Abstract
Background:
Transverse tubules (t-tubules) are important structural elements, derived from sarcolemma, found on all striated myocytes. These specialized organelles create a scaffold for many proteins crucial to the effective propagation of signal in cardiac excitation-contraction coupling. The full protein composition of this region is unknown.
Methods:
We characterized the t-tubule subproteome using 52,033 immunohistochemical images covering 13,203 proteins from the Human Protein Atlas (HPA) cardiac tissue microarrays. We used HPASubC, a suite of Python tools, to rapidly review and classify each image for a specific t-tubule staining pattern. The tools Gene Cards, String 11, and Gene Ontology (GO) Consortium as well as literature searches were used to understand pathways and relationships between the proteins.
Results:
There were 96 likely t-tubule proteins identified by HPASubC. Of these, 12 were matrisome proteins and 3 were mitochondrial proteins. A separate literature search identified 50 known t-tubule proteins. A comparison of the two lists revealed only 17 proteins in common, including 8 of the matrisome proteins. String11 revealed that 94 of 127 combined t-tubule proteins generated a single interconnected network.
Conclusion:
Using HPASubC and the HPA, we identified 78 novel, putative t-tubule proteins and validated 17 within the literature. This expands and improves our knowledge of this important subcellular structure of the cardiac myocyte. This information can be used to identify new structural targets involved in excitation-contraction coupling that may be altered in disease.
Keywords: t-tubule, proteomics, caveolin
1. Introduction
Transverse tubules (t-tubules) are important structural elements found on all striated myocytes. These specialized organelles are invaginations of the myocyte sarcolemma that run perpendicular to the longitudinal axis of myocytes and form a network around sarcomere z-discs.[1] They have historically been visualized by transmission electron microscopy (TEM), which identified them as having a tortuous structure and open lumen.[2, 3] The cardiomyocyte t-tubule structure is maintained by the cytoskeleton, extracellular matrix, and various scaffolding proteins.
T-tubules are primarily known for their function in regulating cardiac excitation-contraction coupling. T-tubules contain many voltage-gated L-type calcium channels (LTCCs), effectively organizing LTCCs and ryanodine receptors (RyRs) into dyads located close to the sarcoplasmic reticulum (SR). This greatly aids in the release of calcium transients following an action potential, allowing fast propagation of electrical signals. T-tubules also serve as a platform to organize membrane specific microdomains such as LTCC-RyR dyads. Other microdomains such as cytoskeleton scaffolding proteins, proteins of the costamere complex, and ankyrin B microdomains can also be found at the t-tubule.[1]
T-tubules are functionally important in disease states. T-tubules undergo remodeling in heart failure, with decreased protein components and calcium transients.[4] This remodeling is a marker of disease severity that correlates with a decrease in contractility and progression from left ventricular hypertrophy to heart failure.[4, 5] Moreover, t-tubule remodeling precedes detectable left ventricular systolic dysfunction by electrocardiogram, demonstrated by areas of t-tubule loss and remodeling.[4, 5] T-tubule remodeling is also seen in ischemic heart disease (IHD), including secondary to diabetes mellitus induced disease. In IHD, changes in the composition of membrane lipids impact the biophysical properties and functions of myocytes by altering t-tubules, gap junctions, and caveoli.[6]
Because of the importance of the t-tubule in human disease, we queried what proteins were localized to the t-tubule based on staining patterns. We utilized the deep image dataset of the Human Protein Atlas (HPA) and HPASubC.[7, 8] HPASubC is a suite of tools that allow the user to download images from HPA from a particular organ and, using a Playstation-type controller, the user can evaluate HPA images quickly for a pattern of interest. The HPA is a proteomic database consisting of immunohistochemical images from tissue microarrays (TMAs) across 44 different tissues and organs. The HPA database has immunohistochemical images of 16,900 proteins using >24,000 antibodies. A benefit of using HPA, as a proteomic vehicle, is the ability to identify subcellular protein localization. The utility of HPASubC has been previously shown in the identification of cardiac endothelial cells and smooth muscle cells proteins, cardiac mosaicism, and sinusoid specific proteins in the liver.[7, 9, 10]
2. Methods
2.1. Data Collection Using HPASubC
In total, 52,033 heart core images covering 13,203 unique proteins were downloaded from the Human Protein Atlas website (proteinatlas.org).[8] Positive staining patterns for t-tubules were based on the staining patterns of known t-tubule proteins caveolin-1 (CAV1), caveolin-2 (CAV2), caveolin-3 (CAV3) which demonstrate membrane staining and dark t-tubule invaginations into the cell (Fig. 1). This included patterns in which the entire cytoplasmic membrane stained and some invaginations were noted. HPASubC was used to rapidly evaluate the heart images for potential t-tubule staining.[7] Images that only showed cytoplasmic membrane staining or intercalated disc staining were often identified at this step, but were generally excluded.
