Abstract
Background/Objectives:
Nearly half of the population living with HIV in the US is now older than fifty years of age with at least 6% over the age of 65. Between 35–50% live with mild to moderate cognitive impairment. Older persons living with HIV (PLWH) also have a substantial burden of HIV-associated non-AIDS medical conditions (HANA) and are at risk for frailty, geriatric syndromes, and early mortality compared to HIV-uninfected peers. We sought to define the magnitude of geriatric conditions and multimorbidity in PLWH over age 60 who are living with symptomatic cognitive impairment. In a subset of participants, we examine associations between these geriatric conditions.
Design:
Retrospective cohort study
Setting:
HIV Elders Study at the UCSF Memory and Aging Center
Participants:
Participants were HIV-infected, virally suppressed, 60 years or older, and clinically diagnosed with Mild Neurocognitive Disorder (MND).
Measurements:
We conducted standardized assessment of geriatric conditions and everyday function and investigated multimorbidity burden using the Veterans Aging Cohort Study (VACS) index.
Results:
Among 141 older PLWH with MND, 58% report incontinence, 55% meet criteria for pre-frailty, and a substantial proportion report dependence with iADLs (52%) or ADLs (41%). The mean (SD) VACS Index score is 33 (14), suggesting a 13.8% 5-year all-cause mortality risk.
Conclusions:
Older PLWH with symptomatic cognitive impairment carry a substantial burden of other geriatric conditions. Our work supports the need for comprehensive geriatric systems of care for cognitively impaired individuals aging with HIV.
Keywords: HIV, Aging, Frailty, Cognition
INTRODUCTION
Nearly half of the population living with HIV in the US is now older than fifty years of age with at least 6% over the age of 65 1,2. People with HIV are living longer due to effective antiretroviral therapy (ART), a trend also seen in resource-limited settings 3. Particularly concerning for older adults with HIV is their increased risk for cognitive impairment. HIV-associated neurocognitive disorder (HAND) persists in older adults with HIV despite combination ART and is associated with overall morbidity 4. It is estimated that between 35–50% of older adults living with HIV suffer from mild to moderate cognitive impairment 4.
Frailty and geriatric syndromes may occur at a younger age in individuals with HIV than they do in those who are HIV-uninfected 5. Older persons living with HIV (PLWH) also have a greater burden of comorbidities, termed HIV-associated non-AIDS medical conditions (HANA),6 and multimorbidity as measured by the Veterans Aging Cohort Study (VACS) index 7. Past work indicates that the VACS index better predicts hospitalization and all-cause mortality than typical severity measures like CD4 t-lymphocyte counts, HIV RNA levels, and age 7. One group identified direct correlations between the VACS index and cognitive impairment 8.
In older adults living with HIV or without ART, geriatric conditions, such as decreased independence in activities of daily living (iADL) and poor medication adherence, are associated with a higher VACS score 9. It is thought that inflammation, particularly monocyte activation, is a key predictor of increased morbidity and all-cause mortality even in the context of viral suppression 10,1,11.
In this study, we sought to define the frequency of geriatric conditions and burden of multimorbidity in adults over the age of 60 with virally suppressed HIV and diagnosed with Mild Neurocognitive Disorder (MND). We also investigate multimorbidity burden using the Veterans Aging Cohort Study (VACS) index.
METHODS
Participants
We recruited participants from the HIV Elders Study at UCSF. To meet inclusion into the parent study, participants were HIV-infected, virally suppressed (<50 HIV copies/mL), 60 years or older, and clinically diagnosed with MND based on 2007 criteria, as previously described 16. Similar to a Mild Cognitive Impairment (MCI) diagnosis in people without HIV infection, people with MND have mild symptoms and remain functionally independent despite performances on cognitive measures that is impaired in two cognitive domains. All were screening for enrollment into an intervention trial testing Mindfulness Based Stress Reduction (MBSR) and thus, were also required to not be a current practitioner of MBSR. For this study, individuals with conditions other than HIV potentially contributing to cognitive change were allowed to enroll provided they otherwise were symptomatic and had documented poor neuropsychological testing performance in at least 2 cognitive domains, and, in the opinion of the investigators, HIV was a likely contributor to the cognitive change. We examined standardized assessment of geriatric conditions and everyday function conducted during the screening visit. All participants were enrolled between March 2013 and August 2017. Using a regional clinical laboratory, we measured current CD4 t-lymphocyte counts, plasma HIV RNA levels, hemoglobin, liver function tests including AST and ALT, platelet count, creatinine, and hepatitis C serostatus. The VACS index was calculated in standard fashion using age, current CD4 t-lymphocyte count, plasma HIV RNA level, hemoglobin, liver function tests including AST and ALT, platelet count, creatinine level, and hepatitis C co-infection.
