Abstract
Acquisition of cell polarity generates signaling and cytoskeletal asymmetry and thus underpins polarized cell behaviors during tissue morphogenesis. In epithelial tissues, both apical basal polarity and planar polarity, which refers to cell polarization along an axis orthogonal to the apical-basal axis, are essential for epithelial morphogenesis and function. A prime example of epithelial planar polarity can be found in the auditory sensory epithelium (or organ of Corti, OC). Sensory hair cells, the sound receptors, acquire a planar polarized apical cytoskeleton which is uniformely oriented along an axis orthogonal to the longitudinal axis of the cochlear duct. Both cell-intrinsic and tissue-level planar polarity are necessary for proper perception of sound. Here we review recent insights into the novel roles and mechanisms of planar polarity signaling gained from genetic analysis in mice, focusing mainly on the OC but also with some discussions on the vestibular sensory epithelia.
Keywords: Planar Cell Polarity, stereocilia, kinocilium, hair bundle, hair cell, cochlea, deafness
A. Intercellular PCP signaling in cochlear morphogenesis
Located in the cochlea, the OC consists of one row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs) interdigitated by intervening supporting cells (SCs), forming a highly stereotyped cellular mosaic that extend along the longitudinal axis of the cochlea. Each hair cell (HC) has an actin-based stereociliary hair bundle on its apical surface that converts sound vibrations into electrical signals [81, 93]. HCs manifest several salient features of planar polarity (Figure 1). First, on a cell-intrinsic level, the hair bundle adopts a polarized structure with rows of stereocilia arranged in a V-shaped staircase pattern and is thus directionally sensitive to mechanical stimuli [81, 93]. On a tissue level, asymmetric hair bundles are uniformly aligned along the medial-lateral (or neural-abneural) axis of the OC, pointing toward the lateral edge of the cochlea duct, and thus respond only to deflections along the medial-lateral axis. The tissue-wide alignment of HC orientation is referred to as planar cell polarity (PCP).
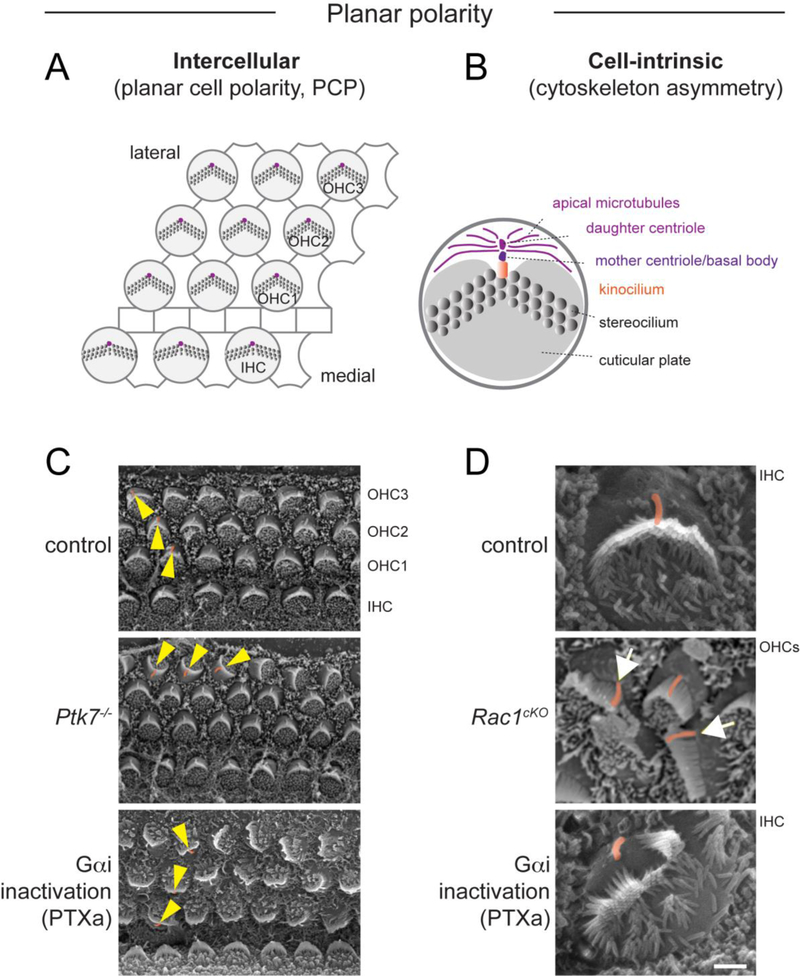
Figure 1. Two levels of planar polarity in the organ of Corti.
A) Diagram depicting tissue-level, intercellular planar cell polarity (PCP), manifested by the uniform orientation of HCs along the medial-lateral axis of the cochlear duct. PCP is regulated by intercellular communication through asymmetric core PCP protein complexes at HC-SC junctions. B) By contrast, cell-intrinsic planar polarity refers to cytoskeleton asymmetry along the planar axis in individual HCs. Different structures displaying intracellular planar polarity are indicated. This behavior involves protein modules acting cell-intrinsically. C) Scanning electron microscopy (SEM) images showing PCP defects in representative mouse mutants. Yellow arrowheads indicate OHC orientation. D) SEM images showing cell-intrinsic planar polarity defects in representative mouse mutants. The kinocilium is highlighted in orange. Note OHCs in Rac1 mutants with off-center kinocilium relative to an abnormally flat hair bundle (arrows). PTXa mutants show IHCs where the kinocilium is disconnected from a split hair bundle. Ptk7 mutants strictly exhibit orientation (PCP) defects, whereas Rac1 and PTXa mutants exhibit both orientation and cell-intrinsic planar polarity defects. PTXa mutants have Cre-induced expression of the catalytic subunit of Pertussis toxin (PTXa) in HCs. Ptk7 and Rac1 SEM images are modified from [36, 66].
A1. Regulation of cochlear extension and hair cell orientation
The OC develops from a pool of progenitor cells in the prosensory domain expressing Sox2 and p27kip1 (reviewed in [76]). Following cell cycle exit at around embryonic day (E) 14, prosensory cells undergo myosin II-dependent cellular rearrangements resulting in thinning and elongation of the OC [20, 112], and HC differentiation begins around E15 and proceeds in a base-to-apex gradient along the cochlear duct. The first physical evidence of planar polarity at the HC apex is the centrifugal migration of the HC primary cilium, the kinocilium, and its associated basal body towards the lateral pole of the cell [18, 74, 104] (Figure 2A). This is followed by the growth of neighboring microvilli into stereocilia, and nascent V-shaped hair bundles form by E17, with the kinocilium tethered to adjacent stereocilia at the vertex. During the same time period, neighboring HCs adopt a similar orientation to align their kinocilium and nascent hair bundle along the medial-lateral axis. This manifestation of PCP is likely influenced by “tug of war” interactions between HCs and SCs as a result of active cellular movements in the OC. This notion is supported by the identification of three major intercellular signaling pathways that act in concert in both HCs and SCs to coordinate HC orientation and control cellular patterning in the OC.
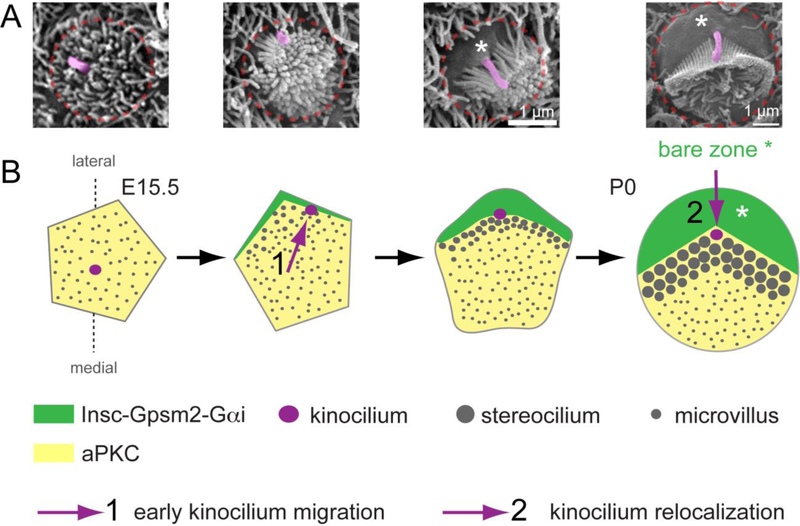
Figure 2. A molecular blueprint for planar polarization of the apical cytoskeleton.
A) SEM images of individual OHCs representative of different stages of apical differentiation. The kinocilium is highlighted in pink and the approximate OHC junction indicated in red. B) Diagram depicting changes at the HC apex from the onset of differentiation (E15.5, left) to around birth (P0, right). Initially, the aPKC kinase is uniformly enriched at the apical membrane, which is covered with microvilli, and the kinocilium occupies a central position. The first morphological evidence of planar asymmetry is the approximately lateral position of the kinocilium, which occurs at about the time the Insc-Gpsm2-Gαi complex becomes planar polarized at the lateral aspect of the cell. The Insc-Gpsm2-Gαi complex expands in surface area and labels the bare zone, the lateral region of apical membrane devoid of stereocilia or microvilli (asterisks). Insc-Gpsm2-Gαi prevents aPKC enrichment at the bare zone, establishing a molecular blueprint at the apical membrane that helps position and coordinate the hair bundle and the kinocilium. The expansion of the bare zone coincides with a relocalization of the kinocilium, from its post-migration position juxtaposed to the lateral junction to a more central position at the vertex of the chevron-shaped hair bundle around birth.
A1.1. The core PCP pathway
Ground-breaking discoveries in 2003 [12, 77] followed by numerous studies have shown that the evolutionarily conserved core PCP pathway regulates OC patterning by coordinating HC orientation in inner ear sensory epithelia. Mammalian core PCP proteins comprise orthologs of Drosophila Frizzled (Fzd3 and Fzd6) [109], Van Gogh (Vangl1–2) [11, 97, 98], Flamingo (Celsr1–3) [12, 22], Dishevelled (Dvl1–3) [24, 108], Prickle (Pk1–2) [16] and Diego (Ankrd6) [48]. Core PCP mutants have severe neural tube closure defect and often die at birth. In inner ear sensory epithelia, the uniform orientation of hair bundles is disrupted, although the asymmetry of the apical cytoskeleton including the polarized structure of the hair bundle appears unaffected. Consistent with a role in cellular rearrangements during OC extension, the cochlear ducts of core PCP mutants are shorter, with HCs in the apex organized into supernumerary rows (reviewed in [34, 67]). Similar to other systems, core PCP proteins form two complexes asymmetrically localized along the medial-lateral axis in both HCs and SCs to propagate tissue polarity information across the entire OC. Another conserved feature of intercellular PCP signaling is the cell non-autonomous function of transmembrane core PCP proteins. Mosaic analysis in the Drosophila wing epithelium showed that Van Gogh and Frizzled mutant clones can repolarize neighboring wild-type cells, a phenomenon coined “domineering non-autonomy” (reviewed in [34]). A recent study showed that Vangl1/2 deletion in the lateral extrastriolar region of the utricle, one of the vestibular sensory organs, can likewise cause misorientation in neighboring striolar wild-type HCs [98].
