Abstract
Background
In patients with peripheral artery disease (PAD), the severity of symptoms correlates poorly with ankle-brachial index ABI. We hypothesized that limb perfusion assessed by contrast-enhanced ultrasound (CEU) during contractile exercise varies according to functional class in PAD patients, particularly those with ankle-brachial index (ABI) in the 0.4–0.6 range whose symptoms vary widely.
Methods
Bilateral quantitative CEU perfusion imaging of the calf was performed in normal controls (n=10), and patients with PAD who had at least one limb with moderately reduced ABI (0.4–0.6) (n=17). Imaging was performed at rest and immediately after 30 seconds of modest periodic (0.3 Hz) plantar flexion (10 Watts).
Results
In PAD patients, Rutherford symptom classification for each limb varied widely, including in limbs with ABI 0.4–0.6 (n=6 with mild or no symptoms, n=14 for moderate-severe symptoms). CEU perfusion imaging parameters at rest were similar between control and PAD subjects irrespective of the ABI. In normal controls, limb flow increased on average by >20-fold after only 30 seconds of moderate exercise. In PAD patients, muscle exercise perfusion for all limbs was reduced compared to controls, and decreased according to the severity of ABI reduction, primarily from reduced microvascular flux rate. Even limbs with an ABI >0.9 in PAD patients had lower exercise perfusion than control subjects (p=0.03). In PAD subjects, exercise perfusion was lower in those with moderate-severe versus mild symptoms when analyzed for all limbs (median: 30 [IQR 21–52] vs 84 [IQR 36–177] IU/s, p=0.01), and limbs with ABI 0.4–0.6 (median: 26 [IQR 14–41] vs 54 [IQR 31–105] IU/s, p=0.05).
Conclusions
In patients with PAD, CEU exercise perfusion imaging detects differences in limb muscle perfusion that are likely to be responsible for differences in symptom severity; and can detect the flow abnormalities from microvascular dysfunction even in limbs with normal ABI.
Keywords: Contrast ultrasound, Microbubbles, Peripheral artery disease
Conventional methods used to evaluate the presence and severity of peripheral artery disease (PAD) include measurement of segmental pressures such as the ankle-brachial index (ABI), impedance measurement of tissue pulse-volume, or direct imaging of the degree of obstruction or focal pressure gradients in conduit arteries. A major clinical gap in vascular medicine is the lack of methods for evaluating impairment in limb microvascular perfusion, which is the fundamental problem responsible for symptoms and tissue injury in PAD.1 In patients with claudication, assessment of limb perfusion during exercise could be used to assess the cumulative effects of sequential or diffuse disease, collateral perfusion, and microvascular dysfunction which is common in those with diabetes mellitus.2 Perfusion imaging could address the poor relationship seen between severity of symptoms and the commonly-used ABI,3–5 and the issue of falsely elevated ABIs from non-compressible vessels.6 Perfusion imaging could also potentially be used as a more accurate method to evaluate novel therapies for PAD, particularly those aimed at improving microvascular function 7.
Contrast-enhanced ultrasound (CEU) has recently been used to evaluate limb skeletal muscle perfusion in patients with PAD.8–12 Methods have recently been developed that allow for robust real-time assessment of perfusion with CEU within seconds using microbubble agents that are resistent to inertial cavitation and produce high signal-to-noise during intermediate power imaging.13 This rapid imaging approach allows for multi-site perfusion imaging that can be performed easily during exercise hyperemia. In this study, CEU was used to test the hypothesis that perfusion, particularly during exercise stress, varies according to functional class in PAD subjects. We specifically focused on PAD patients with an ABI range between 0.4 and 0.6 who are often characterized by a wide range of symptom status ranging from asymptomatic to severe claudication.
METHODS
Study Population
The study was approved by the Investigational Review Board at Oregon Health & Science University and registered on Clinicaltrials.gov (NCT02398266). We studied patients with a history of PAD (based on symptoms and either non-invasive diagnostic testing or angiography) and an ABI between 0.4 and 0.6 in at least one limb (n=21); and healthy control volunteers (n=10). Exclusion criteria for control subjects were history of PAD, any symptoms of lower extremity weakness or claudication, any abnormalities in pulse examination bilateral or ABIs bilaterally, or history of diabetes mellitus. Exclusion criteria for control or PAD subjects included pregnancy, lactation, evidence for large right-to-left shunt, more than mild left ventricular dysfunction, critical limb-threatening ischemia, planned amputation, muscular or neuromuscular disease, or inability to perform plantar flexion calf raise exercise.
