Abstract
Aqueous solutions of chlorpyrifos oxon are used to study the ability of chlorpyrifos oxon to catalyze protein crosslinking. Assays for protein crosslinking can avoid artifacts by using information on the stability of chlorpyrifos oxon in solution. We undertook to determine the half-life of chlorpyrifos oxon in aqueous solution because literature values do not exist. The rate of conversion of chlorpyrifos oxon to 3,5,6-trichloro-2-pyridinol was measured at 23°C in 20 mM TrisCl pH 8 and pH 9 by recording loss of absorbance at 290 nm for chlorpyrifos oxon and increase in absorbance at 320 nm for 3,5,6-trichloro-2-pyridinol. The half-life of chlorpyrifos oxon was 20.9 days at pH 8 and 6.7 days at pH 9. Literature reports for the stability of other organophosphorus toxicants were summarized because our current studies suggest that other organophosphorus toxicants are also crosslinking agents.
Keywords: chlorpyrifos oxon, stability, half-life, extinction coefficient, absorbance spectra
1. Introduction
Neurotoxic symptoms are associated with chronic, low-dose exposure to organophosphorus pesticides (Jamal et al., 2002; Kamel et al., 2007; Wang et al., 2014; Jokanovic, 2018; Naughton and Terry, 2018). Low dose exposure to the organophosphorus nerve agent, sarin, is hypothesized to explain Gulf War Illness (Golomb, 2008). We have developed a mechanism to explain neurotoxicity resulting from low dose exposure to organophosphorus toxicants (OP). We find that organophosphorus esters are crosslinking agents. Mass spectrometry analysis shows that proteins treated with chlorpyrifos oxon form stable covalent crosslinks between lysine and glutamic acid or lysine and aspartic acid (Schopfer and Lockridge, 2018; Schopfer and Lockridge, 2019). We hypothesize that crosslinked proteins form insoluble aggregates that disrupt neuronal function.
Our experimental protocol incubates proteins with OP in aqueous buffer. The question we addressed in this report is the stability of OP in aqueous buffer. We needed to know whether or not OP esters spontaneously degrade in a few hours. The published literature has information on the stability of many OP, but no information on the stability of chlorpyrifos oxon. We report that the half-life of chlorpyrifos oxon in pH 8 and pH 9 buffers at 23°C is 20.9 and 6.7 days, respectively.
2. Materials and Methods
2.1. Materials:
Chlorpyrifos oxon Chem Service MET 11459B
3,5,6-trichloro-2-pyridinol Chem Service MET-674A
Paraoxon ethyl Chem Service N-12816
Paraoxon methyl Chem Service N-11775
Diazoxon Chem Service MET-11621A
Human butyrylcholinesterase purified in house (Schopfer et al., 2019)
YM-10 centrifugal filters, 10,000 MW cut off (Merck Millipore MRCPRT010)
Slide-A-Lyzer cassettes, 7000 MW cut off, 0.5-3 mL capacity (Pierce 66370)
Cellulose dialysis tubing 12,000-14,000 MW cut off (Spectrapor 132700)
2.2. Absorbance spectra of chlorpyrifos oxon and 3,5,6-trichloro-2-pyridinol.
Stock solutions of 2 mM 3,5,6-trichloro-2-pyridinol (TCP) and 2 mM chlorpyrifos oxon (CPO) in acetonitrile were diluted into 20 mM TrisCl pH 8.0 to make 20 μM. Absorbance was read against water in matched 4 ml quartz cuvettes over the range 265 nm to 350 nm on a Gilford single-beam spectrophotometer at 23°C. Extinction coefficients were calculated from absorbance at 290 nm for CPO and 320 nm for 3,5,6-trichloro-2-pyridinol (TCP). Data in Figure 1.
Figure 1.
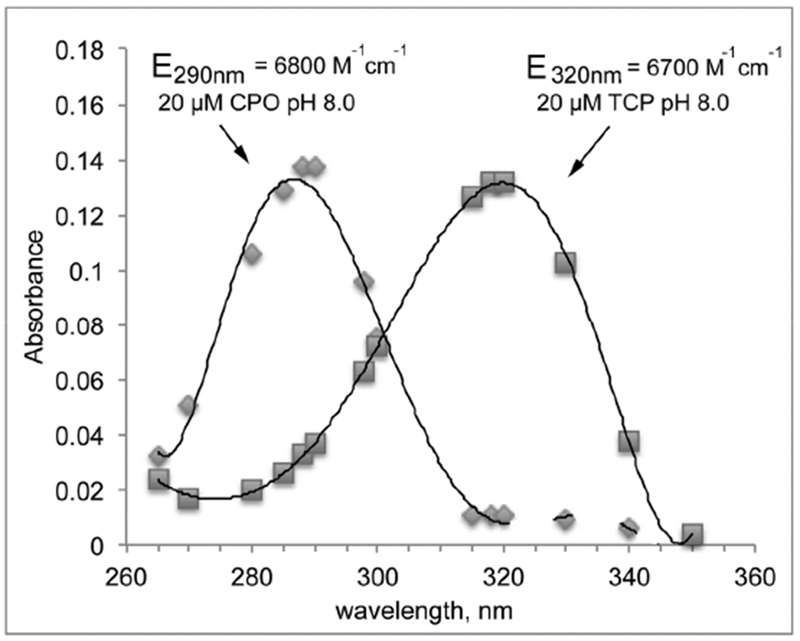
Absorbance spectra of 20 μM chlorpyrifos oxon (CPO) and 20 μM 3,5,6-trichloro-2-pyridinol (TCP) in 20 mM TrisCl pH 8.0 at 23°C, measured in a single-beam Gilford spectrophotometer in 4 ml quartz cuvettes. The symbols indicate the wavelengths at which absorbance was measured manually. Curves were drawn through the points with Excel software. Diamond symbols are absorbance values for 20 μM chlorpyrifos oxon. Squares are absorbance values for 20 μM 3,5,6-trichloro-2-pyridinol.