Besides conserved functional properties, it is worth noting that there are also intriguing differences that hint at functional divergence of core PCP signaling in the OC. For example, Drosophila Van Gogh and Frizzled proteins are enriched on opposite sides of cell junctions where they form intercellular bridges stabilized by Flamingo on both sides. Intracellularly, Frizzled is thought to regulate Rho family GTPases through membrane recruitment of the cytoplasmic scaffold protein Dishevelled. In the OC, Dvl2/3 proteins are asymmetrically localized along the lateral junctions of OHCs [24, 108]. However, Fz3 and Fz6 are enriched on the medial junctions of HCs [109], where they have been proposed to interact with Vangl2 that is asymmetrically enriched at the lateral junction of the neighboring SC [33]. Dvl2 localization on lateral membranes of HCs has been independently demonstrated by electroporation of a Dvl2-EGFP plasmid in cochlear explants [94], while Fzd6 localization on the medial membranes of HCs was demonstrated using genetic mosaics [109]. Phenotypically, Fz3/Fz6 double mutants showed reversal of hair bundle orientation in IHCs without significantly affecting OHCs, whereas Vangl1/2 double mutants and Dvl compound mutants affect both IHCs and OHCs. [24, 97, 109]. These discrepancies in protein localizations and mutant phenotypes suggest that additional Fzd receptors might be involved in PCP in the OC. Fzd2/7 are likely candidates as they have redundant functions in convergent extension during gastrulation, genetically interact with core PCP genes [114] and are expressed in the cochlea, although their protein localizations are unknown [30].
A1.2. A vertebrate specific, Ptk7-mediated PCP pathway
Protein tyrosine kinase 7 (Ptk7) was identified as a vertebrate-specific regulator of PCP signaling [66]. Similar to core PCP mutants, Ptk7 deficiency causes a wide spectrum of developmental defects, including OHC3 misorientation [66, 84, 113]. Ptk7 encodes a conserved receptor-like pseudo-kinase that lacks endogenous kinase activity. In the developing OC, Ptk7 acts in parallel to the core PCP pathway and is required in SCs for hair bundle orientation [59]. Mechanistically, Ptk7 signals in part through Src family kinases (SFKs) to regulate actomyosin contractility and intercellular tension [1, 59]. Unlike core PCP proteins, Ptk7 is uniformly localized at cell junctions, and thus may act as a permissive factor or partner with other PCP regulators. The ligands for Ptk7 in the OC remain unknown.
A1.3. Nectin- and Notch-mediated cell adhesion signaling
Nectins are Ca2+-independent IG-like cell-adhesion molecules that interact in trans to induce intercellular adhesion. The cellular mosaic in the OC is mediated by heterotypic interactions between Nectin-1 and −3, which are differentially expressed in HCs and SCs, respectively [105]. Moreover, in Nectin-3 deficient mice, some HCs are aberrantly in contact with one another, accompanied by hair bundle morphology and orientation defects [28]. The PCP pathways do not overtly affect heterotypic HC-SC adhesion, suggesting that Nectin-mediated cell adhesion signaling is independent of the PCP pathways.
Notch signaling also mediates HC-SC interactions, and some Notch ligand and receptor mutants display patterning defects in line with their role in cell fate regulation, including abnormal contact between HCs. Perhaps as a consequence, these mutants also display hair bundle orientation and shape defects [52, 58, 119].
It is worth noting that defective Nectin and Notch signaling disrupts both hair bundle orientation and shape, possibly indicating a role in both cell-intrinsic and tissue-level planar polarity. How Nectin and Notch signaling regulates HC planar polarity at a molecular level remains to be determined, but likely involves cross-talk with neighboring cells and the recruitement of intrinsic polarity modules in HCs (see section B2).
A1.4. HC reorientation during normal and abnormal development.
Presumably because the cuticular plate that anchors the hair bundle is initially loosely organized [85], the initial orientation of nascent hair bundles is imperfect and undergoes continuous refinement of up to 30 degrees during embryonic and postnatal development. This process might involve Wnt signaling based on in vitro evidence [13]. In principle, core PCP proteins might participate in normal refinement, as asymmetric core PCP protein localization is maintained after birth [11, 95]. However, OHC3 hair bundles in a viable conditional knockout (cKO) for Vangl2 mediated by Pax2-Cre were able to correct their initial misorientation and reorient by as much as ~180 degrees at postnatal stages, except in the apex region of the cochlea [11]. As asymmetric core PCP complexes were disrupted in the Vangl2 cKO cochlea, the core PCP pathway is dispensable or acts redundantly for the correction process. Consequently, it is likely that a still undefined molecular mechanism is involved in postnatal HC reorientation.
A2. Additional roles of PCP signaling in the cochlea
A2.1. Roles in SC cytoarchitecture and type II SGN afferent guidance.
SCs in the OC not only propagate apical PCP information to orient neighboring HCs, but also have a planar polarized cytoarchitecture themselves. There are several types of SCs in the OC, including the inner phalangeal cells, the inner and outer pillar cells and the Deiters’ cells. During cochlear extension, the OC thins from a pseudo-stratified epithelium to a two-layer stratified epithelium with the nuclei of SCs below those of HCs (reviewed in [76]). On the luminal surface, HCs are separated from one another by phalangeal processes projected from the cell body of SCs. Interestingly, the phalangeal processes of outer pillar cells and Deiters’ cells show a slant toward the cochlear apex, contacting OHCs located several cell bodies away along the longitudinal axis [47] (Figure 3). In Vangl2 cKO mutants, the apically-directed slant of the Deiters’ phalangeal processes was lost, accompanied by disorganized HC-SC contacts [11]. Core PCP signaling is therefore involved in the longitudinal mechanical coupling between Deiters’ cells and OHCs, a property essential for OHC electromotility and cochlear amplification [80, 115]. Accordingly, Vangl2 cKO mutants had hearing deficits as shown by elevated ABR thresholds [11].
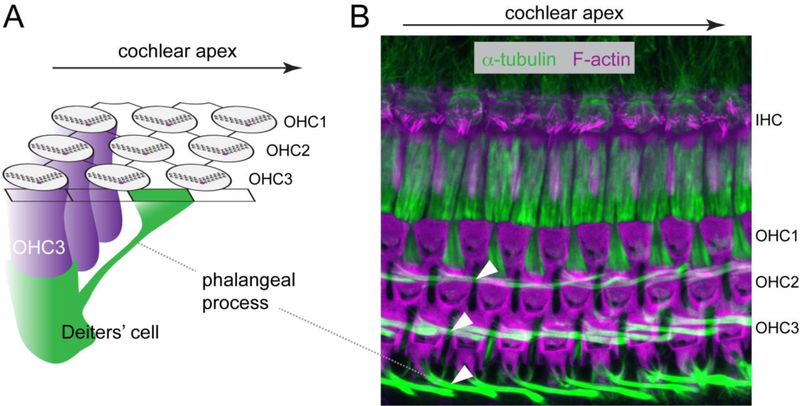
Figure 3. Deiters’ cell phalangeal processes are planar polarized along the longitudinal axis of the cochlea.
A) Diagram depicting one Deiters’ cell (green) that extends a slanted phalangeal process towards the luminal surface further towards the cochlear apex compared to its cell body position. B) A maximum-intensity projection of confocal Z stacks of a P18 cochlea whole-mount stained for F-actin (magenta) and alpha-tubulin (green). Note the planar polarized phalangeal processes of the three rows of Deiters’ cells (arrowheads). Arrows indicate the direction of the cochlear apex.
Besides regulating their cytoarchitecture, PCP signaling in SCs is increasingly recognized to polarize other processes during cochlear development. Early studies in cochlear explants had shown that cochlear extension is unidirectional from the base to the apex and requires myosin II activity [108, 112]. Recent time-lapse imaging experiments showed that SCs, but not HCs, extend dynamic protusions along the basement membrane and migrate toward the cochlear apex, thus providing a cellular basis for the unidirectional extension [20]. Interestingly, Vangl2 is asymmetrically localized at the SC-SC baso-lateral contacts along the longitudinal axis [32], parallel to the trajectory of SC movements and orthogonal to its medial-lateral polarization at apical HC-SC junctions, raising the possibility that SC directional migration is regulated by core PCP signaling. While this remains to be determined, a recent study uncovered a novel, non-autonomous role of PCP signaling in SCs to guide type II spiral ganglion neurons (SGN) afferents [32].
Each SGN extends a peripheral process toward the OC and a central process that branches into the cochlear nuclei. The most abundant type I SGNs form ribbon-like synaptic contacts exclusively at the single row of IHCs. In the remaining 5–10% type II SGNs, the peripheral process crosses the tunnel of Corti into the Deiters’ cell region and turns towards the cochlear base, extending several hundred micrometers to innervate several OHCs [2, 10]. Unlike type I afferents, type II do not encode sound information but are thought to detect acoustic trauma [64]. When Vangl2 was deleted in the cochlear epithelium using Emx2-Cre, type II SGN afferent turning appeared randomized, and about 50% of fibers turned erroneously towards the cochlear apex. By contrast, deletion of Vangl2 in SGNs did not cause turning defects, establishing its non-autonomous role in SCs. Similar but milder SGN afferent turning defects were recorded in Fzd3 and Celsr1 null mutants. Vangl2 is asymmetrically enriched on the SC basolateral surface facing towards the cochlear apex, where it is proposed to recruit and stabilize type II growth cones turning towards the base. It remains unclear whether complementary core PCP complexes mediate growth cone-epithelial substrate interactions, as reported for anterior guidance of spinal commissural axons and migration of hindbrain facial branchiomotor neurons (reviewed in [14]).
A2.2. PCP signaling in Kolliker’s organ orients the apical extracellular matrix
Outside the OC, PCP signaling in a subset of cells in Kolliker’s organ was recently shown to orient the overlying tectorial membrane. The tectorial membrane is a specialized apical extracellular matrix structure secreted by epithelial cells in the ventral cochlear duct, and extending over the OC to contact the hair bundles of OHCs (reviewed in [29]). Collagen fibrils in the tectorial membrane are oriented radially along the medial-lateral axis with a slant towards the cochlear apex, a spatial organization participating in auditory function. Interestingly, Pk2 is asymmetrically localized at apical junctions along the longitudinal axis in Kolliker’s organ, orthogonal to its enrichment at apical SC-HC junctions, but parallel to Vangl2 enrichment at basolateral SC-SC junctions. Intriguingly, Pk2 polarized enrichment is only observed in a band of cells whose radial junctions are aligned with the same apically-directly slant as the overlying collagen fibrils. Moreover, the apically-directed slant of collagen fibrils, as well as asymmetric Pk2 localization, were abolished in Vangl2 and Ptk7 cKO mutants, indicating a requirement for PCP signaling in the planar orientation of collagen fibrils within the tectorial membrane [35]. In summary, core PCP proteins are widely expressed in the developing cochlear epithelium, and their planar polarized distribution serves as a “compass” to orient a range of polarized cellular processes. At junctions between non-sensory cells, core PCP proteins are polarized along the longitudinal axis, orthogonal to the medial-lateral (radial) axis of PCP at HC-SC junctions. In every case, the observed PCP features correspond to the local vector of tissue polarity.
B. Establishment of hair cell-intrinsic planar polarity
Even the most severe PCP mutants [77, 97, 109] retain a normal planar polarization of the apical cytoskeleton in individual HCs at birth. This includes a peripheral basal body/kinocilium situated at the vertex of the V-shaped hair bundle edge, a heart-shaped cuticular plate stabilizing the hair bundle, and apical microtubules (MTs) confined laterally by the cuticular plate (Figure 1B). Therefore, intercellular PCP signaling is not required for planar polarization of individual HCs. In the last decade, several signaling modules have been identified and likely act in concert to control planar asymmetry of the cytoskeleton in single HCs.
