Abstract
Myelinating cells of both the peripheral and central nervous systems undergo dramatic cytoskeletal reorganization in order to differentiate and produce myelin. Myelinating oligodendrocytes in the central nervous system show a periodic actin pattern, demonstrating tight regulation of actin. Furthermore, recent data demonstrate that actin polymerization drives early cell differentiation and that actin depolymerization drives myelin wrapping. Dysregulation of the actin cytoskeleton in myelinating cells is seen in some disease states. This review highlights the cytoskeletal molecules that regulate differentiation of and myelination by cells of the PNS and CNS, informing our understanding of neural development, in particular myelination.
Keywords: actin cytoskeleton, myelin, oligodendrocyte, Schwann cell
Introduction: the cytoskeleton
One of the most basic, yet most complex, tasks in biology is membrane process extension within a diverse extracellular matrix. This intricate task is involved in targeted cellular migration and in polarized membrane extension during cellular differentiation. It involves sensing the local environment, circumventing and/or interacting with extracellular proteins and other cells, ultimately reaching a final destination, and differentiating into a mature cell. The cytoskeleton provides both structure and function, acting as a mechanism for cell motility and as a skeletal framework defining cell morphology. For example, in migrating epithelial cells, the actin cytoskeleton consists of a dense meshwork of filamentous actin and its regulators, which control actin filament turnover at the leading edge of a cell, thereby driving cellular movement.
With recent advances in imaging techniques, there is more detailed understanding of the cytoskeletal architecture of cells in the nervous system. Investigations into neural cell types provide novel insights and hypotheses regarding neuron-glia interactions. Periodic cytoskeletal patterns of actin and spectrin, an actin binding protein, are found in multiple cell types of the nervous system. These patterns are found in both inhibitory and excitatory neurons, as well as differentiating oligodendrocytes, the myelinating cell of the central nervous system (CNS). Surprisingly, astrocytes or microglia do not contain the same cytoskeletal periodicity, suggesting cell-type specific cytoskeletal patterns [1]. The cytoplasmic cytoskeletal periodicity of oligodendrocytes differs from epithelial cell cytoskeletal networks and appears to act as a scaffold to mediate cell-cell interactions. In oligodendrocyte-axon interactions, these cytoskeletal patterns generally align with one another and may be mediated through specific cell adhesion molecules such as L1CAMs [2]. These data suggest that the cytoskeleton is tightly regulated in myelinating cells. How are these patterns determined? Are these patterns an intrinsic pattern of the cell or are there extracellular signals mediating the formation of these cytoskeletal patterns? This review highlights advances in our understanding of role of the actin cytoskeleton in the cell biology of myelinating cells.
Cytoskeleton in myelinating cells
Myelin is a critical component of the nervous system where it facilitates action potential propagation and provides metabolic support to axons. Modulation of cytoskeletal organization is required for oligodendrocyte progenitor cells (OPC) movement during development, when these cells migrate to their appropriate destination and differentiate to mature oligodendrocytes, the myelinating cells of the CNS. There, they respond to axons by extending massive amounts of differentiated plasma membrane to ensheath the axon and generate myelin, thereby allowing for increased action potential propagation along the axon, as well as metabolic support to axons [3]–[6].
In the peripheral nervous system (PNS), myelin is formed by Schwann cells. Neural crest-derived Schwann cell precursor cells migrate to the periphery where they proliferate and move along axons until they reach their final destination at which point they surround bundles of axons. Schwann cells develop a basal lamina, and begin radial sorting of axons, where they separate out large caliber axons, generating a 1:1 relationship with them, and begin myelination [7] [8]. Although oligodendrocytes and Schwann cells are derived from different tissues, they share major similarities in the drastic morphological changes required for myelination that necessitate changes to the cytoskeleton.
Actin cytoskeletal dynamics are a major element driving myelination. Recent discoveries highlight the role of actin polymerization during OPC process extension and of actin filament retraction and depolymerization driving myelin wrapping [9], [10]. Filamentous actin (F-actin) is found in OPCs and OPC processes, but both in vitro and in vivo models show that F-actin is absent from compact myelin except for the outermost myelin layer and the leading edge [1], [9], [10]. Two hypotheses arise from these data. One proposes that during myelination, actin polymerization at the leading edge, the inner tongue of myelin, is the driving force promoting myelin wrapping, while actin depolymerization immediately behind the leading edge allows for myelin compaction [9]. The second hypothesis suggests that actin depolymerization provides the force to drive myelin wrapping [9], [10]. Both hypotheses require a tightly-regulated, localized signal(s) that could rapidly switch from actin polymerization to actin depolymerization. That signal is yet to be determined as technical limitations have constrained high-resolution visualization of actin polymerization at the leading edge in vivo. In vivo models of myelin cytoskeleton phenotypes are summarized in Table 1. The question remains, what regulates the cytoskeleton during cellular differentiation and myelination?
Table 1.
Myelin cytoskeleton phenotypes in in vivo animal models.
