Abstract
Background and objective:
In vivo evaluation of the microstructural differences between asthmatic and non-asthmatic airways and their functional consequences is relevant to understanding and, potentially, treating asthma. In this study we use endobronchial optical coherence tomography to investigate how allergic airways with asthma differ from allergic non-asthmatic airways in baseline microstructure and in response to allergen challenge.
Methods:
A total of n=45 subjects completed the study, including n=20 allergic, mildly asthmatic individuals, n=22 non-asthmatic allergic controls, and n=3 healthy controls. A 3 cm airway segment in the right middle and right upper lobe were imaged in each subject immediately before and 24 hours following segmental allergen challenge to the right middle lobe. Relationships between optical airway measurements (epithelial and mucosal thicknesses, mucosal buckling, and mucus) and airway obstruction (FEV1/FVC and FEV1%) were investigated.
Results:
Significant increases at baseline and in response to allergen were observed for all four of our imaging metrics in the asthmatic airways compared to the non-asthmatic airways. Epithelial thickness and mucosal buckling exhibited a significant relationship to FEV1/FVC in the asthmatic group.
Conclusions:
Simultaneous assessments of airway microstructure, buckling, and mucus revealed both structural and functional differences between the mildly asthmatic and control groups, with airway buckling seeming to be the most relevant factor. The results of this study demonstrate that a comprehensive, microstructural approach to assessing the airways may be important in future asthma studies as well as in the monitoring and treatment of asthma.
Keywords: asthma, radiology and other imaging, respiratory structure and function, bronchoscopy and interventional techniques, respiratory function tests
SUMMARY AT A GLANCE:
Endobronchial optical coherence tomography is used to investigate microstructural features of the airway in allergic individuals with and without asthma, before and after allergen challenge. Quantification of these features reveals significant differences between the asthmatic and non-asthmatic airway both at baseline and in response to allergen.
INTRODUCTION:
Asthma is a disorder of the airways characterized by chronic inflammation, airway remodeling and airway hyperresponsiveness. It is estimated to affect over 300 million people worldwide, and there is a large healthcare burden associated with the portion of the population classified as severe asthmatics who have poorly controlled symptoms1. Among the different types of asthma, allergic asthma is the most common, accounting for approximately 60% of the asthmatic population in the United States2. Although historically asthma has been regarded as an inflammatory disease, the failure of anti-inflammatory treatments to sufficiently control asthma in a crucial subset of patients emphasizes the need to better understand the pathogenesis of airway remodeling in asthma. This highlights the need to investigate more sophisticated approaches to researching and monitoring the microstructural features of airway remodeling.
Some of the key features of asthma include airway wall thickening, bronchoconstriction, excessive mucus production, and epithelial changes2-10. Although there are techniques, such as bronchial biopsy, that allow some of these features to be assessed, the need to remove tissue and sample distortion mitigate their overall usefulness. One possible solution for studying asthma at the microstructural level that has received only minimal attention is endobronchial optical coherence tomography (OCT). OCT is a high-resolution, high-speed, volumetric imaging modality that has been described as the optical analog to ultrasound11, 12. A significant advantage to using endobronchial OCT to study airway remodeling is its compatibility with bronchoscopy. Research techniques such as segmental allergen challenge (SAC)13 as well as therapies such as bronchial thermoplasty14 are bronchoscopic procedures, and incorporating endobronchial OCT imaging into the procedure introduces minimal additional time and risk to the subject.
In this study we use endobronchial OCT to assess airway microstructure, mucosal buckling, and mucus content in mild allergic asthmatic (AA), non-asthma allergic control (AC), and healthy control (HC) human volunteers. The advantage of using endobronchial OCT is that these measurements can be performed from a single volumetric scan. We conduct imaging (1) at baseline to assess airway remodeling; and (2) following a segmental allergen challenge (SAC) to investigate the transient microstructural and functional response to the allergen. We correlate our baseline in vivo measurements with FEV1/FVC and FEV1% measurements obtained at baseline to determine the relationship between these baseline imaging measurements and airway obstruction. This comprehensive study both demonstrates underlying structural and functional differences between mildly asthmatic and non-asthmatic individuals and establishes a methodology for future asthma research with endobronchial OCT.