Study Design and Protocol
Subjects underwent an initial screening including brief medical history and focused physical examination including blood pressure measurement, cardiopulmonary exam, and peripheral vascular exam. Limited echocardiography was performed to evaluate for exclusion criteria. PAD subjects completed the Walking Impairment Questionnaire.14 Symptom severity by Rutherford category was assigned on a per limb basis by agreement of two independent assessments. CEU perfusion imaging of calf was performed in both legs. Perfusion imaging was performed while seated at rest, and immediately upon completion of 30 seconds of modest plantar flexion exercise in each limb. Calf raise plantar flexion was performed on a seated calf raise (Powerline PSC43X, Body-Solid Inc., Forest Park, IL) machine (Supplemental Figure 1) with an approximate work level of 10 Watts every 3 secs for a total duration of 30 seconds. All subjects except for 6 patients in the PAD cohort returned on a separate day within 6 weeks for restudy in order to evaluate test-retest variability of CEU perfusion imaging at rest and during stress.
Ankle-Brachial Index
For ABI measurements, systolic blood pressures for the brachial, dorsalis pedis, and posterior tibialis arteries were measured using a Doppler probe. ABI was calculated by dividing the systolic blood pressure at the ankle by the highest systolic blood pressures in the arm. For each measurement, the highest calculated ABIs from either the dorsalis pedis or posterior tibialis were reported.
Contrast Enhanced Ultrasound Perfusion Imaging
CEU perfusion imaging was performed with a phased-array transducer (S5–1, IE33, Philips Ultrasound, Andover, MA) using a multipulse contrast-specific algorithm imaging at 1.8 MHz and a mechanical index of 0.3. Gain settings were optimized to a level that just eliminated tissue speckle and held constant. Imaging was performed bilaterally using trans-axial imaging planes of the calf one-third the distance from the popliteal fossa to the ankle. Lipid-shelled perfluorobutane microbubbles (Sonazoid™, GE Healthcare, Amersham, United Kingdom) (United States Food and Drug Administration IND 125975) were diluted 2:20 (v:v) in normal saline and infused intravenously at 1.5 mL/min continuously without interruption for the rest and stress images. End-diastolic frames (achieved by ECG gating) were obtained continuously for 30 sec after a 5-frame high-power (mechanical index >1.1) pulse sequence. CEU imaging was performed bilaterally at rest, then sequentially on each limb after 30 seconds of unilateral exercise with initiation of imaging immediately after the final plantar flexion. All post-exercise image sets were initiated within 2 seconds of completing exercise.
Contrast Ultrasound Image Analysis
Video intensity (VI) was measured from regions-of-interest placed over the gastrocnemius and soleus muscle groups. The immediate post-destruction frame was used as background and digitally subtracted from subsequent frames to eliminate signal both from tissue and from rapidly-refilling large-conduit intramuscular arteries or veins. Time versus video intensity data were fit to the function:
where y is VI at pulsing interval t, A is the plateau VI reflecting microvascular blood volume, and β is the rate of microbubble replenishment reflecting microvascular blood flux rate. Microvascular blood flow was quantified by the product of A and β.15,16 We also calculated the time from microbubble destruction to a VI at 80% of the maximal plateau video intensity by:
Qualitative Assessment
Based on results from the quantitative assessment of time to 80% of the maximal plateau, a reader blinded to subject identity or limb data reviewed exercise CEU images and classified each limb as normal versus abnormal according to whether post-destruction contrast replenishment was largely achieved by 2 seconds.
Spatial Heterogeneity of Perfusion
For exercise stages, background-subtracted frames were selected once full replenishment of microbubbles had occurred. Contrast-enhancement during this plateau phase of the destruction-replenishment sequence represents actively perfused microvascular units in skeletal muscle. In regions-of-interest encompassing the soleus and gastrocnemius muscles, the background-subtracted image was then processed by a bandpass filter to eliminate all nonvascular data from the region, and all remaining pixels were converted to binary format. The images were analyzed using a digital texture analysis (MaZda, Institute of Electronics, Technical University of Lodz, Poland) for spatial assessment of wavelet energies which describe geometrically-confined frequency domain patterns based on linear encountering of enhanced pixels or clusters of pixels.17,18 Wavelet analysis was performed only at the coarsest level (energy 1) to determine patterns of flow homogeneity.