| transiently during development | ||||
|---|---|---|---|---|
| optic nerve | CNP−/− | · Fewer cytoplasmic channels | Snaidero et al. 2017 | |
| Ptenfl/fl; Cnp1-Cre, Plp1CreERT2 | · Increased number of cytoplasmic channels | Snaidero et al. 2014 | ||
| ADF−/−; Cfl1fl/fl; Cnp-Cre | · Increased number of cytoplasmic channels | Snaidero et al. 2017 | ||
| ArpC3fl/fl; CNP-Cre or Olig2-Cre | · Fewer myelinated axons · Increased myelin outfoldings |
Zuchero et al. 2015 | ||
| ArpC3fl/fl; Plp1-CreERT | · No myelin phenotype | Zuchero et al. 2015 | ||
| Gsn −/− | · Thinner myelin · No phenotype (Nawaz et al. 2015) |
Zuchero et al. 2015
Nawaz et al. 2015 |
||
| brain | Pak1/Pak3 dKO | · Hypomyelination | Huang et al. 2011 | |
| PAK3−/− | · Decreased OPC differentiation | Maglorius-Renkilaraj et al. 2016 | ||
| spinal cord | ADF/cofilinfl/fl; Cnp-Cre | · Increased F-actin at inner tongue · Increased F/G actin ratio in WM · Thinner myelin |
Nawaz et al. 2015 | |
| Shiverer | · Increased F-actin in white matter | Zuchero et al. 2015 | ||
| Cdc42fl/fl; Cnp-Cre | · Increased inner tongue · Myelin outfoldings |
Thurnherr et al. 2006 | ||
| Rac1fl/fl; Cnp-Cre | · Increased inner tongue · Myelin outfoldings |
Thurnherr et al. 2006 | ||
| Camk2b−/− | · Impaired OPC differentiation · Thinner myelin |
Waggener et al. 2013 | ||
| Camk2b A303R (kinase inactive) | · No myelin phenotype | Waggener et al. 2013 | ||
| EAE (mouse model of MS) | · Oligodendrocyte-specific increase in β-actin gene expression | Locatelli et al. 2015 | ||
| ON, CC, SC | WAVE1−/− | · Fewer OPC processes · Fewer myelinated axons (ON) · No change myelinated axons (SC) |
Kim et al. 2006 | |
| PNS | Cdc42fl/fl; Dhh-Cre | · Impaired radial sorting · Decreased Schwann cell proliferation |
Benninger et al. 2007 | |
| Rac1fl/fl; Dhh-Cre | · Impaired radial sorting · Deficit in Schwann cell process extension and stabilization |
Benninger et al. 2007 | ||
| N-WASPfl/fl; Dhh-Cre or P0-Cre | · Fewer myelinated axons · Thinner myelin · Shorter internodes |
Novak et al. 2011
Jin et al. 2011 |
||
| Profilin1fl/fl; Dhh-Cre or Cnp-Cre | · Delayed radial sorting · Deficits in axon ensheathment |
Montani et al. 2014 | ||
| Pmp22+/− | · Increased F-actin at paranodes · Increased PAK1 |
Hu et al. 2016 | ||
Abbreviations: ON: optic nerve, CC: corpus callosum, SC: spinal cord, EAE: experimental autoimmune encephalomyelitis
Cnp-Cre and Olig2-Cre are used for early promoters in the CNS. Plp-CreERT is used for a late promoter in the CNS. Cnp-Cre and Dhh-Cre are used as PNS promoters.
Actin polymerization in myelinating cells
Myelinating cells in both the CNS and PNS express actin polymerizing proteins, including members of the Arp2/3 complex, N-WASP and WAVE proteins [11], which are also found in myelin sheath preparations. In oligodendrocytes, WAVE1 is the predominate actin polymerizing protein, whereas in Schwann cells, WAVE2 is the major protein [11]. Thus, PNS and CNS myelinating cells share similar but distinct molecular players. Both cell types also express the family of Rho GTPases, which transmit signals from membrane receptors to the cytoskeleton [12]. Rho GTPases regulate signal transduction by acting as molecular switches-quickly alternating between on/off signals- through their rapid change between their GTP bound (active) state and GDP bound (inactive state) [13]. The most studied Rho GTPases are RhoA, Cdc42, and Rac1. RhoA activates ROCK to regulate stress fiber formation, while Cdc42 and Rac1 activate N-WASP and WAVE proteins, which promote actin nucleation through activation of the Arp2/3 complex [14].
In the CNS, in vitro data demonstrate that Cdc42 and Rac1 are positive regulators of OPC morphological differentiation-the change from a simple bipolar morphology to cells with multiple complex processes. RhoA acts in opposition as a negative regulator of process extension [15]. Loss of Cdc42 or Rac1 does not prevent OPC migration or differentiation in vivo, but loss of these GTPases leads to a unique myelin phenotype. Specifically, loss of Cdc42 or Rac1 leads to enlargement of the inner tongue and double knockouts suggest that Cdc42 and Rac1 act synergistically to regulate myelination [16]. In the PNS, comparable GTPases regulate Schwann cell proliferation, radial sorting, and myelination. Cdc42 primarily regulates Schwann cell proliferation while Rac1 promotes Schwann cell lamellipodia formation [17]. Conditional knock out of Rac1 also results in delayed axonal sorting and hypomyelination [18]. Rac1, but not Cdc42, is regulated by AKT in the PNS, where hyperactivation of AKT in Schwann cells leads to increased myelin thickness. Inhibition of the AKT/mTOR pathway via the mTOR inhibitor rapamycin rescues hypermyelination in the CNS [19], [20] but not the PNS [20], suggesting that AKT regulates myelination both through mTOR and Rac1 pathways.
Loss of known targets of these GTPases also impairs OPC development. Loss of the actin nucleator WAVE1 leads to a decrease in the number of OPC processes, as well as hypomyelination, quantified by the number of myelinated axons in the corpus callosum and optic nerve. Mice lacking WAVE1 also have decreased Na+ and K+ channel clustering in the optic nerve [21]. Surprisingly, loss of WAVE1 in the spinal cord does not change the number of myelinated axons. Another downstream target of GTPases, ArpC3, a molecule that promotes actin nucleation, is a positive regulator of OPC morphological differentiation. When ArpC3 is lost before myelination, there is a decrease in the number of myelinated axons in the optic nerve but its loss during myelin wrapping does not impact myelination [10]. Thus, this study would suggest that actin polymerization is required for OPC process extension and initial axon ensheathment but is dispensable during myelin wrapping.
Similarly, downstream GTPase targets also regulate PNS myelination. One of the downstream targets, the actin nucleator N-WASP, is required for proper PNS myelination. Specifically, loss of N-WASP in Schwann cells leads to a decrease in the number of myelinated axons up to P60, and the myelin that is produced is significantly thinner than WT myelin, with shorter internodes. These data suggest that N-WASP is important for the longitudinal extension of myelin, or myelin internode length [22], [23]. Another Rho GTPase target is Profilin1 (Pfn1). Pfn1 promotes actin polymerization by binding to actin monomers and promoting polymerization through the exchange of actin-bound ADP to ATP [24], [25]. Montani and colleagues demonstrate that Pfn1 promotes radial sorting and myelination and is regulated by Rho/ROCK signaling [26]. These data from both the PNS and CNS indicate that Rho GTPases and their downstream targets are required for proper cell differentiation and myelination.
There are also cytoskeletal components not necessarily associated with GTPases that are important regulators of the cytoskeleton. For example, junction mediating and regulatory protein, Jmy, has recently been shown to regulate oligodendrocyte differentiation. In an elegant experiment, Azevedo and colleagues isolated OPC soma and early OPC membrane protrusions in rat primary OPCs, to undertake transcriptomics to characterize mRNAs translocated to OPC/differentiating oligodendrocyte processes. The investigators identified Jmy as one of the mRNAs enriched in the newly formed processes, which increases expression during CNS myelination. Loss of Jmy results in decreased OPC morphological differentiation that is dependent on F-actin assembly. Thus, they conclude that Jmy is a novel regulator of OPC differentiation through actin modulation [27]. Similarly, CamKIIb promotes OPC morphological differentiation, and its loss results in thinner myelin. However, specifically inhibiting the kinase activity of CamKIIb in vivo does not alter myelination. This suggests that CamKIIb may regulate OPC morphological differentiation independent of its kinase activity, potentially through its actin binding domain [28].
