Abstract
Glioblastoma is a hostile brain tumor associated with high infiltration leading to poor prognosis. Anti-cancer chemotherapeutic agents have limited access into the brain due to the presence of the blood brain barrier (BBB). In this study, we designed a dual functionalized liposomal delivery system, surface modified with transferrin (Tf) for receptor mediated transcytosis and a cell penetrating peptide-penetratin (Pen) for enhanced cell penetration. We loaded doxorubicin and erlotinib into liposomes to enhance their translocation across the BBB to glioblastoma tumor. In vitro cytotoxicity and hemocompatibility studies demonstrated excellent biocompatibility for in vivo administration. Co-delivery of doxorubicin and erlotinib loaded Tf-Pen liposomes revealed significantly (p < 0.05) higher translocation (~15%) across the co-culture endothelial barrier resulting in regression of tumor in the in vitro brain tumor model. The biodistribution of Tf-Pen liposomes demonstrated ~12 and 3.3 fold increase in doxorubicin and erlotinib accumulation in mice brain, respectively compared to free drugs. In addition, Tf-Pen liposomes showed excellent antitumor efficacy by regressing ~90% of tumor in mice brain with significant increase in the median survival time (36 days) along with no toxicity. Thus, we believe that this study would have high impact for treating patients with glioblastoma.
Keywords: blood brain barrier, dual functionalized liposomes, combination drug therapy, in vitro brain tumor model, targeted drug delivery, glioblastoma
1. Introduction
Glioblastoma multiforme (GBM) is an aggressive malignant brain tumor arising from astrocytes. The median survival time of the patients from this tumor is less than 15 months with a 5-year survival rate of less than 3 % after diagnosis [1,2]. This is due to rapid growth of tumor cells and their aggressive infiltration into other parts of brain. Currently, therapeutic treatments available for brain tumor are divided into surgical resection, radiation and chemotherapy. Removal of tumor completely by surgical resection is not possible due to its ability to invade and progress in different parts of the brain [3]. Chemotherapy is the major treatment modality which functions by damaging DNA of cancerous cells. However, intravenous administration of chemotherapeutics does not reach the brain in desired concentration due to the presence of a protective barrier called blood brain barrier (BBB). In addition, incompetent tumor targeting of these chemotherapeutic agents leads to several detrimental and toxic effects on healthy brain tissue [4,5]. Therefore, it is crucially important to develop a delivery system with efficient glioblastoma targeting ability which is able to translocate well in high concentration across the BBB as well as deliver the chemotherapeutic agents to the core of glioblastoma tumor including migrating cells [6].
Expression of several receptors on the BBB facilitate the transcytosis of amino acids, glucose, or nucleic acids into brain, which can be used to deliver a carrier with chemotherapeutic agents into the brain tissue [7-10]. Overexpression of transferrin receptors (TfRs) on the surface of brain endothelial cells as well as on glioblastoma cells can be exploited to deliver chemotherapeutic agents in high concentration across the BBB to glioblastoma tumor [11,12]. Receptor targeted drug delivery systems have been studied to enhance the targeting effect. Conversely, drug delivery from such systems have been found to be restricted due to receptor saturation [10,13]. Another limitation of the receptor targeted delivery systems is their endosomal entrapment followed by degradation [14,15]. In order to overcome the above mentioned limitations, there is a need of an additional ligand to improve the delivery across the BBB. Cell penetrating peptides (CPPs) are short chain cationic peptides which facilitate intracellular uptake of delivery carriers such as liposomes or nanoparticles [14-16]. Thereby using two different ligands in our formulation we aim to enhance the translocation of delivery carrier across the BBB into the brain through multiple mechanisms and potentially overcoming receptor saturation. Penetratin (Pen) is an amphiphilic cationic peptide which facilitates internalization of delivery carriers. This peptide is derived from 60 residue Antennapedia homeodomain, a naturally occurring protein. Pen is 16 amino acids sequence from 43-58 residue of the third α-helix of the homeodomain which is important for the translocation activity [17]. The significance of this study lies in designing and developing an efficient dual functionalized liposomal delivery system, surface modified with Tf and Pen (Tf-Pen liposomes), to co-deliver anticancer chemotherapeutic agents doxorubicin (Dox) and erlotinib (Erlo), across the BBB for the treatment of invasive brain gliomas (Fig. 1).
Fig. 1.
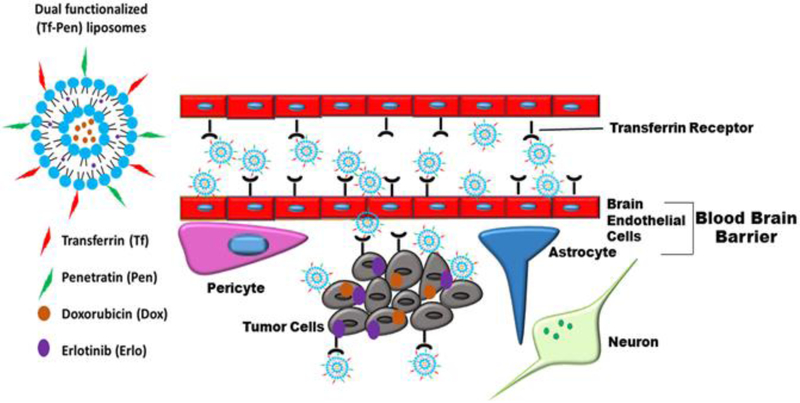
Schematic showing translocation of Dox and Erlo loaded Tf-Pen liposomes across the BBB, followed by endocytosis into glioblastoma tumor cells.
Combination drug therapy is the most effective treatment option for cancer and has a potential to enhance the therapeutic efficacy. The rationale behind combination therapy is to use different mechanisms for targeting cancer cells, thereby decreasing the probability of development of drug resistance. Drug resistance to chemotherapeutic agents, such as doxorubicin, is a common clinical problem limiting their effectiveness, especially as a single agent. Data suggests that conventional therapies exert their cytotoxic activities primarily by inducing apoptosis in tumor cells however, resistant cells adopt mechanisms to evade these apoptotic pathways [18]. Recently, characterization of the adaptive kinome response in the context of Epidermal Growth Factor Receptor (EGFR) therapy has shown that EGFR inhibition promotes the expression of compensatory kinases to allow for continued proliferative signaling in the cell, diminishing the growth inhibitory effect of the drug [19]. Thus, the proposed co-administering of doxorubicin and EFGR inhibitor (erlotinib) is suggested to be an effective approach for attenuating GBM progression in vivo.
In this study, detailed experimental investigations were performed to evaluate the antitumor efficacy of Dox and Erlo loaded Tf-Pen liposomes using in vitro brain tumor model as well as in intracranial glioblastoma bearing mice model. Dual functionalized liposomes were developed by modifying their surface with Tf and Pen for Tf receptor targeting and enhanced cell penetration, respectively. In vitro biocompatibility of liposomes was evaluated to determine their suitability for in vivo administration by performing hemolysis and cell viability assays. We also performed the cellular uptake of dual functionalized liposomes to evaluate their targeting and penetration ability. In vitro antitumor efficacy of Dox and Erlo loaded Tf-Pen liposomes was performed using in vitro brain tumor model. A 3-dimensional in vitro brain tumor model was prepared by growing glioblastoma (U87) cells in a biodegradable PLGA-chitosan scaffold. This model simulates in vivo like condition, where the systemically administered dual functionalized liposomes have to cross the BBB before reaching the tumor sites. The ability of dual functionalized liposomes to translocate across the BBB was demonstrated in vivo quantitatively by measuring the biodistribution in mice brain as well as qualitatively using in vivo fluorescent imaging system. In addition, the antitumor efficacy and survival were evaluated in intracranial glioblastoma bearing nude mice.
2. Materials and methods
2.1. Materials
1,2-dioleoyl-3-trimethylammonium-propane chloride (DOTAP), 1,2-dioleoyl-snglycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (DOPE-lissamine rhodamine), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were obtained from Avanti Polar Limited (Birmingham, Alabama). 3-(N-succinimidyloxyglutaryl) aminopropyl, polyethyleneglycolcarbamyl distearoylphosphatidyl-ethanolamine (NHS-PEG(2000)-DSPE) was procured from Biochempeg Scientific Inc. (Watertown, Massachusetts). Doxorubicin HCl was purchased from MedChem Express (Monmouth Junction, New Jersey). Erlotinib was bought from Cambridge Chemicals (Woburn, Massachusetts). Transferrin (Tf), Cholesterol (Chol) and Chitosan (50 kDa) were obtained from Sigma-Aldrich Company (St. Louis, Missouri). Penetratin (Pen) was purchased from Zhejiang Ontores Biotechnologies Co., Ltd (Zhejiang, China). Fetal bovine serum (FBS) was procured from Omega scientific Inc. (Tarzana, California). Dulbecco’s modified eagle medium (DMEM) and Dulbecco’s phosphate buffered saline (DPBS) were purchased from Mediatech Inc. (Manassas, Virginia). 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was purchased from Alfa Aesar (Ward Hill, Massachusetts). Polyethylene terephthalate (PET) thincerts, cell culture inserts were bought from Greiner Bio-One International (Monroe, North Carolina). Poly (D, L-lactide-co-glycolide) 50:50 was purchased from Polyscitech (West Lafayette, Indiana). All the chemicals used were of analytical grade. Glioblastoma (U87) and Brain endothelial cells (bEnd.3) were procured from American Type Culture Collection (ATCC, Rockville, Maryland).
2.2. Synthesis of DSPE-PEG(2000)-Pen and DSPE-PEG(2000)-Tf:
2.2.1. Synthesis of DSPE-PEG(2000)-Pen:
The coupling of Pen was performed using nucleophilic substitution reaction to the distal end of activated NHS-PEG2000-DSPE. Briefly, NHS-PEG2000-DSPE and Pen at a molar ratio of 3:1 were dissolved in an anhydrous dimethylformamide (DMF) and adjusted pH to 8-9 using triethylamine. The reactant mixture was stirred for 3 days at room temperature. Further, the resulting product was dialyzed using dialysis membrane MWCO 3.5kDa for 48 h to remove free uncoupled Pen. The dialysate was lyophilized and stored at −20 °C until used. The coupling efficiency was determined using micro bicinchoninic acid (BCA) assay in accordance with manufacturer’s protocol. Pen and DSPE-PEG(2000)-NHS were used as standard and control, respectively for the studies.