Statistical Methods
Statistical analysis was performed both with Prism (V.6.02, GraphPad, La Jolla, CA) and R (V.3.3.3). Data are expressed as mean (± standard deviation) unless otherwise stated. Clinical variables were compared between control and PAD subjects using t-test for continuous variables and Fisher’s exact test for proportions. Analysis of the limb-specific values was performed with a Mann-Whitney u-test, but were also performed by linear mixed modelling which accounted for within-subject correlation between the paired measurements. Comparisons between PAD subjects with different ABI classifications and normal subjects were made using Bonferroni’s correction for multiple comparisons. Grubb’s test for outliers was applied and resulted in censuring a single β-value from the ABI 0.4–0.6 group. Test-retest variability was characterized by interclass correlation coefficient (ICC) and by the correlation of coefficient between the repeated and baseline values of log-transformed parameters. For all tests, the two-sided p-value of <0.05 was considered statistically significant.
RESULTS
Clinical characteristics
Clinical characteristics of PAD patients and healthy control subjects are shown in Table 1. Patients with PAD were slightly older, had more co-morbidities including diabetes mellitus, which was present in 30%, had higher systolic blood pressure, and were more likely to be treated with cardiovascular medications. Among the 21 patients with PAD, there were 16 limbs with mild symptoms (Rutherford class 0 or 1), and 26 with moderate to severe symptoms (Rutherford class 2 or 3). In limbs with ABI of 0.4–0.6, there were 6 limbs with no or mild symptoms and 17 limbs with moderate-severe symptoms. For all limbs in the PAD cohort, the ABI in limbs with moderate-severe symptoms by Rutherford classification were lower than those with mild symptoms (Figure 1A). This was not the case for limbs within the ABI 0.4–0.6 category (Figure 1B).
Table 1.
Clinical Characteristics
| Controls (n=10) | PAD (n=21) | |
|---|---|---|
| Age (years) | 58±14 | 69±9* |
| Male gender, n (%) | 7 (70) | 19 (90) |
| Prior Revascularization, n (%) | 13 (62) | |
| Co-morbidities | ||
| Coronary artery disease, n (%) | 0 (0) | 11 (52)* |
| Hypertension, n (%) | 1 (10) | 20 (95)* |
| Hyperlipidemia, n (%) | 1 (10) | 17 (81)* |
| Diabetes, n (%) | 0 (0) | 7 (33) |
| Tobacco use, n (%) | 1 (10) | 21 (100)* |
| Medications | ||
| Aspirin, n (%) | 2 (20) | 16 (76)* |
| Clopidogrel n (%) | 0 (0) | 2 (10) |
| Oral anticoagulant n (%) | 0 (0) | 7 (33) |
| Beta blocker, n (%) | 0 (0) | 16 (76)* |
| Statin, n (%) | 1 (10) | 19 (90)* |
| Insulin, n (%) | 0 (0) | 6 (29) |
| Rutherford Category† | ||
| 0- Asymptomatic, n (%) | 18 (100) | 7 (18) |
| 1- Mild Claudication, n (%) | 9 (23) | |
| 2- Moderate Claudication, n (%) | 13 (33) | |
| 3- Severe Claudication, n (%) | 10 (25) | |
| 4- Ischemic Rest pain, n (%) | 1 (3) | |
| 5- Minor tissue loss, n (%) | 0 | |
| Body mass index (kg/m2) | 24±3 | 28±6 |
| HR (beats per min) | 70±12 | 72±12 |
| Systolic BP (mm Hg) | 122 ± 13 | 142±20* |
| Diastolic BP (mm Hg) | 79±7 | 75±9 |
| ABI- most symptomatic limb | 0.50±0.07 | |
| ABI- least symptomatic limb | 0.89±0.27 | |
| WIQ Score | 0.39±0.21 |
P <0.05 compared to control
n=20 and 34 for limb-based assessment; ABI, ankle brachial index; HR, heart rate; WIQ, walking impairment questionnaire
Figure 1.
Box-whisker plots of median (bar), IQR (box), and range (whiskers) for ankle-brachial index (ABI) in PAD subjects with either mild (Rutherford 0–1) or moderate-severe (Rutherford 2–3) symptoms. Data are shown for (A) all PAD subjects, and (B) PAD subjects with ABI 0.4–0.6.