Geriatric Syndromes
We identified the presence of geriatric syndromes in participants based on both subjective reports and objective measures. These included self-reported responses to structured questionnaires indicative of conditions common in the aging population regarding falls in the past year, any report of urinary incontinence, mobility, and difficulty and dependence with activities of daily living (ADL) and instruments activities of daily living (iADL) 17. Hearing difficulty was determined if the participant was unable to identify whispered numbers or letters in either ear. Vision difficulty was determined if Snellen acuity was worse than 20/40. We defined mild to moderate depression as a Center for Epidemiological Studies depression (CES-D) score of 16 or greater. Difficulty with mobility was defined as reporting difficulty walking across a room or two blocks.
Frailty assessment was based on Fried’s frailty model, which included information on self-reported weight-loss, exhaustion, and low physical activity as well as objective measure of grip strength and walking speed 18. Self-report of exhaustion was based on two items from the CES-D questionnaire and low physical activity was based on the Minnesota Leisure score. We measured walking speed, standardized by height and gender, and weakness using grip strength, standardized by gender and BMI. Frailty was defined as three or more of the above criteria, while pre-frailty was defined as at least one of the criteria met.
Multimorbidity/VACS Index.
We calculated the VACS index in standard fashion using age, current CD4 t-lymphocyte count, plasma HIV RNA level, hemoglobin, liver function tests including AST and ALT, platelet count, creatinine level, and active hepatitis C infection.
Statistical Analysis
Descriptive statistics to characterize the frequency of geriatric conditions included the median and range for continuous variables and frequencies and percentages for categorical data. Statistical significance was defined as p≤0.05 for two-sided hypotheses. SPSS v. 25.1 (IBM, Inc., Chicago, IL) was used for all statistical analyses.
RESULTS
141 participants were enrolled in our study (Table 1). The participant group had a median age of 64 (range: 60–80), were mostly Caucasian (91%), and more than half had at least 16 years of education (57%). About half of participants reported living alone (56%). The mean (SD) CD4 count was 600 (273), the median (range) estimated duration of HIV was 26 years (3–40) and the percentage of participants with a CD4 nadir < 200 cells was 71% (n=100).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Demographics and Clinical Characteristics of Study Participants | |
|---|---|
| Age, median (range) | 64 (60–80) |
| Male (n, %) | 128, 91% |
| Caucasian (n, %) | 110, 78% |
| Education ≥ 16 years (n, %) | 80, 57% |
| Lives Alone (n, %) | 79, 56% |
| Current CD4 (cells/μL), mean (SD) | 600 (273) |
| EDI (years; median [range]) | 26 (3–40) |
| CD4 Nadir < 200 | 100, 71% |
SD: standard deviation; EDI: estimated duration of infection
Frequency of Geriatric Conditions.
Among participants studied, 58% reported incontinence, 55% reported pre-frailty, and 52% reported iADL difficulties (Figure 1). Frailty was noted in 7%. We found that 32% indicated difficulty with mobility and 41% reported a fall in the last year. Mild to moderate depression was present in 39%. In the overall cohort, the mean VACS score was 33 with a standard deviation of 14 (n=138).
Figure 1.
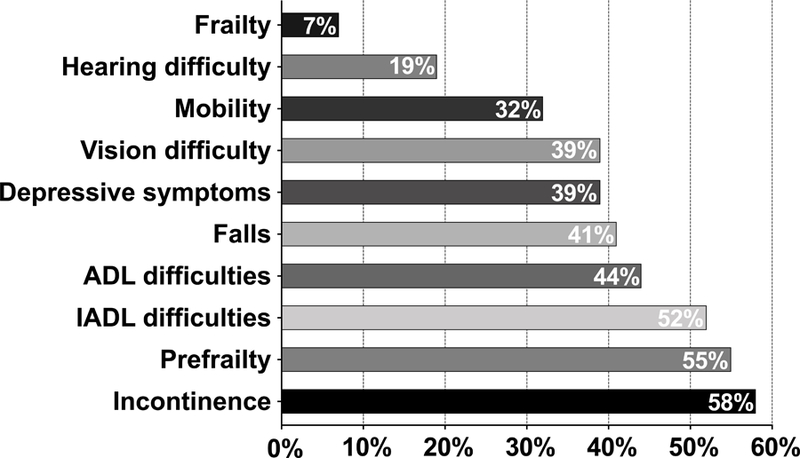
Frequencies of geriatric syndromes among Older HIV-infected Adults with MND (n=141).