2.2.2. Synthesis of DSPE-PEG(2000)-Tf:
Tf was coupled to the terminal end of DSPE-PEG(2000)-NHS using nucleophilic substitution, as described in the previous section [8,20,21]. Briefly, DSPE-PEG(2000)-NHS and Tf (125 μg /μ mole of lipid) were dissolved in an anhydrous DMF. Using triethylamine, the pH was adjusted to 8-9. The mixture was stirred using magnetic stirrer for 24 h at room temperature. The resultant product was passed through G-100 sephadex column to separate uncoupled transferrin. The coupling efficiency was evaluated using micro BCA assay, as per described in the previously published article [8]. Tf and DSPE-PEG(2000)-NHS were used as standard and control, respectively for the studies.
2.3. Preparation of Dual Functionalized Liposomes and Drug Loading:
Pen-liposomes were prepared using thin film hydration method while post-insertion method was used to formulate dual-functionalized liposomes [7,8,21]. Briefly, DSPE-PEG(2000)-Pen, erlotinib and other phospholipids, in the following molar ratio: DOTAP/DOPE/cholesterol/DSPE-PEG(2000)-Pen (45:45:2:4 mole %) were dissolved in chloroform: methanol (2:1, v/v). Dried lipid film was formed after evaporating the solvent mixture using rotary evaporator (Buchi Rotavapor RII, New Castle, DE). Further, the film was hydrated with 300 mM citric acid buffer pH 5.0 to form Pen-liposomes. Then, DSPE-PEG(2000)-Tf micelles were added to this and stirred on magnetic stirrer overnight at room temperature to form Tf-Pen coupled liposomes. pH gradient was used to encapsulate doxorubicin into liposomes. The external pH was exchanged by titrating the liposomes with 300 mM sodium carbonate. Then, doxorubicin was added to the liposomes and incubated for 1 h at 50 °C. After cooling the liposomes were pass through G-100 sephadex column to separate unentrapped Dox and Erlo.
The percent entrapment efficiency of drugs was determined as per previously published reports [8,21]. Briefly, the liposomal formulations before and after passing through column were lysed using methanol and triton X-100 (0.5% v/v). Then, the lysed dispersion was centrifuged for 10 min at 3000 rpm. The supernatant from liposomal lysate was injected into high performance liquid chromatography (HPLC) to quantify the entrapment efficiency of drugs. Analysis of Dox was performed using C-18 column (Thermoscientific Hypersil Gold, 5 μm, 250 × 4.6 mm) at a wavelength 234 nm with some modifications.[8] The mobile phase comprised of 0.2 M phosphate buffer, pH 5.5: acetonitrile (70:30) with a flow rate of 1 ml/min at room temperature. With some modifications, Erlo was analyzed using C-8 column (Thermoscientific Hypersil BDS, 5 μm, 250 × 4.6 mm) at a wavelength of 246 nm. The mobile phase consisting of 0.2 M potassium phosphate, pH 3.0: acetonitrile (50:50) with allow rate of 0.750 ml/min at room temperature.[8].
For preparation of lissamine-rhodamine labeled liposomes, 0.5 mole% of lissamine-rhodamine coupled DOPE as liposomes membrane marker was dissolved along with other phospholipids prior to formation of thin lipid film.
2.4. Characterization of Liposomal Nanoparticles:
The hydrodynamic diameter and zeta potential analysis of the liposomes were conducted after appropriately diluted with phosphate buffer saline (PBS) at 25 °C by zetasizer ZS 90 (Malvern Instruments, Worcestershire, UK). The cuvettes filled with samples were placed in the path of 5 mW He–Ne laser of wavelength 633 nm and the data was collected at a scattering angle of 90°. The shape and morphology of Tf-Pen liposomes were evaluated through atomic force microscopy (AFM) (Veeco DI-3100 Veeco, St paul, MN). After appropriately diluted with D.I. water, the liposomal nanoparticles were placed on a freshly cleaved mica film air drying. Image was capture at scan size of 1 μm and scan rate of 1Hz.
2.5. In Vitro Cytotoxicity:
In vitro cytotoxicity was performed to evaluate the biocompatibility of liposomes. The cytotoxic potential of liposomes was evaluated in U87, bEnd.3, and glial cell lines using MTT assay. Briefly, 1,000 cells per well were seeded in 96 well plates in 200 μl of DMEM supplemented with 10% FBS and 1% pen-strep and incubated at 37 °C under 5% CO2 atmosphere. After attachment for 24 h, cells were incubated with different phospholipid concentrations of liposomes (plain, Tf, Pen, and Tf-Pen liposomes) for 2 h in serum free media. Subsequently, the formulation was removed and cells were further incubated at 37 °C under 5% CO2 atmosphere for 48 h, with fresh serum containing media. After 48 h, the viability of cells was evaluated using MTT assay. Untreated cells were considered as control group under the same cell culture conditions.
2.6. Cellular Uptake Assessment:
Cells (U87, bEnd.3 and glial) were seeded at a density of 6 × 105 cells per well in 6 well plate and incubated at 37 °C under 5% CO2 atmosphere. After 24 h, cells were incubated for 2 h with different Dox and Erlo encapsulated liposomal formulations at a concentration of 200 nMoles of phospholipid. Then, the formulation was removed and the cells were washed and rinsed with DPBS, pH 7.4. For qualitative uptake, the nucleus of cells was stained with 1 ml of Hoechst 33342 (1 μg/mL). The cells were observed under Leica DMi8 fluorescence microscope (Leica Microsystems Inc., Buffalo Grove, IL). For quantitative estimation of Dox and Erlo uptake, triton X-100 (0.5% v/v) was used to lyse the cells. Then, methanol was added to the cell lysate to extract drugs. The solution was centrifuged at 4 °C for 10 min at 10,000 rpm and the supernatant was injected in HPLC and analysis was performed as described above for evaluation of doxorubicin and erlotinib loading [8].
2.7. Hemolysis Study:
Hemolysis study demonstrates the interaction between the negatively charged membrane of erythrocytes and liposomes, which may cause hemolysis. The liposomes are meant to be injected intravenously, therefore, it is important to evaluate in vitro hemocompatibility prior to in vivo studies. Briefly, blood sample was collected from an adult rat into EDTA containing tubes. Then, the blood containing tube was centrifuged for 10 min at 2000 rpm to separate erythrocytes and washed thrice with sterile PBS, pH 7.4. With predetermined number of erythrocytes (1.5 × 107) were incubated with different phospholipid concentrations of liposomes for 60 min at 37 °C. Again, the samples were centrifuged at 2000 rpm for 10 min. The absorbance of the supernatant was determined at 540 nm using spectrophotometer (SpectraMax® M5, Molecular devices, Sunnyvale, CA). Hemolysis of 0% and 100% after treatment with PBS and triton X-100 were taken as controls, respectively. The percent hemolysis was determined by using the following equation:[7,8]
Where OD(treatment), OD(PBS) and OD(T) are the optical densities of treatment groups, PBS and triton X-100, respectively.
2.8. Design of Endothelial Barrier:
The in vitro co-culture endothelial barrier was formed by seeding bEnd.3 cells (37,500 cells/cm2) and glial cells (15,000 cells/cm2) on luminal side and abluminal side of the insert, respectively and cultured for 6 days to form a tight barrier, as per the previously published reports [7,8,22]. The culture medium of barrier was changed in every 2 days and the barrier was checked for cell confluency. The integrity of the barrier was determined by measuring the transendothelial electrical resistance (TEER) using EVOM2 with STX2 (World Precision Instruments, Sarasota, FL) [8,22]. The TEER values were determined for the co-culture endothelial (bEnd.3 and primary glial cells) and monolayer (bEnd.3 cells) barrier models.
2.9. 3-Dimensional Tumor Growth Inside PLGA-Chitosan Scaffold:
Emulsion freeze dry method was used for the preparation of scaffold, as per the previously published reports [8,21]. Concisely, a 0.2 g/ml solution of poly (D, L-lactide-co-glycolide) (50:50) (PLGA) was prepared in dichloromethane. Separately, in 10 ml of acetic acid buffer, pH 4.5, 500 mg of chitosan and 150 mg of poly vinyl alcohol were dissolved. An emulsified paste was prepared by mixing PLGA solution to chitosan-PVA mixture at a rate of 2 ml/min and stirred slowly, followed by addition of 500 μl of 0.1 % w/v of collagen solution. At last, the emulsified paste was poured into a rod shaped mold and freeze dried. Later, the rod shaped scaffold was cut into 2 mm circular disc and soaked in 5 M sodium hydroxide and washed to remove excess of sodium hydroxide. Then, the scaffolds were treated in 70% ethanol and washed, followed by soaking them overnight in 30% FBS containing DMEM. Subsequent day, 5 × 105 U87 cells were added on the surface of scaffold and incubated for 6 h. Then, the fresh media containing 30% FBS was added to the scaffold. The culture media of the scaffold was changed in every 2 days. At predetermined time intervals, histological evaluation of the scaffolds was performed to check the growth of U87 tumor inside scaffolds using hematoxylin and eosin (H&E) staining.
2.10. Liposomal Transport Across the Endothelial Barrier:
The liposomal transport was studied across in vitro brain tumor model, as per previously published report [7,8]. To develop the model, the culture insert containing bEnd.3 and glial cells (co-culture endothelial barrier) were placed above the U87 grown scaffold on day 14 and additional cultured for 7 more days. On day 21, the upper compartment of the co-culture endothelial barrier was treated with 200 nMoles of phospholipid concentration of different liposomal formulations in 500 μl of DPBS with 10% FBS and samples were taken at predetermined intervals. Following the transport, the tumor cells in the scaffold were lysed as per described above and the lysate was centrifuged at 10,000 rpm for 10 min at 4 °C. The transport of liposomes was analyzed in the same way as described above in the cellular uptake using HPLC. Fluorescence analysis of endothelial co-culture barrier after transport of lissamine-rhodamine labeled liposomes was performed. Concisely, the co-culture barrier insert membrane was carefully excised using a scalpel after 24 h treatment with liposomal nanoparticles and stained with Hoechst 33342. Further, the excised membrane was mounted on glass slide and covered with glass cover slip using cytoseal 60 mounting medium. The membrane sections were imaged on a Zeiss Axio observer Z1 LSM 700 confocal laser-scanning microscope (Peabody, MA). The images were processed using Imaris x64 9.0.2 software by Bitplane AG (Concord, MA). MA).