B1. Influence of the basal body and kinocilium on hair bundle morphogenesis
Hair bundle development is both temporally and spatially coupled to the lateral placement of the kinocilium and its associated basal body. The kinocilium recedes around postnatal day (P) 9 when hair bundle maturation is close to complete, and is absent in mature auditory HCs.
B1.1. A microtubule-based mechanism for basal body/kinocilium placement
The lateral migration of the basal body is required to establish the asymmetric shape of the hair bundle, as illustrated by the circular bundles observed in a small fraction of Ift88 mutant HCs where the basal body failed to migrate [49]. While the mechanisms that trigger the early centrifugal migration of the kinocilium are still under debate (see sections B3, F2), accumulating evidence indicates that the final lateral basal body/kinocilium placement is controlled by a MT-based machinery (Figure 1B). The basal body anchors both the kinocilium and the apical MTs, and its placement was proposed to be mediated by interactions between the dynamic plus ends of apical MTs and the HC cortex at the lateral junction. First, the plus end tracking protein EB1 is enriched near the lateral HC junction [26]. Second, proper basal body placement requires both plus-end and minus-end directed MT motors. In addition to its role in intraflagellar transport, the kinesin-II subunits Kif3a mediates coupling between the hair bundle and the basal body as well as cortical Rac1 GTPase signaling via MT-mediated intracellular transport [96]. Deletion of Lis1, an activator of cytoplasmic dynein, disrupted MT organization and cortical attachment, leading to basal body placement and hair bundle defects [95]. These results indicate that dynein-mediated cargo transport and/or pulling forces on apical MTs play a critical function in basal body/kinocilium placement. In contrast, early basal body migration was unaffected by Kif3a or Lis1 inactivation. Lastly, several planar polarized signaling modules appear to stabilize MT-cell cortex interactions to anchor the basal body at the lateral pole, as described in Section B2.
B1.2. A role for the kinocilium and Usher syndrome type I (Ush1) proteins
As a signaling organelle physically attached to the hair bundle (Figure 1B, 2A) the kinocilium may play both a signaling and mechanical role during hair bundle morphogenesis. Its importance was first demonstrated by inactivating genes required for intraflagellar transport, such as Ift88 and Kif3a, thereby preventing ciliogenesis [49, 96]. The mechanical role of the kinocilium was then demonstrated by specifically ablating the CD2 isoform of Pcdh15, a component of the kinociliary links that tether the kinocilium to neighboring stereocilia [111]. In the absence of the kinocilium or kinociliary links, hair bundles lost their chevron shape and had a flattened morphology. On rare occasions, circular hair bundles either with a central or peripheral basal body were also observed [49, 111]. However, most HCs still formed a planar polarized hair bundle in the absence of a kinocilium, even when the remaining basal body was spatially uncoupled from the forming hair bundle [49, 96]. This challenges the popular but unproven assumption that following its migration, the kinocilium somehow prompts neighboring microvilli to grow into stereocilia. Interestingly, core PCP protein localization was largely normal in Ift88, Kif3a and Pcdh15-CD2 mutants, suggesting that core PCP signaling operates independently of the kinocilium to position stereocilia. Of note, trafficking of Pcdh15-CD2 to the kinocilium was recently shown to be mediated by DAB2/clathrin/Ift-B transport particles and regulated by ciliary Fgfr1 signaling, providing one of the first examples of signaling functions of the kinocilium in HC planar polarity [41].
Ciliopathies are developmental disorders generally associated with defects in primary cilia. Accordingly, a number of mouse models for human ciliopathies exhibit a variable spectrum of hair bundle and kinocilium defects, including Mkks/Bbs6, Bbs4 and Alms1 mutants [26, 42, 89]. Importantly however, some ciliopathy proteins were found to have non-ciliary functions that also influence HC planar polarity. For example, unique among Ift and Bbs proteins, Bbs8 and Ift20 were shown to interact with the core PCP protein Vangl2 and to regulate its polarized localization, presumably through MT-based trafficking [73]. In addition, many ciliopathy gene products localize to or around the basal body, and may therefore regulate basal body/centrosome-associated functions such as proteasomal degradation and endosomal trafficking [31, 39].
Besides Pcdh15, other Usher syndrome 1 (Ush1) proteins, including Cdh23, the scaffold proteins harmonin and SANS, myosin VIIa, and the calcium and integrin-binding protein CIB2, are also important for hair bundle structure and kinocilium positioning. In addition to kinociliary links, heterophilic Pcdh15 and Cdh23 adhesion complexes form various inter-stereocilia links that maintain hair bundle cohesion during HC differentiation [60, 111]. Therefore, it is tempting to speculate that kinocilium and hair bundle positioning are regulated in a reciprocal manner; as a cohesive unit, the hair bundle may also regulate the final position of the basal body/kinocilium by pulling on kinociliary links. In Ush1 mutants, this reciprocal regulation is disrupted, leading to planar polarity defects.
B2. Several signaling modules act in concert during HC planar polarization.
While ciliary and Ush1 proteins influence the structural polarity of the hair bundle, they are not themselves planar polarized in HCs. By contrast, several signaling modules with a planar polarized distribution have been identified and likely play a more instructive and broader role in establishing HC cytoskeleton planar polarity, as discussed below.
B2.1. The Par3-Rac1-Pak module
The small GTPase Rac1 and its effector Pak were among the first HC-intrinsic planar polarity regulators identified [36]. Cortical Rac-Pak signaling activity is asymmetrically enriched along the lateral HC junction, where it was proposed to preferentially stabilize interactions between the apical MTs and the HC junction, thereby controlling kinocilium placement and hair bundle shape [36, 96].
How is the polarized domain of Rac signaling established? A recent study revealed a critical function of the PDZ-domain scaffold protein Par3, a conserved regulator of cell polarity [57, 69]. In epithelial cells, Par3 helps define the apical domain by forming a tripartite complex with Par6 and the kinase aPKC. The complex occupies apical cell junctions, while aPKC-Par6 but not Par3 are also enriched at the apical membrane [37, 38, 71, 78]. In HCs, Par6 and aPKC are planar polarized on the medial side while Par3 is polarized on the lateral side at the onset of hair bundle formation [26, 57, 102]. Par3 was shown to have both cell-autonomous and non-autonomous functions and to stimulate Rac1 signaling in both HCs and SCs through interactions with the guanine nucleotide exchange factors (GEFs) Tiam1 and Trio. Strikingly, expression of constitutively active Rac1 rescued most, if not all, planar polarity defects in Par3 cKO, arguing that the key function of Par3 in HC planar polarity is to regulate Rac1 signaling [57]. As a scaffold protein, Par3 can interact with multiple planar polarity regulators and thereby likely participates in signal integration (see Section C).
B2.2. The cdc42-aPKC-Par6 module
Cdc42 is another member of the Rho family GTPases with conserved functions in cell polarity and cytoskeletal regulation. Inactivation of Cdc42 starting at E13.5 in the OC resulted in defects in OHC patterning, MT organization and hair bundle orientation, indicating a requirement of Cdc42 for HC planar polarity. In Cdc42 mutants, the asymmetric localization of aPKC, a known effector of Cdc42, was misoriented in register with the hair bundle, while asymmetric localization of core PCP proteins was unaffected [54]. Although their function in HCs has not been reported, Par6 and aPKC have a similar medial localization in HCs [26] and thus likely form a Cdc42/aPKC/Par6 module.
B2.3. The Insc-Gpsm2-Gαi module establishes a molecular blueprint for hair cell planar polarity
Gpsm2 (also known as LGN) stands for G protein signaling modulator 2, and Gαi is the inhibitory alpha subunit of the heterotrimeric G protein complex. Along with partners, GPSM2-Gαi serves as an evolutionary conserved cortical complex polarizing pulling forces that orient the mitotic spindle during cell division [79]. Mechanistically, Gαi binds Gpsm2 and is myristoylated to anchor the complex at the plasma membrane, while Gpsm2 recruits other partners including Numa (Fig. 4A). Numa in turn recruit effectors including the dynein motor to pull on astral MTs. The net result is control over the spindle axis, daughter cell placement and thus tissue architecture. Biochemically, Gpsm2 is thought to act as a G protein dissociation inhibitor (GDI), sequestering Gαi in a GDP-bound form that is usually considered to be an inactive signaling state.
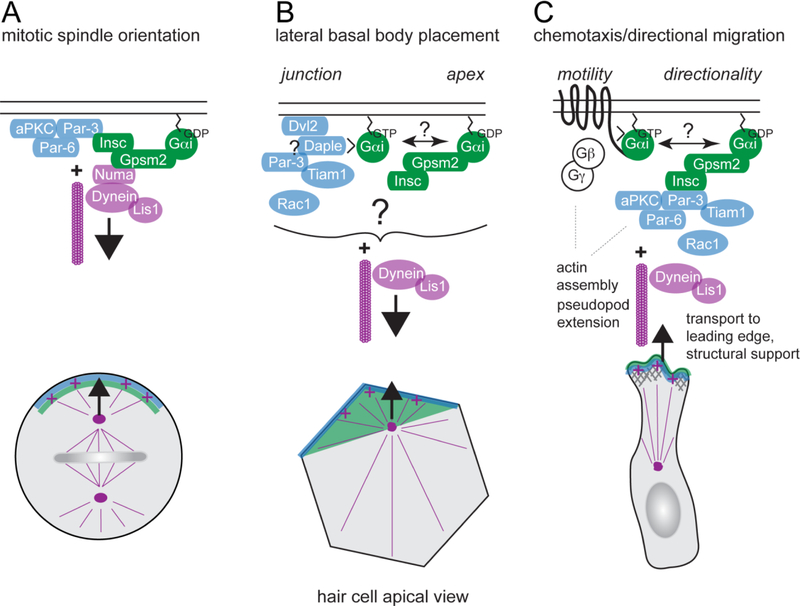
Figure 4. Shared protein modules regulate HC planar polarity and other polarized processes.
A) During oriented cell division, the Gpsm2-Gαi complex, and in some cases the Par3-Par6-aPKC complex, are polarized at the cell cortex and recruit effectors to pull on astral microtubules and position the mitotic spindle. Gαi is myristoylated and anchored at the membrane. Gpsm2-Gαi can interact with the Par3 complex through binding to the Insc adapter, or recruit the minus-end microtubule motor dynein through the Numa adapter. Lis1 is an activator for dynein required for spindle orientation. This evolutionary conserved process controls daughter cell positioning and tissue architecture across multiple tissues. B) During HC planar polarization, dynein-mediated pulling forces on apical microtubules may play a role in the lateral placement of the basal body. Junctional proteins including Dvl2, Daple and Par3, may serve as cortical dynein tethers to anchor pulling forces, and/or stabilize microtubule cortical attachment by activating Rac-Pak signaling. The Insc-Gpsm2-Gαi complex provides a blueprint on the HC apical membrane, and may also plays a role in basal body placement. C) During chemotaxis, for example in neutrophils, motility requires G protein coupled receptors (GPCRs) to sense extracellular cues in the environment and activate heterotrimeric G proteins. Gβγ dissociated from activated, GTP-bound Gαi in turn activates downstream effectors to regulate actin assembly and pseudopod extension at the leading edge. In addition to canonical GPCR signaling, it has been proposed that Gαi-GDP bound to Gpsm2 might more specifically regulate sustained directionality by signaling through Insc and Par3. In other cell types, sustained directionality was shown to require microtubules and Lis1/dynein to regulate transport and polarized activation of signaling molecules such as Rac1 GTPase. It is interesting to speculate that control over Gαi guanine nucleotide exchange involving GEF proteins like GPCRs or Daple might ultimately interconnect different polarity modules that act in concert in HCs.