2.3. Hydrolysis of CPO by sodium hydroxide.
TCP was confirmed as the major hydrolysis product of CPO by acquiring a series of absorbance spectra over time for 28.8 μM CPO in 10 mM sodium hydroxide pH 11.8 at 23°C in a Cary 3 Bio UV-Visible double-beam spectrophotometer. The reference cuvette contained 0.1 M potassium phosphate pH 7.0. Data in Figure 2.
Figure 2.
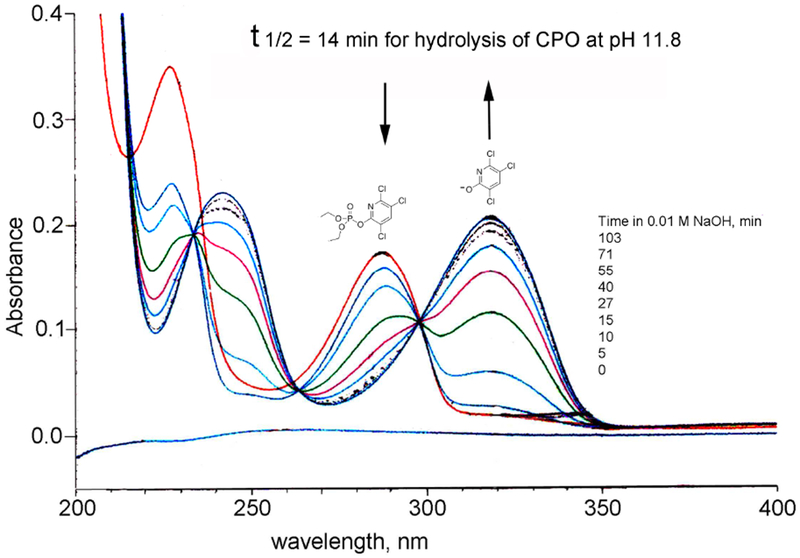
Hydrolysis of 28.8 μM chlorpyrifos oxon (CPO) to 3,5,6-trichloro-2-pyridinol (TCP) by 10 mM sodium hydroxide at 23°C. Arrows indicate disappearance of CPO at 290 nm and appearance of TCP at 320 nm. Conversion to TCP was complete in 103 min. The half-life of CPO at alkaline pH was 14 min. The extinction coefficient for TCP at 320 nm pH 11.8 was 7100 M−1 cm−1.
2.4. Stability of CPO in aqueous buffer
In our hands 300 mM CPO solutions in ethanol or acetonitrile are stable for years at −80°C. However, we suspected that CPO is unstable in aqueous buffers. We measured the stability of 20 μM CPO by recording the increase in absorbance at 320 nm, the wavelength for maximum absorbance of the TCP hydrolysis product. Duplicate 100 mL solutions of 20 μM CPO in 20 mM TrisCl, 0.01% azide pH 8.0 and pH 9.0 were prepared in tightly closed glass bottles stored in the dark at 23°C. Absorbance at 320 nm was read against water over a period of one month for aliquots transferred from the bottles to matched quartz cuvettes.
2.5. Calculation of decay rate constant and half-life
Absorbance at 320 nm of 20 μM TCP was 0.137 at pH 8 and at pH 9. The natural logarithm for the difference between 0.137 and the observed absorbance at 320 nm of the chlorpyrifos oxon solution was plotted as a function of days of incubation at 23°C. The slope of the resulting lines yielded the first order rate constant k for decay of chlorpyrifos oxon. The half-life was calculated using the equation t½ = 0.693/k. Duplicate absorbance readings were essentially identical for each point. Data in Figure 3.
Figure 3.
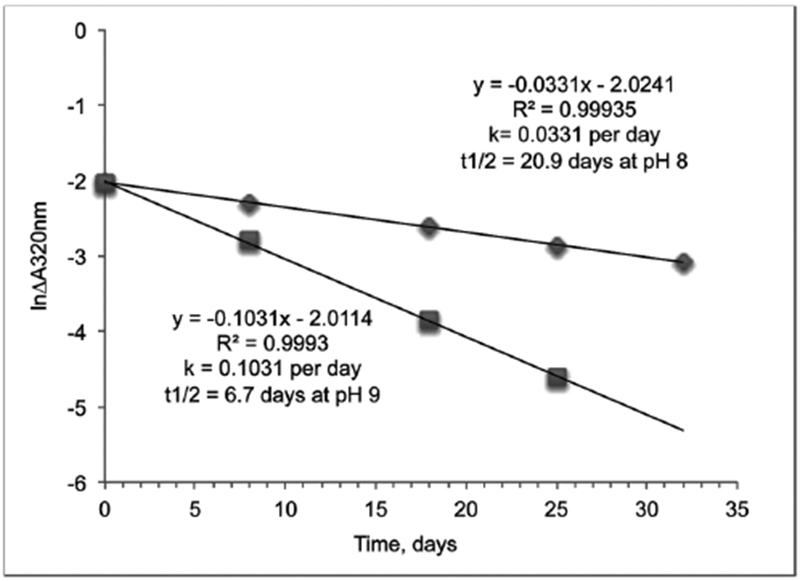
Rate of decay of chlorpyrifos oxon to 3,5,6-trichloro-2-pyridinol (TCP) at 23°C. Chlorpyrifos oxon (20 μM) in 100 mL of 20 mM TrisCl, 0.01% sodium azide pH 8.0 and pH 9.0 was stored in the dark. Spontaneous decay of chlorpyrifos oxon to TCP was monitored by the increase in absorbance at 320 nm. The half-life for chlorpyrifos oxon at pH 8.0 was 20.9 days, and at pH 9.0 was 6.7 days. Duplicate absorbance readings were essentially identical for each point.