2.11. Anti-Tumor Efficacy of Liposomes Using In vitro Brain Tumor Model:
The antitumor efficacy of liposomes was performed in in vitro brain tumor model, as described in previously published report [7,8]. Briefly, the media in the upper compartment of the model was replaced with 200 nMoles of phospholipid concentration of different Dox and Erlo loaded liposomal nanoparticles in 500 μl of DPBS with 10% FBS and treated for 24 h. Following treatment, the scaffolds were further incubated with fresh DMEM supplemented with 30% FBS for 6 days at 37 °C under 5% CO2 atmosphere. The media was changed in every two days. The percent cell viability of U87 tumor cells in scaffold was quantified by using MTT assay. This was further confirmed by performing fluorescence imaging of treatment scaffolds. The scaffolds were stained with live/dead cell staining (Biotum Inc., Fremont, CA) after 6 days of treatment, as per the manufacturer’s protocol. Then, the scaffolds were snap frozen in OCT and 20 μm thick sections of scaffolds were cut using a cryostat. The slides were observed under Leica DMi8 fluorescence microscope (Leica Microsystems Inc., Buffalo Grove, IL) for the images.
2.12. Animal Experiments in Mice:
All animal experiments were performed according to the animal protocol # 17074 approved by the Institutional Animal Care and Use Committee (IACUC) at North Dakota State University. Male/Female nude mice (nu/J; stock # 002019) were used for all animal experiments. Animals were purchased from the Jackson laboratory (Bar Harbor, ME). The animals were housed under controlled temperature conditions with 12 h dark and light cycles. The animals were allowed free access of water and food. The experiments were started after 7 days’ acclimation period.
2.12.1. In Vivo Biodistribution and Biocompatibility of Liposomes:
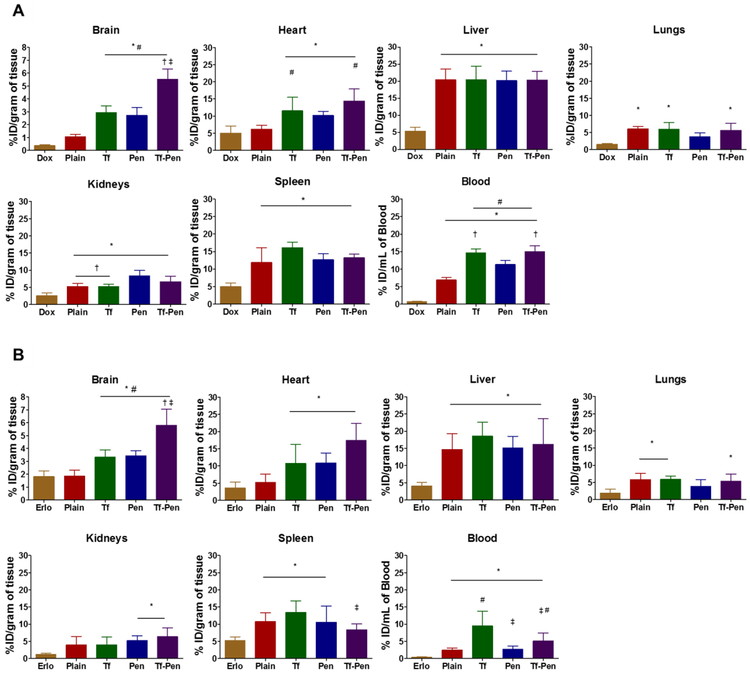
In vivo biodistribution of liposomes was performed qualitatively as well as quantitatively. Male/female mice were randomly divided into six groups each group consisted of 6 mice (3 males and 3 females). Each group was injected with either PBS, free doxorubicin, free erlotinib, doxorubicin and erlotinib loaded plain, Tf, Pen, and Tf-Pen loaded liposomes via tail vein at a dose of 15.2 μmoles/ kg of body weight, calculated based on in vitro biocompatibility studies. Animals injected with only PBS were considered as control group. After 24 h, animals were sacrificed and various organs including brain, heart, lungs, liver, spleen and kidneys were harvested and blood samples were withdrawn. The organs were washed with PBS, weighed and frozen at −80 °C until assayed. To determine the biodistribution of drugs, organs were homogenized and drugs were extracted in acetonitrile: methanol (9:1). Then, the extracted sample was centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was evaporated using vacuum evaporator. The residue extracted sample was reconstituted in methanol: PBS pH 5.5 (1:1) and vortexed followed by centrifugation of the sample at 4 °C for 15 min at 10,000 rpm to separate unwanted proteins. Quantification of drugs was accomplished by HPLC. The quantification of Dox was performed as described in doxorubicin loading, while the quantification of Erlo was performed as described in erlotinib loading with some modifications. The mobile phase consisted of 0.2 M potassium phosphate buffer pH 3.0: acetonitrile (52:48) with a flow rate of 0.600 ml/min at room temperature. All data were normalized and represented in units of percentage of injected dose per gram of the tissue (% ID/gram).
For qualitative distribution of liposomes, mice were injected with lissamine-rhodamine labeled liposomes via tail vein at a dose of 15.2 μmoles/ kg of body weight. At 24 h, mice were sacrificed and whole body as well as ex-vivo fluorescent images of the organs were acquired using Kodak in vivo imaging system FX (Carestream Health Inc., Rochester, NY). The system was equipped with a halogen lamp of 150 W as the excitation light source and rhodamine channel was used to acquire images. The organs were exposure for 2 min. The captured images were processed using Kodak molecular imaging software (4.0). For biocompatibility of liposomes, all the major organs including brain, heart, lungs, liver, spleen, and kidneys were harvested and fixed in 10% neutralized buffer formalin. Then, the organs were embedded in paraffin and sectioned for histopathological analysis with hematoxylin and eosin (H&E) staining. The tissue slides were carefully observed for any sign of toxicity.
2.12.2. Antitumor Efficacy in Mice:
For orthotopic brain tumor model, male/female nude mice were anesthetized (using a mixture of oxygen 1 L/min and 4% of isoflurane for induction and later 1% for maintenance) and carefully placed on a stereotaxic apparatus (David Kopf Instruments, Tujunga, USA). A 10 mm incision was made along the midline and a burr hole was drilled into the frontal lobe of the skull 1.6 mm to the right lateral and 0.7 mm anterior to the bregma using a high speed drill. With the help of a 27 Gauge Hamilton syringe 5 μl of DMEM containing 5 × 105 U87 cells were injected at the junction between the cortex and striatum at a depth of 3.0 mm from the outer border of cranium over a period of 10 min. The needle was kept in place for another 5 min after injection and then slowly removed to prevent a vacuum and cell build-up the needle track. The hole was covered with bone wax to prevent the leakage of cerebrospinal fluid and surgical clips were used to close the incision. After surgery, animals were regularly checked for any sign of pain and distress.
2.12.3. Tumor Regression:
After 10 days of tumor inoculation, the mice were randomly divided into 6 groups and each group consisted of 6 mice. Each group was injected with either PBS, free Dox-Erlo, Dox and Erlo loaded plain, Tf, Pen, and Tf-Pen loaded liposomes at a dose of 15.2 μmoles/ kg of body weight. Dose was administered via tail vein injection, every two days with a total of 3 doses per mouse. At day 16, mice were sacrificed and brains were surgically removed. Brains were fixed in 10% neutralized buffer formalin and embedded in paraffin. Hematoxylin and eosin (H&E) staining was performed on brain sections. Brain tumor diameter was measured and tumor area was calculated using the formula: π (d/2)2. Where d corresponds to the diameter of tumor [23]. Percent body weight of mice were also measured.
2.12.4. Survival Study:
On day 10th of tumor inoculation, the animals were randomly divided into 6 groups. Mice in each group were administered 3 doses of either PBS, free Dox-Erlo, Dox and Erlo loaded Plain, Tf, Pen, and Tf-Pen liposomes at a dose of 15.2 μmoles/ kg of body weight in every two days via tail vein injection. Survival time was calculated from the day of tumor inoculation (day 0) to the day of death of mice. Kaplan-Meier survival curves were plotted for each group using Graphpad Prime 5.0 for windows (GraphPad Software, Inc., La Jolla, CA).
2.12.5. Immunofluorescence staining for Proliferation and Apoptosis:
Brains were harvested from mice after treatments and fixed in 10% neutralized buffer formalin, paraffin-embedded, and sectioned (4 μm thick). The brain tissue sections were incubated with anti-Ki-67 antibody (1:500) (Abcam, Cambridge, MA) for cell proliferation and with anti-cleaved PARP antibody (1:800) (Abcam, Cambridge, MA) for cell apoptosis for 1 h. Thereafter, the tissue sections were incubated with goat anti-rabbit CF®633 (1:200) (Biotum, Inc., Fremont, CA). The slides were observed under Leica DMi8 fluorescence microscope (Leica Microsystems Inc., Buffalo Grove, IL).
2.13. Data Analysis:
All the quantitative data were demonstrated as a mean ± standard deviation (SD). Statistical significant analysis among groups were performed using either Student’s t test, one or two-way ANOVA. A p value of less than 0.05 was considered statistically significant. All quantitative data analysis was performed using Graphpad Prime 5.0 for windows (GraphPad Software, Inc., La Jolla, CA).
3. Results and Discussion
3.1. Synthesis and Characterization of Tf-Pen Liposomes
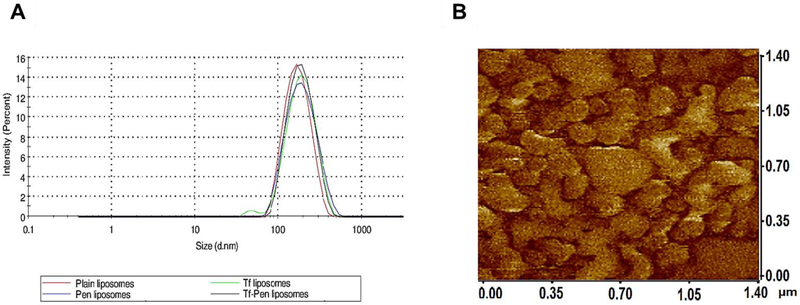
The distal end of the NHS-PEG2000–DSPE was modified by Tf and Pen via nucleophilic substitution reaction at room temperature. The activated NHS ester group of PEG derivatives reacts with primary amine groups in Tf and Pen in slightly alkaline conditions (pH 8 – 9) to form stable amide bonds. The coupling was confirmed using micro BCA assay, which showed more than 80% of coupling efficiency. The Tf and Pen content was approximately 77.78 ± 3.44% and 80.73 ± 4.89 % for Tf-Pen liposomes, respectively. The dual functionalized liposomes were prepared using post-insertion method. Post-insertion is a spontaneous process, where hydrophobic part of PEG derivative interacts with hydrophobic region of lipid membrane and helps in insertion of active ligands into preformed liposomes [24]. This method eliminates the possibility of degradation of encapsulated molecules by conjugating reagents [25,26]. In addition, the post-insertion method ensures appropriate targeting efficiency as well as stable conformation of large targeting proteins [27,28]. As shown in Table 1, the average particle size and zeta potential of Tf-Pen liposomes were found to be less than 200 nm and ~ 10 mV, respectively. A particle size distribution graph of liposomes is shown in Fig. 1A. The mean particle size of plain and Tf-Pen liposomes were 176.68 ± 6.29 nm and 177.95 ± 7.04 nm, respectively. Thus, the surface modification of liposomes did not significantly (p > 0.05) change the particle size of the liposomes. As depicted in figure 1B, the phase image analysis performed using atomic force microscopy (AFM) showed shape, morphology and uniform distribution of liposomal nanoparticles. The zeta potential of Tf-Pen and plain liposomes was found to be 10.43 ± 0.50 mV and 6.76 ± 1.97 mV, respectively. The surface modification of liposomes with Tf changed the zeta potential of liposomes to negative due to the presence of the negative charge of Tf. However, the Tf-Pen liposomes showed near neutral charge (0 – 15 mV) due to the counter balancing of the positive charge of Pen with the negative charge of Tf. The PEGylation of liposomes improves their stability, increases their residence time as well as their accumulation in brain by forming a hydrophilic layer on them [29]. PEGylation and near neutral charge of liposomes also prevent them from being eliminated by macrophage system [30]. However, the PEG chains can hinder the binding and internalization of liposomes to tumor cells. This can be overcome by coupling ligands to the distal end of PEG chains, thereby maintaining long circulation properties [31,32].