METHODS:
Subject Enrollment
The Partners Healthcare Institutional Review Board granted approval for this study (protocol #2007P001050) and participants gave their informed written consent. Prior to enrollment, subjects were screened for eligibility with a full medical history, baseline spirometry, methacholine challenge, and allergen skin testing, as previously described13. All subjects underwent allergen skin prick testing, and all subjects enrolled into the AA or AC groups had a positive skin prick test to either cat hair or dust mite extract, with the threshold level determined by titration. For subjects allergic to both, cat hair extract was selected for use in the study. All HC subjects had a negative reaction to skin test. Asthmatics were classified as previously described15. All asthmatic volunteers had a PC20 (concentration of methacholine needed to produce 20% fall of FEV1 from baseline) of less than 20 mg/mL at baseline. Baseline airway obstruction was assessed with spirometry (FEV1/FVC and FEV1 %). The subjects were asked to refrain from using inhaled steroids for 2 weeks prior to bronchoscopy and bronchodilator for 12 hours prior. A detailed description of the inclusion/exclusion criteria for each of the three study groups can be found in Appendix S1.
Segmental Allergen Challenge
Comprehensive details regarding the SAC procedure have previously been published16-18. Briefly, a baseline bronchoalveolar lavage (BAL) was conducted in the lingula (4 × 30 ml of normal saline) and 2 ml of diluent and allergen (at the lowest concentration of cat hair or dust mite extract that elicited a positive skin prick test) were subsequently administered to the anterior segment of the right upper lobe (RUL) and the lateral segment of the right middle lobe (RML), respectively. The HC group was administered a dose of 500 bioequivalent allergen units per mL (BAU/mL) of cat hair extract in the RML. Twenty-four hours following allergen challenge, a second bronchoscopy procedure was performed and BAL was performed in the diluent and allergen-challenged lung segments. Cell differential counts were obtained from the collected fluid using a cytocentrifuge16.
OCT System
The details of our OCT system has been previously described12, 19. The axial resolution of the system was approximately 10 µm, and the penetration depth in soft tissue 2–3 mm. The fiber optic imaging catheter used a monolithic side-viewing ball lens design with a beam spot size of ~30 µm. 360º cross-sectional images of the airway were acquired at a rate of 33 frames per second, and longitudinal scanning was performed at a rate of 1 mm per second. The resultant longitudinal pitch between frames was ~33 µm.
OCT Procedure
Endobronchial OCT was performed in the RUL and the RML immediately prior to the SAC on day 1 and immediately prior to the BAL on day 2 (Supplementary Figure S1). Imaging was performed by inserting the OCT catheter (1.65 mm outer diameter, ~2 m length) through the working channel of the bronchoscope to extend approximately 3 cm into the selected airway segment. Care was taken to ensure consistency in the generation of the airway imaged, and the placement of the bronchoscope with respect to the airway was used as a reference. Volumetric OCT of each 3 cm segment was subsequently conducted in 30 seconds. Images were displayed in real time and the raw data was saved for post-procedure analysis.
OCT Image Analysis
OCT images were processed according to standard processing techniques20. Four distinct metrics were quantified per dataset: epithelial thickness, mucosal thickness, mucosal buckling (as a measure of bronchoconstriction), and mucus contrast (an optical assessment of mucus content21). Analysis of all excluding mucus contrast were performed using ImageJ (National Institute of Health, Bethesda, Maryland). Measurements for these features were only performed in frames that did not contain any bronchial branching. Epithelial thickness and mucosal thickness were measured at approximately 2mm intervals along each imaged segment, excluding the rare non-cartilaginous region that may have been observed distally in some datasets.