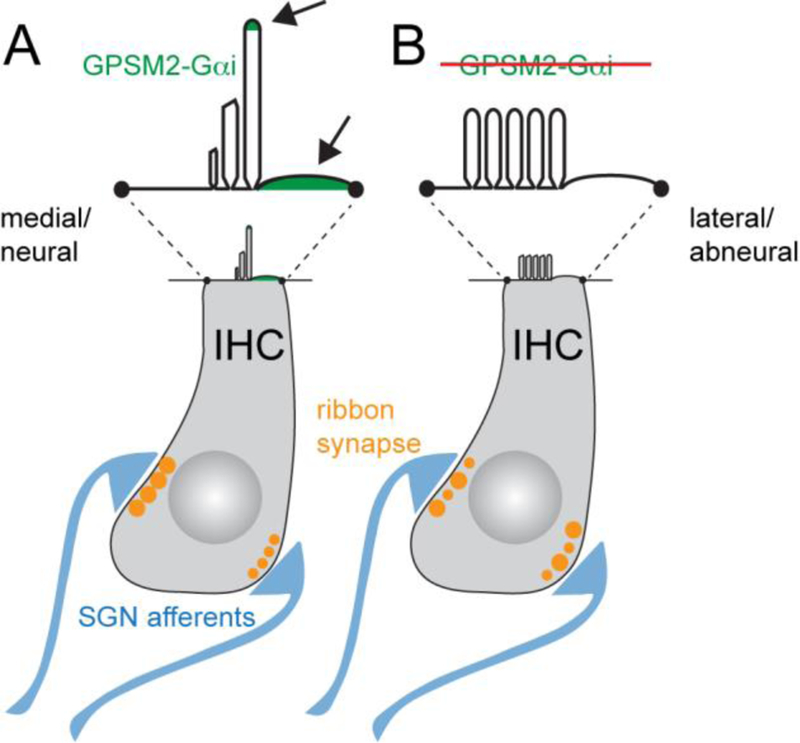
Gpsm2-Gαi is rapidly planar polarized at the lateral aspect of post-mitotic HCs [6, 26, 102] (Figure. 3). This behavior roughly coincides in time with the early lateral migration of the basal body/kinocilium. However these processes appear to be independent, as the position of the kinocilium and the Gpsm2-Gαi crescent are initially only loosely correlated. Gpsm2-Gαi and the Gpsm2 binding partner Insc occupy the “bare zone”, an expanding lateral region of apical membrane devoid of microvilli that appears after the migration of the basal body [102] (Figure 2). There, Insc-Gpsm2-Gαi antagonizes aPKC that initially spans the entire apical membrane, and appear to exclude microvilli previously covering the entire apical surface. Consistent with the notion that segregation of polarity proteins at the apical membrane acts as a blueprint to pattern the cytoskeleton at large, stereocilia, the kinocilium, the cuticular plate and apical MTs are abnormally distributed in Gpsm2 mutants [26, 102].
To circumvent functional redundancy among Gαi proteins, Pertussis toxin (PTX) was used to globally downregulate Gαi activity in HCs. Surprisingly, treating cochlear explants with PTX [26] or expressing PTX catalytic subunit (PTXa) in HCs using transgenic approaches [102, 103] produced cytoskeleton placement defects as in Gpsm2 mutants, but caused also a unique and severe PCP phenotype (Figure 1C). Specifically, PTXa-expressing OHCs showed graded orientation reversal by row where OHC1 were cleanly reversed, OHC2 were variably oriented medially and OHC3 were variably oriented laterally. These results suggest that Gαi is involved independently from Gpsm2 in the Emx2-directed switch of HC orientation recently uncovered in vestibular organs [46] (see section D1).
It is unclear how the polarized localization of Gpsm2-Gαi is initially established. Interestingly, a recent study identified SorCS2, a member of the Vps10 family of sorting receptors involved in protein trafficking, as essential for Gpsm2-Gαi localization at the bare zone. Supporting the blueprint model, the medial aPKC domain was expanded in SorCS2 mutants, coinciding with severe hair bundle defects [27]. New studies are needed to identify other components of the trafficking machinery underlying cell-intrinsic planar polarity.
B3. Common signaling modules polarize diverse cellular processes
Regulation of HC intrinsic planar polarity by several components of the mitotic spindle orientation machinery has suggested mechanistic parallels between these processes (Figure 4A). Based on their shared lateral bias at the HC apex, it was proposed that Insc-Gpsm2-Gαi and Par3, similar to their role as cortical landmarks in mitotic progenitors, might recruit partners including Lis1/dynein to exert pulling forces on centriolar MTs. By analogy with mitotic spindle orientation, this mechanism could trigger the early lateral migration of the basal body/kinocilium to break radial symmetry at the HC apex, and/or regulate their placement at later stages of HC differentiation [26, 95]. These compelling hypotheses are discussed in Future Directions (see section F2, F3).
Another polarized cellular process that shares striking parallels with HC planar polarity is directional migration/chemotaxis (Figure 4C). Persistent directional migration towards a source of chemoattractant requires a protrusive leading edge and a retracting trailing edge that define the front-to-rear polarity axis. During directed migration, MTs are oriented and show persistent growth towards the leading edge. MTs and the dynein motor were shown to regulate direction persistence by maintaining front-rear polarity [21, 117]. In addition to a structural role, MT trafficking can deliver a number of GEFs to activate Rac and Cdc42 at the leading edge. Reciprocally, the Par3 complex links Cdc42 and Rac signaling to MT regulation (reviewed in [25]). The Insc-Gpsm2-Gαi module is also enriched at the leading edge and can recruit Par3-aPKC via Insc during neutrophil chemotaxis [50](Figure 4C). Importantly, in that case, the Gpsm2-Gαi complex regulates actin dynamics, underscoring that Gpsm2-Gαi has MT-independent functions, as also evident during stereocilia elongation (see section E1). While “canonical” G protein receptor-coupled (GPCR) signaling is well-known to detect chemoattractants and promote cell motility (reviewed in [51]), non-canonical signaling involving Gpsm2-Gαi was proposed to stabilize the leading edge and thus ensure sustained directionality in neutrophils (Figure 4C). It is interesting to speculate that core PCP signaling in HCs may play an analogous role as chemokine gradients in migrating cells to orient cell-intrinsic polarity modules in the tissue.
C. Integration of intercellular and cell-intrinsic polarity signaling
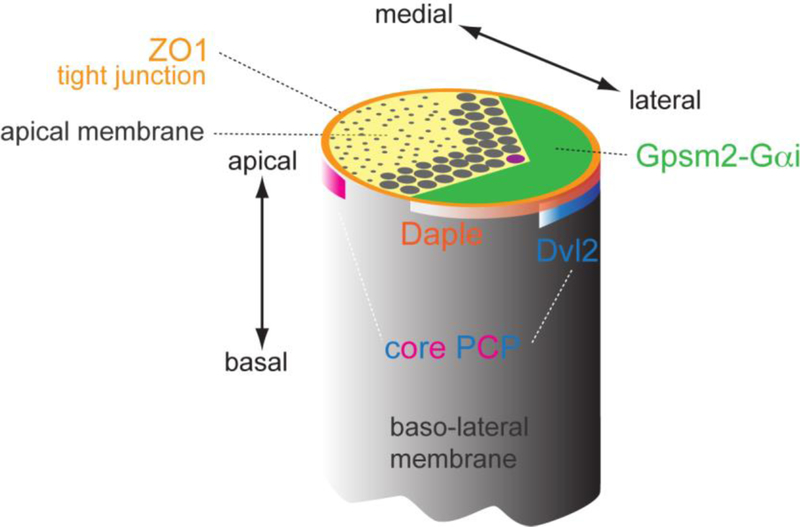
In core PCP mutants, intrinsic polarity modules including planar polarized Rac activity and Insc-Gpsm2-Gαi domains are intact in individual HCs, but misoriented at the tissue level [26, 36, 102]. Reciprocally, core PCP protein distribution is also unaffected in Gpsm2 or Gαi mutants [26, 102]. Furthermore, Gpsm2-Gαi is only planar polarized in HCs, while core PCP proteins are polarized in both HCs and SCs to propagate polarity information in the OC. Finally, Gpsm2-Gαi are planar polarized at the apical membrane above tight junctions [94], whereas core PCP proteins are planar polarized at cell-cell contacts below tight junctions [26], two distinct yet abutting subcellular domains (Figure 5). Taken together, intercellular (PCP) and cell-intrinsic planar polarity appear to function independently, but must be coordinated during development to ensure that asymmetric hair bundles are all oriented laterally. A strategy to functionally bridge the two systems may employ proteins that can physically bind both junctional core PCP proteins and cell-intrinsic players at the apical membrane. Two candidates fulfilling some of these requirements have been reported to date.
Figure 5. Distinct subcellular localizations for different polarity modules at the HC apex.
Proteins identified as planar polarized at the apical HC surface have been shown to occupy distinct subcellular domains. Core PCP proteins are enriched at adherens junctions and form antagonistic lateral (e.g. Dvl2, blue band) or medial (pink band) protein complexes. In contrast, Par3 and Daple proteins largely coincide with lateral tight junctions (orange), slightly more apical than core PCP proteins. Finally, Insc-Gpsm2-Gαi are enriched above tight junctions at the bare zone, the region of apical membrane devoid of stereocilia or microvilli (green). Close vicinity between Gpsm2-Gαi, Daple-Par3 and Dvl2 at the lateral aspect of HCs suggests that cross-talk between these protein modules and compartments may integrate intercellular PCP and cell-intrinsic cytoskeleton polarization.
Daple (Dishevelled-associating protein with a high frequency of leucines), also known as Ccdc88c, is a regulator of Wnt signaling that can bind Dvl with its C-terminal PDZ-binding domain [83] and bind and regulate Gαi, acting as a non-receptor GEF in vitro [3]. Interestingly, Daple mutant HCs show severe apical cytoskeleton defects including a grossly mishappen hair bundle and randomized kinocilium position [94]. This complex phenotype can be interpreted as a mixture of PCP and planar asymmetry defects. Early kinocilium migration defects similar to those of Vangl2 mutants [77] suggest disrupted PCP signaling. By contrast, complementary but aberrant Gpsm2-Gαi and aPKC domains at the apex suggest defects in the apical blueprint. At the molecular level, Daple is planar polarized laterally near the HC tight junction, sitting at the interface between Gαi at the bare zone and Dvl2 at the adherens junction [94] (Figure 5). Although the exact relationship between Dvl2, Daple and Gαi remains to be solved, they show co-dependence for their normal enrichment and are all spontaneously planar polarized and spatially coordinated in ectopic HC-like cells induced in Kolliker organ [94]. Together, this suggests that Daple could bridge intercellular and cell-intrinsic planar polarity modules by binding to Dvl2 and Gαi.
