four Asian countries. Like period-based variations, results were divided into two parts by age groups 20–49 years and 50–84 years as illustrated in Figure 3. Incidence rates slightly fluctuated in lower age groups (20 to 49), whereas higher incidence rates were observed in upper age groups (50 to 84) in all Asian countries under study. In general, incidence rates observed in the earlier 1950s cohorts were consistently increased with age but decreased after that. Birth cohort rates observed in the age group including women aged 60–64 years increased steadily with cohort years in China. In India, incidence rates observed in the age group 70–74 and 80–84 years consistently increased with cohort years.
Figure 3.
Trends in rates of female breast cancer incidence by birth cohort. The figure shows trends in four Asian countries in different age bands (A) 20 to 49 years (B) 50 to 84 years.
Moreover, women who were born after 1960–1964 birth cohort showed a higher rate of incidence relative to previous birth cohort. Birth cohort-related incidence trends in Pakistan showed a fluctuated pattern with changes in cohort year within same age groups excluding the age group 65–84 years. Women belonged to age groups 65–69, 70–74, 75–79, and 80–84 years showed a consistently increasing trend of BCI with increasing cohort years. Earlier 1950s birth cohorts showed an upward trend, and after that trend began to decline downward. Whereas, cohort-related trend of incidence in Thailand fluctuated over the whole birth cohort. Specifically, birth cohort 1951–1955 exhibited greater incidence rate in the age group of 55–59 years (Figure 3).
Estimates of age, period, and birth cohort using APC model
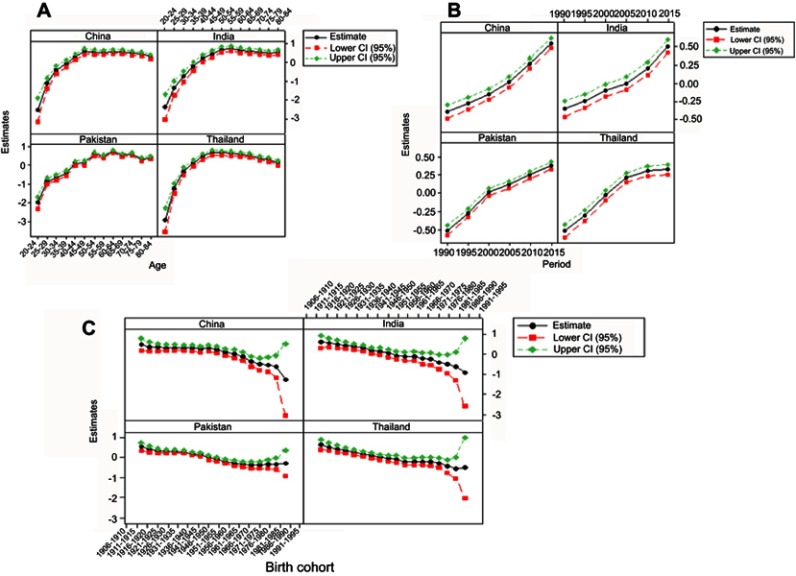
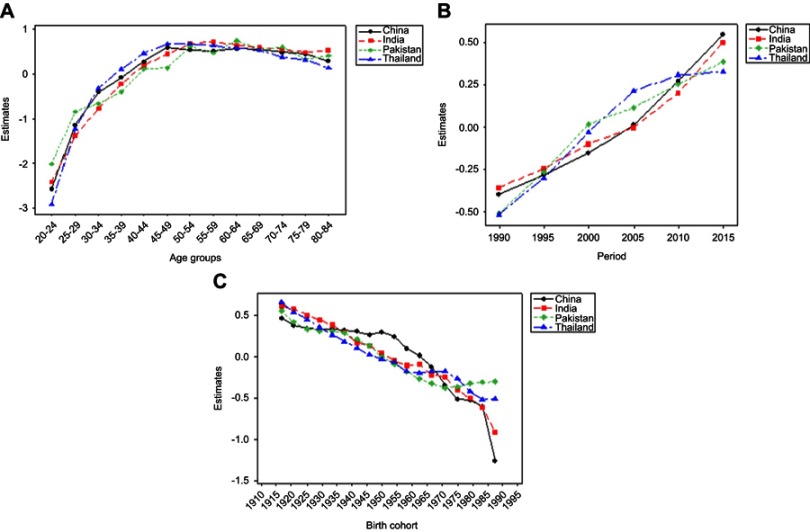
Effect of age, period, and birth cohort was estimated using IE by APC model. The goodness of fit of the APC model for BCIR in four Asian countries is demonstrated in Table 1. Estimated coefficients with 95% CI for age, period, and birth-cohort effect for four Asian countries are presented in Figure 4.