OPCs are highly dynamic in their membrane extension and retraction and they make myriad connections with axons. However, only some of these processes remain in place and produce stable myelin sheaths [3], leading to the question of the regulation of process stabilization vs retraction during myelination. Thus, further investigation is needed into the cell biology of OPC process retraction. While it is well established that actin polymerization is required to extend OPC processes, what are the signals that regulate OPC process retraction? Is there a local decrease in actin polymerization factors or local activation of actin depolymerizing factors at the leading edge? Evidence of local control of actin polymerization exists in other systems. For example, in the PNS, exposure of Schwann cells to low levels of hydrogen peroxide triggers local translation of cytoskeletal regulators such as Annexin2 [29]. Local translation and phosphorylation of Annexin2 accompanies significant cytoskeletal reorganization and Schwann cell polarization [29]. It is likely that local signaling from the environment, from axons or interacting neurons, may promote local translation of cytoskeletal regulators that alter actin polymerization and myelination.
Actin depolymerization in myelinating cells
In addition to actin polymerization, actin depolymerization is a crucial element driving myelination. Early downregulation of actin depolymerizing proteins including cofilin and gelsolin allow for OPC morphological differentiation [30]. Surprisingly, exposure to the actin depolymerizing agent LatrunculinA during late differentiation in vitro increases oligodendrocyte membrane production [9], [10]. Consistent with this positive impact of actin depolymerization, loss of the actin severing proteins ADF and cofilin1 in vivo inhibits myelination. Importantly, this increases the size of the inner tongue, suggesting that actin depolymerization regulates the size of the inner tongue to drive myelin wrapping [9]. One actin depolymerizing protein is cofilin, and one suggested model of myelination proposes that MBP competes with cofilin for binding to phosphatidyl inositol 4,5 bisphosphate, PIP2. The more MBP outcompetes cofilin and binds to PIP2, the more cofilin is released into the cytoplasm where it acts to depolymerize actin and thereby drive myelin wrapping [10]. In Schwann cells, cofilin is downstream of neuregulin (Nrg1) signaling. It is recruited to the leading edge after stimulation with Nrg1 in vitro, and is required for normal Schwann cell-axon interactions and proper myelination in vivo [31].
It must be noted that the role of cofilin in myelination is potentially quite complex. The ratio of activated (unphosphorylated) cofilin to actin influences cofilin activity. Active cofilin binds to F-actin, inducing a twist to the filament, which leads to actin severing. However, if high levels of cofilin bind to F-actin, this can induce a twist throughout the entire filament and act as an actin filament stabilizing agent [32]. It will be informative to determine the localization of cofilin within the myelin sheath. Does activated cofilin localize to the inner tongue? Investigating the ratio of cofilin to actin will also inform the function of cofilin during myelin sheath formation.
Another major actin depolymerizing protein is gelsolin, a calcium-sensitive protein that severs filamentous actin [33]. Importantly, gelsolin is expressed in both oligodendrocytes and Schwann cells [34]. In vitro, gelsolin is expressed in oligodendrocyte cell bodies and in cytoplasmic processes but not in the membrane sheets [35]. In vivo, gelsolin is expressed in compact myelin and is specifically enriched at the node of Ranvier [34]. Gelsolin protein expression in the forebrain increases during development reaching a peak between 20–30 days, coincident with increasing MBP expression. However, gelsolin expression in the forebrain starts to decrease while MBP expression continues to increase and both gelsolin and MBP are expressed in compact myelin [34]. Gelsolin regulates myelin wrapping in the optic nerve as loss of gelsolin leads to a small decrease in myelin thickness without altering axon caliber [10]. Given the observation that multiple depolymerizing factors are highly expressed in myelinating oligodendrocytes [36], it is possible that gelsolin is one of several actin regulators but there is redundancy to ensure proper regulation of the actin cytoskeleton during myelin wrapping. Overall, these data indicate that expression of actin depolymerizing factors such as cofilin and gelsolin play an important role during OPC differentiation and myelination.
P21-activated kinases
One family of molecules that can regulate the switch between actin polymerization and depolymerization is the family of P21-activated kinases (PAKs). PAKs are small molecule serine/threonine kinases implicated in several diseases, including multiple cancers and schizophrenia, as well as cortical development [37]–[39]. PAKs are divided into two groups, Group I PAKs (PAK1–3), share a high degree of homology and are more studied than Group II PAKs (PAK4–6). PAK activity depends greatly on their upstream activators. GTPases are known activators of PAKs that promote PAK regulation of the actin cytoskeleton [40]–[42]. When a GTPase binds to PAK, this induces a conformational change, releasing PAK from its homodimerization state, causing autophosphorylation, and subsequent PAK activation. PAK can then phosphorylate Lim kinase (LIMK), which then phosphorylates and inactivates the actin-severing protein cofilin [43]. However, PAKs may also be directly activated by PI-3 kinase, and PAK then directly phosphorylates actin, resulting in cytoskeletal rearrangement [42], further demonstrating that PAK function depends on the mechanisms of its upstream activation.
PAKs are intriguing regulators because in addition to their relatively direct impact on the structure of the actin cytoskeleton, PAKs regulate multiple signaling pathways that are known regulators of oligodendrocyte development and myelination, including the AKT and MAP kinase (MAPK) pathways. For example, PAKs can directly phosphorylate MEK1, driving the MAPK pathways [44], [45]. Additionally, while PAKs are kinases, in some cells they can regulate the AKT pathway through kinase-independent mechanisms. For example, Higuchi and colleagues demonstrate that PAK1 acts as a scaffold for AKT so that AKT can be translocated to the membrane and subsequently activated by 3-phosphoinositide-dependent kinase 1 (PDK1) [46].