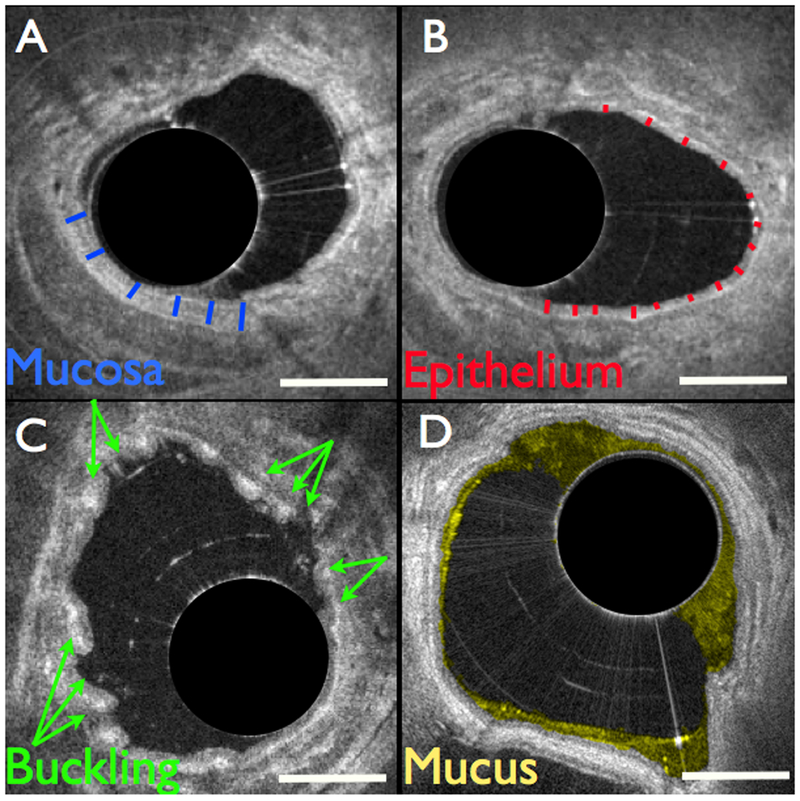
Mucosal thickness was measured from lumen to cartilage perichondrium (Fig. 1a). Epithelial thickness was measured from lumen to basement membrane22 (Fig. 1b). Mucosal buckling was identified by the visible presence of at least 3 distinct folds in the airway wall (Fig. 1c). For quantification purposes, we defined a fold as a bend in the airway wall where the width perpendicular to the wall was (approximately) at least as great as its width tangential to the wall. Mucosal buckling was quantified as the percentage of frames (from the entire dataset) where at least 3 distinct folds were observed. Mucus (Fig. 1d) was segmented and assessed automatically21. The final values are reported as the average of each of the measurements obtained per segment. Volumetric images were generated with Osirix (Pixmeo, Bernex, Switzerland).
Figure 1.
Structural airway features measured with OCT. (a) Mucosal thickness was measured from lumen to cartilage perichondrium, indicated with blue lines. (b) Epithelial thickness was measured from lumen to basement membrane, indicated with red lines. (c) Mucosal buckling was calculated as the percentage of frames in the dataset that exhibited buckling, indicated with arrows. (d) Mucus was automatically segmented and color-coded in yellow. Scale bars, 1 mm.
Statistical Analysis
Data are means +/− SD. Statistical analysis was performed in Prism (Graphpad Software, La Jolla, CA). Baseline differences between study groups were assessed using Kruskal-Wallis with multiple comparisons performed using Dunn’s test. Differences in response to allergen were assessed using a two-way ANOVA with multiple comparisons performed using Sidak’s test. The factors assessed in the allergen response analyses were group (AA, AC, and HC) and time (pre- and post-allergen), the latter of which were assessed using repeated measures. Correlations between spirometry and imaging metrics were assessed on the strength of Pearson’s correlation coefficients. Comparisons were considered statistically significant for p < 0.05.