Table 1.
Particle size distribution, polydispersity index, zeta potential and entrapment efficiency of various liposomal formulations
| Liposomes | Particle size (nm) |
PDIa | Zeta Potential (mV) |
Dox EEb (%) | Erlo EEb (%) | |||
|---|---|---|---|---|---|---|---|---|
| Plain | 176.68 ± 6.29 | 0.134 ± 0.030 | 6.76 ± 1.97 | 64.59 ± 2.11 | 53.65 ± 1.11 | |||
| Tf | 180.60 ± 1.76 | 0.236 ± 0.023 | −7.15 ± 0.67 | 66.55 ± 2.81 | 53.28 ± 1.61 | |||
| Pen | 177.98 ± 4.46 | 0.217 ± 0.020 | 16.25 ± 0.49 | 65.89 ± 2.85 | 53.94 ± 1.46 | |||
| Tf-Pen | 177.95 ± 7.04 | 0.193 ± 0.025 | 10.43 ± 0.50 | 65.80 ± 1.13 | 52.57 ± 1.70 | |||
Polydispersity index (PDI).
Entrapment efficiency (EE). The data represented as mean ± SD, (n=4).
The pH gradient method was used to entrap Dox into liposomes. The hydration of thin film of lipids with 300 mM citric acid pH 5.0, protonates Dox intra-liposomally. According to concentration gradient, the protonation of Dox by acidic buffer further helps in diffusion of the unionized Dox from outside. The core needs to be highly buffered to endure the pH gradient for the high entrapment of Dox. Erlo is entrapped in the exterior lipid bilayer due to its strong hydrophobicity. The entrapment efficiencies of Dox and Erlo for all liposomal formulations were approximately 66% and 53%, respectively (Table 1). The surface modification of liposomes did not significantly (p > 0.05) affect the entrapment of drugs. The percent cumulative release of doxorubicin and erlotinib from Tf-Pen liposomes was more than 37% and 39% for liposomes, respectively, over the period of 24 h (Fig. S1A and B).
3.2. In vitro Biocompatibility Studies
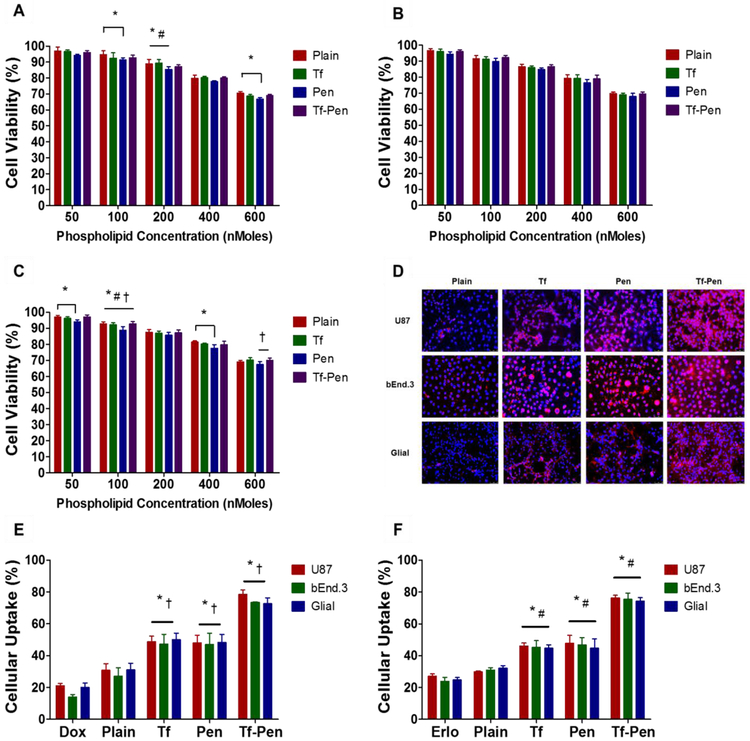
In vitro biocompatibility of liposomes was performed in glioblastoma (U87), brain endothelial (bEnd.3), and primary glial cell lines using MTT assay to demonstrate that liposomes are biocompatible and non-toxic. After exposure to different phospholipid concentrations of liposomes, MTT assay revealed the cell viability was more than 85%, relative to the untreated control group, up to a phospholipid concentration of 200 nMoles. However, the cell viability decreased at 600 nMoles phospholipid concentration to 69.04 ± 0.75%, 69.51 ± 1.27%, and 69.95 ± 1.43%, in U87, bEnd.3, and primary glial cells, respectively (Fig. 3A, B and C). This can be attributed to the cationic charge of Pen at the surface of liposomes [7]. In addition, irrespective of the type of cells, the positive charge of Pen liposomes demonstrated higher cytotoxicity compared to plain, Tf and Tf-Pen liposomes.
Fig. 3.
In vitro biocompatibility and cellular uptake of liposomes. In vitro cell viability plots of plain, Tf, Pen, and Tf-Pen liposomes on (A) U87, (B) bEnd.3 and (C) Glial cells. Statistically significant (p < 0.05) differences are shown as (*) with plain liposomes and (†) with Pen-liposomes. Data represented as mean ± SD, (n=4). (D) Fluorescence images (10X magnification) demonstrated uptake of lissamine rhodamine labeled liposomes (red; excitation/emission wavelengths: 560/583 nm) in U87, bEnd.3 and Glial cells after 2 h incubation. Nuclei were stained with Hoechst 33342 (blue; excitation/emission wavelengths: 350/461 nm). Cellular uptake plots of (E) Dox and (F) Erlo encapsulated liposomes in U87, bEnd.3 and Glial cells after 2 h incubation. Data represented as mean ± SD, (n=4). Statistically significant (p < 0.05) differences are shown as (*) with plain liposomes, with (#) Erlo and (†) Dox.
3.2. Cellular Uptake Assessment
Liposomal uptake was determined in three different type of cells, qualitatively as well as quantitatively. As shown in the Fig. 3D, Tf-Pen liposomes labeled with lissamine rhodamine demonstrated greater and stronger fluorescence pattern throughout the cytoplasm as well as nucleus in comparison to plain liposomes in all three different cell lines. The uptake of Tf liposomes was not significantly (p > 0.05) different than Pen liposomes. The quantitative estimation of the uptake of Dox and Elro loaded liposomes showed more than 73% of cellular uptake in all three types of cells, which further confirmed the efficacy of Tf-Pen liposomes over single ligand or plain liposomes (Fig. 3E and F). This can be explained by the dual mechanism (receptor targeting or cell penetration) for liposomal uptake over single mechanism. The uptake of Tf-Pen liposomes is believed to be a combination effect of both Tf receptor and adsorptive mediated transcytosis that enabled increased cellular uptake. Electrostatic interactions enable the uptake of positively charged Pen-liposomes by binding with the negatively charged heparin sulfate proteoglycans on the cell membrane via adsorptive mediated transcytosis [33]. Thus, the cellular uptake of Tf-Pen liposomes displayed a synergistic approach of binding of Pen to cell membrane followed by binding of Tf to Tf receptor led to enhanced cellular uptake. The results show a rapid and higher uptake of Tf-Pen liposomes in U87 cells compared to bEnd.3 and glial cells, this could be due to the presence of higher density ofTf receptor on U87 cells.
3.3. Hemocompatibility Study
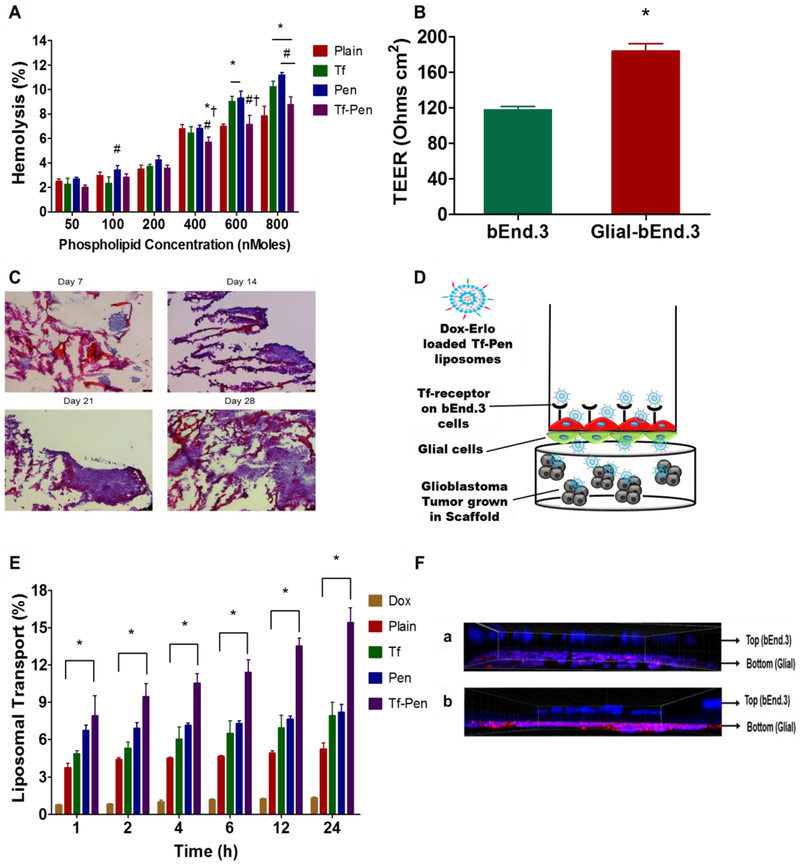
Our liposomal formulation is designed for intravenous administration, therefore it is important to determine its hemocompatibility prior to in vivo administration. The nonspecific interactions of cationic charged liposomes with erythrocytes may trigger lysis of red blood cells and consequently release of hemoglobin upon damage to these cells. Such nonspecific interactions result in various adverse effects such as embolization, thrombosis, as well as reduced half-life and reproducibility of medication [34,35]. The biocompatibility of liposomes was determined by measuring the absorbance of hemoglobin released from erythrocytes after incubating with different concentrations of phospholipid. The results showed increase in the percent hemolysis at higher concentrations of phospholipid. The Tf-Pen liposomes demonstrated only 9% of hemolysis at 800 nMoles of phospholipid concentration (Fig. 4A). However, at the same phospholipid concentration, the Pen-liposomes demonstrated significantly higher hemolysis. This can be attributed to the greater interaction of cationic charged Pen with the negatively charged erythrocytes membrane [7]. Tf-liposomes demonstrated lower hemolysis at low phospholipid concentrations as compared to Pen-liposomes. This is probably due to the presence of negative charge of Tf present on the surface of liposomes, which thereby decreases the interactions with the erythrocyte membrane [36]. Conversely, at high phospholipid concentrations the Tf-liposomes revealed high hemolytic activity in comparison to plain liposomes. This can be explained by the aggregation of Tf-liposomes at high concentration, which results in nonspecific interactions and destabilization of erythrocyte membrane [36]. Over-all, up to 10% of hemolysis is considered as non-toxic and biocompatible [37]. Therefore, Tf-Pen liposomes are considered as non-toxic, biocompatible, and safe for in vivo administration.