Skeletal Muscle Quantitative Blood Flow
Irrespective of the ABI value, CEU perfusion imaging parameters at rest were similar between PAD subjects and controls for total perfusion (Aβ) and the parametric components of functional microvascular blood volume (A-value) and microvascular blood flux rate (β) (Supplemental Figure 2). For exercise conditions, all subjects were able to complete the 30 second plantar flexion exercise and only one PAD patient reported onset of mild unilateral claudication. Moderate level plantar flexion exercise for 30 seconds resulted on average in a >20-fold increase in microvascular perfusion (Aβ), mediated mostly through and increase in microvascular blood flux rate (β) with a smaller relative increase in microvascular blood volume (A-value) (Supplemental Figure 2). During exercise, CEU-derived microvascular perfusion and flux rate were significantly different in the limbs of PAD subjects compared to controls, and varied according to ABI classification in the PAD cohort (Figure 2). Microvascular blood volume in the limbs of PAD subjects with reduced ABI (<0.9) were significantly lower than in controls, but these values did not vary according to ABI classification. Interestingly, exercise perfusion and microvascular flux rate in limbs with a normal ABI (>0.9) in patients with PAD were lower than in normal controls. On continuous analysis (Figure 3), within the PAD group a significant relationship was found between the ABI values and log-transformed CEU data on exercise perfusion (Aβ) and microvascular flux rate (β). Within the narrow ABI range of between 0.4 and 0.6, there was substantial variability in exercise perfusion and microvascular flux rate.
Figure 2.
Box-whisker plots for contrast-enhanced ultrasound (CEU) perfusion data obtained from all limbs at during stress for (A) microvascular blood volume (A-value), (B) microvascular flux rate (β), and (C) microvascular blood flow (product of A-value and β-value). *p<0.05 versus control; †p<0.05 versus >0.9 ABI classification. Rest data are illustrated in Supplemental Figure 1, which also displays data as mean±SD since some datasets were found to be normally distributed. (D) Examples of background-subtracted color-coded CEU images from the calf muscle during exercise, and examples of time-intensity curves from a control subject and two limbs from PAD patients with Rutherford classification of 1 (ABI 0.55) or 2 (ABI 0.58). The images show frames obtained immediately after the destructive pulse sequence (baseline) where background non-linear tissue signal in grey scale is visualized, and at 1, 2, 3, and 10 seconds (end-diastole) where enhancement beyond the background tissue signal intensity is displayed according to the color scales shown at the left of each row. Approximate regions-of-interest for quantitative data are illustrated on the baseline images by the dashed lines.
Figure 3.
Graphs depict the relationship between ABI and CEU parameters of (A) microvascular blood volume; (B) microvascular flux rate (β); and (C) microvascular blood flow (Aβ); for exercise conditions. Data for control subjects is shown at the far right in each graph. CEU data for microvascular flux rate and flow were log-transformed to display relationship, for which linear regression was made using log-linear fit.
When classified according to symptomatic disease severity, CEU-derived microvascular blood flow and flux rate during exercise were different between limbs with mild versus moderate-to-severe symptoms by Rutherford classification, both for the entire PAD cohort and also only for limbs within the target ABI range of 0.4–0.6 (Figure 4A–B). Data for microvascular flux rate detected differences only within the entire cohort (Figure 4C–D). With regards to symptoms classified by the WIQ questionnaire, CEU-derived microvascular perfusion during exercise incrementally increased with functional class by WIQ tertile (Figure 5).
Figure 4.
Box-whisker plots illustrating exercise CEU data on microvascular blood flow from (A) all subjects, and (B) patients with PAD and ABI 0.4–0.6; and for microvascular flux rate from (C) all subjects, and (D) patients with PAD and ABI 0.4–0.6. *p<0.01 versus control subjects; †p<0.05 versus Rutherford 0–1 by Mann-Whitney test.
Figure 5.
Box and whisker plots demonstrating CEU-derived (A) microvascular blood flow, and (B) microvascular blood velocity according to tertiles of symptom classification scores on the WIQ Questionnaire.
Qualitative Assessment of Perfusion
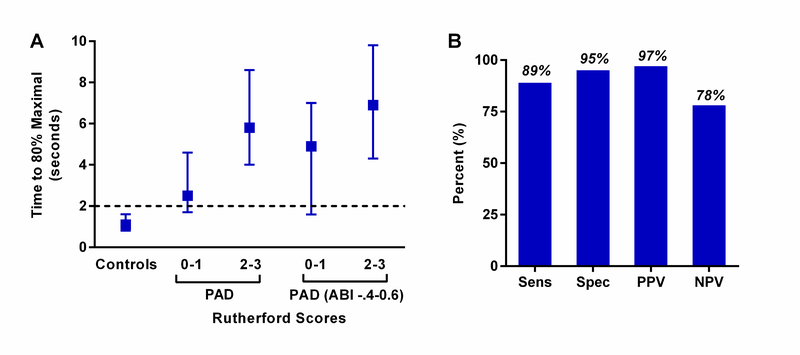
Quantitative data of time to reach 80% of maximal replenishment (τ80) during exercise indicated that near complete muscle filling by two seconds could provide a reasonable threshold for differentiating limbs from normal subjects from PAD patients (Figure 6). Accordingly, qualitative analysis by a blinded reviewer was performed for each limb according to whether post-destruction contrast replenishment was achieved by two seconds during exercise. The diagnostic accuracy for discriminating limbs from control subjects versus PAD patients was 89%, with sensitivity of 89%, specificity of 95%, and positive and negative predictive values of 97% and 78%, respectively. Of the 5 limbs from PAD subjects that were categorized as normal, three had an ABI >0.90.