RESULTS:
From 2011 to 2016, n = 22 AA, n = 22 AC, and n = 3 HC study volunteers were enrolled in this study. Two AA subjects were unable to undergo the second day of imaging due to excessive coughing, and FEV1<50% predicted, respectively. The results presented in this manuscript represent data obtained from the remaining study subjects; n = 20 AAs, n = 22 ACs, and n = 3 HCs. In two cases an individual from the AA group received a bronchodilator on the second day of the study as recommended by a physician but were able to complete the study. Patient characteristics and pulmonary function tests are provided in Table 1.
Table 1.
Subject Characteristics
| AA | AC | HC | p value | |
|---|---|---|---|---|
| Subjects, n | 20 | 22 | 3 | |
| Age, yr, mean (SD) | 28.7 (9.3) | 27.2 (7.7) | 24 (2.3) | .935 |
| Female, n (%) | 11 (61.1) | 10 (45.5) | 2 (66.6) | .546 |
| FEV1, mean (SD) | ||||
| -Liters | 3.31 (0.79) | 4.02 (0.68) | 3.76 (0.34) | .071 |
| -% predicted | 88.9 (10.6)† | 103.1 (12.9) | 102.7 (7.77) | .004 |
| FVC, mean (SD) | ||||
| -Liters | 4.46 (1.31) | 4.83 (0.82) | 4.30 (0.34) | .306 |
| -% predicted | 100.3 (12.2) | 104.9 (14.0) | 101.7 (7.51) | .294 |
| FEV1/FVC, mean (SD) | 0.76 (0.08)†‡ | 0.84 (0.05) | 0.87 (0.02) | .0005 |
| PC20, mg/mL, mean (SD) | 3.50 (5.38)†‡ | > 25 | > 25 | <.0001 |
| Allergen, n (%) | ||||
| -D. pteronyssinus | 12 (60) | 10 (45.5) | - | |
| -Cat hair extract | 8 (40) | 12 (54.5) | - | |
| Allergen Dose, BAU/mL, mean (SD) | ||||
| -D. pteronyssinus | 226.2(339.0) | 110.1(108.2) | - | 0.847* |
| -Cat hair extract | 158.7(172.0) | 751.7(418.6) | 500 (0) | 0.0042* |
Definition of abbreviations: AA = allergic asthmatic; AC = allergic control; BAU = bioequivalent allergen unit; PC20 = concentration of methacholine needed to produce a 20% fall in FEV1 from baseline.
p < 0.05 for post hoc comparison with AC.
p < 0.05 for post hoc comparison with HC.
HC group excluded and Mann-Whitney test used for statistical analysis.
Baseline Airway Morphology and Mucus
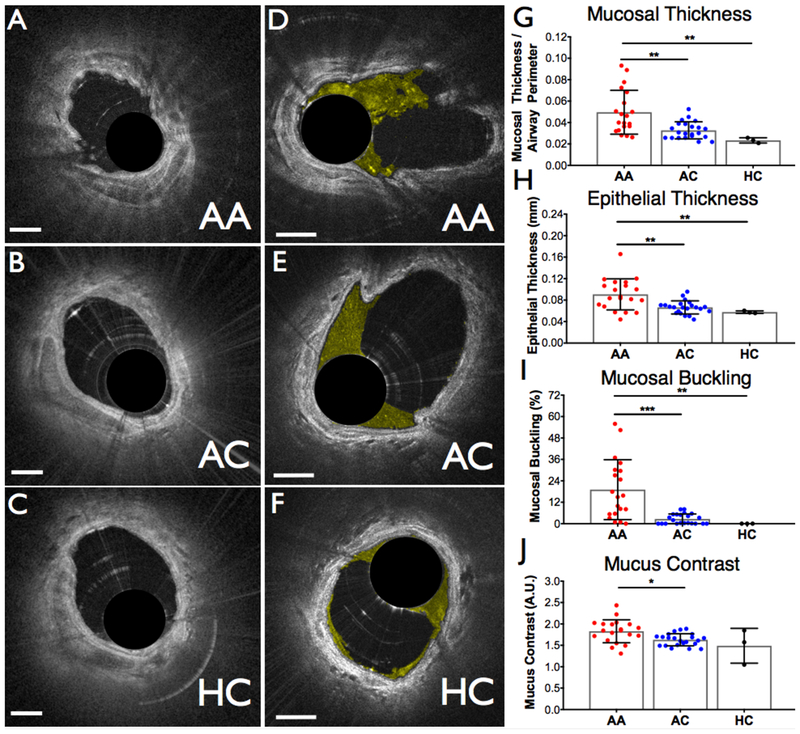