Fig. 4.
Hemolysis study and transport of liposomes using in vitro brain tumor model. (A) Percent hemolysis plot of various liposomes on RBCs after 60 min incubation. Significant (p < 0.05) differences are shown as with (*) plain liposomes, (#) Tf-liposomes, and (‡) Pen-liposomes. (B) TEER value for the co-culture (glial and endothelial cells) and endothelial monolayer model only. Significant (p < 0.05) difference is shown, with (*) endothelial monolayer. (C) The histological evaluation of tumor cell proliferation in PLGA-chitosan scaffold at different time points (10X magnification). (D). Schematic of in vitro brain tumor model showing the transport of Tf-Pen liposomes across, the co-culture endothelial barrier, into the glioblastoma tumor growing in the scaffold. (E) Plot demonstrates the percent transport of different liposomes loaded with doxorubicin, across the in vitro brain tumor model. Significant (p < 0.05) difference is shown in the transport of Tf-Pen liposomes in comparison to (*) free drug. Data represented as mean ± SD, (n=4). (F) Confocal fluorescent images of co-culture endothelial barrier demonstrated the evidence of transport of (a) plain liposomes and (b) Tf-Pen liposomes (40X magnification).
3.4. Endothelial Barrier Integrity and Tumor growth
In the current study, an in vitro brain tumor model was designed to evaluate the transport as well as anti-tumor efficacy of the liposomes. The endothelial co-culture barrier was constructed by seeding brain endothelial cells and glial cells on the luminal and abluminal side of polyethylene terephthalate (PET) membrane of culture inserts, respectively. The transendothelial electrical resistance (TEER) method measures the electrical resistance across the monolayer and co-culture models to evaluate their integrity and permeability. The above non-invasive method can be used to determine the various stages of growth and differentiation of live cells. The TEER values for co-culture model (183.97 ± 8.23 Ω cm2) was found significantly (p < 0.05) higher than monolayer model (117.95 ± 3.67 Ω cm2) (Fig. 4B). This can be attributed to the upregulation and uniform localization of the junctional proteins around cell borders and the physical support by the glial cells, which are associated with high resistance of the co-culture model [7,38,39]. The high TEER value of co-culture model demonstrates the formation of a tighter barrier compared to the monolayer model. Therefore, the endothelial co-culture barrier model was used to study transport as well as anti-tumor efficacy of liposomes. In addition, the microscopic images of tumor grown scaffold demonstrated the gradual growth of U87 tumor in PLGA-chitosan scaffold, as depicted in Fig. 4C. The images showed the formation of tumor spheroids on day 21 of tumor growth. The hematoxylin-eosin staining of the tumor-scaffold showed the biocompatible and porous nature of the scaffold thus enabling attachment of U87 cells leading to tumor growth in a 3-dimensional environment.
3.5. Liposomal Transport Across In Vitro Brain Tumor Model
The transport of liposomes was studied across the endothelial co-culture barrier layer using in vitro brain tumor model (Fig. 4D). The study was performed in 10% FBS to simulate in vivo like conditions. As shown in (Fig. 4E), the drug loaded Tf-Pen liposomes revealed 15.39 ± 1.19% transport across the endothelial co-culture barrier into the tumor grown scaffold which is significantly higher (p < 0.05) than single ligand (8.21 ± 0.62% for Pen liposomes and 7.91 ± 1.09 % for Tf liposomes) or plain liposomes (5.25 ± 0.46%), over a period of 24 h. Tf liposomes demonstrated the transport via receptor mediated transcytosis after binding to Tf receptor, while Pen liposomes were postulated to transport by the electrostatic interaction of cationic charged Pen liposomes to negatively charged cell membrane via adsorptive mediated transcytosis. In addition, detailed investigation into the uptake mechanism revealed the major pathway of the transport of Tf-Pen and Tf liposomes is clathrin mediated uptake, while for Pen-liposomes it is macropinocytosis and clathrin coated vesicles mediated uptake [21]. Conversely, the presence of serum protein can interfere with the initial binding of the Pen liposomes. In addition, the specific binding of Tf liposomes with its receptor may eliminate the nonspecific interaction with serum protein. Thus, Tf-Pen liposomes demonstrated increased transport and emphasized the significance of dual mechanism of uptake via receptor as well as adsorption mediated transcytosis across the in vitro brain tumor model. The transport of Tf-Pen liposomes across in vitro brain tumor model was further confirmed by incubating the model with lissamine rhodamine labeled liposomes for 24 h. The endothelial co-culture barrier was fixed with cold methanol. The z-stacks of confocal microscope demonstrated the uptake of liposomes by bEnd.3 cells (luminal side) on transwell culture insert and transported across the PET membrane or the glial cells (abluminal side). Apart from the cellular uptake of liposomes, the fluorescence of lissamine rhodamine labeled Tf-Pen liposomes demonstrated excellent evidence of transcytosis across the in vitro brain tumor model in comparison to plain liposomes, as depicted in Fig. 4E.
3.6. In Vitro Anti-Tumor Efficacy of Liposomes
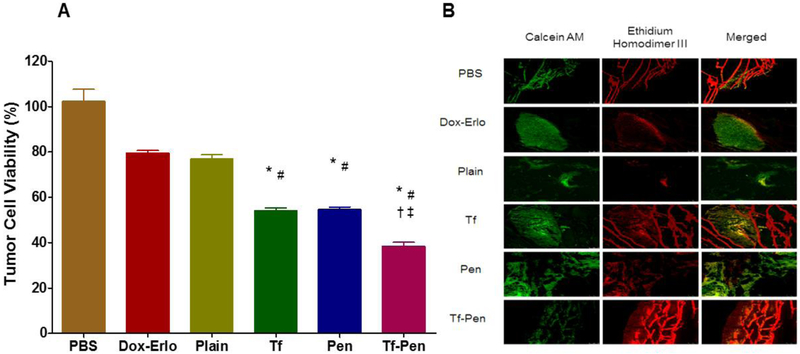
The anti-tumor efficacy of Tf-Pen liposomes was evaluated using a robust in vitro brain tumor model by evaluating the glioblastoma tumor regression housed in the scaffold. The percent tumor cell viability was quantified using MTT assay and confirmed qualitatively by live/dead cell imaging. The in vitro brain tumor model was comprised of tightly packed endothelial barrier (co-culture) placed on glioblastoma housed in PLGA-chitosan scaffold. The model demonstrated in vivo like conditions, where the formulation has to cross the endothelial barrier prior reaching the glioblastoma tumor inside scaffold. The porous nature of PLGA-chitosan scaffold helps glioblastoma cells to attach inside scaffold in a 3-dimensional environment. The model was treated with Dox and Erlo loaded liposomes on day 21 of tumor inoculation for a period of 24 h. Subsequently, the tumor housed scaffold was incubated with fresh DMEM containing 30% FBS, replaced every other day. On day 28 of tumor inoculation, the percent tumor cell viability in the scaffold was found to be decreased to 76.88 ± 3.80% and 38.5 ± 3.3% for Dox-Erlo loaded plain liposomes and Dox-Erlo loaded Tf-Pen liposomes, respectively. In addition, the co-delivery of Dox and Erlo from Tf-Pen liposomes demonstrated significantly (p < 0.05) lower the percent tumor cell viability as compared to single drug loaded Tf-Pen liposomes (Fig. S2). Thus, the results revealed the potential of combination therapy in enhancing anticancer activity, thereby showing better regression of glioblastoma tumor. As depicted in Fig. 5A, Tf-Pen liposomes demonstrated significant decrease (p < 0.05) in the percent cell viability compared to single ligand or plain liposomes. The results showed Tf-Pen liposomes were efficiently translocated across the endothelial barrier through dual mechanisms of receptor facilitated and increased cell penetration, thereby reaching the glioblastoma tumor and co-delivering Dox and Erlo from liposomes to the glioblastoma tumor in the scaffold. In addition, the antitumor efficacy of Tf-Pen liposomes was further confirmed through live/dead cells imagining. The fluorescence images of the treated scaffold sections showed mostly dead tumor cells (Fig. 5B). Based on the results from this experiment, it can be concluded that the Tf-Pen liposomes demonstrated excellent antitumor efficacy by translocating across the endothelial barrier and co-delivering Dox and Erlo to the glioblastoma tumors inside the scaffold.
Fig. 5.
In vitro anti-tumor efficacy of liposomes. (A) Plot demonstrates the percent tumor cell viability after 24 h treatment with different Dox and Erlo loaded liposomes using an in vitro brain tumor model. Statistically significant (p < 0.05) differences with (*) plain liposomes, (#) free Dox-Erlo, (‡) Tf-liposomes, and (†) Pen-liposomes were observed. Data represented as mean ± SD, (n=4). (B) The fluorescence images show tumor cell death in scaffold after treatment.