Mice with global loss of PAK1 and PAK3 have impaired postnatal brain growth, although the double knockouts are born with normal neonatal brain sizes. The adult double knockout mice show altered behaviors, including increased travel distance in open field tests and increased latency to platform in the water maze test, which likely result from altered neuronal function as they have decreased neuronal dendritic complexity. Interestingly, they also have a decrease in myelin production, indicating that PAK1 and PAK3 may be important for myelin production [47]. Maglorius Renkilaraj and colleagues show that PAK3 is highly expressed early in OPC development and decreases with differentiation; in a model with global loss of PAK3, they show that PAK3 regulates OPC differentiation. In cultured PAK3-deleted OPCs, the loss of PAK3 does not change OPC morphology, migration or proliferation but does decrease OPC differentiation. By P14 in vivo, PAK3 KO mice have a reduced number of myelinated axons in the corpus callosum, but by adulthood there is recovery, as the number of myelinated axons are found. This suggests that mice can compensate for the loss of PAK3 [48]; for example, other PAK family members may be able to compensate for loss of one PAK. PAK1 is also expressed in OPCs and its activated form, phospho-PAK1/2 is expressed in both the cytoplasm and in OPC processes (Fig. 1). Thus, multiple PAK family members may be important for oligodendrocyte development.
Figure 1.
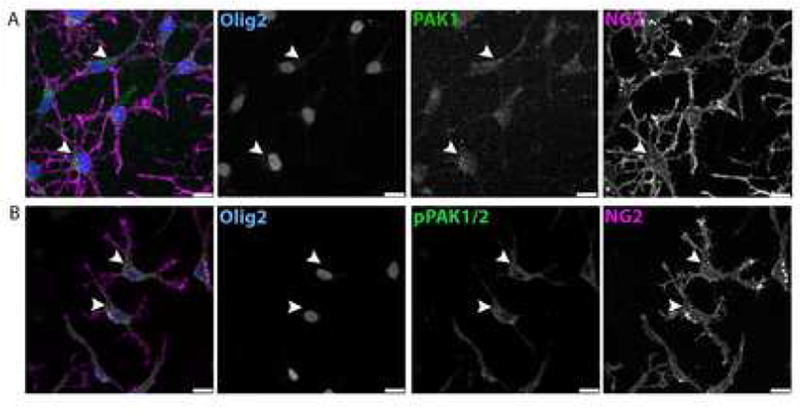
PAK1 is expressed in rat OPCs. A) Blue labels the oligodendrocyte lineage marker Olig2, green labels PAK1, and magenta labels NG2. Arrows point to PAK1 expression in the cytoplasm of the cell body and into the OPC processes. B) pPAK1/2 is expressed in rat OPCs. Blue labels the oligodendrocyte lineage marker Olig2, green labels pPAK1ser144/pPAK2ser141, and magenta labels NG2. Arrows point to pPAK1/2 expression in the cytoplasm of rOPCs in the cell body and into the OPC processes. Scale bars are 25μM.
Fine tuning of PAK function is important for myelination, and thereby axonal function. In a PNS mouse model of hereditary neuropathy with liability of pressure palsies (HNPP) generated by heterozygous deletion of Pmp22, the compound muscle action potential amplitude is decreased with no axonal loss, which suggests that the change in action potentials may result from changes in myelin. Indeed, myelin is somewhat decompacted with increased F-actin at the paranodes. This is accompanied by an increase in PAK1 and increased MEK1S298 phosphorylation in the sciatic nerves, and importantly, PAK inhibition decreased the F-actin accumulation and restored nerve conduction [49].
These studies in both the CNS and PNS indicate that the PAKs play a role in myelination and that fine-tuning PAK activity is important for proper myelination, as either loss or overactivity of PAKs can cause abnormal myelin phenotypes. PAKs can regulate multiple signaling pathways important for oligodendrocyte development, including the cytoskeleton pathway through LIMK and cofilin as well as the AKT and ERK pathways. Additionally, there are multiple drugs available that target the PAK pathway. It will be important for future investigations to determine the cell-specific role for the PAK family members.
Cytoskeleton involvement in adult myelin function
As our knowledge of the oligodendrocyte cytoskeleton increases, it provides insights into investigations of known regulators of the oligodendrocyte development program and their novel interactions with the cytoskeleton. Clearly the cytoskeleton is important in developing and actively myelinating oligodendrocytes. One of the proteins required for proper myelination, CNP, is present in CNS myelin and recent data suggest its interaction with F-actin may impact cytoplasmic openings in the myelin [50]. Cytoplasmic openings remain present in mature CNS myelin, reminiscent of Schmidt-Lanterman incisures of the PNS, where F-actin is localized [51], [52], and they may allow for increased transport within myelin between the oligodendrocyte soma and axon. Although loss of CNP1 does not grossly alter CNS myelination or myelin structure, it eventually leads to axonal swellings, progressive motor deficits, and premature death [53]. Snaidero and colleagues suggest that CNP normally antagonizes the compaction of CNS myelin by MBP, by interacting with F-actin directly, increasing actin bundling, and this maintains cytoplasmic openings within myelin, thereby allowing communication to the axon [50]. This suggests that in addition to its role during myelination, F-actin plays an important role in adult myelin by maintaining cytoplasmic openings in uncompacted myelin domains (Fig. 2). Important questions remain as to what signaling molecules determine F-actin localization, and whether other myelin proteins promote F-actin stabilization or disassociation.
Figure 2.