Figure 2(a-c) shows representative OCT cross-sectional images obtained from each of the study groups. Baseline measurements are reported as an average of both the RML and the RUL measurements per subject, as there was no statistically significant difference observed between locations within each study subject. The mean diameter of all airways imaged was 2.8 mm (+/− 0.7 mm). An increase in epithelial and mucosal thickness and mucosal buckling was observed in AA study subjects compared to AC subjects (mucosal thickness: p = 0.0073; epithelial thickness: p = 0.0082; mucosal buckling: p = 0.0003) and compared to HC subjects (mucosal thickness: p = 0.0029; epithelial thickness: p = 0.0376; mucosal buckling: p = 0.0031) (Fig. 2g-i). Representative airway cross sectional images highlighting the mucus are shown for the three groups in Figure 2(d-f). Mucus contrast was found to be higher for the AA group compared to the AC group (p = 0.0154) (Fig. 2j).
Figure 2.
Representative cross-sectional images comparing pre-challenge airway wall morphology (a-c) and mucus (d-f) for the three groups included in the study. Mucus was color-coded in yellow. (a,c) Allergic asthmatic, (b,e) allergic controls, and (c,f) healthy controls. (g-j) Airway morphology and mucus metrics measured pre-challenge for each of the three groups. (g) Mucosal thickness, (h) epithelial thickness, (i) percentage of frames exhibiting mucosal buckling, and (j) mucus contrast. AA, allergic asthmatic; AC, allergic control; HC, healthy control; A.U, arbitrary units. Scale bars, 1 mm. * p < 0.05, * p < 0.01, *** p < 0.001.
OCT and Spirometry
To determine if OCT measures of airway morphology at baseline are reflected in global airway obstruction we investigated the relationship between these metrics and both FEV1/FVC and FEV1% for the AA and AC groups. No correlation was observed for any of the metrics and FEV1%; however, both epithelial thickness and mucosal buckling were found to be correlated to FEV1/FVC in the AA group (p = 0.03 for epithelial thickness, and p = 0.003 for mucosal buckling). Figure 3 shows the relationship between FEV1/FVC and the AA and AC measurements.
Figure 3.
Correlations between FEV1/FVC and (a) mucosal thickness, (b) epithelial thickness, (c) mucosal buckling, and (d) mucus contrast, using baseline measurements for each. Values from both the AA (red dots) and AC (blue dots) group are included. Correlations are presented both AA and AC, separately. Trend line is included for AA only. A.U, arbitrary units.