The first order rate constant for decay of chlorpyrifos oxon at pH 11.8 and the half-life at pH 11.8 in 10 mM sodium hydroxide were calculated from the absorbance spectra in Figure 2.
The rate constants and half-lives for decay of paraoxon ethyl, paraoxon methyl, and diazoxon were measured in 100 mM sodium hydroxide pH 13.
2.6. Methods for removing excess CPO
Our publications on the crosslinking action of CPO have used dialysis and diafiltration to remove excess CPO. Small protein volumes, less than 3 mL, were dialyzed in Slide-A-Lyzer cassettes against 3 x 4 L of 10 mM ammonium bicarbonate pH 8 at 4°C. Alternatively 0.5 ml protein volumes were centrifuged through YM-10 centrifugal filters that allowed CPO to pass through, but retained protein. Repeated dilution and centrifugation cleared the protein sample of CPO. Large protein volumes, 3 to 10 mL, were placed into cellulose dialysis bags and dialyzed at 4°C against 3 x 4 L of 10 mM ammonium bicarbonate pH 8.
2.7. Test for successful removal of excess CPO
The activity of human and equine butyrylcholinesterase is inhibited by CPO concentrations as low as 0.5 x 10−9 M (Amitai et al., 1998; Heilmair et al., 2008). Spent dialysis buffer and the final dialyzed protein were tested for the presence of CPO by adding an aliquot to purified human butyrylcholinesterase and comparing butyrylcholinesterase activity before and after addition of the test sample. For example, 100 μl of purified human butyrylcholinesterase with an activity of 1.1 u/ml (18 × 10−9 M) was incubated with 100 μl of spent dialysis buffer for 30 min to 2 h at room temperature. A 50 μl aliquot of the incubation mixture was added to a 4 ml quartz cuvette containing 2 ml of 1 mM butyrylthiocholine and 0.5 mM 5,5’-dithiobis(2-nitrobenzoic acid) in 0.1 M potassium phosphate pH 7.0. Increase in absorbance at 412 nm was recorded for 1 min in a Gilford spectrophotometer at 25°C. The absorbance change per min was converted to μmoles butyrylthiocholine hydrolyzed per min using the Extinction coefficient of 13,600 M−1 cm−1 for the reaction product (Ellman et al., 1961). The ΔAbsorbance at 412 nm per min was 0.177 for control and for samples that were free of OP, but was zero for samples that contained more than 18 nM OP.
3. Results
3.1. Background information for measuring stability of chlorpyrifos oxon in aqueous solution
Absorbance spectra of freshly prepared 20 μM solutions of CPO and 3,5,6-trichloro-2-pyridinol (TCP) in 20 mM TrisCl pH 8.0 distinguished between the two compounds. Figure 1 shows a peak absorbance for CPO at 290 nm with an extinction coefficient of 6800 M−1 cm−1. Peak absorbance for TCP was at 320 nm with an extinction coefficient of 6700 M−1 cm−1
The time course for hydrolysis of CPO at alkaline pH in Figure 2 shows that CPO is converted to a product with the characteristic absorbance spectrum of TCP, in an isosbestic manner indicating that there are no intermediate species. The half-life of CPO at pH 11.8 was 14 min. The extinction coefficient of TCP was pH sensitive, being slightly higher at pH 11.8 (7100 M−1 cm−1) than at pH 8.0 (6700 M−1 cm−1).
3.2. Stability of chlorpyrifos oxon in aqueous buffers
The information in Figures 1 and 2 led to the conclusion that the stability of CPO can be monitored by measuring increase in absorbance at 320 nm. We wanted to know the stability of CPO in more-neutral, aqueous buffers to allow us to estimate the concentration of intact CPO in protein solutions treated with CPO for extended periods of time. The results in Figure 3 show that CPO has a half-life of 20.9 days at pH 8.0 and 6.7 days at pH 8.0 and 6.7 days at pH 9.0. We treat proteins with CPO in pH 8 to pH 9 buffers. The long half-life of CPO in pH 8 and pH 9 buffers makes it important to remove excess CPO from CPO-treated protein solutions before processing samples for SDS gel electrophoresis and mass spectrometry. Our protocol for studying the crosslinking action of CPO and other organophosphorus toxicants always includes removal of excess toxicant before analyzing proteins for crosslinked peptides.
3.3. Stability of OP at alkaline pH
The first order rate constant of 0.0499 per min and half-life of 13.9 min for chlorpyrifos oxon in 10 mM sodium hydroxide pH 11.8 were calculated by plotting the natural log of absorbance change versus time in Figure 4. We used a similar protocol to determine the first order rate constant of decay and the half-life of diazoxon (t½ = 2.5 min), paraoxon-ethyl (t½ = 11.5 min), and paraoxon methyl (t½ = 2.3 min) in 100 mM sodium hydroxide pH 13, see Table 1. Our values for paraoxon ethyl are similar to the results of Ginjaar and Vel (Ginjaar and Vel, 1958). Table 1 includes literature values for decay of chlorpyrifos and dichlorvos at alkaline pH. It was concluded that organophosphorus esters are rapidly inactivated by 10 to 100 mM sodium hydroxide solutions in water.
Figure 4.