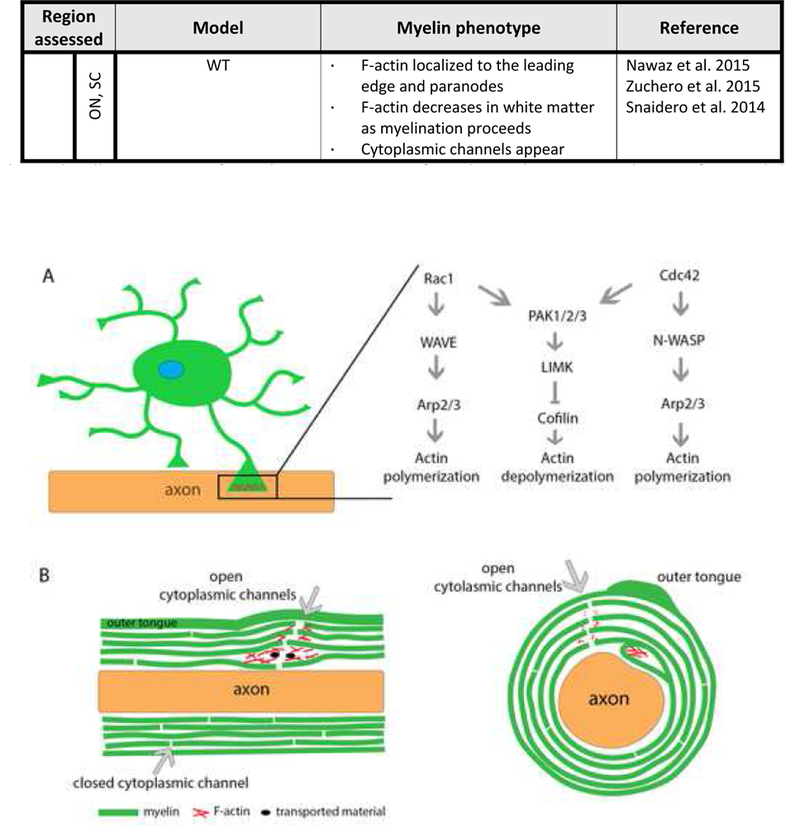
Predicted cytoskeletal signaling pathways at the leading edge of myelinating cells. (A) The leading edge of an OPC process is the cytoplasmic rich expansion that comes into contact with an axon, which then becomes the inner tongue during myelin wrapping. The inner tongue remains in close contact with the axon as it ensheathes the axon and continues to wrap during active myelination. RhoGTPases Rac1 and Cdc42 signal through WAVE or N-WASP and then the Arp2/3 complex to promote actin polymerization. RhoGTPases can also signal through the PAK proteins to phosphorylate LIMK, that then phosphorylates and inactivates cofilin to regulate actin turnover. Active (unphosphorylated) cofilin binds to f-actin, induces a local twist in the filament, leading to actin severing. High levels of cofilin bound to f-actin can induce twisting throughout the entire filament, changing cofilin activity to an actin stabilizing protein. (B) Longitudinal view of a myelinated axon with areas of open cytoplasmic channels that close upon myelin compaction. (C) Cross section of myelinated axon. F-actin localizes to cytoplasmic channels, allowing access from the outer tongue of myelin to the axon-myelin interface and the inner tongue.
Cytoskeleton in disease states
Cytoskeletal changes occur in multiple diseases, in both the CNS and PNS. For example, in the Shiverer mouse, there is increased F-actin in myelin [10]. Also, after CNS damage such as diphtheria toxin-induced oligodendrocyte death or experimental autoimmune encephalitis (EAE), a mouse model of multiple sclerosis, oligodendrocytes increase cytoskeletal β-actin gene expression [54]. Thus, CNS injury leads to changes in the cytoskeleton with increases in F-actin.
Additionally, after sciatic nerve injury in the PNS, Schwann cells increase expression of Cdc42. In this injury context, Cdc42 promotes Schwann cell proliferation and migration through the Wnt/β-catenin and p38 MAPK pathways [55]. In the Trembler J mouse, a model for Charcot Marie Tooth disease (CMT), F-actin localization remains unchanged, but it accumulates at the nodes of Ranvier and Schmidt-Lanterman incisures [56]. In a mouse model of HNPP there is loss of Schmidt-Lanterman incisures and abnormal accumulation of F-actin within myelin, as well as decreases in myelin internode length [57]. When compression induces injury in the peripheral nerve in this HNPP model, F-actin organization is changed and impairs Schwann cell migration. Other forms of CMT result from mutations in frabin/FGD4, a guanine nucleotide exchange factor that promotes Cdc42 activity by stimulating the exchange of GDP for GTP. This leads to PNS myelin pathology, in particular resulting in myelin outfoldings [58]. These studies indicate that while changes to cytoskeletal regulators can lead to disease states, additionally, disease states can induce changes in the actin cytoskeleton in the PNS.
Important questions in the field
Historically, electron microscopy has been the standard to quantify the amount of myelin produced by measuring myelin thickness through g-ratio. As imaging technology advances, so does the knowledge of different parameters of myelin, such as myelin internode length. Insights from the PNS suggest that the actin cytoskeleton regulates myelin internode length. In vitro inhibition of the Rho/ROCK pathway in Schwann cells leads to shorter and sometimes thicker myelin internodes [59] while in vivo deletion of N-WASP in Schwann cells leads to shorter internodes in teased sciatic fibers [23]. In the CNS, it is known that axons in the neocortex have distinct, intermittent myelin distributions and variations in internode length [60]. It will be important for future investigations to determine how the cytoskeleton regulates this process of internode length and intermittent myelination in the CNS.
A broader question is the relationship between myelin thickness and internode length. The g ratio is a relative constant that correlates axon diameter with myelin thickness, and multiple investigations demonstrate a relationship between axon diameter and internode length, with smaller diameter axons tending to have shorter myelin internodes [61]–[63]. However, the correlation between axon diameter and internode length is relatively weak, with correlation coefficients of 0.13–0.3 [63], suggesting that other variables contribute to the variation of myelin internode length. Is it possible to separate out differences in myelin thickness from differences in internode length and are there different cytoskeletal molecules regulating each myelin feature?
Since many cytoskeletal regulators are quickly activated/inactivated, are signals from the axon regulating myelin production by local changes in the inner tongue driving actin polymerization and depolymerization? Myelin-associated glycoprotein (MAG) is an immunoglobulin family protein expressed in myelin and localizing to the inner wraps of myelin at the interface between myelin and axon [64]. MAG mutants have delayed myelination as well as redundant myelin [65], [66]. MAG has also been shown to regulate RhoA activity [67], suggesting that MAG may be one of the molecules transducing signals from the axon throughout myelin and back to the oligodendrocyte soma, potentially through the cytoskeleton.
Concluding remarks and future directions
There is much to learn about the cell biology of myelination. The field is rapidly increasing its understanding of the cytoskeleton throughout differentiation and myelination, yet there is much more to investigate. It will be useful to determine whether the cytoskeleton provides the force pushing cytoplasm laterally to increase myelin internode length and where the cytoskeletal proteins, such as actin polymerizing/depolymerizing proteins, RhoGTPases and PAKs, localize in OPC branches (Fig 2), where they are localized in Schwann cells, and whether/where they localize within the myelin sheath.
Many questions remain, for example, how does the cytoskeleton maintain cytoplasmic channels? How is information transmitted from the inner tongue of the myelin sheath to the cell body? Is there local translation of actin regulators at the inner tongue or are there small molecules that can act as molecular switches to rapidly regulate the change from actin polymerization to actin depolymerization at the leading edge? The cytoskeleton of myelinating cells is an amazingly complex structure with myriad functions-providing structure, motility, and signaling throughout normal development, in the normal adult nervous system and in disease pathology. Increasing our knowledge of the fundamentals of oligodendrocyte and Schwann cell biology will improve our understanding of neural development and function.