3.7. In Vivo Biocompatibility Study
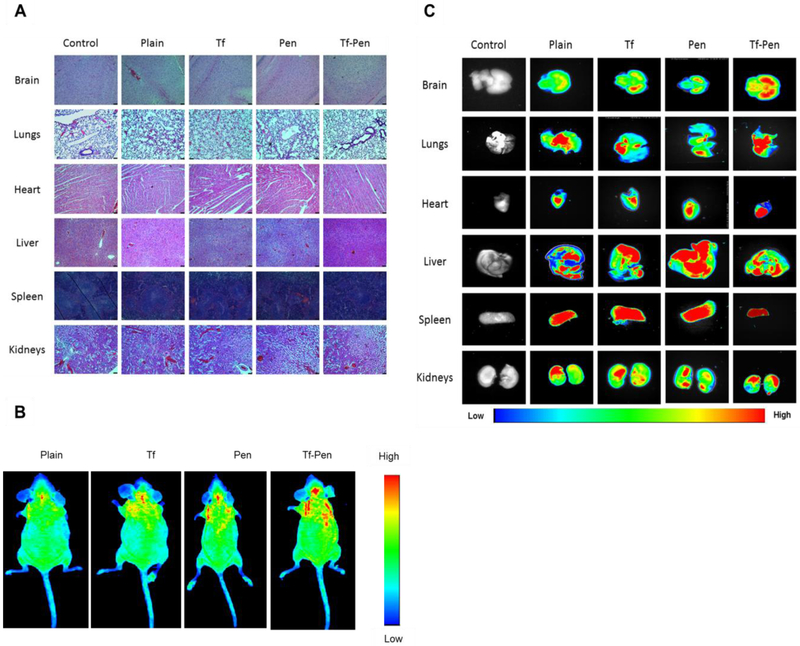
Non-specific interaction and higher penetration of cell penetrating peptides have been reported toxicity in highly perfused organs [40]. Therefore, in vivo biocompatibility of various liposomal formulation was evaluated by histological examination of tissues. The tissue sections from the mice administered with PBS were used as a control. As depicted in Fig. 6A, tissue sections stained with hematoxylin-eosin demonstrated no evidence of change in morphological appearance. Furthermore, there were no signs of tissue necrosis, inflammation or nuclei enlargement after examining tissue sections of different organs as compared to the tissue sections from control group. Liver, spleen and heart sections were carefully examined for histological changes. The histological examination of liver showed no signs of inflammation, ballooning of hepatocytes or enlargement of nuclei while spleen sections confirmed no evidence of necrosis. In addition, the histological examination of heart demonstrated no signs of myofibrillar loss or diffuse fibrosis of myocardium. Other organs such as brain, lungs and kidneys were also histologically examined for any signs of necrosis, lesions or inflammation. The dose of liposomes was calculated based on the in vitro biocompatibility study, 200 nMoles of phospholipid concentration demonstrated more than 85% of cell viability. We estimated the blood volume in mouse using Lee and Blaufox equation: BV = 0.06 × BW + 0.77, Where, BV is the blood volume in mouse and BW is the mouse body weight [41]. The blood volume was found to be in the range from 1.97 to 2.27 ml for 5 weeks old mice (20 – 25 g). The injected dose of liposomes was approximately 304 – 380 nMoles for mice weighing 20 – 25 g with approximate 2 ml of blood volume. Additionally, the results from the hemocompatibility study demonstrated that the Tf-Pen liposomes up to 800 nMoles of phospholipid concentration was observed to be safe in a volume of 1 ml of PBS with 1.5 × 107 erythrocytes. Therefore, the dose of the liposomes injected in mice was significantly below the hemolytic concentration of phospholipid and are suitable for in vivo administration. Thus, the Tf-Pen liposomes administered at a dose of 15.2 μmoles of phospholipid/kg of body weight demonstrated no signs of toxicity in any of the tissues from mice.
Fig. 6.
In vivo biocompatibility and biodistribution of liposomes. (A) Histological examination of different tissues after injected with different liposomes. The images were taken at 10X magnification. The tissue sections from mice administered PBS were considered as controls. (B) In vivo fluorescence imaging of mice at 24 h post intravenous injection. (C) Ex-vivo fluorescence imaging of different organs isolated from mice after 24 h invtravenous injection.
3.8. In Vivo Biodistribution of Liposomes
The biodistribution of Tf-Pen liposomes was studied qualitatively as well as quantitatively after 24 h of intravenous administration of liposomes. The lissamine rhodamine labeled liposomes were tracked by an in vivo imaging system which images the whole mice body as well as various organs to study the biodistribution. The results from in vivo imaging of mice (Fig. 6B) showed the higher fluorescent intensity of Tf-Pen liposomes in the brain as compared to plain or single ligand liposomes which demonstrated the accumulation of liposomes. In addition, the ex vivo images of organs also demonstrated the strong fluorescence of Tf-Pen liposomes in the brain (Fig. 6C). As expected, a strong fluorescent signal was observed in liver and spleen after 24 h. Further, the liposomes accumulation in various organs were quantified by homogenization of organs followed by extraction of drugs. Drugs loaded plain liposomes were used as a passive control. Accumulation of drugs loaded liposomal tissue samples were analyzed using HPLC. Doxorubicin, a hydrophilic drug molecule is unable to cross the BBB in vivo, while erlotinib, a hydrophobic drug is able to cross the BBB. The dual functionalized, Tf-Pen liposomes demonstrated maximum brain penetration of Dox (~5.5 % ID/gram of tissue) and Erlo (~5.79 % ID/gram of tissue) as compared to free drugs (~0.45 % ID/gram of tissue for Dox and ~1.70 % ID/gram of tissue for Erlo). Therefore, the biodistribution of Dox and Erlo loaded Tf-Pen liposomes showed more than 12 and 3.3-fold increase in Dox and Erlo accumulation in mice brain, respectively which is significantly (p < 0.05) higher compared to administration of free drugs (Fig. 7A and B). This showed that incorporation of Pen to liposomes significantly increased the accumulation of Tf-Pen liposomes in brain by translocating across the BBB effectively and more efficiently compared to other liposomal formulations. The Tf-Pen liposomes also showed higher accumulation in liver, spleen and heart 24 h post intravenous injection. The percent injected dose per gram of tissue accumulated in liver, spleen, heart, kidneys and lungs were found to be ~ 20%, 10%, 14%, 7% and 6% respectively. The Tf receptors are also present in liver, spleen and heart which triggered the uptake of Tf-liposomes in these organs [42,43]. Moreover, liver and spleen are considered to be the major macrophage organs and thus, the intravenously injected liposomes were eliminated rapidly through these organs [42,43]. However, the surface modification of liposomes with Tf and Pen improved the transport of liposomes to brain. It can be seen from the results that the negatively charged Tf-liposomes circulated longer in the blood however, the accumulation of drugs in the brain is significantly lesser (p < 0.05) than Tf-Pen liposomes. Pen-liposomes were accumulated in liver and spleen due to non-specific interactions and cationic charge of penetratin. In addition, free drugs (Dox and Erlo) and plain liposomes were majorly transported to liver, spleen, kidneys and heart, and demonstrated less accumulation in brain. In conclusion, the incorporation of Pen in combination with the Tf receptor targeting ability of Tf into the Tf-Pen liposomes, resulted in higher transport across the BBB and higher accumulation in the brain through dual mechanisms of receptor mediated transcytosis and enhanced cell penetration.
Fig. 7.
Bar graphs representing the biodistrubition of Dox and Erlo loaded liposomes at 24 h time point after intravenous injection. (A) The biodistribution of Dox and (B) the biodistribution of Erlo. The data are expressed as percent injected dose (% ID)/gram of tissue; (mean ± SD; n = 6). Statistically significant (p < 0.05) differences with (#) plain liposomes, (*) free drugs, (‡) Tf-liposomes, and (†) Pen-liposomes were observed.