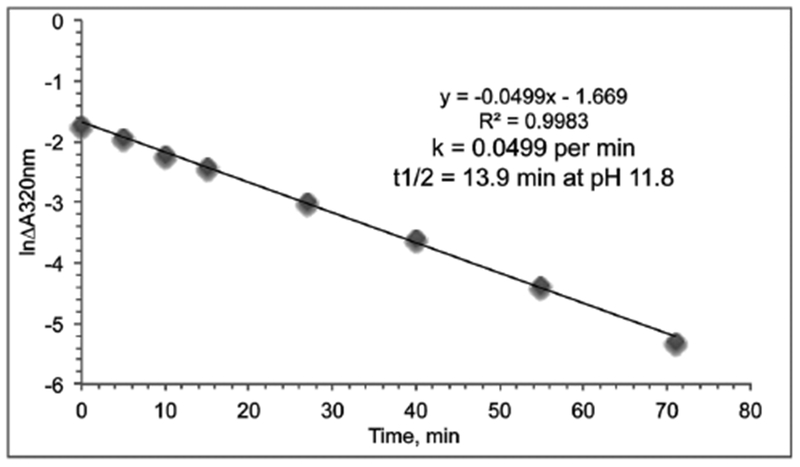
Rate of decay of chlorpyrifos oxon to 3,5,6-trichloro-2-pyridinol at pH 11.8 and 23°C. Absorbance change at 320 nm as a function of time in 10 mM sodium hydroxide was from Figure 2. The half-life of chlorpyrifos oxon was 13.9 min at pH 11.8.
Table 1.
OP half-life in sodium hydroxide
| OP | pH | t½, min | k, min−1 | °C | Solvent | Reference |
|---|---|---|---|---|---|---|
| Chlorpyrifos | 12.9 | 18.2 | 0.038 | 25 | NaOH in water | (Macalady and Wolfe, 1983) |
| Chlorpyrifos oxon | 11.8 | 13.9 | 0.05 | 23 | 10 mM NaOH | Present report |
| Dichlorvos | 11.5 | 46.2 | 0.015 | 25 | NaOH in water | (Grechko et al., 1983) |
| Diazoxon | 13 | 2.5 | 0.28 | 23 | 100 mM NaOH | Present report |
| Paraoxon-ethyl | 13 | 11.5 | 0.06 | 23 | 100 mM NaOH | Present report; |
| Paraoxon-methyl | 13 | 2.3 | 0.3 | 23 | 100 mM NaOH | Present report; |
4. Discussion
4.1. Chlorpyrifos oxon
Our study shows that chlorpyrifos oxon, the toxic metabolite of the pesticide chlorpyrifos, persists for days in aqueous solution. This finding necessitates removal of excess CPO before CPO-treated protein samples are processed for SDS gel electrophoresis to look for aggregation, and before CPO-treated proteins are digested with trypsin for mass spectrometry analysis of protein crosslinks. Excess OP could accelerate crosslinking during the heat-denaturation step of proteins prior to SDS gel electrophoresis. Excess OP could induce random crosslinks between tryptic peptides that would not represent crosslinks between peptides in native proteins.
4.2. Inactivation at high pH
OP pesticides and nerve agents are rapidly destroyed at pH 10 to 13 as shown in Figures 5 and 6. Fifty percent of an aqueous solution of paraoxon methyl degrades to nontoxic products in 2.3 min and 99% in about 30 min in the presence of 100 mM sodium hydroxide. The most stable OP in Table 1 is dichlorvos which is 50% degraded in 46.2 min and is 99% inactivated in about 4 hours at pH 11.5.
Figure 5.
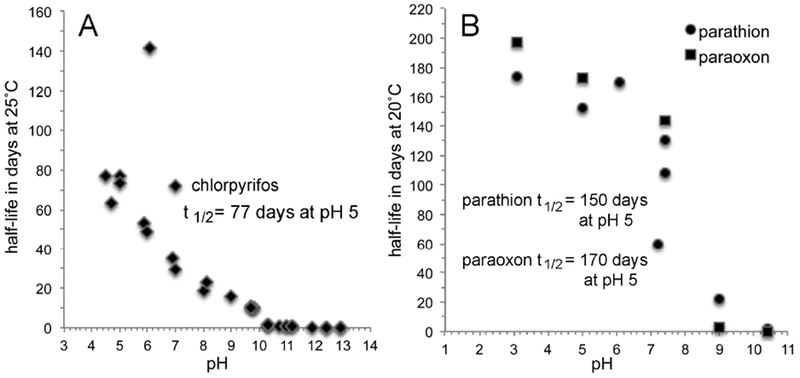
Chlorpyrifos (Panel A), parathion ethyl, and paraoxon ethyl (Panel B) are stable at acid to neutral pH (4 to 7) for days, but decay rapidly at alkaline pH (Gomaa and Faust, 1972; Freed et al., 1979; Chapman and Cole, 1982; Macalady and Wolfe, 1983; Solomon et al., 2014).
Figure 6.
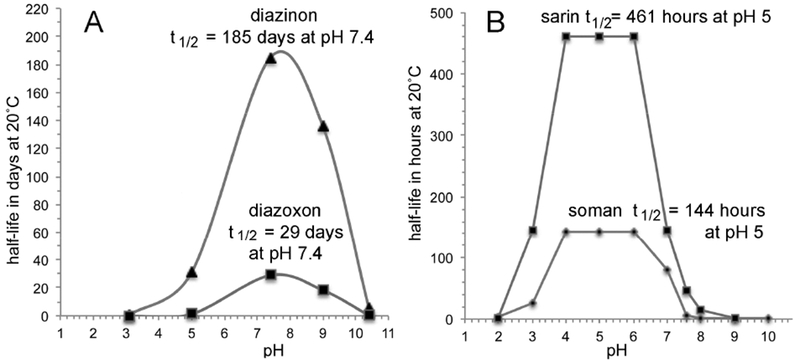
Diazinon and diazoxon (Panel A), sarin and soman (Panel B) are unstable at pH 3 and pH 10. Sarin and soman are most stable at pH 4-6 where sarin has a half-life of 461 hours, and soman 144 hours. Note the half-life data for sarin and soman are in hours in the figure, but in days in Table 1. Diazinon and diazoxon are most stable at pH 7-8. Figure 6A was constructed from information in (Gomaa et al., 1969). Figure 6B was constructed from information in (Epstein, 1974) and (Clark, 1989).