Fig. 1.
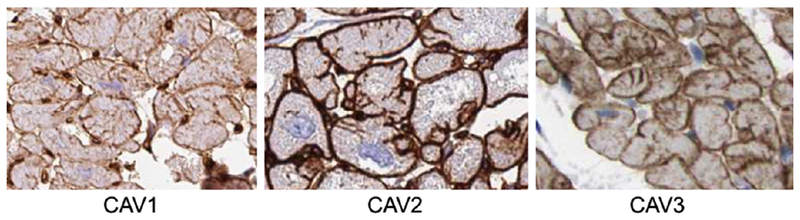
Caveolin patterns of t-tubule staining. These patterns are representative of the patterns seen across all of the reported proteins. Images from the Human Protein Atlas.
For the secondary review, images were scored on a scale of 0-3. A 0 indicated a negative t-tubule staining pattern, 1 indicated that the image was unlikely for t-tubule staining, 2 indicated that the image was likely positive for t-tubule staining, and 3 indicated that the image was positive for t-tubule staining. Proteins that were selected in the initial fast evaluation that only showed cell membrane staining were scored 0.5 (Supplemental Fig. 1).
2.2. Heart Matrisome
Matrisome genes were taken from the MIT Matrisome Project website (http://matrisomeproject.mit.edu/, access date 08/14/2018) using their master list for the human matrisome.[11] The matrisome contains 1027 genes broken up into 274 “Core matrisome” genes and 753 “Matrisome-associated” genes.
To find cardiac matrisome genes, publicly available RNA-seq data from the GTEx database (heart left ventricle) was used.[12] We filtered out genes with less than 0.1 transcripts per million (TPM) median expression to remove noise leaving 19,893 genes. Gene names were compared between GTEx and the matrisome project and mismatched names (8) were properly reassigned using GeneCards’ previous aliases.[13]
2.3. Analysis tools
To analyze the protein lists generated from the HPASubC search, we utilized literature searches, Gene Cards (www.genecards.com), Stringl 1, and the Gene Ontology Consortium.[14, 15] Gene expression level data was taken from HPA and normalized as transcripts per million (TPM). Cellular localization of these proteins and searches of known t-tubule proteins was performed using Google Scholar, PubMed, and Genecards.org as references.
2.4. Genome Wide Association Study Comparison
The GWAS Catalog at https://www.ebi.ac.uk/gwas/home was searched for all t-tubule genes/proteins (from this study or the literature). All associated phenotypes were recorded for each gene. Genes were considered positive if they were in a locus for a cardiac trait related to the conducting system (EKG traits) or heart rate. Non-cardiac, coronary artery-related, and hypertension-related GWAS hits were excluded.
3. Results
3.1. Identification of t-tubule staining proteins
A total of 52,533 heart core images for 13,203 proteins were evaluated. A total of 643 images were extracted for secondary review. Two investigators (J.X.C. and M.K.H.) scored the images. A final list of 96 proteins with positive t-tubule staining was generated (Supplemental Table 1, Supplemental Fig. 2). The average score across all 96 proteins was 1.4 on the 0-3 scale. Sixty-seven proteins had at least one core scored as a 3. Fifty-two proteins had staining for two or more antibodies with 50% (26) showing staining for two or more of these antibodies. This variability was indicative of staining heterogeneity across tissues and antibodies as further explained in the discussion.