Table 1.
Goodness of fit of APC model for BCIR in four Asian countries
| China | Pakistan | India | Thailand | |
|---|---|---|---|---|
| Deviance | 4.738 | 14.189 | 5.494 | 7.869 |
| df | 44 | 44 | 44 | 44 |
| AIC | 6.303 | 7.142 | 5.968 | 6.474 |
| BIC | −186.956 | −177.505 | −186.200 | −183.826 |
Abbreviations: APC, age−period−cohort; BCIR, breast cancer incidence rate.
Figure 4.
Trends of estimated age−period−cohort model coefficients with 95% CI in four Asian countries separately for (A) age, (B) period, and (C) birth cohort effect.
Influence of age, period, and birth cohort
Continuously increasing effect of age was observed in the age group 20–49 years; thereafter the effect began to decline in China and Thailand. The higher rate of change (0.672, OR=1.958) was observed in Thailand comparatively to China for population belonging to age group 45–49 years, which indicated that the group of women aged 45–49 years had higher cancer incidence risk. Whereas, in the upper age groups from 50 to 84 years, a more significant net rate of change (0.238=0.520–0.282, OR=1.269) was maintained in China, which showed a higher cancer incidence risk in upper age groups in China as compared to Thailand. In India, a continuously increasing age effect was observed throughout the age groups from 20 to 55 years which started to decline slightly in the age group of 60–79 years, with higher rate of change 0.717 (OR 2.048; 95% CI 1.786–2.34), which revealed that women belonging to the age group 55–59 years had a relatively higher risk of incidence in India. Overall age effect in Pakistan showed moderately fluctuated increasing trend over the whole age groups, especially the age group 60–64 years showed 22% more increase (OR 2.09; 95% CI 1.95–2.246) compared to the age group 50–54 years (OR 1.522, 95% CI 1.679–1.991), which illustrated that this group of women had a higher risk of incidence as compared to other aforementioned Asian countries (Figure 4A).
The period effect presented progressively upward trend in China and India during the entire period, which indicated that period effect contributed to the perceived increase in linear trend of BCI. In the same way, an approximately linear trend identified in Pakistan and Thailand with a cut point at the 2000-time period. The upward trend with negative coefficients was observed before the cut point, which presented that although the effect was linear in that period at lower risk, later periods contributed higher effect to increasing BCIR. Overall, all countries showed an increasing trend of time effects which indicated that period effect significantly contributed to raised BCI in all countries especially far ahead the 2000 period (Figure 4B).
Estimated cohort effect in China and Pakistan presented almost similar upward to down ward variable trends in association with cohort year. In China, the cohort born between 1906 and 1955 years showed a rising trend among the cohorts, while subsequent cohorts depicted a sharp decrease with a negative rate of change, which represented that the women born before 1955 had a higher risk of breast cancer compared to those born later where negative coefficients showed a weak effect. Whereas, in Pakistan, the higher breast cancer risk was observed among the group of women born between 1906 to 1945 birth cohorts. For India, birth cohort 1940 and below showed a higher rate of change (range 0.61–0.16), which indicates that women born in this and previous cohorts had a higher risk of breast cancer relative to those born in other cohorts. In Thailand, women born in 1906–1935 birth cohorts suffered a higher risk of incidence rate with a net increase (0.472=0.658–0.186), which indicated that birth cohort effect alone increased the BCI risk by 72% from 1906 to 1935. Overall, the birth cohort effect declined later than1950 in China, Pakistan, India, and Thailand (Figure 4C).
Comparison of estimated APC effects among four Asian countries
To compare the age, period, and cohort effects of four countries, a comprehensive plot of these effects was constructed and presented in Figure 5. Figure 5A compares the estimated effect of age among China, India, Pakistan, and Thailand. It can be easily seen that all these countries had almost continuously increasing trend with increasing age, whereas, trends in Pakistan had shown gradually fluctuated trend from lower to upper age groups. Overall, a higher degree of change with increasing age was observed in the age groups 45–49 years in Thailand, 55–59 years in India, 60–64 years in Pakistan, and 45–49 years in China; among these groups higher rate was observed in Pakistan (0.738, OR=2.092, P<0.0001).