4.3. OP stability information in the literature
To date only chlorpyrifos oxon and the nerve agent VX have been described as crosslinking agents (Schmidt et al., 2014; Schopfer and Lockridge, 2018; Schopfer and Lockridge, 2019). The toxicants listed in Table 2 are under investigation as crosslinking agents and therefore information on their stability in aqueous solution is needed.
Table 2.
Half-life of OP in aqueous solution
| OP | pH | t½, days | °C | Solvent | Reference | |
|---|---|---|---|---|---|---|
| Chlorpyrifos CAS 2921-88-2 |
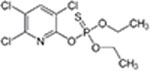 |
1 1.2 1.2 5.9 6.1 9.8 9.8 9.7 9.7 10.3 10.3 10.3 10.7 11 11 11.2 11.2 11.9 12.4 12.4 12.9 12.9 |
89.1 123.4 54.7 53.5 141.5 10.1 10.2 10.5 10 1.6 1.4 1.6 0.8 0.5 0.5 0.4 0.4 0.2 0.04 0.04 0.01 0.01 |
25 25 25 25 25 25 25 25 25 25 25 25 25 25 25 25 25 25 25 25 25 25 |
Sterile water or carbonate buffer | (Macalady and Wolfe, 1983) |
| Chlorpyrifos | 5 7 9 |
73 72 16 |
25 25 25 |
Not specified | (Solomon et al., 2014 | |
| Chlorpyrifos | 4.5 5 6 7 8 |
77 77 49 29.4 18.9 |
25 25 25 25 25 |
Sterile 0.2 M phosphate | (Chapman and Cole, 1982) | |
| Chlorpyrifos | 4.7 6.9 8.1 |
63 35 23 |
25 25 25 |
0.02 M phosphate | (Meikle and Youngson, 1978) | |
| Chlorpyrifos | 6.9 7.3 |
51.8 76.6 |
22 22 |
Pond water | (Lu et al., 2006) | |
| Chlorpyrifos | 7.1 | 110 | 22 | water | (Mansour et al., 1999) | |
| Chlorpyrifos | 7.1 | 70 | 21 | Distilled water | (Sharom et al., 1980) | |
| Chlorpyrifos | 6.1 7.4 |
120 53 |
20 20 |
Phosphate buffer | (Freed et al., 1979) | |
| Chlorpyrifos | 5.7 7.66 7.93 7.99 8.15 |
45.9 56 126 26 24 |
Deionized water River water |
(Liu et al., 2001) | ||
| Chlorpyrifos | 6 8.5 8.7 6 8.5 8.7 |
16.8 2.5 9.7 1 0.1 0.2 |
28 28 28 28 28 28 |
Tap water River water Brackish water Aerated tap Aerated river Aerated brackish |
(Thomas and Mansingh, 2002) | |
| Chlorpyrifos | 8 | 62 | 29 | Sterile 0.02 M phosphate | (Noblet et al., 1996) | |
| Chlorpyrifos oxon CAS 5598-15-2 |
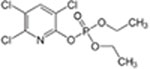 |
8 9 |
20.9 6.7 |
23 23 |
20 mM TrisCl, 0.01% NaN | Present report |
| Chlorpyrifos oxon | 11.8 | 0.01 | 23 | 10 mM NaOH for pH 11.8 | Present work | |
| Diazinon CAS 333-41-5 |
 |
3.1 5.0 7.4 9.0 10.4 |
0.5 31 185 136 6 |
20 20 20 20 20 |
0.02 M phosphate | (Gomaa et al., 1969) |
| Diazinon | 5 7 9 |
12 138 77 |
Not specified | (EPA, 2006) | ||
| Diazinon | 7.3 | 171 | 21 | Not specified | (Mansour et al., 1999) | |
| Diazinon | 7.1 | 70 | 21 | Distilled water | (Sharom et al., 1980) | |
| Diazinon | 6.1 7.3 |
69 52 |
22 22 |
MilliQ water filtered river water | (Lartiges and Garrigues, 1995) | |
| Diazinon | 6.9 7.3 |
69.5 134.4 |
22 22 |
Pond water Pond water |
(Lu et al., 2006) | |
| Diazinon | 4.5 5 6 7 8 |
3.1 14 54.6 70 53.9 |
25 25 25 25 25 |
Sterile 0.2 M phosphate | (Chapman and Cole, 1982) | |
| Diazinon | 2.2 3.2 4.2 5.2 7.5 |
0.25 0.5 8 16.6 16.6 16.6 |
28 28 28 28 28 28 |
Mineral growth medium | (Drufovka et al., 2008) | |
| Diazinon | 8 | 130 | 29 | Sterile 0.02 M phosphate | (Noblet et al., 1996) | |
| Diazoxon CAS 962-58-3 |
 |
3.1 5.0 7.4 9.0 10.4 |
0.017 1.27 29 18 0.42 |
20 20 20 20 20 |
0.02 M phosphate | (Gomaa et al., 1969) |
| Diazoxon | 13 | 0.002 | 23 | 100 mM NaOH | Present report | |
| Dichlorvos CAS 62-73-7 |
 |
5 7 9 |
12 5 0.9 |
25 25 25 |
Buffered water | (Lim, 1996) |
| Dichlorvos | 2.6 5.6 7.0 9.0 |
>1.7 0.31 0.07 0.40 |
25 25 25 25 |
Water Cacodylate Cacodylate Tetraborate |
(Benoit-Marquié et al., 2004) | |
| Dichlorvos | 6.1 | <81 | 22 | Not specified | (Lartiges and Garrigues, 1995) | |
| Dichlorvos | 6 7 8.5 |
12.7 13.6 |
25 25 25 |
Well water near Ljubljana | (Druzina and Stegu, 2007) | |
| Dichlorvos | 4.0 6.9 9.3 11.5 |
30 4.5 0.9 0.03 |
25 25 25 25 |