Figure 6.
(A) Median (±95% CI) for time to reach 80% of the maximum intensity during exercise derived by natural log-transformation of time-intensity data from controls and patients with PAD subdivided into all limbs and limbs with ABI in the study target range. The threshold of two seconds (dashed line) for time to achieve near maximal replenishment on visual qualitative analysis discriminated between normal and PAD subjects and was used prospectively for qualitative analysis. (B) Diagnostic accuracy for qualitative analysis of normal versus abnormal based on whether post-destructive replenishment had visually occurred by two seconds during exercise CEU. NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
Heterogeneity of Flow Distribution
The spatial distribution of actively perfused microvascular units during exercise was assessed with CEU perfusion imaging using wavelet analysis of binary “flow, no-flow” data. In the PAD patient cohort, flow distribution by the smallest wavelet energy, which examines the finest resolvable detail, was less homogenous than in controls (p<0.001); however, there was only a non-significant trend for further differences between those with mild versus moderate-severe symptoms (Supplemental Figure 3).
Test- retest variability
In the 21 subjects who underwent repeat testing on a separate day, the degree of variability for rest and stress CEU perfusion imaging was low (Figure 7). When combined for both resting and exercise conditions, both CEU perfusion (Aβ) and microvascular blood flux rate (β) demonstrated very good interclass correlation coefficients (ICC 0.88 and 0.84, respectively). Most of the repeated measurements (approximately 70%) were within a factor of 2 of the measurements at baseline.
Figure 7.
Contrast-enhanced ultrasound test-retest variability measured on separate days. Relationships for test-retest measurements are shown for (A) microvascular blood flow and (B) microvascular flux rate. Data are combined for rest and stress, and are accordingly displayed on log-scale. The red line denotes the line-of-identity while the blue line represents the fitted regression line.
DISCUSSION
Non-invasive imaging for detecting myocardial ischemia has been used for decades in the management of patients with known or suspected coronary artery disease. These methods which rely on stress-rest assessment of regional perfusion or the functional consequences of ischemia (i.e. regional contractile function) are integrated into guidelines for patient care.19 In patients with known or suspected PAD, perfusion imaging is not routinely used. Lack of adoption of perfusion imaging in PAD can be explained by several obstacles for implementation, including: (i) the need for quantitative assessment of flow since simple assessment of regional heterogeneity in perfusion, such as with SPECT myocardial imaging, cannot be relied on in those with large artery PAD, (ii) difficulty acquiring quantitative data during exercise stress in those with claudication, (iii) inability to rely on vasodilator stress,9 and (iv) healthcare costs associated with new uses of imaging technologies in PAD management.
The approach used to image limb perfusion with CEU in this study addresses many of the concerns above. The destruction-replenishment tracer kinetic analysis provides quantitative data that have been previously validated.20 The combination of intermediate-power imaging with acoustically stable microbubbles generates robust blood pool signal and allows rapid completion of imaging, within 10–15 seconds during stress. Accordingly, imaging can be performed immediately upon cessation of exercise before resolution of hyperemia, even after the brief modest-intensity exercise that was used in this study. The brevity of these protocols are important for the ability to perform multi-level assessment of limb perfusion which may be important for angiosomal assessment of perfusion deficits.21
The primary aim of the study was to test whether perfusion imaging with CEU would provide information on the severity of disease in patients with PAD judged to be moderate by ABIs within the 0.4–0.6 range. The a priori selection of these patients was based on the wide variation in clinical status that is seen within this somewhat narrow range of ABIs, with symptoms that range from none to critical limb ischemia (CLI).22,23 There are many reasons why symptoms vary widely within a narrow ABI grouping. Pressure gradients may not accurately reflect tissue perfusion because of the confounding impact of small vessel disease, microvascular dysfunction and collateral flow. Also, the non-linear relationship between conduit arterial stenosis and pressure gradient is steep in this ABI range, and is influenced by flow.24 Hence, ABIs in a narrow range can be reflective of a wide variety of relationships between stenosis severity and resting flow.