Figure 5.
Comparison of estimated age−period−cohort model trends among four Asian countries. The effect of (A) age, (B) period, and (C) birth cohort illustrated separately.
Comparison between estimated period effects among the four Asian countries is demonstrated in Figure 5B. Results showed continuously decreasing to increasing trend of period effect with a cut point during the period 2005 where a sharp increased trend was observed up to the end year in China and India. Whereas, Pakistan and Thailand showed an almost rising trend throughout the period. In the observation period, 2000–2010, a higher rate of change was observed in Thailand, whereas thereafter, a higher slope observed in China, India, Pakistan, and Thailand, respectively.
Results showed a fluctuated pattern of birth effect in China, India, Pakistan, and Thailand, which are depicted in Figure 5C. Overall, birth cohort 1910–1935 showed a higher rate of change relative to other birth cohorts. It indicated that the women born during the cohort (1910–1935) had experienced a higher risk of BCI, and a systematically higher association of this cohort with BCI was discovered in India, Thailand, Pakistan, and China, respectively.
General visualization of BCI change in four Asian regions
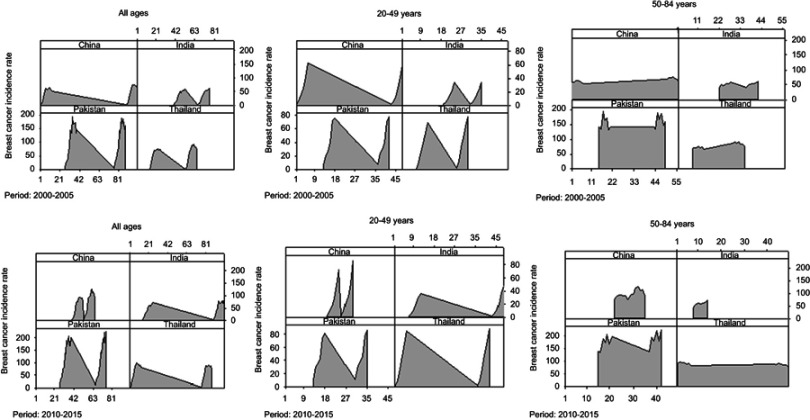
Furthermore, to assess the extent and variation of BCIR in three age groups (20–49 and 50–84 and the third group included all ages) in two time spans 2000–2005 and 2010–2015, the area charts were constructed (Figure 6). After visual inspection, the highest rates were observed in the last 5 years (2010–2015), indicating an increased BCI in representative four Asian countries. Countries that previously had low BCI had a significant increase with a different spread in the next years. First, the population of women belonging to all age groups showed significantly increased BCI in the years (2010–2015). In China, BCI increased in the period 2010–2015 and then in the 2000–2005 period, with least variation toward approximately symmetric bell-shaped curve, indicating that in these years there was a significantly consistent higher rate of incidence perceived from previous years. While India and Thailand showed an increased rate in the latest years, and almost consistent but thin curve was observed in both periods in Pakistan.
Figure 6.
Comparison of general incidence rates (per 100,000) between two observation periods (2000–2005, 2010–2015) for three age groups, that is, all ages, 20–49 years and 50–84 years. This area chart shows the change in breast cancer incidence rate in women over time in four Asian countries separately.
Moreover, a higher rate of breast cancer incidence was observed in recent years (2010-2015) in the age group 20-49 years in China and Pakistan.. Furthermore, the upper age group 50–84 years showed the highest BCI with reduced variation in successive years in all regions except Thailand where the widespread curve was found relative to previous years. Generally, it was observed that although BCIR raised in all the mentioned countries, a quite higher rate was reported in Pakistan (Figure 6).