3.9. In vivo Anti-Tumor Efficacy
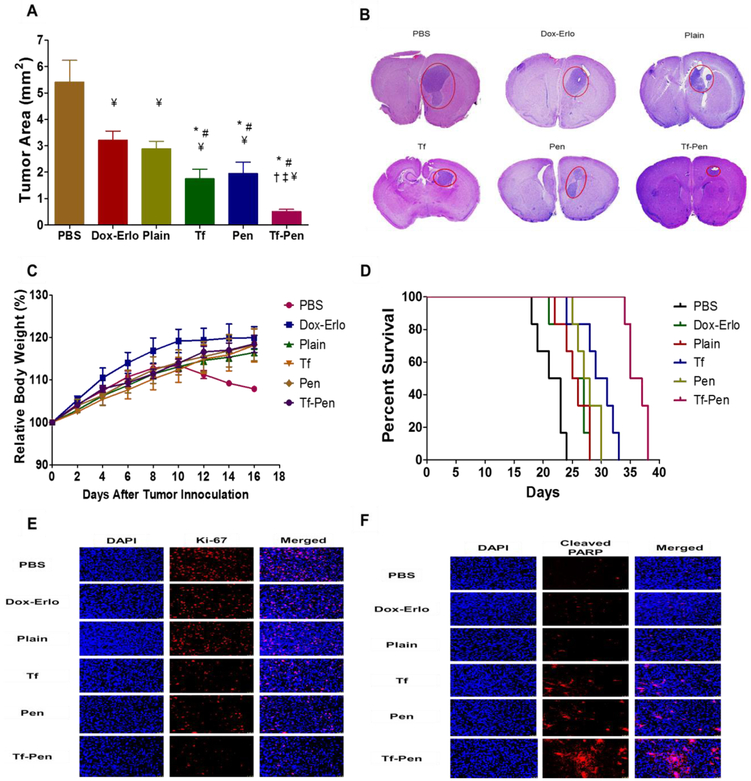
The orthotopic brain tumor mice model is an appropriate and efficient model to evaluate the antitumor efficacy for targeted therapies against GBM. This model replicates both relevant signaling pathway alterations and the histopathological features of human GBM. Furthermore, in orthotopic implanted tumors the process of metastasis is efficient and mimics human metastasis [44]. Therefore, we believe that by using this model we were able to mimic human GBM and our results are likely to resemble the activity of the delivery system in patients with GBM. The antitumor efficacy of Dox and Erlo loaded Tf-Pen liposomes was evaluated in intracranial glioblastoma bearing nude mice. As shown in Fig. 8A, Dox and Erlo loaded Tf-Pen liposomes demonstrated significant (p < 0.05) regression of tumor area (0.54 ± 0.07 mm2) as compared to free drugs (3.2 ± 0.33 mm2), plain (2.8 ± 0.28 mm2) or single ligand liposomes (1.75 ± 0.356 mm2 for Tf-liposomes and 1.95 ± 0.43 mm2 for Pen-liposomes). The tumor area of mice administered with PBS was considered as a control. These results demonstrate that the Dox-Erlo loaded Tf-Pen liposomes efficiently translocated across the BBB and co-delivered drugs to glioblastoma tumor in vivo, thereby achieving significant reduction in tumor burden in comparison to plain or single ligand liposomes. The H&E stained tumor sections of mice brain in Figure 8B, confirmed that the Tf-Pen liposomes were more efficient in regressing tumor with a tumor inhibition of ~ 90% as compared to control (PBS). There are several published reports demonstrating therapeutic efficacy using Tf modified delivery systems to treat gliomas [45-48]. However, the translocation was limited due to receptor saturation, endosomal entrapment and loss of specificity by formation of a protein corona on ligand by other proteins present in a complex biological microenvironment, resulting in restricted transport of delivery system across the BBB [10,13,45,48,49]. Additionally, several other published reports with single drug loaded dual functionalized delivery systems have also shown therapeutic efficacy in treating glioblastoma [50-52]. Furthermore, they were also restricted with drug resistance in the tumor cells. Thus, we believe that the co-delivery of drugs (Dox and Erlo) through dual functionalized liposomal nanoparticulate system is superior and efficient in overcoming all the above mentioned limitations, without eliciting undesired toxicity. Moreover, the biodistribution of Tf-Pen liposomes showed more than 12 and 3.3-fold increase in Dox and Erlo accumulation in mice brain, respectively compared to tree-drugs (Fig. 7A & B). This demonstrated that the translocation of Tf-Pen liposomes was not affected by either receptor saturation, endosomal entrapment or loss of specificity. The high translocation of Tf-Pen liposomes across the BBB was followed by their excellent targeting and penetrating ability into glioblastoma tumors which led to significant decrease in tumor burden (Fig. 8A & B). Furthermore, the percent relative body weight of mice showed no significant difference in the treatment groups while PBS group demonstrated higher weight loss due to the aggressive invasion of GBM into the brain and deteriorating health of the animal (Fig. 8C). In addition, the results demonstrate no weight loss and maintained body conditioning, which are the signs of normal liver function regardless of high distribution of liposomes in liver [53]. Kaplan-Meier survival curve (Figure 7D) demonstrated that the median survival of mice treated with Dox-Erlo loaded Tf-Pen liposomes (36 days) was significantly (p < 0.05) longer as compared to Dox-Erlo loaded Tf liposomes (30 days) and Dox-Erlo loaded Pen liposomes (27.5 days). In contrast, the animals in the control group (PBS) survived only 22 days. The Dox-Erlo loaded plain liposomes (25.5days) and free drugs, Dox-Erlo (25 days) showed slight improvement than PBS group in the median survival time of mice. These results indicate the superior efficacy of Tf-Pen liposomes to achieve receptor and adsorptive mediated transcytosis across the BBB and accumulate at the glioblastoma tumor site, thereby achieving tumor control and survival in glioblastoma bearing mice. Additionally, the co-delivery of Dox and Erlo from Tf-Pen liposomes demonstrated excellent potential of combination therapy in enhancing the antitumor efficacy on tumor regression and significant (p < 0.05) increase in the median survival time in comparison to single drug therapy (Fig. S3-S5). The potential of combination therapy is in targeting different pathways, thereby demonstrating their effect on the proliferation and death rates of tumor cells as well as on the number of point mutations which are responsible for resistance. Thus, our results revealed the efficient targeting ability of Tf-Pen liposomes and co-delivery of Dox and Erlo appear to be an excellent strategy than single ligand or single drug approach.
Fig. 8.
Antitumor efficacy in intracranial glioblastoma bearing nude mice. (A). Graph demonstrates the tumor regression in mice brain after 3 doses of treatment. Data represented as mean ± SD; n = 6. Statistically significant (p < 0.05) differences with (*) plain liposomes, (#) free Dox-Erlo, (‡) Tf-liposomes, and (†) Pen-liposomes were observed. (B) Histological sections of brain display the tumor regression (in red circle) after treatment. The images were taken at 20X magnification. The brain section from mice administered PBS was considered as a control. (C) Graph represents the relative body weight of mice during treatment after tumor inoculation. Data represented as mean ± SD; n = 6. (D) Kaplan-Meier survival curves of mice after treatment (n = 6). (E) Immunofluorescence staining for Ki-67 on mice brain glioblastoma site for tumor cell proliferation. (F) Immunofluorescence staining for cleaved PARP on mice brain glioblastoma site for tumor cell apoptosis. The images were taken at 20X magnification.
Immunofluorescence staining for Ki-67 and cleaved poly (ADP-ribose) polymerase (PARP) for glioblastoma bearing mice brain sections of treated groups were also observed for assessing tumor cells proliferation and apoptosis, respectively. As shown in Fig. 8E, glioblastoma bearing mice brain sections treated with Dox-Erlo loaded Tf-Pen liposomes showed significantly lesser Ki-67 positive cells in comparison to PBS control group demonstrating the presence of fewer number of proliferating cells. In addition, the cleaved PARP is an established marker to detect apoptosis [54,55]. The glioblastoma bearing mice group treated with Dox-Erlo loaded Tf-Pen liposomes produced significantly higher numbers of cleaved PARP apoptotic cells on the surface as well as at the core of the GBM than the control (PBS) group (Fig. 8F). These images revealed the efficient binding of Tf-Pen liposomes on tumors which resulted in effective release of drugs to tumors, thereby showing increased apoptosis with decreased proliferation of tumors as compared to single ligand or plain liposomes. Therefore, these results demonstrate the excellent antitumor efficacy of the Tf-Pen liposomes in crossing the BBB and co-delivering anticancer chemotherapeutics to brain in achieving tumor regression and survival in mice bearing glioblastoma.
4. Conclusion
In this study, we successfully prepared and characterized the dual functionalized liposomes by modifying their surface with Tf for receptor targeting and Pen for enhanced cell penetration for the co-delivery of Dox and Erlo for the treatment of invasive brain gliomas. Insertion of Pen to Tf-liposomes revealed excellent biocompatibility as well as high cellular uptake, in vitro. This study demonstrates the efficient translocation of the dual functionalized liposomes across the BBB, thereby showing high concentration of anti-cancer chemotherapeutic drugs in mice brain. In addition, these liposomes displayed excellent antitumor efficacy in treating invasive brain gliomas by significantly increasing the mice survival time as well as significant regression of glioblastoma tumor in mice brain. Therefore, we believe that this study would have high impact for co-delivering of chemotherapeutics across the BBB for treating patients with glioblastoma.
Supplementary Material
Fig. 2.
(A). Size distribution of different liposomes as obtained from dynamic light scattering. (B) Morphological analysis of Tf-Pen liposomes observed using atomic force microscopy.
Highlights:
Transferrin-Penetratin (Tf-Pen) liposomes were prepared by post-insertion method.
Tf-Pen liposomes showed excellent biocompatibility for in vivo administration.
Higher translocation of Tf-Pen liposomes across the co-culture endothelial barrier.
Several fold increase in the concentration of anticancer drugs in mice brain.
Increase in survival time and regression in glioblastoma tumor in mice brain.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant RO1 AG051574.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS, Breast Cancer Metastasis to the Central Nervous System, Am. J. Pathol 167 (2005) 913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Johnson DR, O’Neill BP, Glioblastoma survival in the United States before and during the temozolomide era., J. Neurooncol 107 (2012) 359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- [3].Donahue MJ, Blakeley JO, Zhou J, Pomper MG, Laterra J, van Zijl PCM, Evaluation of human brain tumor heterogeneity using multiple T1-based MRI signal weighting approaches., Magn. Reson. Med 59 (2008) 336–344. doi: 10.1002/mrm.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lichter AS, Lawrence TS, Recent Advances in Radiation Oncology, N. Engl. J. Med 332 (1995) 371–379. doi: 10.1056/NEJM199502093320607. [DOI] [PubMed] [Google Scholar]
- [5].Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R, Nanocarriers as an emerging platform for cancer therapy., Nat. Nanotechnol 2 (2007) 751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- [6].Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF, Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain, Expert Rev. Mol. Med 13 (2011) e17. doi: 10.1017/S1462399411001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lakkadwala S, Singh J, Dual Functionalized 5-Fluorouracil Liposomes as Highly Efficient Nanomedicine for Glioblastoma Treatment as Assessed in an In Vitro Brain Tumor Model, J. Pharm. Sci 107 (2018) 2902–2913. doi: 10.1016/j.xphs.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lakkadwala S, Singh J, Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model, Colloids Surfaces B Biointerfaces. 173 (2019) 27–35. doi: 10.1016/j.colsurfb.2018.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qian ZM, Li H, Sun H, Ho K, Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway., Pharmacol. Rev 54 (2002) 561–587. [DOI] [PubMed] [Google Scholar]