We evaluated each protein individually using Google Scholar, Pubmed, and GeneCards to classify each’s likelihood of being found at the t-tubule. We identified and subsetted 12 proteins that are known members of the matrisome (extracellular matrix).[11] We also removed 3 proteins that localize to the mitochondria. Based on known information for each, of the remaining 81 proteins, 20 (~25%) were likely to be found at the t-tubule, 37 (~46%) were unlikely to be found in the t-tubule, and 24 (~30%) were of unknown cellular location. We compared the protein data with gene expression. Expression data available from HPA identified a median expression level of 18.8 transcripts per million (TPM) for the entire set and 60.2 TPM for the “likely” t-tubule proteins.
3.2. Exclusion of Z-line and junctional SR membrane patterns
Due to the large size and variable structural components of a cardiomyocyte, it may be it possible that perceived specific t-tubule staining may represent a different cell organelle or structure. Therefore, we specifically attempted to identify and exclude patterns that matched the Z-line and junctional sarcoplasmic reticulum (SR) membrane. We evaluated actinin 2 (ACTN2), cypher (LDB3), and capping actin protein of muscle Z-line subunit beta (CAPZB) as markers of the Z-line.[16–18] Triadin (TRDN) and calsequestrin (CASQ1) were used as markers of the junctional SR.[19] Z-line proteins gave a rigid parallel staining pattern and junctional SR gave a granular/heterogeneous cytoplasmic staining pattern (Supplemental Fig. 3). There is no evidence of an overlapping pattern of these regions and the patterns found at the t-tubule lending further support for the specificity of t-tubule staining.
3.3. Comparing new t-tubule proteins with previously identified t-tubule proteins
A literature search for known or suspected t-tubule proteins identified 50 proteins (Supplemental Table 2).[20–42] Of these, only 17 (31%) were identified in our HPA search. Three had no images in HPA to be evaluated. The remaining had a variety of non-t-tubule patterns including generalized cytoplasmic staining, intercalated disc staining and nuclear staining. The group of literature-based t-tubule proteins not found in the HPA includes such well-described t-tubule proteins as bridging integrator 1/amphophysin 2 (BIN1), ryandodine receptors 1 and 2 (RYR1, RYR2), and ankyrin B (ANK2).
We were surprised by the lack of overlap and wondered if any ancillary data could indicate which dataset was more robust. We queried the NHGRI-EBI catalog of genome-wide association studies (GWAS) for each of the 129 total genes. We hypothesized that there would be more GWAS hits in the loci of proteins localized to the t-tubule, a structural region involved in cardiac polarization.[43–45] We found that 6 of the 17 shared genes (35%) were in loci related to cardiac traits. Interestingly, similar percentages were noted in the exclusive literature list (3 of 33; 9%) and those proteins first reported herein (7 of 79; 9%) as being within cardiac GWAS loci (Supplemental Table 3). As an outgroup, we investigated the GWAS loci for “breast cancer” and saw percentages of 6% (1/17), 6% (2/33), and 4% (3/79) for the three groups respectively. Thus, GWAS data lends strong support to the 17 shared proteins/genes being the most robust t-tubule proteins and equivalent support to the other two lists.
We also used the Online Mendelian Inheritance in Man (OMIM) tool to determine how often these genes are involved in cardiac diseases with EKG-related traits. Eight genes, all in our novel group of 79 were not found in OMIM. Among the 17 shared genes, 6 (35%) were mutated in a cardiac disease. Of the literature only group, 9 genes (28%) had associated cardiac diseases, while 12 genes (17%) were found in our new dataset.
3.4. Extracellular matrix and mitochondrial proteins of the t-tubule
The human matrisome lists 274 core matrisome proteins and 753 matrisome-associated proteins.[46] Of these, 205 and 455 are present in the human left ventricular heart based on GTEx expression data. We identified 9 core matrisome and 3 matrisome-associated proteins having t-tubule staining patterns (Table 1, Fig. 2). Some of these have been previously described, such as laminin, annexins 6 and 7 and collagen IV.[26, 27, 30] Other annexins, described in the literature as being t-tubule associated, did not have a t-tubule pattern in the HPA data.[24, 25] Versican (VCAN) and adiponectin, C1Q and collagen domain containing (ADIPOQ) were new matrisome proteins found to be part of the t-tubule matrix. The 3 mitochondrial proteins identified as having a t-tubule pattern were MTCH1, RAB32 and ETFRF1 (LYRM5).[47–49]
Table 1.