Our data revealed that, quantitative CEU perfusion imaging during exercise not only differentiates normal controls from PAD subjects, but also discriminates between those with mild versus moderate to severe symptoms. Discrimination based on symptom class was possible even in the target group with ABI 0.4–0.6, although the p-value in this group was of borderline significance after applying advanced statistical methods to account for two possibly inter-related limbs in each individual. While all three of the parameters derived from CEU differentiated PAD limbs with reduced ABI from controls; only muscle perfusion (Aβ) and microvascular flux rate (β) varied according to symptoms. The finding that flow was influence more by β than microvascular blood volume is aligned with the concept that flow reduction from both conduit artery stenosis and microvascular dysfunction are a results of reduced pre-capillary driving pressures.25 Although we examined parameters of perfusion reserve (β-reserve and Aβ-reserve), we did not present these data since their predictive value was much lower owing to the fact that resting limb perfusion is very low so that very small absolute changes in resting perfusion lead to large changes in calculated reserve.
In the PAD population with claudication, limb perfusion at rest was not different between PAD subjects and controls, regardless of PAD symptom status. Instead, exercise perfusion was required. Yet the degree of exercise needed to achieve large increases in blood flow in control subjects, and to discriminate between PAD symptom classes was brief (30 seconds), modest, and well tolerated by all PAD subjects. We believe that only modest exercise was required only because it was possible to perform CEU perfusion imaging rapidly before any decay in exercise hyperemia. Abnormal flow in PAD patients was primarily attributable to the microvascular flux rate component of perfusion, which is consistent with current understanding that either atherosclerotic narrowing or microvascular dysfunction will reduce pre-capillary pressure.26
Interestingly, we found that exercise perfusion in limbs with completely normal ABIs from PAD patients was lower than that in normal subjects. This finding could be explained by the co-morbidities of PAD such as hyperlipidemia and diabetes mellitus that predispose to microvascular dysfunction, but probably not age since subject age does not influence perfusion when the degree of exercise is controlled (Supplemental Figure 4). It is possible that perfusion imaging is more sensitive than ABIs for detecting mild disease. This issue may be important since, while major organizations have differed regarding screening asymptomatic individuals, there is concensus that PAD is frequently under diagnosed with patients with exertional leg symptoms because of poor sensitivity of ABI in mild disease.27 For example, in a study of elderly patients (age >70), an ABI <0.9 was present in only 15–20% of limbs with disease defined by ≥50% stenosis by magnetic resonance angiography.28
Our evaluation of spatial distribution of perfusion was based on the notion that the adequacy of nutritive delivery to a tissue relies not only the movement of a volume of blood over a given time period but also on the spatial distribution of that flow across the tissue. One could imagine that if total muscle flow was equal between two limbs, there would be greater symptoms and potentially tissue loss for a limb where muscle blood flow was inhomogeneous, resulting in small areas of severe ischemia. Our wavelet analysis, however, did not provide any major discriminatory ability to explain differences in symptoms within the target ABI range. It is worth noting that other approaches for assessing flow distribution may fare better.
In myocardial contrast echocardiography perfusion imaging, qualitative analysis based on time-to-replenishment is often used to differentiate normal from abnormal perfusion. Accordingly, we analyzed images qualitatively according to whether contrast replenishment had occurred by 2 seconds. This threshold value was selected based on analysis of time-domain information from quantitative flux rate data. Qualitative analysis performed quite well with a diagnostic accuracy of 89% for identification of limbs as being from PAD versus control subjects, with most false negatives attributable to limbs of PAD subjects with completely normal ABI (>0.9).
Aside from its potential to impact clinical management of PAD, CEU could also be an asset in clinical trials of new PAD therapeutics, particularly those directed at the microvasculature. ABIs have been recognized to have limitations in quantifying physiologic changes despite the benefit of medical and physical therapies that improve claudication.5,29,30 The use of ABIs as an endpoint is particularly problematic in patients with non-compressible vessels or diabetic angiopathy. In this context, our test-retest data suggests that CEU perfusion imaging is reproducible despite known variation in skeletal muscle blood flow.31 It should be noted, however, that our selection of both the ultrasound contrast agent and intermediate mechanical index was based on a foundation of work that clearly demonstrated that quantitative data is inaccurate and reproducibility is low if contrast signal-to-noise ratio is low.13,32
There are several limitations of this study. The samples size for the PAD cohort was relatively small, largely because of our requirement for ABIs within a narrow range. We did not attempt to examine different degrees of exercise. Instead, we limited exercise to a regime that we thought would augment flow several fold but could also be performed in those with symptomatic PAD. Perfusion was also evaluated in only a single short axis view of the calf muscles (soleus and gastrocnemius) which may have potentially been misleading if hypo-perfusion in other areas of the limb musculature were the major source of symptoms with exercise. Because of this issue, we believe that future efforts should focus on a multilevel assessment of perfusion, even within the same muscle groups. We did not compare our CEU results to exercise ABI or to TBI, although we have previously demonstrated that CEU fares better than exercise ABI for symptom discrimination.8 Finally patients did not undergo a conventional vascular imaging study that would have allowed correlation with the vascular anatomy and location of stenoses.