Buffered water | (Grechko et al., 1983) | |
| Methamido phos CAS 10265-92-6 |
 |
7 9 |
5 2.9 |
22 22 |
Not specified | (Tomlin, 1997) |
| Monocrotop hos CAS 6923-22-4 |
 |
5 7 9 |
96 66 17 |
20 20 20 |
Not specified | (Tomlin, 1997) |
| Parathion ethyl CAS 56-38-2 |
 |
3.1 5 7.4 9 10.4 |
174 153 108 22 1.4 |
20 20 20 20 20 |
0.2 M phosphate | (Gomaa and Faust, 1972) |
| Parathion ethyl | 6.1 7.4 |
170 130 |
20 20 |
Phosphate buffer | (Freed et al., 1979) | |
| Parathion ethyl | 7.2 | 60 | 20 | Not specified | (Mansour et al., 1999) | |
| Parathion ethyl | 4 7 9 |
272 260 130 |
22 22 22 |
Not specified | (Tomlin, 1997) | |
| Parathion ethyl | 6.1 7.3 |
84 33 |
22 22 |
Milli-Q water Filtered river water |
(Lartiges and Garrigues, 1995) | |
| Parathion ethyl | 4.5 5 6 7 8 |
273 301 231 168 105 |
25 25 25 25 25 |
Sterile 0.2 M phosphate | (Chapman and Cole, 1982) | |
| Parathion ethyl | 7 | 120 | 25 | Not specified | (Coates, 1949) | |
| Parathion ethyl | 6 7 8.5 |
23.9 33.5 31.4 |
25 25 25 |
Well water near Ljubljana | (Druzina and Stegu, 2007) | |
| Parathion-methyl CAS 298-00-0 |
 |
6 7 8.5 |
45.5 47.2 47.6 |
25 25 25 |
Well water near Ljubljana | (Druzina and Stegu, 2007) |
| Paraoxon ethyl CAS 311-45-5 |
 |
3.1 5 7.4 9 10.4 |
197 173 144 2.9 0.25 |
20 20 20 20 20 |
0.2 M phosphate | (Gomaa and Faust, 1972) |
| Paraoxon ethyl | 7 | 87 | 25 | Not specified | (Coates, 1949) | |
| Paraoxon ethyl | 13 | 0.008 | 23 | 100 mM NaOH | Present report | |
| Paraoxon-methyl CAS 950-35-6 |
 |
13 | 0.002 | 23 | 100 mM NaOH | Present report |
| Sarin CAS 107-44-8 |
 |
2.0 4.0 6.5 7.0 7.5 8.0 9.0 |
0.1 19.2 19.2 6.1 1.9 0.6 0.06 |
20 20 20 20 20 20 20 |
Buffered water | (Epstein, 1974) |
| Soman CAS 96-64-0 |
 |
2 3 4 5 6 7 7.6 9 10 |
0.1 1 6 6 6 3.3 0.28 0.02 0.02 |
20 20 20 20 20 20 20 20 20 |
Buffered water | (Clark, 1989) |
| Soman | 4.5 4.5 4.5 |
18.5 15.9 2.6 |
25 25 25 |
Dilute acid Water Acetate |
(Ellin et al., 1981) | |
| Soman | 6.4 8.0 8.6 |
6-14 0.3 0.1 0.1 |
27 27 27 27 |
0.15 M NaCl 0.02 M phosphate 0.02 M phosphate 0.02 M phosphate |
(Broomfield et al., 1986) | |
| Tabun CAS 77-81-6 |
 |
7 | 0.35 | 20 | water | (Clark, 1989) |
| VX CAS 50782-69-9 |
 |
7 | 16-42 | 25 | Not specified | (National.Research.Council.US, 1999) |
Table 2 gives half-lives as a function of OP structure, pH, temperature, and buffer composition. As can be seen from the table, the reaction condition information is incomplete in several publications. This is likely the reason for differences in the half-life values reported by different authors. For example, chlorpyrifos at pH 7 and 25°C was reported to have a half-life of 29 days by Chapman and Cole and 72 days by Solomon et al. (Chapman and Cole, 1982; Solomon et al., 2014), but the reaction buffer was not stipulated by Solomon et al. Reaction conditions clearly affect the measured hydrolysis rates. For example, dichlorvos at pH 7 and 25°C has a half-life of 5 days in buffered water, but 0.07 days in cacodylate buffer (Lim, 1996; Benoit-Marquié et al., 2004). Spontaneous hydrolysis of O-hexyl 2,5-dichlorophenyl phosphoramidate was significant in 100 mM phosphate buffer pH 7.4, but negligible in 100 mM Tris/citrate pH 7.4 (Sogorb et al., 1993). The presence of copper (II) ions increases the rate of hydrolysis for OP pesticides and nerve agents (Epstein, 1974; Meikle and Youngson, 1978; Smolen and Stone, 1997; Liu et al., 2001).
Figure 5A shows that chlorpyrifos is relatively stable below pH 6, with a half-life at pH 5 of 77 days. Figure 5B shows that parathion ethyl and paraoxon ethyl are stable in aqueous solution at pH below 7. The half-life at pH 5 for parathion ethyl is 150 days, and for paraoxon ethyl is 170 days. However at pH 10 the half-lives of all three OP are a few minutes.