Par3 is another candidate signal integrator of planar polarity. Par3 is enriched at the lateral HC junction similar to Daple and Dvl2, and was previously found to be a binding partner of Nectins [57, 94, 101]. Par3 is also enriched at low levels at the bare zone along with Insc-Gpsm2-Gαi [102], and can bind Insc in the context of spindle orientation [79]. However, Par3 is not required for asymmetric Gpsm2-Gαi localization in HCs. Instead, Par3 regulates several GEFs that activate Rac signaling in both HCs and SCs, thereby likely influencing both intercellular and cell-intrinsic planar polarity [57]. Interestingly, Par3 was found to bind Daple in a yeast assay and their early distribution is very similar [94], suggesting potential crosstalk between the Dvl2-Daple-Gαi-Gpsm2 and Par3-Rac-Pak modules (Figure 4). Moreover, Par3 may dynamically interact with aPKC-Par6 to establish a crosstalk between the lateral Par3-Rac-Pak and medial Cdc42-aPKC-Par6 modules.
In addition to physical interactions between PCP and cell intrinsic polarity proteins, signal integration may also occur through mechanical cues, as both PCP and front-rear polarity can be reoriented by tissue and cell mechanics [56, 68]. In support of this notion, the Par3-Rac1 [23] signaling axis has been shown to reinforce asymmetric core PCP protein localization in the OC, likely indirectly through cytoskeletal remodeling [36, 57].
D. Transcriptional regulation of hair cell planar polarity
D1. Transcription factors known to be required for hair cell planar polarity
As a component of the HC differentiation program, planar polarization is probably under the control of transcription factors that drive OC development. Although transcriptomic analysis of FACS-purified HCs and other cochlear cell types have been performed [8, 23, 91], presently very little is known about specific regulation of planar polarity genes by key TFs that control OC differentiation. Through genetic analyses, two TFs, namely Six1 and Emx2, have been shown to influence HC planar polarity. The homeodomain TF Six1 and its cofactor Eya1 play essential roles of in prosensory cell differentiation. Deletion of Six1 in OC progenitor cells disrupted the OC cellular mosaic and HC planar polarity [118], although it is unclear whether Six1 directly regulates planar polarity genes, or whether polarity defects are an indirect effect of impaired OC differentiation.
Of particular interest, the homeobox transcription factor Emx2 has emerged as a global regulator of HC orientation in the vestibular system. HCs in the crista ampullae detect angular head acceleration and adopt a uniform orientation matching the spatial trajectory of the associated semicircular canal. HCs in the macular organs (the utricle and saccule in mammals) detect linear head acceleration and gravity, and adopt a radiating range of orientations where HCs are aligned locally. PCP in the maculae also includes an abrupt transition that separates each organ in two distinct HC populations with opposite orientations. HCs across this virtual line of polarity reversal (LPR) thus show opposite responses to the same head movement (reviewed in [15]).
Interestingly, core PCP proteins are not reversed in their asymmetric enrichment across the LPR, demonstrating that the PCP vector of tissue polarity is not sufficient to define HC orientation [16]. Expression of the transcription factor Emx2 was recently shown to be limited to one orientation domain, and to be necessary and sufficient to reverse HC orientation [40, 46]. Remarkably, Emx2 restriction to one orientation domain is conserved in chicken macular organs and in the zebrafish lateral line, where HCs grouped in neuromasts respond to bi-directional water movement. In zebrafish, as in the mouse, Emx2 is necessary and sufficient to reverse HC orientation.
Presumably, HCs respond to Emx2 by reversing how cell-intrinsic planar polarity modules associate to invariant organ-level core PCP patterning at cell junctions. Effectors in the Emx2 transcriptional program remain to be identified, but Gαi activity appears to be required. PTXa expression can indeed reverse HC orientation in the lateral utricle, and thus phenocopy the loss of Emx2 [46]. As PTXa expression can prevent HC reversal induced by ectopic Emx2 expression, Gαi clearly functions downstream of Emx2. Interestingly, the auditory epithelium is Emx2-positive but cristae are Emx2-negative [46], and PTXa was previously shown to provoke the graded orientation reversal of OHCs in the cochlea (see section B2.3) [26, 102]. These results suggest that Emx2-based patterning might be an evolutionary novelty used to reverse a “ground” state of HC orientation present in ancestral vestibular organs. Emx2 is required for OHC differentiation however [40], precluding direct testing of this hypothesis in the cochlea.
D2. Approaches to identify TF target genes crucial for cochlear morphogenesis
Along with many other TFs, Six1 and Emx2 form an intricate and dynamic gene regulatory network for hair cell specification, differentiation and survival. Only a handful of direct target genes are known, in part because popular techniques for genomic mapping of TF binding sites such as chromatin immunoprecipitation (ChIP)-seq and ATAC-seq have not been widely applied to cochlear tissues due to the small number of HCs in the cochlea [44, 118]. To overcome this limitation, investigators have used a multi-pronged approach by identifying differentially expressed gene in loss- and gain-of function TF mutants, and cross-referencing with available ChIP-seq and TF-binding site datasets in other tissues and cell lines [8, 23]. With the rapid advance of next-generation sequencing technologies, genome-wide mapping of TF targets in space and time during cochlear morphogenesis will be achievable in the near future, which will shed light on the mechanisms underlying global patterns of planar polarity in inner ear sensory epithelia.
E. Novel roles of cell-intrinsic planar polarity factors in hair cells
E1. Role of Gpsm2-Gαi in stereocilia elongation and hair bundle maturation
While Insc-Gpsm2-Gαi proteins are first planar polarized at the bare zone (Figure 2; see section B2.3), Gpsm2-Gαi, but not Insc, is then also enriched in the hair bundle during embryogenesis already [99, 103]. At postnatal stages, Gpsm2-Gαi is enriched at the distal tip of stereocilia in the first, tallest row. Enrichment in the bundle is first observed in central stereocilia adjacent to the kinocilium, and then spread to more peripheral ones. Broad and premature Gpsm2-Gαi enrichment at stereocilia tips was observed in Insc and Gpsm2 mutants, with a concomitant loss of enrichment at the bare zone. This imbalance in protein distribution suggests that protein anchoring at the bare zone prevents premature trafficking to stereocilia tips, establishing a relationship between these subcellular compartments [103]. Based also on the close segregation of Gpsm2-Gαi at the bare zone with the base of row 1 stereocilia, it was proposed that intrinsic planar polarity information is used to establish the characteristic asymmetric architecture of vertebrate hair bundles in the apicobasal axis, with stereocilia rows of graded heights.
This idea is attractive because in absence of Gpsm2 or Gαi function, postnatal stereocilia elongation is compromised, a phenotype most striking in IHC stereocilia [72, 103]. IHC hair bundles are largely flat and maintain supernumerary rows, very reminiscent of defects reported previously for Myo15a, Whrn and Eps8 mutants [70, 75, 86, 116] (Figure 6). Myo15a, Whrn and Eps8 localize to stereocilia tips and were proposed to promote actin incorporation to stimulate postnatal stereocilium elongation [5, 17, 70, 90]. In fact, genetic interaction studies demonstrated that Gpsm2-Gαi and Myo15a-Whrn function in the same elongation/maturation pathway [99]. Stereocilia stunting is a likely cellular etiology for profound congenital hearing loss reported when Gpsm2-Gαi function or trafficking is defective in humans or mouse models [19, 27, 72, 103, 106].
Figure 6. Novel roles for Gpsm2-Gαi and planar polarity in hair cells.
A) At the IHC apical membrane, Gpsm2-Gαi is required for stereocilia elongation and graded heights across stereocilia rows. Gpsm2 and Gαi proteins (green, arrows) are localized at the tip and near the base of stereocilia in the first row, being planar polarized at the lateral bare zone. This dual distribution suggests that planar polarity might play a role in the restriction of Gpsm2-Gαi trafficking to first row stereocilia, establishing their tallest identity and regulating hair bundle staircase-like architecture. At the basolateral IHC membrane, both Gpsm2 and Gαi are required for the medial-lateral gradient of ribbon synapse (orange) size and synaptic activity. The IHC ribbon synapse releases neurotransmitters onto spiral ganglion neuron (SGN) afferent terminals (blue). B) In absence of Gpsm2-Gαi function, stereocilia fail to elongate at postnatal stages and the hair bundle retains an immature morphology with an excess number of rows, and the gradient of ribbon synapse size is abolished. It remains unclear how Gpsm2-Gαi proteins influence synapse size and activity.
Teasing apart protein localization in normal and mutant contexts revealed that Myo15a-Eps8 forms an early complex at stereocilia tips, with a later Whrn-Gpsm2-Gαi module added in first row stereocilia only [99]. Myo15a and Whrn are required for Gpsm2-Gαi localization at tips, and Whrn can bind both Gpsm2 and Gαi [72, 99]. This suggests that Whrn acts as an adapter to include Gpsm2-Gαi to Myo15a cargo [72]. In contrast, Myo15a-Eps8 were still trafficked to tips in absence of Gpsm2-Gαi function, but protein amounts never built up to high levels observed in normal row 1 [99]. In addition, Myo15a-Eps8 lost its characteristic graded enrichment mirroring stereocilia height across rows. As protein markers for shorter rows were found in the first row when Gpsm2-Gαi function was disrupted, and reciprocally, markers for the first row (Gpsm2-Gαi themselves) were found in all rows in PTXa-expressing HCs, mutant stereocilia appear to maintain a generic molecular and morphological identity. The Whrn-Gpsm2-Gαi module thus appears to be added to an ubiquitous Myo15a-Eps8 stereocilia tip complex in order to specify the unique tallest identify of row 1 stereocilia, and to promote differential row identity in the planar-asymmetric hair bundle [99].
E2. Role of Gpsm2-Gαi and Emx2 in hair cell synaptic properties
Each IHC forms ribbon synapses with multiple SGN type I afferent fibers to transmit auditory signals to the brain. Although they innervate the same sound receptor, these afferents have different firing properties and contact heterogeneous active zones on the IHC body, which helps explain the astounding range of sound pressure processed by the inner ear. Heterogeneity is spatial in nature and potentially includes planar polarized features. For example, ribbon synapses are larger on the medial than the lateral side of the cell [61, 63]. This coincides with graded electrophysiological synaptic properties, and could potentially instruct well-established graded afferent behaviors at the post-synaptic level [62]. Interestingly, Gpsm2 or Gαi3 inactivation and PTXa expression collapsed the medio-lateral gradient of ribbon synapse size while maintaining overall ribbon size and number [43] (Figure 6). In addition, PTXa provoked enlarged pre- and post-synaptic clusters, increased Ca2+ influx, and the collapse of the spatial gradient of maximal synaptic Ca2+ influx. These results suggest that the Gpsm2-Gαi complex polarizes not only the cytoskeleton at the HC apex, but also the spatial organization and the properties of ribbon synapses at the basal end of the IHC. An independent study showed that Gαi2 and Gαi3 are redundantly required for the normal maturation of the ribbon synapses in IHCs [4]. More work is needed to determine how Gpsm2-Gαi influences the assembly and function of heterogeneous active zones. It would also be interesting to test a potential role of PCP proteins.
A link between cell-intrinsic planar polarity and synapse development was also found in HCs in the zebrafish neuromasts. Single neuromast afferents neurons exhibit directional selectivity, contacting only HCs that share the same orientation. Remarkably, recent work demonstrated that a single neuron either contacts Emx2-positive or Emx2-negative HCs [45, 65]. Emx2 inactivation or Emx2 ectopic expression in HCs led to an increase or decrease in the number of HCs contacted, depending on the afferent selectivity. As Emx2-based selectivity was retained in a core PCP mutant with randomized hair bundle orientation, afferent synaptic choices strictly reflect Emx2 expression in HCs, and not HC orientation. It remains unknown however how navigating fibers discriminate between Emx2-positive or Emx2-negative HCs to establish synaptic contacts. In conclusion, as Gpsm2-Gαi, Emx2 influences both the polarization of the apical cytoskeleton and basal synaptic properties in HCs.