Matrisome proteins that localize to the t-tubule
| Gene Symbol | Gene Name | Gene Expression (TPM) | Matrisome type | Previously Known |
|---|---|---|---|---|
| ADIPOQ | adiponectin, C1Q and collagen domain containing | 4.6 | Core matrisome | No |
| COL4A1 | collagen type IV alpha 1 chain | 49.3 | Core matrisome | Yes |
| COL4A2 | collagen type IV alpha 2 chain | 50.3 | Core matrisome | Yes |
| COL6A2 | collagen type VI alpha 2 chain | 93 | Core matrisome | No |
| LAMA2 | laminin subunit alpha 2 | 31.5 | Core matrisome | No |
| LAMB1 | laminin subunit beta 1 | 40.1 | Core matrisome | Yes |
| LAMB2 | laminin subunit beta 2 | 110.8 | Core matrisome | No |
| LAMC1 | laminin subunit gamma 1 | 39.8 | Core matrisome | Yes |
| VCAN | versican | 9.6 | Core matrisome | No |
| ANXA6 | annexin A6 | 129 | Matrisome-associated | Yes |
| ANXA7 | annexin A7 | 74.1 | Matrisome-associated | Yes |
| ANXA11 | annexin A11 | 137.1 | Matrisome-associated | No |
Fig. 2.
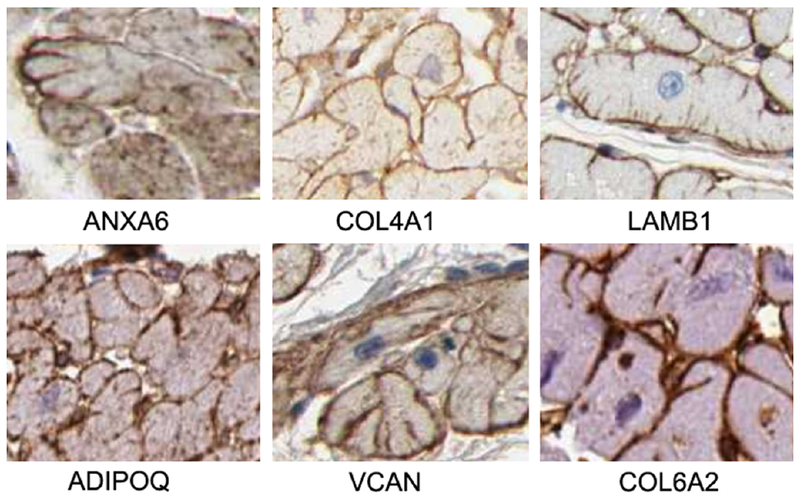
Representative matrisome protein expression at the t-tubule. Key: ANXA6: Annexin 6, COL4A1: Collagen type IV alpha 1 chain; LAMB1: laminin subunit beta 1; ADIPOQ: adiponectin, C1Q and collagen domain containing; VCAN: versican; COL6A2: Collagen type VI alpha 2 chain. Images from the Human Protein Atlas.
3.5. Gene Ontology Validation of t-Tubule Proteins
We performed Gene Ontology (GO) validation on the 96 FIPASubC-identified, 50 literature-identified, or the 127 combined t-tubule proteins. We queried for biological process and cellular compartment and ranked the responses by P value. For the 96 FIPASubC-identified proteins, when queried for biological process, the top GO terms were “cell differentiation,” “cellular developmental process,” and “muscle contraction.” The top cellular components were sarcolemma and membrane microdomain, while T-tubule ranked 31st on the list by p value (Table 2A). Among the known (literature-identified) t-tubules, the top GO biological processes were “regulation of localization, ion membrane transport and heart contraction.” In this list, T-tubule was only the 14th ranked cellular component (Table 2B). In the combined list, the top biological process was regulation of heart contraction and the t-tubule was the 18th ranked cellular component by P-value (Table 2C). We also assessed GO terms to further define protein groups. We found a median of 25 GO terms associated with each protein. Ranking and reporting the mostly commonly identified GO terms identified typical terms such as “protein binding,” “plasma membrane,” and “cytosol.” Other more frequent terms being found in 10 or more proteins included “calcium ion binding,” collagen-containing extracellular matrix,” and “membrane raft.” (Supplemental Table 4).