In summary, we have demonstrated that CEU exercise perfusion imaging parameters in limbs of patients with PAD varies according to symptoms, thereby providing potentially important information on causes of symptom variation in this population. Our data also suggests that, in patients with PAD, exercise flow is abnormal in limbs with normal ABIs contralateral to those with moderately reduced ABIs, implying either superior sensitivity or the ability to detect microvascular impairment. While larger clinical trials may be needed to ascertain incremental benefit of CEU to standards of care in terms of patient outcomes, our study which included reproducibility data provides strong justification for using perfusion imaging as an endpoint for clinical trials evaluating benefit of new therapies targeted to patients with claudication.
Supplementary Material
Acknowledgments
FUNDING SOURCES
The study was sponsored by GE Healthcare and Pfizer, Inc. Dr. Moccetti is supported by a grant from the Swiss National Science Foundation. Dr. Lindner is supported by grants R01-HL078610, R01-HL130046, and P51-OD011092 from the National Institutes of Health, Bethesda, MD.
DISCLOSURES
This study was partially supported by a clinical research collaborative agreement with Pfizer, Inc. and by an investigator-initiated research grant from GE Healthcare.
ABBREVIATIONS AND ACRONYMS
- ABI
Ankle brachial index
- CEU
Contrast-enhanced ultrasound
- ICC
Intra-class correlation coefficient
- MBF
Microvascular blood flow
- PAD
Peripheral artery disease
- WIQ
Walking impairment questionnaire
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, et al. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol (1985). 2011;111:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marso SP and Hiatt WR. Peripheral Arterial Disease in Patients With Diabetes. J Am Coll Cardiol. 2006;47:921–929. [DOI] [PubMed] [Google Scholar]
- 3.Jain A, Liu K, Ferrucci L, Criqui MH, Tian L, Guralnik JM, et al. The Walking Impairment Questionnaire stair-climbing score predicts mortality in men and women with peripheral arterial disease. J Vasc Surg. 2012;55:1662–73.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart KJ, Hiatt WR, Regensteiner JG and Hirsch AT. Exercise training for claudication. N Engl J Med. 2002;347:1941–51. [DOI] [PubMed] [Google Scholar]
- 5.Szuba A, Oka RK, Harada R and Cooke JP. Limb hemodynamics are not predictive of functional capacity in patients with PAD. Vasc Med. 2006;11:155–63. [DOI] [PubMed] [Google Scholar]
- 6.Stein R, Hriljac I, Halperin JL, Gustavson SM, Teodorescu V and Olin JW. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vasc Med. 2006;11:29–33. [DOI] [PubMed] [Google Scholar]
- 7.Kinlay S Outcomes for clinical studies assessing drug and revascularization therapies for claudication and critical limb ischemia in peripheral artery disease. Circulation. 2013;127:1241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindner JR, Womack L, Barrett EJ, Weltman J, Price W, Harthun NL, et al. Limb stressrest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging. 2008;1:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson BP, Belcik JT, Landry G, Linden J and Lindner JR. Exercise versus vasodilator stress limb perfusion imaging for the assessment of peripheral artery disease. Echocardiography. 2017;34:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duerschmied D, Zhou Q, Rink E, Harder D, Freund G, Olschewski M, et al. Simplified contrast ultrasound accurately reveals muscle perfusion deficits and reflects collateralization in PAD. Atherosclerosis. 2009;202:505–12. [DOI] [PubMed] [Google Scholar]
- 11.Amarteifio E, Wormsbecher S, Krix M, Demirel S, Braun S, Delorme S, et al. Dynamic contrast-enhanced ultrasound and transient arterial occlusion for quantification of arterial perfusion reserve in peripheral arterial disease. Eur J Radiol. 2012;81:3332–8. [DOI] [PubMed] [Google Scholar]
- 12.Meneses AL, Nam MCY, Bailey TG, Magee R, Golledge J, Hellsten Y, et al. Leg Blood Flow and Skeletal Muscle Microvascular Perfusion Responses to Submaximal Exercise in Peripheral Arterial Disease. Am J Physiol Heart Circ Physiol. 2018. [DOI] [PubMed] [Google Scholar]