Acknowledgements:
This work was supported by the National Science Foundation Graduate Research Fellowship Program DGE-1553798 (TLB) and National Institute of Health RO1 #82203 (WBM). The authors thank the members of the Macklin lab for valuable discussion and feedback on this manuscript.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- [1].D’Este E, Kamin D, Velte C, Göttfert F, Simons M, and Hell SW, “Subcortical cytoskeleton periodicity throughout the nervous system.,” Sci Rep, vol. 6, p. 22741, Mar. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hauser M, Yan R, Li W, Repina NA, Schaffer DV, and Xu K, “The Spectrin-Actin-Based Periodic Cytoskeleton as a Conserved Nanoscale Scaffold and Ruler of the Neural Stem Cell Lineage.,” Cell Rep, vol. 24, no. 6, pp. 1512–1522, Aug. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hines JH, Ravanelli AM, Schwindt R, Scott EK, and Appel B, “Neuronal activity biases axon selection for myelination in vivo,” Nature neuroscience, Apr. 2015. [DOI] [PMC free article] [PubMed]
- [4].Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, and Lyons DA, “Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo,” Nature neuroscience, Apr. 2015. [DOI] [PMC free article] [PubMed]
- [5].Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, and Rothstein JD, “Oligodendroglia metabolically support axons and contribute to neurodegeneration,” Nature, vol. 487, no. 7408, pp. 443–448, Jul. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, and Nave K-A, “Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity.,” Nature Publishing Group, vol. 485, no. 7399, pp. 517–521, Apr. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ackerman SD and Monk KR, “The scales and tales of myelination: using zebrafish and mouse to study myelinating glia.,” Brain Res, vol. 1641, no. Pt A, pp. 79–91, Jun. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jessen KR and Mirsky R, “The origin and development of glial cells in peripheral nerves.,” Nature reviews. Neuroscience, vol. 6, no. 9, pp. 671–682, Sep. 2005. [DOI] [PubMed] [Google Scholar]
- [9].Nawaz S, Sanchez P, Schmitt S, Snaidero N, Mitkovski M, Velte C, Bruckner BR, Alexopoulos I, Czopka T, Jung SY, Rhee JS, Janshoff A, Witke W, Schaap IA, Lyons DA, and Simons M, “Actin Filament Turnover Drives Leading Edge Growth during Myelin Sheath Formation in the Central Nervous System,” Developmental cell, vol. 34, no. 2, pp. 139–151, Jul. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zuchero JB, Fu M-M, Sloan SA, Ibrahim A, Olson A, Zaremba A, Dugas JC, Wienbar S, Caprariello AV, Kantor C, Leonoudakis D, Leonoudakus D, Lariosa Willingham K, Kronenberg G, Gertz K, Soderling SH, Miller RH, and Barres BA, “CNS myelin wrapping is driven by actin disassembly.,” Developmental cell, vol. 34, no. 2, pp. 152–167, Jul. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bacon C, Lakics V, Machesky L, and Rumsby M, “N-WASP regulates extension of filopodia and processes by oligodendrocyte progenitors, oligodendrocytes, and Schwann cells-implications for axon ensheathment at myelination.,” Glia, vol. 55, no. 8, pp. 844–858, Jun. 2007. [DOI] [PubMed] [Google Scholar]
- [12].Etienne-Manneville S, “Polarity proteins in glial cell functions.,” Current opinion in neurobiology, vol. 18, no. 5, pp. 488–494, Oct. 2008. [DOI] [PubMed] [Google Scholar]
- [13].Jaffe AB and Hall A, “Rho GTPases: biochemistry and biology.,” Annu. Rev. Cell Dev. Biol, vol. 21, no. 1, pp. 247–269, 2005. [DOI] [PubMed] [Google Scholar]
- [14].Etienne-Manneville S and Hall A, “Rho GTPases in cell biology.,” Nature, vol. 420, no. 6916, pp. 629–635, Dec. 2002. [DOI] [PubMed] [Google Scholar]
- [15].Liang X, Draghi NA, and Resh MD, “Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes,” J. Neurosci, vol. 24, no. 32, pp. 7140–7149, Aug. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thurnherr T, Benninger Y, Wu X, Chrostek A, Krause SM, Nave KA, Franklin RJ, Brakebusch C, Suter U, and Relvas JB, “Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS,” J. Neurosci, vol. 26, no. 40, pp. 10110–10119, Oct. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave K-A, Franklin RJM, Meijer D, Brakebusch C, Suter U, and Relvas JB, “Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development.,” The Journal of cell biology, vol. 177, no. 6, pp. 1051–1061, Jun. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nodari A, Zambroni D, Quattrini A, Court FA, D’Urso A, Recchia A, Tybulewicz VLJ, Wrabetz L, and Feltri ML, “Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination.,” The Journal of cell biology, vol. 177, no. 6, pp. 1063–1075, Jun. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Narayanan SP, Flores AI, Wang F, and Macklin WB, “Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination.,” J. Neurosci, vol. 29, no. 21, pp. 6860–6870, May 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Domènech-Estévez E, Baloui H, Meng X, Zhang Y, Deinhardt K, Dupree JL, Einheber S, Chrast R, and Salzer JL, “Akt Regulates Axon Wrapping and Myelin Sheath Thickness in the PNS.,” J. Neurosci, vol. 36, no. 16, pp. 4506–4521, Apr. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim H-J, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, and Vartanian TK, “WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination.,” J. Neurosci, vol. 26, no. 21, pp. 5849–5859, May 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jin F, Dong B, Georgiou J, Jiang Q, Zhang J, Bharioke A, Qiu F, Lommel S, Feltri ML, Wrabetz L, Roder JC, Eyer J, Chen X, Peterson AC, and Siminovitch KA, “N-WASp is required for Schwann cell cytoskeletal dynamics, normal myelin gene expression and peripheral nerve myelination.,” Development, vol. 138, no. 7, pp. 1329–1337, Apr. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Novak N, Bar V, Sabanay H, Frechter S, Jaegle M, Snapper SB, Meijer D, and Peles E, “N-WASP is required for membrane wrapping and myelination by Schwann cells.,” The Journal of cell biology, vol. 192, no. 2, pp. 243–250, Jan. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Finkel T, Theriot JA, Dise KR, Tomaselli GF, and Goldschmidt-Clermont PJ, “Dynamic actin structures stabilized by profilin.,” Proceedings of the National Academy of Sciences of the United States of America, vol. 91, no. 4, pp. 1510–1514, Feb. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Witke W, Podtelejnikov AV, Di Nardo A, Sutherland JD, Gurniak CB, Dotti C, and Mann M, “In mouse brain profilin I and profilin II associate with regulators of the endocytic pathway and actin assembly.,” EMBO J, vol. 17, no. 4, pp. 967–976, Feb. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Montani L, Buerki-Thurnherr T, de Faria JP, Pereira JA, Dias NG, Fernandes R, Gonçalves AF, Braun A, Benninger Y, Böttcher RT, Costell M, Nave K-A, Franklin RJM, Meijer D, Suter U, and Relvas JB, “Profilin 1 is required for peripheral nervous system myelination.,” Development, vol. 141, no. 7, pp. 1553–1561, Apr. 2014. [DOI] [PubMed] [Google Scholar]
- [27].Azevedo MM, Domingues HS, Cordelières FP, Sampaio P, Seixas AI, and Relvas JB, “Jmy regulates oligodendrocyte differentiation via modulation of actin cytoskeleton dynamics.,” Glia, vol. 138, no. 20, p. 4443, May 2018. [DOI] [PubMed] [Google Scholar]
- [28].Waggener CT, Dupree JL, Elgersma Y, and Fuss B, “CaMKIIβ regulates oligodendrocyte maturation and CNS myelination.,” J. Neurosci, vol. 33, no. 25, pp. 10453–10458, Jun. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Negro S, Stazi M, Marchioretto M, Tebaldi T, Rodella U, Duregotti E, Gerke V, Quattrone A, Montecucco C, Rigoni M, and Viero G, “Hydrogen peroxide is a neuronal alarmin that triggers specific RNAs, local translation of Annexin A2, and cytoskeletal remodeling in Schwann cells.,” RNA, vol. 24, no. 7, pp. 915–925, Jul. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu A, Muggironi M, Marin-Husstege M, and Casaccia-Bonnefil P, “Oligodendrocyte process outgrowth in vitro is modulated by epigenetic regulation of cytoskeletal severing proteins.,” Glia, vol. 44, no. 3, pp. 264–274, Dec. 2003. [DOI] [PubMed] [Google Scholar]
- [31].Sparrow N, Manetti ME, Bott M, Fabianac T, Petrilli A, Bates ML, Bunge MB, Lambert S, and Fernandez-Valle C, “The actin-severing protein cofilin is downstream of neuregulin signaling and is essential for Schwann cell myelination.,” J. Neurosci, vol. 32, no. 15, pp. 5284–5297, Apr. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Andrianantoandro E and Pollard TD, “Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin.,” Mol. Cell, vol. 24, no. 1, pp. 13–23, Oct. 2006. [DOI] [PubMed] [Google Scholar]
- [33].Nag S, Larsson M, Robinson RC, and Burtnick LD, “Gelsolin: the tail of a molecular gymnast.,” Cytoskeleton, vol. 70, no. 7, pp. 360–384, Jul. 2013. [DOI] [PubMed] [Google Scholar]
- [34].Tanaka J and Sobue K, “Localization and characterization of gelsolin in nervous tissues: gelsolin is specifically enriched in myelin-forming cells.,” Journal of Neuroscience, vol. 14, no. 3, pp. 1038–1052, Mar. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lena JY, Legrand CH, Faivre-Sarrailh C, Sarlieve LL, Ferraz C, and Rabie A, “High gelsolin content of developing oligodendrocytes.,” Int. J. Dev. Neurosci, vol. 12, pp. 375–386, Apr. 1994. [DOI] [PubMed] [Google Scholar]
- [36].Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, and Wu JQ, “An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex,” J. Neurosci, vol. 34 , no. 36, pp. 11929–11947, Sep. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hayashi-Takagi A, Araki Y, Nakamura M, Vollrath B, Duron SG, Yan Z, Kasai H, Huganir RL, Campbell DA, and Sawa A, “PAKs inhibitors ameliorate schizophrenia-associated dendritic spine deterioration in vitro and in vivo during late adolescence,” Proceedings of the National Academy of Sciences of the United States of America, vol. 111, no. 17, pp. 6461–6466, Apr. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mira JP, Benard V, Groffen J, Sanders LC, and Knaus UG, “Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway.,” Proceedings of the National Academy of Sciences of the United States of America, vol. 97, no. 1, pp. 185–189, Jan. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pan X, Chang X, Leung C, Zhou Z, Cao F, Xie W, and Jia Z, “PAK1 regulates cortical development via promoting neuronal migration and progenitor cell proliferation.,” Mol Brain, vol. 8, no. 1, p. 36, Jun. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zenke FT, King CC, Bohl BP, and Bokoch GM, “Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity.,” The Journal of biological chemistry, vol. 274, no. 46, pp. 32565–32573, Nov. 1999. [DOI] [PubMed] [Google Scholar]
- [41].Tang Y, Zhou H, Chen A, Pittman RN, and Field J, “The Akt proto-oncogene links Ras to Pak and cell survival signals.,” The Journal of biological chemistry, vol. 275, no. 13, pp. 9106–9109, Mar. 2000. [DOI] [PubMed] [Google Scholar]
- [42].Papakonstanti EA and Stournaras C, “Association of PI-3 kinase with PAK1 leads to actin phosphorylation and cytoskeletal reorganization.,” Molecular biology of the cell, vol. 13, no. 8, pp. 2946–2962, Aug. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Edwards DC, Sanders LC, Bokoch GM, and Gill GN, “Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics.,” Nature cell biology, vol. 1, no. 5, pp. 253–259, Sep. 1999. [DOI] [PubMed] [Google Scholar]
- [44].Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, and Cobb MH, “Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins.,” EMBO J, vol. 16, no. 21, pp. 6426–6438, Nov. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Slack-Davis JK, Eblen ST, Zecevic M, Boerner SA, Tarcsafalvi A, Diaz HB, Marshall MS, Weber MJ, Parsons JT, and Catling AD, “PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation.,” The Journal of cell biology, vol. 162, no. 2, pp. 281–291, Jul. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Higuchi M, Onishi K, Kikuchi C, and Gotoh Y, “Scaffolding function of PAK in the PDK1-Akt pathway.,” Nature cell biology, vol. 10, no. 11, pp. 1356–1364, Nov. 2008. [DOI] [PubMed] [Google Scholar]