- [10].Sharma G, Modgil A, Sun C, Singh J, Grafting of cell-penetrating peptide to receptor-targeted liposomes improves their transfection efficiency and transport across blood-brain barrier model, J. Pharm. Sci 101 (2012) 2468–2478. doi: 10.1002/jps.23152. [DOI] [PubMed] [Google Scholar]
- [11].Prabhakar K, Afzal SM, Kumar PU, Rajanna A, Kishan V, Brain delivery of transferrin coupled indinavir submicron lipid emulsions-Pharmacokinetics and tissue distribution, Colloids Surfaces B Biointerfaces. 86 (2011) 305–313. doi: 10.1016/j.colsurfb.2011.04.013. [DOI] [PubMed] [Google Scholar]
- [12].Skarlatos S, Yoshikawa T, Pardridge WM, Transport of [125I]transferrin through the rat blood-brain barrier., Brain Res. 683 (1995) 164–171. [DOI] [PubMed] [Google Scholar]
- [13].Kibria G, Hatakeyama H, Ohga N, Hida K, Harashima H, Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery, J. Control. Release. 153 (2011) 141–148. doi 10.1016/j.jconrel.2011.03.012. [DOI] [PubMed] [Google Scholar]
- [14].Zong T, Mei L, Gao H, Cai W, Zhu P, Shi K, Chen J, Wang Y, Gao F, He Q, Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals, Mol. Pharm 11 (2014) 2346–2357. doi: 10.1021/mp500057n. [DOI] [PubMed] [Google Scholar]
- [15].Bolhassani A, Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer, Biochim. Biophys. Acta - Rev. Cancer. 1816 (2011)232–246. doi: 10.1016/j.bbcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- [16].Sharma G, Lakkadwala S, Modgil A, Singh J, The Role of Cell-Penetrating Peptide and Transferrin on Enhanced Delivery of Drug to Brain, Int. J. Mol. Sci 17 (2016) 806. doi: 10.3390/ijms17060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Derossi D, Joliot AH, Chassaing G, Prochiantz A, The third helix of the Antennapedia homeodomain translocates through biological membranes., J. Biol. Chem 269 (1994) 10444–10450. [PubMed] [Google Scholar]
- [18].Odabaei G, Chatterjee D, Jazirehi AR, Goodglick L, Yeung K, Bonavida B, Raf-1 kinase inhibitor protein: structure, function, regulation of cell signaling, and pivotal role in apoptosis., Adv. Cancer Res 91 (2004) 169–200. doi: 10.1016/S0065-230X(04)91005-6. [DOI] [PubMed] [Google Scholar]
- [19].Stuhlmiller TJ, Miller SM, Zawistowski JS, Nakamura K, Beltran AS, Duncan JS, Angus SP, Collins KAL, Granger DA, Reuther RA, Graves LM, Gomez SM, Kuan P-F, Parker JS, Chen X, Sciaky N, Carey LA, Earp HS, Jin J, Johnson GL, Inhibition of Lapatinib-Induced Kinome Reprogramming in ERBB2-Positive Breast Cancer by Targeting BET Family Bromodomains., Cell Rep. 11 (2015) 390–404. doi: 10.1016/j.celrep.2015.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li X, Ding L, Xu Y, Wang Y, Ping Q, Targeted delivery of doxorubicin using stealth liposomes modified with transferrin, Int. J. Pharm 373 (2009) 116–123. doi: 10.1016/j.ijpharm.2009.01.023. [DOI] [PubMed] [Google Scholar]
- [21].Sharma G, Modgil A, Zhong T, Sun C, Singh J, Influence of short-chain cell-penetrating peptides on transport of doxorubicin encapsulating receptor-targeted liposomes across brain endothelial barrier, Pharm. Res 31 (2014) 1194–1209. doi: 10.1007/s11095-013-1242-x. [DOI] [PubMed] [Google Scholar]
- [22].Ying X, Wen H, Lu W-L, Du J, Guo J, Tian W, Men Y, Zhang Y, Li R-J, Yang T-Y, Shang D-W, Lou J-N, Zhang L-R, Zhang Q, Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals., J. Control. Release. 141 (2010) 183–192. doi: 10.1016/j.jconrel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- [23].Wang S-C, Yu C-F, Hong J-H, Tsai C-S, Chiang C-S, Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis., PLoS One. 8 (2013) e69182. doi: 10.1371/journal.pone.0069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nag O, Awasthi V, Surface Engineering of Liposomes for Stealth Behavior, Pharmaceutics. 5 (2013) 542–569. doi: 10.3390/pharmaceutics5040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iden DL, Allen TM, In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion approach., Biochim. Biophys. Acta. 1513 (2001) 207–216. [DOI] [PubMed] [Google Scholar]
- [26].Moreira JN, Ishida T, Gaspar R, Allen TM, Use of the post-insertion technique to insert peptide ligands into pre-formed stealth liposomes with retention of binding activity and cytotoxicity., Pharm. Res 19 (2002) 265–269. [DOI] [PubMed] [Google Scholar]
- [27].Torchilin VP, Khaw BA, Smirnov VN, Haber E, Preservation of antimyosin antibody activity after covalent coupling to liposomes, Biochem. Biophys. Res. Commun 89 (1979) 1114–1119. doi: 10.1016/0006-291X(79)92123-5. [DOI] [PubMed] [Google Scholar]
- [28].Marqués-Gallego P, De Kroon AIPM, Ligation strategies for targeting liposomal nanocarriers, Biomed Res. Int 2014 (2014) 12. doi 10.1155/2014/129458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barenholz Y. (Chezy), Doxil® — The first FDA-approved nano-drug: Lessons learned, J. Control. Release. 160 (2012) 117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- [30].Chen Y, Liu L, Modern methods for delivery of drugs across the blood-brain barrier, Adv. Drug Deliv. Rev 64 (2012) 640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- [31].Zhao B-X, Zhao Y, Huang Y, Luo L-M, Song P, Wang X, Chen S, Yu K-F, Zhang X, Zhang Q, The efficiency of tumor-specific pH-responsive peptide-modified polymeric micelles containing paclitaxel., Biomaterials. 33 (2012) 2508–2520. doi: 10.1016/j.biomaterials.2011.11.078. [DOI] [PubMed] [Google Scholar]
- [32].Wang Z, Yu Y, Dai W, Lu J, Cui J, Wu H, Yuan L, Zhang H, Wang X, Wang J, Zhang X, Zhang Q, The use of a tumor metastasis targeting peptide to deliver doxorubicin-containing liposomes to highly metastatic cancer, Biomaterials. 33 (2012) 8451–8460. doi: 10.1016/j.biomaterials.2012.08.031. [DOI] [PubMed] [Google Scholar]
- [33].Trabulo S, Cardoso AL, Mano M, De Lima MCP, Cell-Penetrating Peptides-Mechanisms of Cellular Uptake and Generation of Delivery Systems, Pharmaceuticals (Basel). 3 (2010) 961–993. doi: 10.3390/ph3040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Antohi S, Brumfeld V, Polycation-cell surface interactions and plasma membrane compartments in mammals. Interference of oligocation with polycationic condensation., Zeitschrift Fur Naturforschung. Sect. C, Biosci 39 (1984) 767–775. [DOI] [PubMed] [Google Scholar]
- [35].Zhu S, Qian F, Zhang Y, Tang C, Yin C, Synthesis and characterization of PEG modified N-trimethylaminoethylmethacrylate chitosan nanoparticles, Eur. Polym. J 43 (2007) 2244–2253. doi: 10.1016/j.eurpolymj.2007.03.042. [DOI] [Google Scholar]
- [36].Sharma G, Modgil A, Layek B, Arora K, Sun C, Law B, Singh J, Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: Biodistribution and transfection, J. Control. Release. 167 (2013) 1–10. doi: 10.1016/j.jconrel.2013.01.016. [DOI] [PubMed] [Google Scholar]
- [37].Amin K, Dannenfelser R-M, In vitro hemolysis: Guidance for the pharmaceutical scientist, J. Pharm. Sci 95 (2006) 1173–1176. doi: 10.1002/jps.20627. [DOI] [PubMed] [Google Scholar]
- [38].Janzer RC, Raff MC, Astrocytes induce blood–brain barrier properties in endothelial cells, Nature. 325 (1987) 253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- [39].Arthur FE, Shivers RR, Bowman PD, Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model, Dev. Brain Res 36 (1987) 155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- [40].Ramana LN, Sethuraman S, Ranga U, Krishnan UM, Development of a liposomal nanodelivery system for nevirapine., J. Biomed. Sci 17 (2010)57. doi: 10.1186/1423-0127-17-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lee HB, Blaufox MD, Blood volume in the rat., J. Nucl. Med 26 (1985) 72–76. [PubMed] [Google Scholar]
- [42].Deaglio S, Capobianco A, Cali A, Bellora F, Alberti F, Righi L, Sapino A, Camaschella C, Malavasi F, Structural, functional, and tissue distribution analysis of human transferrin receptor-2 by murine monoclonal antibodies and a polyclonal antiserum., Blood. 100 (2002) 3782–3789. doi: 10.1182/blood-2002-01-0076. [DOI] [PubMed] [Google Scholar]
- [43].Jefferies WA, Brandon MR, V Hunt S, Williams AF, Gatter KC, Mason DY, Transferrin receptor on endothelium of brain capillaries., Nature. 312 (1984) 162–163. [DOI] [PubMed] [Google Scholar]
- [44].Killion JJ, Radinsky R, Fidler IJ, Orthotopic models are necessary to predict therapy of transplantable tumors in mice., Cancer Metastasis Rev 17 (1999) 279–284. [DOI] [PubMed] [Google Scholar]
- [45].Jhaveri A, Deshpande P, Pattni B, Torchilin V, Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma, J. Control. Release. 277 (2018) 89–101. doi: 10.1016/j.jconrel.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cui Y, Xu Q, Chow PK-H, Wang D, Wang C-H, Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment, Biomaterials. 34 (2013) 8511–8520. doi: 10.1016/j.biomaterials.2013.07.075. [DOI] [PubMed] [Google Scholar]
- [47].Gan CW, Feng S-S, Transferrin-conjugated nanoparticles of Poly(lactide)-d-α-Tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the bloodαbrain barrier, Biomaterials. 31 (2010) 7748–7757. doi: 10.1016/j.biomaterials.2010.06.053. [DOI] [PubMed] [Google Scholar]
- [48].Chen H, Qin Y, Zhang Q, Jiang W, Tang L, Liu J, He Q, Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas., Eur. J. Pharm. Sci 44 (2011) 164–173. doi: 10.1016/j.ejps.2011.07.007. [DOI] [PubMed] [Google Scholar]
- [49].Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Åberg C, Mahon E, Dawson KA, Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface, Nat. Nanotechnol 8 (2013) 137. [DOI] [PubMed] [Google Scholar]
- [50].Qin L, Wang C-Z, Fan H-J, Zhang C-J, Zhang H-W, Lv M-H, Cui S-D, A dual-targeting liposome conjugated with transferrin and arginine-glycine-aspartic acid peptide for glioma-targeting therapy., Oncol. Lett 8 (2014) 2000–2006. doi: 10.3892/ol.2014.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang P, Hu L, Yin Q, Feng L, Li Y, Transferrin-modified c[RGDfK]-paclitaxel loaded hybrid micelle for sequential blood-brain barrier penetration and glioma targeting therapy., Mol. Pharm 9 (2012) 1590–1598. doi: 10.1021/mp200600t. [DOI] [PubMed] [Google Scholar]
- [52].Wei X, Gao J, Zhan C, Xe C, Chai Z, Ran D, Ying M, Zheng P, Lu W, Liposome-based glioma targeted drug delivery enabled by stable peptide ligands., J. Control. Release. 218 (2015) 13–21. doi: 10.1016/j.jconrel.2015.09.059. [DOI] [PubMed] [Google Scholar]
- [53].Lam FC, Morton SW, Wyckoff J, Vu Han T-L, Hwang MK, Maffa A, Balkanska-Sinclair E, Yaffe MB, Floyd SR, Hammond PT, Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles, Nat. Commun 9 (2018) 1991. doi: 10.1038/s41467-018-04315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jo GH, Bögler O, Chwae Y-J, Yoo H, Lee SH, Park JB, Kim Y-J, Kim JH, Gwak H-S, Radiation-induced autophagy contributes to cell death and induces apoptosis partly in malignant glioma cells, Cancer Res. Treat. 47 (2015) 221–241. doi: 10.4143/crt.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Casao A, Mata-Campuzano M, Ordas L, Cebrian-Perez JA, Muino-Blanco T, Martinez-Pastor F, Cleaved PARP-1, an Apoptotic Marker, can be Detected in Ram Spermatozoa., Reprod. Domest. Anim 50 (2015) 688–691. doi: 10.1111/rda.12549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.