- 13.Davidson BP, Hodovan J, Belcik JT, Moccetti F, Xie A, Ammi AY, et al. Rest-Stress Limb Perfusion Imaging in Humans with Contrast Ultrasound Using Intermediate-Power Imaging and Microbubbles Resistant to Inertial Cavitation. J Am Soc Echocardiogr. 2017;30:503–510 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regensteiner JGSJ, Panzer RJ, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. Journal of Vascular Medicine Biology. 1990;2:142–152. [Google Scholar]
- 15.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, et al. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. American Journal of Physiology - Endocrinology and Metabolism. 2002;282:E714E720. [DOI] [PubMed] [Google Scholar]
- 16.Davidson BP, Belcik JT, Mott BH, Landry G and Lindner JR. Quantification of residual limb skeletal muscle perfusion with contrast-enhanced ultrasound during application of a focal junctional tourniquet. J Vasc Surg. 2016;63:148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szczypiński PM, Strzelecki M, Materka A and Klepaczko A. MaZda—A software package for image texture analysis. Comput Methods Programs Biomed. 2009;94:66–76. [DOI] [PubMed] [Google Scholar]
- 18.Szczypinski PM, Strzelecki M and Materka A. Mazda - a software for texture analysis. 2007 International Symposium on Information Technology Convergence (ISITC 2007). 2007:245–249. [Google Scholar]
- 19.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:e354–471. [DOI] [PubMed] [Google Scholar]
- 20.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM and Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–83. [DOI] [PubMed] [Google Scholar]
- 21.Kret MR, Cheng D, Azarbal AF, Mitchell EL, Liem TK, Moneta GL, et al. Utility of direct angiosome revascularization and runoff scores in predicting outcomes in patients undergoing revascularization for critical limb ischemia. J Vasc Surg. 2014;59:121–8. [DOI] [PubMed] [Google Scholar]
- 22.Lozano FS, March JR, Gonzalez-Porras JR, Carrasco E, Lobos JM and Ros E. Relative value of the Ankle-Brachial Index of intermittent claudication. Int J Clin Pract. 2014;68:147882. [DOI] [PubMed] [Google Scholar]
- 23.Sukul D, Grey SF, Henke PK, Gurm HS and Grossman PM. Heterogeneity of AnkleBrachial Indices in Patients Undergoing Revascularization for Critical Limb Ischemia. JACC Cardiovascular interventions. 2017;10:2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bragadeesh T, Sari I, Pascotto M, Micari A, Kaul S and Lindner JR. Detection of peripheral vascular stenosis by assessing skeletal muscle flow reserve. J Am Coll Cardiol. 2005;45:780–5. [DOI] [PubMed] [Google Scholar]
- 25.Kaul S and Jayaweera AR. Myocardial capillaries and coronary flow reserve. J Am Coll Cardiol. 2008;52:1399–401. [DOI] [PubMed] [Google Scholar]
- 26.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. [DOI] [PubMed] [Google Scholar]
- 27.Wikström J, Hansen T, Johansson L, Ahlström H and Lind L. Lower extremity artery stenosis distribution in an unselected elderly population and its relation to a reduced anklebrachial index. J Vasc Surg. 2009;50:330–334. [DOI] [PubMed] [Google Scholar]
- 28.Wikström J, Hansen T, Johansson L, Lind L and Ahlström H. Ankle brachial index <0.9 underestimates the prevalence of peripheral artery occlusive disease assessed with whole-body magnetic resonance angiography in the elderly. Acta Radiol. 2008;49:143–149. [DOI] [PubMed] [Google Scholar]
- 29.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatr Soc. 2001;49:755–62. [DOI] [PubMed] [Google Scholar]
- 30.Hiatt WR, Hirsch AT, Regensteiner JG and Brass EP. Clinical trials for claudication. Assessment of exercise performance, functional status, and clinical end points. Vascular Clinical Trialists. Circulation. 1995;92:614–21. [DOI] [PubMed] [Google Scholar]
- 31.Goh V, Halligan S, Hugill JA and Bartram CI. Quantitative assessment of tissue perfusion using MDCT: comparison of colorectal cancer and skeletal muscle measurement reproducibility. AJR Am J Roentgenol. 2006;187:164–9. [DOI] [PubMed] [Google Scholar]
- 32.Seol SH, Davidson BP, Belcik JT, Mott BH, Goodman RM, Ammi A, et al. Real-time contrast ultrasound muscle perfusion imaging with intermediate-power imaging coupled with acoustically durable microbubbles. J Am Soc Echocardiogr. 2015;28:718–26 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.