SAC Response
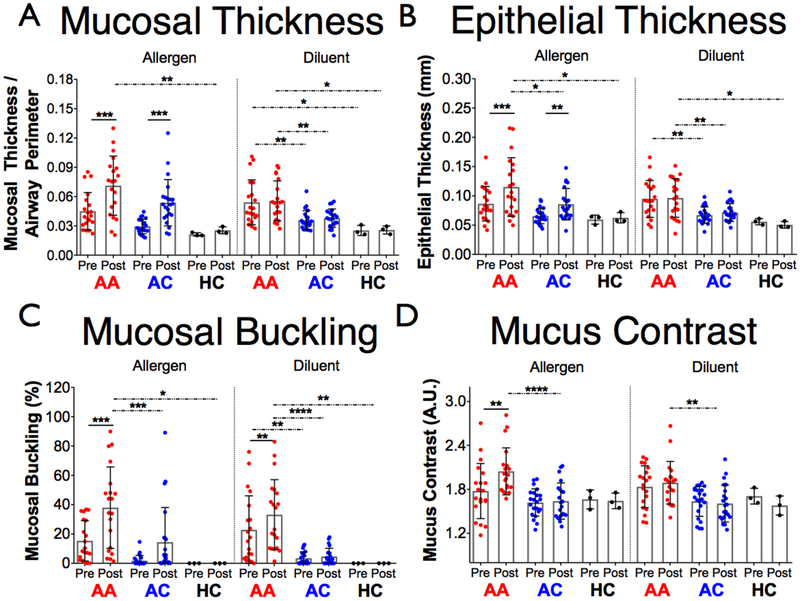
BAL results for some potentially relevant cellular data are shown in Supplementary Figure S2. An increase in epithelial thickness, mucosal thickness, and mucosal buckling measured in the RML compared to baseline values was observed in response to allergen in both AA and AC groups (Fig. 4a-c). While epithelial and mucosal thicknesses increased in both the AA and AC groups (p = 0.0003 AA, p = 0.0004 AC for mucosal thickness; p = 0.0002 AA, p= 0.0075 AC for epithelial thickness), mucosal buckling was only increased in the AA group (p = 0.0006 AA). An increase in mucosal buckling was also observed in response to diluent in the AA group (p = 0.0054). No change was in the HC subjects.
Figure 4.
The four OCT metrics included in the study measured pre- and post- allergen (right middle lobe) and diluent (right upper lobe) for each of the three groups. (a) Mucosal thickness. (b) Epithelial thickness. (c) Mucosal buckling. (d) mucus contrast. AA, allergic asthmatic; AC, allergic control; HC, healthy control; A.U, arbitrary units. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
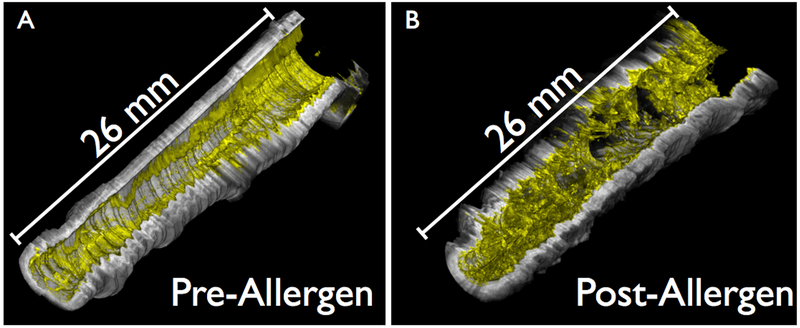
The results of the mucus measurements are depicted in Figure 4(d). Post-allergen, mucus contrast was increased in the AA group relative to both the baseline of the AA group (p = 0.0011) and the post-allergen AC group (p < 0.0001). A volumetric representation of an allergen-challenged AA airway segment highlighting the change in both mucus volume and heterogeneity compared to the pre-allergen airway is depicted in Figure 5.
Figure 5.
Volumetric renderings of mucus in a right middle lobe airway segment of an allergic asthmatic study subject pre-challenge and 24 hours post-challenge. The airway wall was rendered in grayscale and the mucus false-colored in yellow. (a) Pre-allergen. (b) Post-allergen.