DISCUSSION
Results from this study show that virally suppressed older PLWH with symptomatic cognitive impairment also have a substantial burden of other geriatric conditions—particularly, incontinence (58%), pre-frailty (55%), and difficulty with iADLs (52%) and ADLs (41%). The burden of depressive symptoms was also quite high, at 39% and similar to prior reports 19,20. We identified a mean VACS index score of 33, supportive of higher risk for mortality. Based on prior reports, this score is associated with a 13.8% all-cause five-year mortality risk 21,22. This mortality risk should be considered in the context of our selection with all participants having well-controlled HIV with suppressed HIV RNA in plasma and most had CD4 counts > 200 cells (n=133/139). The risk in those who are unsuppressed and, particularly those with lower CD4 counts can be expected to be higher.
A little more than half of participants live alone (56%) raising concerns for the availability of social support of older adults with HIV. Older adults with HIV tend to have higher rates of social isolation than younger adults 24. Social isolation in combination with difficulty with performing activities of daily living contributes to feelings of social loneliness, and has been associated with depressive symptoms 25. The combination of cognitive impairment, social isolation and other geriatric syndromes highlights the substantial vulnerability of this aging group of individuals.
We conducted our study in a convenience sample of individuals with MND enrolling into an MBSR intervention, limiting generalizability of our findings to the broader population living with HIV. Those with MND may already be more vulnerable to adverse clinical outcomes than individuals without cognitive symptoms and those without objective cognitive impairment. A comparison group of HIV-uninfected adults of comparable age and those without cognitive impairment could have provided added clarity to our findings. Since 39% or our participants reported symptoms of depression, we cannot exclude the possibility that depression impacted rates of self-reporting symptoms related to geriatric syndromes.
Geriatric syndromes are useful in assessing the trajectory of patients’ physical function and functional status as they age. Assessing geriatric syndromes can predict how patients will respond to environmental stressors and situational challenges 26,17. Early and accurate identification of geriatric syndromes may aid clinicians in focusing resources on assisting the most vulnerable patients.
Our work has important implications for clinicians managing older PLWH who report cognitive symptoms. In the context of well-managed HIV care, these individuals remain vulnerable with a high burden of multimorbidity and geriatric conditions. We add to the literature that geriatric conditions and early functional decline persist in the era of combination ART. In older adults suffering with MND, cognitive symptoms are just the “tip of the iceberg” of conditions that would benefit from careful clinical management. Future studies should look more closely at the role that in monocyte activation plays in functional decline.
In an era of HIV and aging, multimorbidity, polypharmacy, and functional decline are primary concerns 1. A geriatrics-focused approach of functional preservation will become increasingly important in caring for older adults with HIV living in the community 5. Taken together, there remains an urgent need to develop systems of care that better address the holistic needs of older adults living with HIV despite optimal management of HIV, itself.
ACKNOWLEDGEMENTS:
Contents were presented at the Hawaii American College of Physicians (2019) and submitted and accepted for presentation at the American Geriatrics Society annual meeting (2019). This work supported by R01NR015223 (VV). We thank the American Federation for Aging (MSTAR program) for their collaboration and support. Funding sources: this work funded by the National Institutes of Health Grant Numbers: R01 NR015223 and R01 MH113406 (MSTAR: T35AG026736).
Footnotes
Conflict of Interest:
Dr. Valcour has served as a consultant to ViiV Healthcare and Merck on issues related to HIV and aging. Dr. Ndhlovu has served as an advisory board member to ViiV Healthcare related to ART and HIV.
Sponsor’s role: none
REFERENCES
- 1.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012;60 Suppl 1:S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HIV Among People Aged 50 And Over 2018; https://www.cdc.gov/hiv/group/age/olderamericans/index.html, 2018.