Table 2.
Gene Ontology results for t-tubule proteins.
| A. HPASubC-identified t-tubule proteins (N=96) | ||
|---|---|---|
| GO Biological Process | Fold Enrichment | P value |
| 1. Cell differentiation | 2.63 | 3.55E-06 |
| 2. Cellular developmental process | 2.58 | 6.88E-06 |
| 3. Muscle contraction | 10.05 | 1.64E-04 |
| GO Cellular Component | Fold Enrichment | P value |
| 1. Sarcolemma | 31.50 | 2.49E-19 |
| 2. Membrane microdomain | 11.78 | 1.17E-09 |
| 31. T-tubule | 21.52 | 7.70E-03 |
| B. Literature-identified t-tubule proteins (N=50) | ||
| GO Biological Process | Fold Enrichment | P value |
| 1. Regulation of localization | 6.09 | 1.27E-20 |
| 2. Regulation of ion transmembrane transport | 20.67 | 1.20E-19 |
| 3. Regulation of heart contraction | 37.5 | 1.23E-19 |
| GO Cellular Component | Fold Enrichment | P value |
| 1. Contractile fiber part | 41.30 | 2.79E-25 |
| 2. Myofibril | 40.73 | 3.67E-25 |
| 14. T-tubule | 80.93 | 2.97E-13 |
| C. Combined t-tubule proteins (N=129) | ||
| GO Biological Process | Fold Enrichment | P value |
| 1. Regulation of heart contraction | 18.04 | 9.85E-17 |
| 2. Regulation of localization | 3.66 | 4.50E-16 |
| 3. Muscle system process | 13.62 | 6.63E-16 |
| GO Cellular Component | Fold Enrichment | P value |
| 1. Sarcolemma | 35.59 | 1.24E-31 |
| 2. Contractile fiber | 17.93 | 3.39E-20 |
| 18. T-tubule | 35.05 | 1.56E-10 |
We then used String 11 to evaluate protein-protein interactions across the 127 combined literature-based and HPASubC identified t-tubule proteins. All 50 literature-identified and 44 HPASubC-identified t-tubule proteins together made a highly connected single integrated interactome map (Fig. 3; https://version-11-0.string-db.org/cgi/network.pl?networkId=2rIliZbUYTTY).
Fig. 3.

The entire t-tubule interactome map (String 11). Red boxes indicate t-tubule proteins new to this study.
3.6. Expression of cardiac t-tubule proteins in skeletal muscle
The 96 HPASubC-discovered cardiac t-tubule proteins were investigated within HPA skeletal muscle images for similar features. The clear cardiac t-tubule pattern of deep invaginations was not noted in skeletal muscle. Rather, the most common pattern seen for the 96 proteins was myocyte cell membrane staining (n=36) followed by cytoplasmic (n=24) and endothelial (n=19) staining patterns. (Supplemental Fig. 4, Supplemental Table 1).
4. Discussion
The t-tubule is a subcellular region of the cardiac myocyte important for excitation-contraction coupling. While the general function and many proteins of the t-tubule are known, others have remained to be discovered. Using an agnostic pattern-centric approach upon 13,203 proteins, we have identified 96 proteins that have a pattern of IHC staining suggesting they reside within or adjacent to the t-tubule. Only 17 of these proteins were previously reported at the t-tubule, thus we have potentially added a significant number of new targets to be considered in pathologic and physiologic analysis of this region.
While at least 50 proteins have previously been reported in the t-tubule, only 31% of these overlapped with our findings. It may be that some of these proteins are found in the t-tubule and elsewhere in the cardiomyocyte, such that their staining patterns are not distinctly of the t-tubule. Indeed, many had generalized cytoplasmic staining and several well-characterized t-tubule proteins such as BIN1 appeared in this list. This indicates that the invagination pattern that we used (Supplemental Fig. 1) was not wholly inclusive of all t-tubule proteins. However, using a second agnostic approach, GWAS trait loci, literature-based proteins were not favored over those described here. The only enrichment for GWAS loci was among the 17 proteins found in both datasets.