F. Future Directions
Despite significant advances in the last decade, our understanding of the mechanisms of planar polarity and their functions in the inner ear is still fragmentary. Below, we discuss several incompletely understood areas that warrant future investigations.
F1. Unsolved questions in intercellular PCP signaling
Despite conservation of the principle features of core PCP signaling, a number of lingering questions remain about intercellular PCP signaling in the inner ear. First, the puzzling opposite localization of Fzd3/6 and Dvl2/3 in HCs suggest that additional Frizzled proteins may regulate Dvl at the lateral junction, and/or that Fzd3/6 may partner with Dvl1 or function independently of Dvl. Second, it remains unknown how the vector of tissue polarity changes in different parts of the cochlear epithelium. While tissue mechanics likely plays a role [53], junctional rearrangement and actomyosin dynamics have not been analyzed outside the OC. It also remains unclear how the uniformly distributed Ptk7 protein can generate signaling and cytoskeletal asymmetry. To solve these questions, it would be important to catalog the precise localization of all core PCP proteins in the auditory and vestibular epithelia. This is a challenging task for several reasons. 1) The OC alone includes 2 HC and at least 6 SC types, and therefore the heterogeneity of bi-cellular junctions could influence core PCP protein distributions differently. 2) The cell(s) of origin for junctional enrichment has proved difficult to determine, requiring high-resolution microscopy or mosaic analyses. 3) Difference of core PCP protein organization in a single cell type could exist between the auditory and vestibular epithelia, or even among vestibular organs. Although a comprehensive catalog is work-intensive and might appear a “low impact” descriptive goal, it would greatly facilitate future studies required to fully understand intercellular PCP. An important future direction is to probe dynamic protein localization and its links to junctional actomyosin contractility in space and time, as has been initiated in other systems using quantitative live imaging [7]. Finally, mapping the protein interactomes of known PCP genes in the inner ear may identify novel PCP regulators and/or modes of signaling integration.
F2. Is Gαi function required for the early peripheral migration of the basal body/kinocilium?
Gαi was proposed to be the driving force for the centrifugal migration of the basal body (see B3), but to date limited evidence supports this model. Treating E16-E18 cochlear explant cultures with PTX resulted in a centrally positioned basal body and consequently a circular hair bundle in a small fraction of HCs [26]. However PTXa expression in vivo, even with an early Cre driver (Foxg1-Cre), did not recapitulate this phenotype [46, 102, 103]. Furthermore, HCs were apparently able to break radial symmetry in conditional double Gαi2/3 mutants using Foxg1-Cre [4], and when the Gαi regulator Gpsm2 was missing [26, 102]. Another caveat is the subcellular localization of Gpsm2-Gαi, which occupies the lateral apical membrane and only abutt the lateral junction where a planar pulling force would be expected to originate. In summary, it remains unclear whether the MT-pulling role of Gpsm2-Gαi during mitotic spindle orientation is conserved in HCs. As Gpsm2-Gαi was closely associated to actin dynamics [72, 99, 103], notably in stereocilia (see section E1) and during chemotaxis (Figure 4), Gαi activity might not be required for the early basal body migration, and alternative mechanisms yet to be identified. Alternatively, it is also possible that we still lack adequate tools to fully block Gαi function in HCs. Definitive support for a mitotic spindle-like role in HCs awaits an experimental model where the basal body remains central in HCs in vivo.
F3. Role of dynein-mediated pulling force in subsequent kinocilium positioning?
While there is no evidence for dynein mediating the early migration of the basal body, dynein is required for its subsequent positioning at the lateral apex. Dynein’s precise mechanism of action is not fully understood, but likely involves both cargo transport and force generation. Specifically, both dynein and Lis1 are concentrated around the basal body where many ciliary and polarity proteins are localized, suggesting a possible cargo transport role. In addition, dynein is also localized near the cell junctions, consistent with an involvement in generating pulling force [26, 95]. In the latter case, how dynein is tethered at the HC cortex remains a key unresolved question. In mitotic cells, dynein is recruited through binding to NuMA that is in complex with Gpsm2-Gαi (Figure 4A). Of note, NuMA is a nuclear protein and is only relocalized to the spindle poles and the cell cortex upon nuclear envelope breakdown [87]. In post-mitotic HCs, NuMA is found sequestered in the nucleus as expected, and is therefore unlikely to function in HC planar polarization [6]. What protein(s), then, could tether dynein at the cell cortex? Par3 and Daple again emerge as possible candidates, as both have been shown to interact with dynein in other systems [88, 92]. Moreover, both Par3 and Daple can bind MTs [9, 100], and they colocalize at lateral junctions from early stages of HC differentiation [94], an appropriate location to anchor planar pulling forces.
F4. Additional roles of the kinocilium during hair bundle morphogenesis?
While kinocilium tethering to central stereocilia in the first row is important to impart a chevron shape to the early hair bundle, the potential role of the kinocilium during postnatal hair bundle maturation remains relatively unexplored. In light of recent results, it would be interesting to ask whether the kinocilium is somehow implicated in the restricted trafficking of Gpsm2-Gαi to first row stereocilia, or the fact that these proteins are first enriched in central stereocilia in the kinocilium vicinity before being enriched in more peripheral stereocilia. Interestingly, cross-talk between the kinocilium and the Gαi domain seems essential for hair bundle integrity, as hair bundle fragmentation was only observed in Daple mutants when the aberrantly medial kinocilium was outside of the Gαi domain [94]. Alternatively, it also remains possible that the kinocilium only plays a limited early role to shape the hair bundle. A comprehensive understanding of the kinocilium contribution would greatly benefit from in vivo models that disrupt kinocilium function without confounding non-ciliary defects.
F5. Molecular mechanism(s) of Gαi function during apical HC morphogenesis?
In the context of mitotic spindle orientation, Gαi is thought to be sequestered in its GDP form by Gpsm2. By analogy, Gαi activity in HCs at the bare zone and at stereocilia tips is likely to be non-canonical, perhaps serving as a cortical anchor point rather than a bona fide signaling relay for a GPCR. However, the citical role of the non-receptor GEF Daple suggests that guanine nucleotide exchange could be important for Gαi function during HC planar polarization. Daple might compete with Gpsm2 for Gαi binding at the apical membrane (bare zone)/apical junction interface, for example. In addition, Gαi appears to function in a Gpsm2-independent pathway to regulate HC orientation reversal downstream of Emx2. While PTX is best known to disrupt GPCR signaling by preventing heterotrimeric Gαi-Gβγ complex dissociation, PTX appears to effectively inhibit Gpsm2-Gαi function as well, as illustrated by stunted stereocilia, for example. Nevertheless, it remains possible that some aspects of Gαi function are insensitive to PTX. Further studies need to clarify 1) what possibly independent purposes Gαi serves during HC differentiation, 2) what partners are regulating Gαi activity in each case, and 3) to what extent each role involves classic signaling or a more structural function.
F6. Solving and leveraging planar polarity mechanisms for hair cell regeneration
Studying developmental mechanisms underlying auditory HC development has both fundamental and translational significance. In particular, recent insights indicate that planar polarity signaling regulates many aspects of auditory HC and SC development critical for mechanotransduction (section E1) and synaptic transmission (section E2). Thus, reactivation of planar polarity signaling pathways is absolutely necessary for regeneration of fully functional auditory HCs. Indeed, stem cell derived and experimentally regenerated HC-like cells often form vestibular-like or misshapen hair bundles with limited potential to function properly as sound receptors [55, 82, 107]. While restoration of intercellular PCP has been observed during HC regeneration of non-mammalian species [110], it will be important to probe and manipulate both PCP and cell-intrinsic signaling in mammalian models of HC regeneration.
Highlights.
Intercellular and cell-intrinsic signaling coordinately regulates hair cell planar polarity.
Planar polarity of supporting and other non-sensory cells is also crucial for hearing.
Hair bundle stereocilia elongation is coupled to hair cell planar polarity.
Epithelial planar polarity influences hair cell-spiral ganglion neuron connectivity.