- 3.Negin J, Wariero J, Cumming RG, Mutuo P, Pronyk PM. High rates of AIDS-related mortality among older adults in rural Kenya. J Acquir Immune Defic Syndr 2010;55(2):239–244. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene M, Covinsky KE, Valcour V, et al. Geriatric Syndromes in Older HIV-Infected Adults. J Acquir Immune Defic Syndr 2015;69(2):161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Justice A, Falutz J. Aging and HIV: an evolving understanding. Curr Opin HIV AIDS 2014;9(4):291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Justice Amy C. MSF, Tracy Russ, Kuller Lew, Tate Janet p., Goetz Matthew Bidwell, Fiellin David A., Vanasse Gary J., Butt Adeel A., Rodriguez-Barradas Maria C., Gibert Cynthia, Oursler Kris Ann, Deeks Steven G., Bryant Kendall. Does an Index Composed of Clinical Data Reflect Effects of Inflammation, Coagulation, and Monocyte Activation on Mortality Among Those Aging with HIV? Clinical Infectious Diseases 2012;54(7):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marquine Maria J. JLM, Umlauf Anya, Fazeli Pariya L., Gouaux Ben, Heaton Robert, ellis Ronald, Letendre Scott, Grant Igor, and Moore David J.. The Veterans Aging Cohort Study (VACS) Index and Neurocognitive Change: A Longitudinal Study. Clinical Infectious Diseases 2016;63(5):694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John MD, Greene M, Hessol NA, et al. Geriatric Assessments and Association With VACS Index Among HIV-Infected Older Adults in San Francisco. J Acquir Immune Defic Syndr 2016;72(5):534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knudsen TB, Ertner G, Petersen J, et al. Plasma Soluble CD163 Level Independently Predicts All-Cause Mortality in HIV-1-Infected Individuals. J Infect Dis 2016;214(8):1198–1204. [DOI] [PubMed] [Google Scholar]
- 11.Sandler NG, Wand H, Roque A, et al. Plasma Levels of Soluble CD14 Independently Predict Mortality in HIV Infection. The Journal of Infectious Diseases 2011;203(6):780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uysal HK, Sohrabi P, Habip Z, et al. Neopterin and Soluble CD14 Levels as Indicators of Immune Activation in Cases with Indeterminate Pattern and True Positive HIV-1 Infection. PLoS One 2016;11(3):e0152258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdo TH AW, Woods SP et al. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013;27(9):1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Antoni ML, Byron MM, Chan P, et al. Normalization of Soluble CD163 Levels After Institution of Antiretroviral Therapy During Acute HIV Infection Tracks with Fewer Neurological Abnormalities. The Journal of Infectious Diseases 2018;218(9):1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imp BM, Rubin LH, Tien PC, et al. Monocyte Activation Is Associated With Worse Cognitive Performance in HIV-Infected Women With Virologic Suppression. J Infect Dis 2017;215(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene Meredith ACJ, and Covinsky Kenneth E.. Assessment of geriatric syndromes and physicial function in people living with HIV. Virulence 2016;8(5):586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 19.Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Curr Psychiatry Rep 2015;17(1):530. [DOI] [PubMed] [Google Scholar]
- 20.Greene M, Hessol NA, Perissinotto C, et al. Loneliness in Older Adults Living with HIV. AIDS Behav 2018;22(5):1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown ST, Tate JP, Kyriakides TC, et al. The VACS index accurately predicts mortality and treatment response among multi-drug resistant HIV infected patients participating in the options in management with antiretrovirals (OPTIMA) study. PLoS One 2014;9(3):e92606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr 2013;62(2):149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer ME, Jain A, Matteini A, et al. Serum levels of the immune activation marker neopterin change with age and gender and are modified by race, BMI, and percentage of body fat. J Gerontol A Biol Sci Med Sci 2010;65(8):858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webel AR, Longenecker CT, Gripshover B, Hanson JE, Schmotzer BJ, Salata RA. Age, stress, and isolation in older adults living with HIV. AIDS Care 2014;26(5):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge L, Yap CW, Ong R, Heng BH. Social isolation, loneliness and their relationships with depressive symptoms: A population-based study. PLoS One 2017;12(8):e0182145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inouye SK SS, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatric Society 2007;55(5):780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]