It is interesting that only a very small percent (1.8%) of all matrisome proteins known to localize to the heart (based on gene expression) had a t-tubule staining pattern. This suggests a very specific interplay of elements of the extracellular matrix and t-tubule proteins to either create and maintain these structures or assist in the function of the t-tubule. While we confirmed some known matrisome proteins of the t-tubule (COL4A1, LAMB1, ANXA6, etc.), VCAN, COL6A2, ADIPOQ and others are identified here for the first time.
The GO terms identified for the HPASubC-discovered t-tubules, while consistent with location and function, were not as statistically strong as the literature-determined t-tubule proteins. One reason is the novelty of some proteins discovered in this work (FAM187A, ZNF750, C15orf59) for which a priori functions and interaction are unknown. This is why the GWAS approach, which is agnostic to function, may better highlight the similarity of the lists.
There are important limitations to this project. As we have stated in every survey of the HPA, IHC-based proteomic data has the potential for error.[7, 9, 10] There are both false positive and false negative predictions based on antibody non-specificity or non-optimized conditions for appropriate antibodies. Often the same antibody will give different patterns of staining across different tissue cores, as noted by an average score of only 1.4 for the images reviewed. Random myocyte orientation and postmortem changes complicate the determination of t-tubule staining patterns. Also, specifically known for BIN1, only one isoform (cBIN1) localizes to the t-tubule membrane so that an antibody to a different isoform of a protein would not show the t-tubule pattern, as we observed. Isoforms, the result of alternative splicing may be important for other proteins, in which only one antibody epitope showed a t-tubule pattern. Additionally, many of these proteins are expressed across multiple tissues, yet specific isoforms might be relevant only for localization to the t-tubules.
However, advantageous to this project was the distinct invagination pattern which cannot be caused by random and non-specific antibody binding. We specifically assessed whether t-tubule staining could be mistaken for junctional SR membrane or Z-lines and excluded this. It is difficult to explain away false positive t-tubule staining as anything more than a shared epitope across proteins, due to the distinctness of the pattern. Regardless, all of these results are putative t-tubule proteins until confirmation by additional methods. Also, the HPA continues to increase its protein coverage and some potential t-tubule proteins (including three identified in the literature) do not yet have image coverage.
Another advantage of the HPA resources is that all of the antibodies used undergo robust testing for IHC and other uses including western blotting, protein array and immunocytochemistry. All of these antibodies are commercially available with links directly to the vendor. Therefore, the tools to continue investigations of the proteins exist for laboratories interested in further functional assays. It is possible these newly described proteins will ultimately become useful diagnostic markers of disease.
The 79 new, putative t-tubule proteins represents a marked increase of proteins that function at the t-tubule. It will be interesting to determine how many of these proteins integrate with known functional pathways at this important subcellular structure. Our HPASubC tool continues to provide new understanding of protein expression in the heart.
Supplementary Material
Highlights.
The first, thorough, agnostic image—based proteomic investigation of the cardiac t-tubule.
The discovery of 96 proteins with immunohistochemistry-based patterns of t-tubule staining
A fully integrated collection of t-tubule localized proteins with informatic analysis.
Acknowledgements
The authors thank Drs. Robin Shaw and Dan Arking for helpful critique of the manuscript.