The oxons (chlorpyrifos oxon, paraoxon, and diazoxon) are orders of magnitude more reactive with acetylcholinesterase than the parent pesticides chlorpyrifos, parathion, and diazinon. This brings up the question of whether the oxons are also more reactive with water. Figure 5B supports the observation of Faust and Gomaa that parathion hydrolysis is slightly faster than paraoxon hydrolysis under acidic or neutral conditions, while paraoxon hydrolysis is 7 times faster than parathion at pH 9.0 and five times faster at pH 10.4 (Faust and Gomaa, 1972). Figure 6A shows that an aqueous solution of diazinon is more stable at all pH values than an aqueous solution of diazoxon. Thus, though there is a tendency for oxons to be more susceptible to aqueous hydrolysis than thions, the differences in reactivity are only a few fold not orders of magnitude as is seen with reactivity with acethycholinesterase.
Figures 6A and 6B show the stability patterns for diazinon, diazoxon, sarin, and soman in aqueous solution. These patterns are different from those in Figures 5A and 5B for chlorpyrifos, parathion, and paraoxon. Figures 6A and 6B display bell shaped stability curves. Diazinon and diazoxon are most stable at pH 7 to 8. The nerve agents sarin and soman are most stable at pH 4-6. All of these organophosphylates show decreasing stability at both acidic and basic pH extremes. Even under the most stable conditions, sarin is less stable than diazinon, i.e. at pH 5, an aqueous solution of sarin is about 10-fold less stable than an aqueous solution of diazinon at pH 7.4.
The foregoing observations illustrate that stability of OP in aqueous solution is highly dependent on the pH of the solution, is markedly dependent upon the structure of the OP, and is susceptible to buffer catalysis.
5. Conclusion
Organophosphorus toxicants, including chlorpyrifos oxon, persist in aqueous buffers at neutral pH and 20-25°C for days to months. They are completely degraded in minutes by 10 to 100 mM sodium hydroxide.
Highlights.
Chlorpyrifos oxon has a half-life of 21 days in pH 8 buffer at 23°C
Chlorpyrifos oxon has a half-life of 7 days in pH 9 buffer at 23°C
Nerve agents and pesticides are degraded within minutes in sodium hydroxide
Acknowledgement
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the French Ministry of Armed Forces or the U.S. Department of Health and Human Services. Use of trade names is for identification only and does not imply endorsement by the French Ministry of Armed Forces or the U.S. Department of Health and Human Services.
Funding
Supported by Fred & Pamela Buffett Cancer Center Support Grant P30CA036727 from NIH, and Direction Générale de l’Armement (DGA) under grant PEA ENVAU2.
Abbreviations
- CPO
chlorpyrifos oxon
- OP
organophosphorus toxicants
- TCP
3,5,6-trichloro-2-pyridinol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no competing interests
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Amitai G, Moorad D, Adani R and Doctor BP (1998) Inhibition of acetylcholinesterase and butyrylcholinesterase by chlorpyrifos-oxon. Biochem Pharmacol 56:293–299. [DOI] [PubMed] [Google Scholar]
- Benoit-Marquié F, de Montety C, Gilard V, Martino R, Maurette MT and Malet-Martino M (2004) Dichlorvos degradation studied by 31P-NMR. Environ Chem Lett 2:93–97. [Google Scholar]
- Broomfield CA, Lenz DE and Maclver B (1986) The stability of soman and its stereoisomers in aqueous solution: toxicological considerations. Arch Toxicol 59:261–265. [DOI] [PubMed] [Google Scholar]
- Chapman RA and Cole CM (1982) Observations on the influence of water and soil pH on the persistence of insecticides. Journal of environmental science and health Part B, Pesticides, food contaminants, and agricultural wastes 17:487–504. [DOI] [PubMed] [Google Scholar]
- Clark DN (1989) Review of reactions of chemical agents in water. Defense Technical Information Center, Alexandria, VA AD-A213: 287. [Google Scholar]
- Coates H (1949) The chemistry of phosphorus insecticides. The Annals of applied biology 36:156–159. [PubMed] [Google Scholar]
- Drufovka K, Danevcic T, Trebse P and Stopar D (2008) Microorganisms trigger chemical degradation of diazinon. International Biodeterioration & Biopdegradation 62:293–296. [Google Scholar]
- Druzina B and Stegu M (2007) Degradation study of selected organophosphorus insecticides in natural waters. Intern J Environ Anal Chem 87:1079–1093. [Google Scholar]
- Ellin RI, Groff WA and Kaminskis A (1981) The stability of sarin and soman in dilute aqueous solutions and the catalytic effect of acetate ion. Journal of environmental science and health Part B, Pesticides, food contaminants, and agricultural wastes 16:713–717. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V Jr. and Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. [DOI] [PubMed] [Google Scholar]
- EPA (2006) Reregistration eligibility decision for diazinon US Environmental Protection Agency Office of Pesticide Programs EPA 738-R-04-006:23. [Google Scholar]
- Epstein J (1974) Properties of GB in water. J Am Water Works Assoc 66:31–37. [Google Scholar]
- Faust SD and Gomaa HM (1972) Chemical hydrolysis of some organic phosphorus and carbamate pesticides in aquatic environments. Environmental letters 3:171–201. [DOI] [PubMed] [Google Scholar]
- Freed VH, Chiou CT and Schmedding DW (1979) Degradation of selected organophosphate pesticides in water and soil. J Agric Food Chem 27:706–708. [Google Scholar]
- Ginjaar L and Vel S (1958) Reactivity of organophosphorous compounds. I. Alkaline hydrolysis of dialkyl p-nitrophenyl phosphates. Recueil des Travaux Chimiques des Pays Bas 77:929–956. [Google Scholar]