Discussion
The current study provided an exclusive analysis of long-term BCI in four different Asian countries including China, India, Pakistan, and Thailand. To the best of our knowledge, APC analysis representing cancer incidence trends has been applied in different developed Asian countries like China,20 Japan, Korea, and Singapore.19 However, systematic analysis of BCI using APC model among China, India, Pakistan, and Thailand was rarely reported before. All these selected Asian countries are facing the increased breast cancer burden due to somewhat similar conditions including sociocultural background, poverty, increased population, and limited access to advanced screening, diagnosis, and treatment. Lee et al conducted a study in 2014 comparing mortality trends of gynecologic cancers among East Asian women using join point regression.21 In the current study, we have conducted an APC analysis using IE algorithm to investigate the trends of BCI during the observed period (1990–2015) in specific age groups and compared the trends among women of four Asian countries (China, India, Pakistan, and Thailand). Furthermore, age-specific, time period, and birth cohort effects on trends of BCI were also investigated.
The current study has illustrated variable trends of BCI among four Asian countries. An overall increasing trend was observed in BCIRs in four regions with different speed and degree as illustrated in Figure 1. Highest BCIRs were found in Pakistan, and the increasing trend was observed in this incidence rate over time being lowest in 1990 and the highest in 2015. Few other studies on local data and reports at BCI available in Pakistan also reported similar findings that Pakistan has the highest rate of BCI in Asia.22,23 China found to be the second most country with increased BCI. Similar trends were observed in a study conducted for Wuhan, China.20 Increasing BCIRs were also observed in India concordant with the already reported study.24 Although the increasing trend was observed in Thailand over time, it was found to be much less as compared to other selected countries. Another study also has reported highly variable trends in BCI among different Asian countries being highest in less developed or developing countries.13 Decreasing trends were observed in the latest years among some European countries7,25 probably due to altered lifestyle and availability of advanced treatment options like surgery, radiotherapy and respective hormonal interventions.26
Furthermore, it was found that the BCIR increases with increased age in three of the selected Asian countries (China, Pakistan, India, and Thailand) as shown in Figures 1 and 2. Continuously increasing effect of age was observed from 20 to 49 years of age, which began to decline gently after that in China and Thailand. Increased rate of change was observed in Thailand relative to China for population belonging to age group 45–49 years, which illustrated that women aged 45–49 years had higher cancer incidence risk. Whereas, in upper age groups from 50 to 84 years, a greater compound rate of change was maintained in China, which showed higher cancer incidence risk in upper age groups in China as compared to Thailand. In India, a continuously increasing age effect was observed in the age groups from 20 to 55 years which started to decline slightly after the age group of 60–79 years, with a higher rate of change 0.717, which revealed that women belonging to the age group 55–59 years had a relatively higher risk of incidence in India. Overall age effect in Pakistan showed increased moderately fluctuated trend over all age groups, especially age group 60–64 showed 22% more increase relative to age group 50–54 years, which illustrated that this group of women had a higher risk of incidence as compared to other Asian countries. Similar findings were reported for other Asian countries.2,27 DNA methylation might be one of the reasons behind increased BCI with age, as it is a normal part of the aging process. Most essential reasons behind increased BCI with age among Asians are poverty, poor dietary habits, and unhealthy lifestyle.
Results from APC analysis illustrated that period effects were increased in all four Asian countries throughout the study period, consistent with results of BCIR in four selected Asian countries. Therefore, it was concluded that period effect played an important role in BCIR through a series of screening programs, treatment, and disease management. Increasing period effect showed that disease diagnosis and treatment programs were not much successful among selected Asian countries as compared to well-developed Asian and European countries concordant with available literature for some Asian countries.19,28
BCI risk by birth cohort in all four Asian countries showed a declining trend during most of the birth cohorts, illustrating those women of earlier 1950s had a higher risk of BCI relative to other cohorts. The decreasing trend of BCI in the birth cohort over the 1950s was possibly due to the development of public health policies and improved treatment options. China is the most developed country among selected Asian countries, whereas health conditions are still not well-developed particularly in India and Pakistan. With time advancements being been made in the health department, there is still a lot more to do. Similar kind of results was reported by other studies.19 Real causes of the decrease in birth cohorts are still not fully explored yet and more studies are needed to confirm the accuracy of results. Availability of cancer screening programs and advanced treatments were possible reasons behind declining birth cohort trends in China. Whereas, declining trends in India and Pakistan were probably due to higher pregnancies and associated reproductive factors.29 Other possible reasons may include the use of oral contraceptives and hysterectomy in younger women.19 In Thailand, increased BCI in 1906–1935 might be due to the adoption of a westernized lifestyle and various sociodemographic and environmental risk factors. Early menarche and late menopause also contribute to increased BCI.12,30
One of the study limitations is the unavailability of breast cancer mortality analysis, as it would be a better predictor of effects caused by any disease. Asian countries included in the current study do not have proper records, and most of the incidence and mortality data were collected from hospital-based registries and records, which challenges its credibility. Therefore, most of the time, researchers avoid to word on such countries. Although results may vary to some extent as compared to the original scenario, even then, it is good to at least give a perspective of disease diagnosis and incidence to provoke government and local bodies so that they should maintain accurate records, which is the ultimate requirement for proper disease diagnosis and management. Furthermore, despite the nonbiased and greater estimation of the IE method, studies with a more significant period and risk factors are required to explain more accurate findings in the future.