Funding
This work was supported by the National Institutes of Health, National Heart Lung Blood Institute (grant number 1R01HL137811) and the American Heart Association (grant number 17GRNT33670405).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Hong T, Shaw RM. Cardiac T-Tubule Microanatomy and Function. Physiological reviews. 2017;97:227–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Forssmann WG, Girardier L. A study of the T system in rat heart. The Journal of cell biology. 1970;44:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lindner E [Submicroscopic morphology of the cardiac muscle]. Zeitschrift fur Zellforschung und mikroskopische Anatomie. 1957;45:702–46. [PubMed] [Google Scholar]
- [4].Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, et al. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Crossman DJ, Jayasinghe ID, Soeller C. Transverse tubule remodelling: a cellular pathology driven by both sides of the plasmalemma? Biophys Rev. 2017;9:919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Russell J, Du Toit EF, Peart JN, Patel HH, Headrick JP. Myocyte membrane and microdomain modifications in diabetes: determinants of ischemic tolerance and cardioprotection. Cardiovascular diabetology. 2017;16:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cornish TC, Chakravarti A, Kapoor A, Halushka MK. HPASubC: A suite of tools for user subclassification of human protein atlas tissue images. Journal of pathology informatics. 2015;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based Human Protein Atlas. Nature biotechnology. 2010;28:1248–50. [DOI] [PubMed] [Google Scholar]
- [9].Anene DF, Rosenberg AZ, Kleiner DE, Cornish TC, Halushka MK. Utilization of HPASubC for the identification of sinusoid-specific proteins in the liver. Journal of proteome research. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang TY, Lee D, Fox-Talbot K, Arking DE, Chakravarti A, Halushka MK. Cardiomyocytes have mosaic patterns of protein expression. Cardiovasc Pathol. 2018;34:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harbor perspectives in biology. 2012;4:a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Current protocols in bioinformatics. 2016;54:1 30 1-1 3. [DOI] [PubMed] [Google Scholar]
- [14].Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Papa I, Astier C, Kwiatek O, Raynaud F, Bonnal C, Lebart MC, et al. Alpha actinin-CapZ, an anchoring complex for thin filaments in Z-line. Journal of muscle research and cell motility. 1999;20:187–97. [DOI] [PubMed] [Google Scholar]
- [17].Sorimachi H, Freiburg A, Kolmerer B, Ishiura S, Stier G, Gregorio CC, et al. Tissue-specific expression and alpha-actinin binding properties of the Z-disc titin: implications for the nature of vertebrate Z-discs. Journal of molecular biology. 1997;270:688–95. [DOI] [PubMed] [Google Scholar]
- [18].Lopez-Ayala JM, Ortiz-Genga M, Gomez-Milanes I, Lopez-Cuenca D, Ruiz-Espejo F, Sanchez-Munoz JJ, et al. A mutation in the Z-line Cypher/ZASP protein is associated with arrhythmogenic right ventricular cardiomyopathy. Clinical genetics. 2015;88:172–6. [DOI] [PubMed] [Google Scholar]
- [19].Muller FU, Kirchhefer U, Begrow F, Reinke U, Neumann J, Schmitz W. Junctional sarcoplasmic reticulum transmembrane proteins in the heart. Basic research in cardiology. 2002;97 Suppl 1 :I52–5. [DOI] [PubMed] [Google Scholar]
- [20].Hong T, Yang H, Zhang SS, Cho HC, Kalashnikova M, Sun B, et al. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nature medicine. 2014;20:624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, et al. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 2010;107:747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Leroy J, Richter W, Mika D, Castro LR, Abi-Gerges A, Xie M, et al. Phosphodiesterase 4B in the cardiac L-type Ca(2)(+) channel complex regulates Ca(2)(+) current and protects against ventricular arrhythmias in mice. The Journal of clinical investigation. 2011;121:2651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS biology. 2005;3:e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Waddell LB, Lemckert FA, Zheng XF, Tran J, Evesson FJ, Hawkes JM, et al. Dysferlin, annexin A1, and mitsugumin 53 are upregulated in muscular dystrophy and localize to longitudinal tubules of the T-system with stretch. Journal of neuropathology and experimental neurology. 2011;70:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Spreca A, Rambotti MG, Giambanco I, Pula G, Bianchi R, Ceccarelli P, et al. Immunocytochemical localization of annexin V (CaBP33), a Ca(2+)-dependent phospholipid- and membrane-binding protein, in the rat nervous system and skeletal muscles and in the porcine heart. Journal of cellular physiology. 1992;152:587–98. [DOI] [PubMed] [Google Scholar]
- [26].Barrientos G, Hidalgo C. Annexin VI is attached to transverse-tubule membranes in isolated skeletal muscle triads. The Journal of membrane biology. 2002;188:163–73. [DOI] [PubMed] [Google Scholar]