- Golomb BA (2008) Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci USA 105:4295–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa HM and Faust SD (1972) Chemical hydrolysis and oxidationof parathion and paraoxon in aquatic environments. Fate of Organic Pesticides in the Aquatic Environment, Advances in Chemistry Series 111:189–209. [Google Scholar]
- Gomaa HM, Suffet IH and Faust SD (1969) Kinetics of hydrolysis of diazinon and diazoxon. Residue reviews 29:171–190. [DOI] [PubMed] [Google Scholar]
- Grechko AV, Nabolotnaya OM and Marchenko PV (1983) The hydrolysis of dichlorvos. Soviet journal of water chemistry and technology (Khimiya i Tekhnologiya Vody) 5:164–165; translated 187–189. [Google Scholar]
- Heilmair R, Eyer F and Eyer P (2008) Enzyme-based assay for quantification of chlorpyrifos oxon in human plasma. Toxicol Lett 181:19–24. [DOI] [PubMed] [Google Scholar]
- Jamal GA, Hansen S and Julu PO (2002) Low level exposures to organophosphorus esters may cause neurotoxicity. Toxicology 181–182:23–33. [DOI] [PubMed] [Google Scholar]
- Jokanovic M (2018) Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: A review. Toxicology 410:125–131. [DOI] [PubMed] [Google Scholar]
- Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC and Sandler DP (2007) Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Human & experimental toxicology 26:243–250. [DOI] [PubMed] [Google Scholar]
- Lartiges SB and Garrigues PP (1995) Degradation Kinetics of Organophosphorus and Organonitrogen Pesticides in Different Waters under Various Environmental Conditions. Environ Sci Technol 29:1246–1254. [DOI] [PubMed] [Google Scholar]
- Lim LO (1996) Dichlorvos Risk Characterization Document. California Environmental Protection Agency:8. [Google Scholar]
- Liu B, McConnell LL and Torrents A (2001) Hydrolysis of chlorpyrifos in natural waters of the Chesapeake Bay. Chemosphere 44:1315–1323. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu L, Newman J, Faber B and Gan J (2006) Degradation of pesticides in nursery recycling pond waters. J Agric Food Chem 54:2658–2663. [DOI] [PubMed] [Google Scholar]
- Macalady DL and Wolfe NL (1983) New perspectives on the hydrolytic degradation of the organophosphorothioate insecticide chlorpyrifos. J Agric Food Chem 31:1139–1147. [Google Scholar]
- Mansour M, Feicht EA, Behechti A, Schramm KW and Kettrup A (1999) Determination photostability of selected agrochemicals in water and soil. Chemosphere 39:575–585. [DOI] [PubMed] [Google Scholar]
- Meikle RW and Youngson CR (1978) The hydrolysis rate of chlorpyrifos, O-O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate, and its dimethyl analog, chlorpyrifos-methyl, in dilute aqueous solution. Arch Environ Contam Toxicol 7:13–22. [DOI] [PubMed] [Google Scholar]
- National.Research.Council.US (1999) Review of the US Army’s Health Risk Assessments for oral exposure to six chemical-warfare agents. National Academies Press (US) Washington, DC. [PubMed] [Google Scholar]
- Naughton SX and Terry AV Jr. (2018) Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 408:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noblet JA, Smith LA and Suffet IH (1996) Influence of natural dissolved organic matter, temperature, and mixing on the abiotic hydrolysis of triazine and organophosphate pesticides. J Agric Food Chem 44:3685–3693. [Google Scholar]
- Schmidt C, Breyer F, Blum MM, Thiermann H, Worek F and John H (2014) V-type nerve agents phosphonylate ubiquitin at biologically relevant lysine residues and induce intramolecular cyclization by an isopeptide bond. Anal Bioanal Chem 406:5171–5185. [DOI] [PubMed] [Google Scholar]
- Schopfer LM and Lockridge O (2018) Chlorpyrifos oxon promotes tubulin aggregation via isopeptide cross-linking between diethoxyphospho-Lys and Glu or Asp: Implications for neurotoxicity. The Journal of biological chemistry 293:13566–13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer LM and Lockridge O (2019) Mass Spectrometry Identifies Isopeptide Cross-Links Promoted by Diethylphosphorylated Lysine in Proteins Treated with Chlorpyrifos Oxon. Chem Res Toxicol 32:762–772. [DOI] [PubMed] [Google Scholar]
- Schopfer LM, Lockridge O, David E and Hinrichs SH (2019) Purification of human butyrylcholinesterase from frozen Cohn fraction IV-4 by ion exchange and Hupresin affinity chromatography. PLoS One 14:e0209795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharom MS, Miles JRW, Harris CR and McEwen FL (1980) Persistence of 12 insecticides in water. Water Research 14:1089–1093. [Google Scholar]
- Smolen JM and Stone AT (1997) Divalent metal ion-catalyzed hydrolysis of phosphorothionate ester pesticides and their corresponding oxonates. Environ Sci Technol 31:1664–1673. [Google Scholar]
- Sogorb MA, Vilanova E and Diaz-Alejo N (1993) The kinetics of O-hexyl O-2,5-dichlorophenyl phosphoramidate hydrolysing activity in hen plasma. Chem Biol Interact 87:117–125. [DOI] [PubMed] [Google Scholar]
- Solomon KR, Williams WM, Mackay D, Purdy J, Giddings JM and Giesy JP (2014) Properties and uses of chlorpyrifos in the United States. Reviews of environmental contamination and toxicology 231:13–34. [DOI] [PubMed] [Google Scholar]
- Thomas C and Mansingh A (2002) Dissipation of chlorpyrifos from tap, river and brackish waters in glass aquaria. Environmental technology 23:1219–1227. [DOI] [PubMed] [Google Scholar]
- Tomlin CDS (1997) (ed) The Pesticide Manual. The British Crop Protection Council, 11th ed Surrey, UK. [Google Scholar]
- Wang A, Cockburn M, Ly TT, Bronstein JM and Ritz B (2014) The association between ambient exposure to organophosphates and Parkinson’s disease risk. Occup Environ Med 71:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]