Acknowledgments
We thank Tingting Du for technical assistance. This work was supported by NIH grant R01DC015242 (to B.T.) and R01DC013773 (to X.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Andreeva A, Lee J, Lohia M, Wu X, Macara IG, Lu X, PTK7-Src signaling at epithelial cell contacts mediates spatial organization of actomyosin and planar cell polarity, Dev Cell 29 (2014) 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Appler JM, Goodrich LV, Connecting the ear to the brain: Molecular mechanisms of auditory circuit assembly, Prog Neurobiol 93 (2011) 488–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aznar N, Midde KK, Dunkel Y, Lopez-Sanchez I, Pavlova Y, Marivin A, Barbazán J, Murray F, Nitsche U, Janssen K-P, Willert K, Goel A, Abal M, Garcia-Marcos M, Ghosh P, Daple is a novel non-receptor GEF required for trimeric G protein activation in Wnt signaling, eLife 4 (2015) e07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beer-Hammer S, Lee SC, Mauriac SA, Leiss V, Groh IAM, Novakovic A, Piekorz RP, Bucher K, Chen C, Ni K, Singer W, Harasztosi C, Schimmang T, Zimmermann U, Pfeffer K, Birnbaumer L, Forge A, Montcouquiol M, Knipper M, Nurnberg B, Ruttiger L, Galphai Proteins are Indispensable for Hearing, Cell Physiol Biochem 47 (2018) 1509–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB, Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia, Nat Cell Biol 7 (2005) 148–156. [DOI] [PubMed] [Google Scholar]
- [6].Bhonker Y, abu rayyan A, Ushakov K, Amir-Zilberstein L, Shivatzki S, Yizhar Barnea O, Elkan-Miller T, tayeb-fligelman E, Myoung Kim S, Landau M, Kanaan M, Chen P, Matsuzaki F, Sprinzak D, Avraham KB, The GPSM2/LGN GoLoco motifs are essential for hearing, Vol. 27, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Butler MT, Wallingford JB, Spatial and temporal analysis of PCP protein dynamics during neural tube closure, Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cai T, Jen HI, Kang H, Klisch TJ, Zoghbi HY, Groves AK, Characterization of the transcriptome of nascent hair cells and identification of direct targets of the Atoh1 transcription factor, J Neurosci 35 (2015) 5870–5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen S, Chen J, Shi H, Wei M, Castaneda-Castellanos DR, Bultje RS, Pei X, Kriegstein AR, Zhang M, Shi SH, Regulation of microtubule stability and organization by mammalian Par3 in specifying neuronal polarity, Dev Cell 24 (2013) 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Coate TM, Kelley MW, Making connections in the inner ear: recent insights into the development of spiral ganglion neurons and their connectivity with sensory hair cells, Semin Cell Dev Biol 24 (2013) 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Copley CO, Duncan JS, Liu C, Cheng H, Deans MR, Postnatal refinement of auditory hair cell planar polarity deficits occurs in the absence of Vangl2, J Neurosci 33 (2013) 14001–14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN, Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse, Curr Biol 13 (2003) 1129–1133. [DOI] [PubMed] [Google Scholar]
- [13].Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC, Kelley MW, Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea, Development 130 (2003) 2375–2384. [DOI] [PubMed] [Google Scholar]
- [14].Davey CF, Moens CB, Planar cell polarity in moving cells: think globally, act locally, Development 144 (2017) 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Deans MR, A balance of form and function: planar polarity and development of the vestibular maculae, Semin Cell Dev Biol 24 (2013) 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Deans MR, Antic D, Suyama K, Scott MP, Axelrod JD, Goodrich LV, Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear, J Neurosci 27 (2007) 3139–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Delprat B, Michel V, Goodyear R, Yamasaki Y, Michalski N, El-Amraoui A, Perfettini I, Legrain P, Richardson G, Hardelin JP, Petit C, Myosin XVa and whirlin, two deafness gene products required for hair bundle growth, are located at the stereocilia tips and interact directly, Human molecular genetics 14 (2005) 401–410. [DOI] [PubMed] [Google Scholar]
- [18].Denman-Johnson K, Forge A, Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse, J Neurocytol 28 (1999) 821–835. [DOI] [PubMed] [Google Scholar]
- [19].Doherty D, Chudley AE, Coghlan G, Ishak GE, Innes AM, Lemire EG, Rogers RC, Mhanni AA, Phelps IG, Jones SJ, Zhan SH, Fejes AP, Shahin H, Kanaan M, Akay H, Tekin M, F.C. Consortium, Triggs-Raine B, Zelinski T, GPSM2 mutations cause the brain malformations and hearing loss in Chudley-McCullough syndrome, Am J Hum Genet 90 (2012) 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Driver EC, Northrop A, Kelley MW, Cell migration, intercalation and growth regulate mammalian cochlear extension, Development 144 (2017) 3766–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dujardin DL, Barnhart LE, Stehman SA, Gomes ER, Gundersen GG, Vallee RB, A role for cytoplasmic dynein and LIS1 in directed cell movement, J Cell Biol 163 (2003) 1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duncan JS, Stoller ML, Francl AF, Tissir F, Devenport D, Deans MR, Celsr1 coordinates the planar polarity of vestibular hair cells during inner ear development, Dev Biol 423 (2017) 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Elkon R, Milon B, Morrison L, Shah M, Vijayakumar S, Racherla M, Leitch CC, Silipino L, Hadi S, Weiss-Gayet M, Barras E, Schmid CD, Ait-Lounis A, Barnes A, Song Y, Eisenman DJ, Eliyahu E, Frolenkov GI, Strome SE, Durand B, Zaghloul NA, Jones SM, Reith W, Hertzano R, RFX transcription factors are essential for hearing in mice, Nat Commun 6 (2015) 8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, Chen P, Wynshaw-Boris A, Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development, PLoS Genet 4 (2008) e1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Etienne-Manneville S, Microtubules in Cell Migration, Annual Review of Cell and Developmental Biology 29 (2013) 471–499. [DOI] [PubMed] [Google Scholar]
- [26].Ezan J, Lasvaux L, Gezer A, Novakovic A, May-Simera H, Belotti E, Lhoumeau AC, Birnbaumer L, Beer-Hammer S, Borg JP, Le Bivic A, Nurnberg B, Sans N, Montcouquiol M, Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton, Nat Cell Biol 15 (2013) 1107–1115. [DOI] [PubMed] [Google Scholar]
- [27].Forge A, Taylor RR, Dawson SJ, Lovett M, Jagger DJ, Disruption of SorCS2 reveals differences in the regulation of stereociliary bundle formation between hair cell types in the inner ear, PLoS Genet 13 (2017) e1006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fukuda T, Kominami K, Wang S, Togashi H, Hirata K, Mizoguchi A, Rikitake Y, Takai Y, Aberrant cochlear hair cell attachments caused by Nectin-3 deficiency result in hair bundle abnormalities, Development 141 (2014) 399–409. [DOI] [PubMed] [Google Scholar]
- [29].Gavara N, Manoussaki D, Chadwick RS, Auditory mechanics of the tectorial membrane and the cochlear spiral, Curr Opin Otolaryngol Head Neck Surg 19 (2011) 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Geng R, Noda T, Mulvaney JF, Lin VY, Edge AS, Dabdoub A, Comprehensive Expression of Wnt Signaling Pathway Genes during Development and Maturation of the Mouse Cochlea, PLoS One 11 (2016) e0148339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gerhardt C, Leu T, Lier JM, Ruther U, The cilia-regulated proteasome and its role in the development of ciliopathies and cancer, Cilia 5 (2016) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ghimire SR, Ratzan EM, Deans MR, A non-autonomous function of the core PCP protein VANGL2 directs peripheral axon turning in the developing cochlea, Development 145 (2018) dev159012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Giese AP, Ezan J, Wang L, Lasvaux L, Lembo F, Mazzocco C, Richard E, Reboul J, Borg JP, Kelley MW, Sans N, Brigande J, Montcouquiol M, Gipc1 has a dual role in Vangl2 trafficking and hair bundle integrity in the inner ear, Development 139 (2012) 3775–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Goodrich LV, Strutt D, Principles of planar polarity in animal development, Development 138 (2011) 1877–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Goodyear RJ, Lu X, Deans MR, Richardson GP, A tectorin-based matrix and planar cell polarity genes are required for normal collagen-fibril orientation in the developing tectorial membrane, Development 144 (2017) 3978–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Grimsley-Myers CM, Sipe CW, Geleoc GS, Lu X, The small GTPase Rac1 regulates auditory hair cell morphogenesis, J Neurosci 29 (2009) 15859–15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Harris TJ, Peifer M, The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila, J Cell Biol 170 (2005) 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hayase J, Kamakura S, Iwakiri Y, Yamaguchi Y, Izaki T, Ito T, Sumimoto H, The WD40 protein Morg1 facilitates Par6-aPKC binding to Crb3 for apical identity in epithelial cells, J Cell Biol 200 (2013) 635–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hehnly H, Chen CT, Powers CM, Liu HL, Doxsey S, The centrosome regulates the Rab11- dependent recycling endosome pathway at appendages of the mother centriole, Curr Biol 22 (2012) 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Holley M, Rhodes C, Kneebone A, Herde MK, Fleming M, Steel KP, Emx2 and early hair cell development in the mouse inner ear, Dev Biol 340 (2010) 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Honda A, Kita T, Seshadri SV, Misaki K, Ahmed Z, Ladbury JE, Richardson GP, Yonemura S, Ladher RK, FGFR1-mediated protocadherin-15 loading mediates cargo specificity during intraflagellar transport in inner ear hair-cell kinocilia, Proceedings of the National Academy of Sciences (2018). [DOI] [PMC free article] [PubMed]
- [42].Jagger D, Collin G, Kelly J, Towers E, Nevill G, Longo-Guess C, Benson J, Halsey K, Dolan D, Marshall J, Naggert J, Forge A, Alstrom Syndrome protein ALMS1 localizes to basal bodies of cochlear hair cells and regulates cilium-dependent planar cell polarity, Hum Mol Genet 20 (2011) 466–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jean P, Ozcete OD, Tarchini B, Moser T, Intrinsic planar polarity mechanisms influence the position-dependent regulation of synapse properties in inner hair cells, Proc Natl Acad Sci U S A (2019). [DOI] [PMC free article] [PubMed]
- [44].Jen HI, Hill MC, Tao L, Sheng K, Cao W, Zhang H, Yu HV, Llamas J, Zong C, Martin JF, Segil N, Groves AK, Transcriptomic and epigenetic regulation of hair cell regeneration in the mouse utricle and its potentiation by Atoh1, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ji YR, Warrier S, Jiang T, Wu DK, Kindt KS, Directional selectivity of afferent neurons in zebrafish neuromasts is regulated by Emx2 in presynaptic hair cells, eLife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jiang T, Kindt K, Wu DK, Transcription factor Emx2 controls stereociliary bundle orientation of sensory hair cells, Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jones C, Chen P, Planar cell polarity signaling in vertebrates, Bioessays 29 (2007) 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jones C, Qian D, Kim SM, Li S, Ren D, Knapp L, Sprinzak D, Avraham KB, Matsuzaki F, Chi F, Chen P, Ankrd6 is a mammalian functional homolog of Drosophila planar cell polarity gene diego and regulates coordinated cellular orientation in the mouse inner ear, Developmental Biology 395 (2014) 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P, Ciliary proteins link basal body polarization to planar cell polarity regulation, Nat Genet 40 (2008) 69–77. [DOI] [PubMed] [Google Scholar]
- [50].Kamakura S, Nomura M, Hayase J, Iwakiri Y, Nishikimi A, Takayanagi R, Fukui Y, Sumimoto H, The Cell Polarity Protein mInsc Regulates Neutrophil Chemotaxis via a Noncanonical G Protein Signaling Pathway, Dev Cell (2013). [DOI] [PubMed]
- [51].Kamp ME, Liu Y, Kortholt A, Function and Regulation of Heterotrimeric G Proteins during Chemotaxis, Int J Mol Sci 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T, The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear, Development 132 (2005) 4353. [DOI] [PubMed] [Google Scholar]
- [53].Kim EJY, Korotkevich E, Hiiragi T, Coordination of Cell Polarity, Mechanics and Fate in Tissue Self-organization, Trends in Cell Biology 28 (2018) 541–550. [DOI] [PubMed] [Google Scholar]
- [54].Kirjavainen A, Laos M, Anttonen T, Pirvola U, The Rho GTPase Cdc42 regulates hair cell planar polarity and cellular patterning in the developing cochlea, Biol Open 4 (2015) 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Koehler KR, Nie J, Longworth-Mills E, Liu X-P, Lee J, Holt JR, Hashino E, Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells, Nature Biotechnology 35 (2017) 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ladoux B, Mège R-M, Trepat X, Front–Rear Polarization by Mechanical Cues: From Single Cells to Tissues, Trends in Cell Biology 26 (2016) 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Landin Malt A, Dailey Z, Holbrook-Rasmussen J, Zheng Y, Hogan A, Du Q, Lu X, Par3 is essential for the establishment of planar cell polarity of inner ear hair cells, Proc Natl Acad Sci U S A 116 (2019) 4999–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW, Notch signalling pathway mediates hair cell development in mammalian cochlea, Nat Genet 21 (1999) 289–292. [DOI] [PubMed] [Google Scholar]