Conclusion
Based on the results presented, it can be concluded that BCIR increases with age in all four selected Asian countries, illustrating that increased age might be a breast cancer-associated risk factor. Highest BCIR was found in Pakistan. Increased incidence showed an urgent need for strengthening the existing screening, diagnostic, and treatment facilities because available medical facilities are inadequate and unable to bear the current increasing load of breast cancer in Asian countries.
Acknowledgments
The National Natural Science Foundation of China (Grant NO. 81773552 & 81602937) and the National Key R&D Program of China “Research on Disease Burden, Prevention and Control Strategies of Major Chronic Diseases” (No. 2018YFC1315302) funded this research. The authors would like to thank the funding foundations for their supports.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med. 2014;11(2):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization & International Union against Cancer. Global action against cancer, Updated ed. World Health Organization. 2005. Available from: https://apps.who.int/iris/handle/10665/43203. Accessed August 01, 2019. [Google Scholar]
- 4.Wang P, Xu C, Yu C. Age-period-cohort analysis on the cancer mortality in rural China: 1990–2010. Int J Equity Health. 2014;13(1):1. doi: 10.1186/1475-9276-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. doi: 10.3322/caac.21320 [DOI] [PubMed] [Google Scholar]
- 6.Gomez SL, Von Behren J, McKinley M, et al. Breast cancer in Asian Americans in California, 1988–2013: increasing incidence trends and recent data on breast cancer subtypes. Breast Cancer Res Treat. 2017;164(1):139–147. doi: 10.1007/s10549-017-4229-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung H, Rosenberg PS, Chen W-Q, et al. Female breast cancer incidence among Asian and Western populations: more similar than expected. J Natl Cancer Inst. 2015;107(7):djv107. doi: 10.1093/jnci/djv107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization, Cancer IAfRo. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Lung Cancer GLOBOCAN. 2012. Available from: https://publications.iarc.fr/Databases/Iarc-Cancerbases/GLOBOCAN-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1.0-2012. Accessed August 01, 2019. [Google Scholar]
- 9.Gupta A, Shridhar K, Dhillon P. A review of breast cancer awareness among women in India: cancer literate or awareness deficit? Eur J Cancer. 2015;51(14):2058–2066. doi: 10.1016/j.ejca.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathew A, George P, Arjunan A, et al. Temporal trends and future prediction of breast cancer incidence across age groups in trivandrum, South India. APJCP. 2016;17(6):2895–2899. doi: 10.7314/apjcp.2016.17.4.2119 [DOI] [PubMed] [Google Scholar]
- 11.Masood K, Masood A, Zafar J, et al. Trends and analysis of cancer incidence for common male and female cancers in the population of Punjab province of Pakistan during 1984 to 2014. Asian Pac J Cancer Prev. 2015;16(13):5297–5304. doi: 10.7314/APJCP.2015.16.13.5297 [DOI] [PubMed] [Google Scholar]
- 12.Malik SS, Mubarik S, Masood N, Khadim MT. An insight into clinical outcome of XPG polymorphisms in breast cancer. Mol Biol Rep. 2018;45(6):2369–2375. doi: 10.1007/s11033-018-4401-7 [DOI] [PubMed] [Google Scholar]
- 13.Ghoncheh M, Momenimovahed Z, Salehiniya H. Epidemiology, incidence and mortality of breast cancer in Asia. Asian Pac J Cancer Prev. 2016;17(S3):47–52. doi: 10.7314/apjcp.2016.17.4.2119 [DOI] [PubMed] [Google Scholar]