- [27].Selbert S, Fischer P, Pongratz D, Stewart M, Noegel AA. Expression and localization of annexin VII (synexin) in muscle cells. J Cell Sci. 1995;108 ( Pt 1):85–95. [DOI] [PubMed] [Google Scholar]
- [28].Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, et al. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS biology. 2010;8:e1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Robenek H, Weissen-Plenz G, Severs NJ. Freeze-fracture replica immunolabelling reveals caveolin-1 in the human cardiomyocyte plasma membrane. Journal of cellular and molecular medicine. 2008;12:2519–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kostin S, Scholz D, Shimada T, Maeno Y, Mollnau H, Hein S, et al. The internal and external protein scaffold of the T-tubular system in cardiomyocytes. Cell and tissue research. 1998;294:449–60. [DOI] [PubMed] [Google Scholar]
- [31].Hofhuis J, Bersch K, Bussenschutt R, Drzymalski M, Liebetanz D, Nikolaev VO, et al. Dysferlin mediates membrane tubulation and links T-tubule biogenesis to muscular dystrophy. J Cell Sci. 2017;130:841–52. [DOI] [PubMed] [Google Scholar]
- [32].Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Progress in biophysics and molecular biology. 2008;98:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Al-Qusairi L, Laporte J. T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skeletal muscle. 2011;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ibrahim M, Gorelik J, Yacoub MH, Terracciano CM. The structure and function of cardiac t-tubules in health and disease. Proceedings Biological sciences. 2011;278:2714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bennett V, Healy J. Membrane domains based on ankyrin and spectrin associated with cell-cell interactions. Cold Spring Harbor perspectives in biology. 2009;1:a003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Demonbreun AR, Rossi AE, Alvarez MG, Swanson KE, Deveaux HK, Earley JU, et al. Dysferlin and myoferlin regulate transverse tubule formation and glycerol sensitivity. The American journal of pathology. 2014;184:248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Flucher BE, Conti A, Takeshima H, Sorrentino V. Type 3 and type 1 ryanodine receptors are localized in triads of the same mammalian skeletal muscle fibers. The Journal of cell biology. 1999;146:621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci U S A. 2002;99:4073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Thomas MJ, Sjaastad I, Andersen K, Helm PJ, Wasserstrom JA, Sejersted OM, et al. Localization and function of the Na+/Ca2+-exchanger in normal and detubulated rat cardiomyocytes. Journal of molecular and cellular cardiology. 2003;35:1325–37. [DOI] [PubMed] [Google Scholar]
- [40].Meyers MB, Puri TS, Chien AJ, Gao T, Hsu PH, Hosey MM, et al. Sorcin associates with the poreforming subunit of voltage-dependent L-type Ca2+ channels. J Biol Chem. 1998;273:18930–5. [DOI] [PubMed] [Google Scholar]
- [41].Vlahovich N, Kee AJ, Van der Poel C, Kettle E, Hernandez-Deviez D, Lucas C, et al. Cytoskeletal tropomyosin Tm5NM1 is required for normal excitation-contraction coupling in skeletal muscle. Molecular biology of the cell. 2009;20:400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kee AJ, Gunning PW, Hardeman EC. Diverse roles of the actin cytoskeleton in striated muscle. Journal of muscle research and cell motility. 2009;30:187–97. [DOI] [PubMed] [Google Scholar]
- [43].Huang H, Chanda P, Alonso A, Bader JS, Arking DE. Gene-based tests of association. PLoS genetics. 2011;7:e1002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].van Setten J, Brody JA, Jamshidi Y, Swenson BR, Butler AM, Campbell H, et al. PR interval genome-wide association meta-analysis identifies 50 loci associated with atrial and atrioventricular electrical activity. Nature communications. 2018;9:2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cantor RM, Lange K, Sinsheimer JS. Prioritizing GWAS results: A review of statistical methods and recommendations for their application. American journal of human genetics. 2010;86:6–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Molecular & cellular proteomics : MCP. 2012;11:M111 014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Floyd BJ, Wilkerson EM, Veling MT, Minogue CE, Xia C, Beebe ET, et al. Mitochondrial Protein Interaction Mapping Identifies Regulators of Respiratory Chain Function. Molecular cell. 2016;63:621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lamarca V, Sanz-Clemente A, Perez-Pe R, Martinez-Lorenzo MJ, Halaihel N, Muniesa P, et al. Two isoforms of PSAP/MTCH1 share two proapoptotic domains and multiple internal signals for import into the mitochondrial outer membrane. American journal of physiology Cell physiology. 2007;293:C1347–61. [DOI] [PubMed] [Google Scholar]
- [49].Bui M, Gilady SY, Fitzsimmons RE, Benson MD, Lynes EM, Gesson K, et al. Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J Biol Chem. 2010;285:31590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