- [47].Huang W, Zhou Z, Asrar S, Henkelman M, Xie W, and Jia Z, “p21-Activated Kinases 1 and 3 Control Brain Size through Coordinating Neuronal Complexity and Synaptic Properties,” Mol. Cell. Biol, vol. 31, no. 3, pp. 388–403, Jan. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maglorius Renkilaraj MRL, Baudouin L, Wells CM, Doulazmi M, Wehrlé R, Cannaya V, Bachelin C, Barnier J-V, Jia Z, Nait Oumesmar B, Dusart I, and BouslamaOueghlani L, “The intellectual disability protein PAK3 regulates oligodendrocyte precursor cell differentiation,” Neurobiol. Dis, vol. 98, pp. 137–148. [DOI] [PubMed] [Google Scholar]
- [49].Hu B, Arpag S, Zhang X, Möbius W, Werner H, Sosinsky G, Ellisman M, Zhang Y, Hamilton A, Chernoff J, and Li J, “Tuning PAK Activity to Rescue Abnormal Myelin Permeability in HNPP.,” PLoS Genet, vol. 12, no. 9, p. e1006290, Sep. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Snaidero N, Velte C, Myllykoski M, Raasakka A, Ignatev A, Werner HB, Erwig MS, Möbius W, Kursula P, Nave K-A, and Simons M, “Antagonistic Functions of MBP and CNP Establish Cytosolic Channels in CNS Myelin.,” Cell Rep, vol. 18, no. 2, pp. 314–323, Jan. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Velumian AA, Samoilova M, and Fehlings MG, “Visualization of cytoplasmic diffusion within living myelin sheaths of CNS white matter axons using microinjection of the fluorescent dye Lucifer Yellow.,” Neuroimage, vol. 56, no. 1, pp. 27–34, May 2011. [DOI] [PubMed] [Google Scholar]
- [52].Snaidero N, Mobius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave KA, and Simons M, “Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue,” Cell, vol. 156, no. 1, pp. 277–290, Jan. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, and Nave K-A, “Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination.,” Nat. Genet, vol. 33, no. 3, pp. 366–374, Mar. 2003. [DOI] [PubMed] [Google Scholar]
- [54].Locatelli G, Baggiolini A, Schreiner B, Palle P, Waisman A, Becher B, and Buch T, “Mature oligodendrocytes actively increase in vivo cytoskeletal plasticity following CNS damage.,” J Neuroinflammation, vol. 12, no. 1, p. 62, Apr. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Han B, Zhao J-Y, Wang W-T, Li Z-W, He A-P, and Song X-Y, “Cdc42 Promotes Schwann Cell Proliferation and Migration Through Wnt/β-Catenin and p38 MAPK Signaling Pathway After Sciatic Nerve Injury.,” Neurochem. Res, vol. 42, no. 5, pp. 1317–1324, May 2017. [DOI] [PubMed] [Google Scholar]
- [56].Kun A, Canclini L, Rosso G, Bresque M, Romeo C, Hanusz A, Cal K, Calliari A, Silveira JS, and Sotelo JR, “F-actin distribution at nodes of Ranvier and Schmidt-Lanterman incisures in mammalian sciatic nerves,” Cytoskeleton, vol. 69, no. 7, pp. 486–495, Jul. 2012. [DOI] [PubMed] [Google Scholar]
- [57].Lee S, Amici S, Tavori H, Zeng WM, Freeland S, Fazio S, and Notterpek L, “PMP22 is critical for actin-mediated cellular functions and for establishing lipid rafts.,” J. Neurosci, vol. 34, no. 48, pp. 16140–16152, Nov. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Stendel C, Roos A, Deconinck T, Pereira J, Castagner F, Niemann A, Kirschner J, Korinthenberg R, Ketelsen U-P, Battaloglu E, Parman Y, Nicholson G, Ouvrier R, Seeger J, De Jonghe P, Weis J, Krüttgen A, Rudnik-Schöneborn S, Bergmann C, Suter U, Zerres K, Timmerman V, Relvas JB, and Senderek J, “Peripheral nerve demyelination caused by a mutant Rho GTPase guanine nucleotide exchange factor, frabin/FGD4.,” American journal of human genetics, vol. 81, no. 1, pp. 158–164, Jul. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Melendez-Vasquez CV, Einheber S, and Salzer JL, “Rho kinase regulates schwann cell myelination and formation of associated axonal domains.,” J. Neurosci, vol. 24, no. 16, pp. 3953–3963, Apr. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tomassy GS, Berger DR, Chen H-H, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, and Arlotta P, “Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex.,” Science, vol. 344, no. 6181, pp. 319–324, Apr. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].HESS A and YOUNG JZ, “Correlation of internodal length and fibre diameter in the central nervous system.,” Nature, vol. 164, no. 4168, p. 490, Sep. 1949. [DOI] [PubMed] [Google Scholar]
- [62].Murray JA and Blakemore WF, “The relationship between internodal length and fibre diameter in the spinal cord of the cat.,” J. Neurol. Sci, vol. 45, no. 1, pp. 29–41, Feb. 1980. [DOI] [PubMed] [Google Scholar]
- [63].Ibrahim M, Butt AM, and Berry M, “Relationship between myelin sheath diameter and internodal length in axons of the anterior medullary velum of the adult rat.,” J. Neurol. Sci, vol. 133, no. 1, pp. 119–127, Nov. 1995. [DOI] [PubMed] [Google Scholar]
- [64].Webster HD, Palkovits CG, Stoner GL, Favilla JT, Frail DE, and Braun PE, “Myelin-associated glycoprotein: electron microscopic immunocytochemical localization in compact developing and adult central nervous system myelin.,” J. Neurochem, vol. 41, no. 5, pp. 1469–1479, Nov. 1983. [DOI] [PubMed] [Google Scholar]
- [65].Montag D, Giese KP, Bartsch U, Martini R, Lang Y, Blüthmann H, Karthigasan J, Kirschner DA, Wintergerst ES, and Nave KA, “Mice deficient for the myelinassociated glycoprotein show subtle abnormalities in myelin.,” Neuron, vol. 13, no. 1, pp. 229–246, Jul. 1994. [DOI] [PubMed] [Google Scholar]
- [66].Bartsch U, Montag D, Bartsch S, and Schachner M, “Multiply myelinated axons in the optic nerve of mice deficient for the myelin-associated glycoprotein.,” Glia, vol. 14, no. 2, pp. 115–122, Jun. 1995. [DOI] [PubMed] [Google Scholar]
- [67].Palandri A, Salvador VR, Wojnacki J, Vivinetto AL, Schnaar RL, and Lopez PHH, “Myelin-associated glycoprotein modulates apoptosis of motoneurons during early postnatal development via NgR/p75(NTR) receptor-mediated activation of RhoA signaling pathways.,” Cell Death Dis, vol. 6, no. 9, pp. e1876–e1876, Sep. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