- 14.Virani S, Bilheem S, Chansaard W, et al. National and subnational population-based incidence of cancer in Thailand: assessing cancers with the highest burdens. Cancers. 2017;9(8):108. doi: 10.3390/cancers9080108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotepui M, Chupeerach C. Age distribution of breast cancer from a Thailand population-based cancer registry. Asian Pac J Cancer Prev. 2013;14(6):3815–3817. doi: 10.7314/APJCP.2013.14.6.3815 [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Bao J, Yu C, Wang J, Li C. Secular trends of breast cancer in China, South Korea, Japan and the United States: application of the age-period-cohort analysis. Int J Environ Res Public Health. 2015;12(12):15409–15418. doi: 10.3390/ijerph121214993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Yu C, Wang P. An age-period-cohort analysis of female breast cancer mortality from 1990–2009 in China. Int J Equity Health. 2015;14(1):76. doi: 10.1186/s12939-015-0211-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Schulhofer-Wohl S, Fu WJ, Land KC. The intrinsic estimator for age-period-cohort analysis: what it is and how to use it. Am J Sociol. 2008;113(6):1697–1736. doi: 10.1086/587154 [DOI] [Google Scholar]
- 19.Wang J, Lv H, Xue Z, Wang L, Bai Z. Temporal trends of common female malignances on breast, cervical, and ovarian cancer mortality in Japan, Republic of Korea, and Singapore: application of the age-period-cohort model. Biomed Res Int. 2018;2018:13p. Article ID 5307459. doi: 10.1155/2018/5307459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Y, Yan Y, Gong J, Yang N, Nie S. Trends in incidence and mortality of female breast cancer during transition in Central China. Cancer Manag Res. 2018;10:6247. doi: 10.2147/CMAR.S182510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J-Y, Kim E-Y, Jung K-W, et al. Trends in gynecologic cancer mortality in East Asian regions. J Gynecol Oncol. 2014;25(3):174–182. doi: 10.3802/jgo.2014.25.3.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanif M, Zaidi P, Kamal S, Hameed A. Institution-based cancer incidence in a local population in Pakistan: nine year data analysis. Asian Pac J Cancer Prev. 2009;10(2):227–230. [PubMed] [Google Scholar]
- 23.Menhas R, Umer S. Breast cancer among Pakistani women. Iran J Public Health. 2015;44(4):586–587. [PMC free article] [PubMed] [Google Scholar]
- 24.Badwe RA, Dikshit R, Laversanne M, Bray F. Cancer incidence trends in India. Jpn J Clin Oncol. 2014;44(5):401–407. doi: 10.1093/jjco/hyu040 [DOI] [PubMed] [Google Scholar]
- 25.DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2015;24:1495–1506. doi: 10.1158/1055-9965.EPI-15-0535 [DOI] [PubMed] [Google Scholar]
- 26.Rosso T, Malvezzi M, Bosetti C, Bertuccio P, Negri E, La Vecchia C. Cancer mortality in Europe, 1970–2009: an age, period, and cohort analysis. Eur J Cancer Prev. 2018;27(1):88–102. doi: 10.1097/CEJ.0000000000000282 [DOI] [PubMed] [Google Scholar]
- 27.Shin HR, Boniol M, Joubert C, et al. Secular trends in breast cancer mortality in five East Asian populations: Hong Kong, Japan, Korea, Singapore and Taiwan. Cancer Sci. 2010;101(5):1241–1246. doi: 10.1111/j.1349-7006.2010.01605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito Y, Ioka A, Nakayama T, Tsukuma H, Nakamura T. Comparison of trends in cancer incidence and mortality in Osaka, Japan, using an age-period-cohort model. Asian Pac J Cancer Prev. 2011;12(4):879–888. [PubMed] [Google Scholar]
- 29.Khan M, Hossain M, Khan A, et al. Ovarian cancer mortality among women aged 40–79 years in relation to reproductive factors and body mass index: latest evidence from the Japan Collaborative Cohort study. J Gynecol Oncol. 2013;24(3):249–257. doi: 10.3802/jgo.2013.24.3.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamińska M, Ciszewski T, Łopacka-Szatan K, Miotła P, Starosławska E. Breast cancer risk factors. Przeglad Menopauzalny. 2015;14(3):196. doi: 10.5114/pm.2015.54346 [DOI] [PMC free article] [PubMed] [Google Scholar]