- [59].Lee J, Andreeva A, Sipe CW, Liu L, Cheng A, Lu X, PTK7 regulates myosin II activity to orient planar polarity in the mammalian auditory epithelium, Curr Biol 22 (2012) 956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lefevre G, Michel V, Weil D, Lepelletier L, Bizard E, Wolfrum U, Hardelin JP, Petit C, A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth, Development 135 (2008) 1427–1437. [DOI] [PubMed] [Google Scholar]
- [61].Liberman LD, Wang H, Liberman MC, Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses, J Neurosci 31 (2011) 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liberman MC, Morphological differences among radial afferent fibers in the cat cochlea: an electron-microscopic study of serial sections, Hear Res 3 (1980) 45–63. [DOI] [PubMed] [Google Scholar]
- [63].Liberman MC, Single-neuron labeling in the cat auditory nerve, Science 216 (1982) 1239–1241. [DOI] [PubMed] [Google Scholar]
- [64].Liu C, Glowatzki E, Fuchs PA, Unmyelinated type II afferent neurons report cochlear damage, Proceedings of the National Academy of Sciences 112 (2015) 14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lozano-Ortega M, Valera G, Xiao Y, Faucherre A, Lopez-Schier H, Hair cell identity establishes labeled lines of directional mechanosensation, PLoS biology 16 (2018) e2004404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M, PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates, Nature 430 (2004) 93–98. [DOI] [PubMed] [Google Scholar]
- [67].Lu X, Sipe CW, Developmental regulation of planar cell polarity and hair-bundle morphogenesis in auditory hair cells: lessons from human and mouse genetics, Wiley Interdiscip Rev Dev Biol 5 (2016) 85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Luxenburg C, Geiger B, Multiscale View of Cytoskeletal Mechanoregulation of Cell and Tissue Polarity. In: Jockusch BM (Ed.), The Actin Cytoskeleton, Springer International Publishing, Cham, 2017, pp. 263–284. [DOI] [PubMed] [Google Scholar]
- [69].Macara IG, Parsing the Polarity Code, Nature Reviews Molecular Cell Biology 5 (2004) 220. [DOI] [PubMed] [Google Scholar]
- [70].Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, Scita G, Kachar B, Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8, Curr Biol 21 (2011) 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K, PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42, Cell 128 (2007) 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mauriac SA, Hien YE, Bird JE, Carvalho SD, Peyroutou R, Lee SC, Moreau MM, Blanc JM, Geyser A, Medina C, Thoumine O, Beer-Hammer S, Friedman TB, Ruttiger L, Forge A, Nurnberg B, Sans N, Montcouquiol M, Defective Gpsm2/Galphai3 signalling disrupts stereocilia development and growth cone actin dynamics in Chudley-McCullough syndrome, Nat Commun 8 (2017) 14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].May-Simera HL, Petralia RS, Montcouquiol M, Wang YX, Szarama KB, Liu Y, Lin W, Deans MR, Pazour GJ, Kelley MW, Ciliary proteins Bbs8 and Ift20 promote planar cell polarity in the cochlea, Development 142 (2015) 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mbiene JP, Sans A, Differentiation and maturation of the sensory hair bundles in the fetal and postnatal vestibular receptors of the mouse: a scanning electron microscopy study, J Comp Neurol 254 (1986) 271–278. [DOI] [PubMed] [Google Scholar]
- [75].Mburu P, Mustapha M, Varela A, Weil D, El-Amraoui A, Holme RH, Rump A, Hardisty RE, Blanchard S, Coimbra RS, Perfettini I, Parkinson N, Mallon AM, Glenister P, Rogers MJ, Paige AJ, Moir L, Clay J, Rosenthal A, Liu XZ, Blanco G, Steel KP, Petit C, Brown SD, Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31, Nat Genet 34 (2003) 421–428. [DOI] [PubMed] [Google Scholar]
- [76].Montcouquiol M, Kelley MW, Development and Patterning of the Cochlea: From Convergent Extension to Planar Polarity, Cold Spring Harb Perspect Med; (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW, Identification of Vangl2 and Scrb1 as planar polarity genes in mammals, Nature 423 (2003) 173–177. [DOI] [PubMed] [Google Scholar]
- [78].Morais-de-Sa E, Mirouse V, St Johnston D, aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells, Cell 141 (2010) 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Morin X, Bellaiche Y, Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development, Dev Cell 21 (2011) 102–119. [DOI] [PubMed] [Google Scholar]
- [80].Motallebzadeh H, Soons JAM, Puria S, Cochlear amplification and tuning depend on the cellular arrangement within the organ of Corti, Proceedings of the National Academy of Sciences 115 (2018) 5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ó Maoiléidigh D, Ricci AJ, A Bundle of Mechanisms: Inner-Ear Hair-Cell Mechanotransduction, Trends in Neurosciences 42 (2019) 221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S, Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells, Cell 141 (2010) 704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Oshita A, Kishida S, Kobayashi H, Michiue T, Asahara T, Asashima M, Kikuchi A, Identification and characterization of a novel Dvl-binding protein that suppresses Wnt signalling pathway, Genes Cells 8 (2003) 1005–1017. [DOI] [PubMed] [Google Scholar]
- [84].Paudyal A, Damrau C, Patterson VL, Ermakov A, Formstone C, Lalanne Z, Wells S, Lu X, Norris DP, Dean CH, Henderson DJ, Murdoch JN, The novel mouse mutant, chuzhoi, has disruption of Ptk7 protein and exhibits defects in neural tube, heart and lung development and abnormal planar cell polarity in the ear, BMC Dev Biol 10 (2010) 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pollock LM, McDermott BM Jr, The cuticular plate: A riddle, wrapped in a mystery, inside a hair cell, Birth Defects Research Part C: Embryo Today: Reviews 105 (2015) 126–139. [DOI] [PubMed] [Google Scholar]
- [86].Probst FJ, Fridell RA, Raphael Y, Saunders TL, Wang A, Liang Y, Morell RJ, Touchman JW, Lyons RH, Noben-Trauth K, Friedman TB, Camper SA, Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene, Science 280 (1998) 1444–1447. [DOI] [PubMed] [Google Scholar]
- [87].Radulescu AE, Cleveland DW, NuMA after 30 years: the matrix revisited, Trends Cell Biol 20 (2010) 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Redwine WB, DeSantis ME, Hollyer I, Htet ZM, Tran PT, Swanson SK, Florens L, Washburn MP, Reck-Peterson SL, The human cytoplasmic dynein interactome reveals novel activators of motility, eLife 6 (2017) e28257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL, Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates, Nat Genet 37 (2005) 1135–1140. [DOI] [PubMed] [Google Scholar]
- [90].Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B, An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal, J Cell Biol 164 (2004) 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Scheffer DI, Shen J, Corey DP, Chen ZY, Gene Expression by Mouse Inner Ear Hair Cells during Development, J Neurosci 35 (2015) 6366–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Schmoranzer J, Fawcett JP, Segura M, Tan S, Vallee RB, Pawson T, Gundersen GG, Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration, Curr Biol 19 (2009) 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Schwander M, Kachar B, Muller U, Review series: The cell biology of hearing, J Cell Biol 190 (2010) 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Siletti K, Tarchini B, Hudspeth AJ, Daple coordinates organ-wide and cell-intrinsic polarity to pattern inner-ear hair bundles, Proc Natl Acad Sci U S A 114 (2017) E11170–E11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sipe CW, Liu L, Lee J, Grimsley-Myers C, Lu X, Lis1 mediates planar polarity of auditory hair cells through regulation of microtubule organization, Development 140 (2013) 1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sipe CW, Lu X, Kif3a regulates planar polarization of auditory hair cells through both ciliary and non-ciliary mechanisms, Development 138 (2011) 3441–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y, Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning, Nature 466 (2010) 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Stoller ML, Roman O, Deans MR, Domineering non-autonomy in Vangl1;Vangl2 double mutants demonstrates intercellular PCP signaling in the vertebrate inner ear, Developmental Biology 437 (2018) 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Tadenev ALD, Akturk A, Devanney N, Mathur PD, Clark AM, Yang J, Tarchini B, GPSM2-GNAI Specifies the Tallest Stereocilia and Defines Hair Bundle Row Identity, Current Biology 29 (2019) 921–934.e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Takagishi M, Sawada M, Ohata S, Asai N, Enomoto A, Takahashi K, Weng L, Ushida K, Ara H, Matsui S, Kaibuchi K, Sawamoto K, Takahashi M, Daple Coordinates Planar Polarized Microtubule Dynamics in Ependymal Cells and Contributes to Hydrocephalus, Cell Reports 20 (2017) 960–972. [DOI] [PubMed] [Google Scholar]
- [101].Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, Monden M, Takai Y, Direct Binding of Cell Polarity Protein PAR-3 to Cell-Cell Adhesion Molecule Nectin at Neuroepithelial Cells of Developing Mouse, Journal of Biological Chemistry 278 (2003) 5497–5500. [DOI] [PubMed] [Google Scholar]
- [102].Tarchini B, Jolicoeur C, Cayouette M, A molecular blueprint at the apical surface establishes planar asymmetry in cochlear hair cells, Dev Cell 27 (2013) 88–102. [DOI] [PubMed] [Google Scholar]
- [103].Tarchini B, Tadenev AL, Devanney N, Cayouette M, A link between planar polarity and staircase-like bundle architecture in hair cells, Development 143 (2016) 3926–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Tilney LG, Tilney MS, DeRosier DJ, Actin filaments, stereocilia, and hair cells: how cells count and measure, Annu Rev Cell Biol 8 (1992) 257–274. [DOI] [PubMed] [Google Scholar]
- [105].Togashi H, Kominami K, Waseda M, Komura H, Miyoshi J, Takeichi M, Takai Y, Nectins establish a checkerboard-like cellular pattern in the auditory epithelium, Science 333 (2011) 1144–1147. [DOI] [PubMed] [Google Scholar]
- [106].Walsh T, Shahin H, Elkan-Miller T, Lee MK, Thornton AM, Roeb W, Abu Rayyan A, Loulus S, Avraham KB, King MC, Kanaan M, Whole exome sequencing and homozygosity mapping identify mutation in the cell polarity protein GPSM2 as the cause of nonsyndromic hearing loss DFNB82, Am J Hum Genet 87 (2010) 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Walters BJ, Coak E, Dearman J, Bailey G, Yamashita T, Kuo B, Zuo J, In Vivo Interplay between p27Kip1, GATA3, ATOH1, and POU4F3 Converts Non-sensory Cells to Hair Cells in Adult Mice, Cell Reports 19 (2017) 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P, Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway, Nat Genet 37 (2005) 980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang Y, Guo N, Nathans J, The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells, J Neurosci 26 (2006) 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Warchol ME, Montcouquiol M, Maintained expression of the planar cell polarity molecule Vangl2 and reformation of hair cell orientation in the regenerating inner ear, J Assoc Res Otolaryngol 11 (2010) 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Webb SW, Grillet N, Andrade LR, Xiong W, Swarthout L, Della Santina CC, Kachar B, Muller U, Regulation of PCDH15 function in mechanosensory hair cells by alternative splicing of the cytoplasmic domain, Development 138 (2011) 1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yamamoto N, Okano T, Ma X, Adelstein RS, Kelley MW, Myosin II regulates extension, growth and patterning in the mammalian cochlear duct, Development 136 (2009) 1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A, PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation, Development 136 (2009) 2039–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Yu H, Ye X, Guo N, Nathans J, Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: evidence for a network of interacting genes, Development 139 (2012) 4383–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yu N, Zhao H-B, Modulation of Outer Hair Cell Electromotility by Cochlear Supporting Cells and Gap Junctions, PLOS ONE 4 (2009) e7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zampini V, Ruttiger L, Johnson SL, Franz C, Furness DN, Waldhaus J, Xiong H, Hackney CM, Holley MC, Offenhauser N, Di Fiore PP, Knipper M, Masetto S, Marcotti W, Eps8 regulates hair bundle length and functional maturation of mammalian auditory hair cells, PLoS biology 9 (2011) e1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhang J, Guo W-H, Wang Y-L, Microtubules stabilize cell polarity by localizing rear signals, Proceedings of the National Academy of Sciences 111 (2014) 16383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhang T, Xu J, Maire P, Xu PX, Six1 is essential for differentiation and patterning of the mammalian auditory sensory epithelium, PLoS Genet 13 (2017) e1006967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Zine A, Van De Water TR, de Ribaupierre F, Notch signaling regulates the pattern of auditory hair cell differentiation in mammals, Development 127 (2000) 3373–3383. [DOI] [PubMed] [Google Scholar]