DISCUSSION:
In this study, we assessed airway remodeling in asthma on a microscopic scale, in vivo, using endobronchial OCT. Three groups were included in this study: allergic asthmatic (AA), allergic non-asthmatic (AC), and healthy control (HC) subjects. Fewer HC subjects were enrolled in this study as we were primarily interested in comparing AA and AC individuals. The measurements for the HC group demonstrated less variability than those of the AA and AC groups, and are included to provide some context for our data and so that possible baseline values may be inferred. The features that we measured were epithelial thickness, mucosal thickness, airway buckling, and mucus. Baseline differences between groups were observed for each of the features. Correlations with FEV1/FVC in the AA group alone were observed for both epithelial thickness and mucosal buckling. The fact that our microstructural assessments have a stronger relationship with FEV1/FVC than FEV1% is consistent with previous observations involving wall thickness as a measure of remodeling with CT23.
Post-allergen, there were significant differences between the AA and AC group for every metric except mucosal thickness. While significance was observed in both the AA and AC groups for mucosal and epithelial thickness, mucosal buckling and mucus contrast were only significantly different in the AA group. Mucus contrast demonstrated the most significant post-allergen difference between the AA and AC groups (p < 0.0001). Although mucus contrast is likely influenced by factors such as cellular debris, we have already demonstrated that mucus contrast correlates with mucin content obtained from BAL21. Although further tests are necessary to establish correlations, this metric seems to accurately capture the appearance of mucus in our images (refer to the more solid appearing mucus in the AA airways of Figures 2 and 5).
Perhaps the most interesting and unique aspect of this study was the differences in mucosal buckling. Previously it has been shown that airway wall thickness assessed with OCT was strongly correlated with lung function24, but in our study the granularity of the distinct microstructural metrics shows mucosal buckling to be the strongest correlate. Additionally, buckling exhibited the most significant difference post-allergen between the two groups. Here we make the reasonable assumption that the buckling is primarily a consequence of bronchoconstriction25, and therefore consider it a surrogate measure for airway smooth muscle tone. In this study we observed several indications that suggest the possibility of airway smooth muscle abnormality26 in the mild asthmatic group: increase in the baseline tone, hyperresponsiveness as indicated by the increased buckling in the diluent airway, and resistance to relaxation as indicated by the increased buckling 24 hours following the allergen administration. Our results, coupled with the results of a pivotal study which found that bronchoconstriction promotes airway remodeling in asthma27 as well as our own pilot study that demonstrated increased muscle thickness in n=3 mild asthmatics19, add to the increasingly compelling evidence that abnormalities of airway smooth muscle can be a crucial component in the pathogenesis of asthma. Further investigation into the relationship between bronchoconstriction and airway smooth muscle in asthma, along with the continued development of technology which can directly assess airway smooth muscle19, may therefore be important for ASM-targeting therapies such as bronchial thermoplasty that aim to mitigate asthma symptoms14, 28-30.
In this study, we used endobronchial OCT to measure mucus and airway microstructure in allergic individuals with and without asthma to examine alterations in mild asthma and determine how each correlated to airflow obstruction. We observed evidence of airway remodeling in individuals with mild asthma, and a difference in reaction to allergen challenge between the mildly asthmatic and non-asthmatic airways. Even with only two 3 cm airway segments imaged per individual, a relationship was observed between baseline remodeling and baseline airway obstruction, highlighting the physiologic relevance of even mild airway remodeling. The results of this study demonstrate the potential of endobronchial OCT for both asthma research and monitoring airway remodeling in asthma, offering a minimally-invasive approach for measuring multiple important features of remodeling simultaneously.
Supplementary Material
ACKNOWLEDGEMENTS:
The authors would like to thank Dr. Joseph Locascio for his guidance and assistance regarding our statistical analyses. The authors would also like to thank Carol Leary, Matthew Murphy, and Lloyd Liang for their participation in the clinical study. This work was supported by the National Institutes of Health (NIH) (R01CA167827, R01HL133664, U19AI095261, R37AI040618).
Abbreviations:
- AA
Allergic Asthmatics
- AC
Allergic Controls
- BAL
Bronchoalveolar Lavage
- BAU
Bioequivalent Allergen Unit
- FEV1
Forced Expiratory Volume in 1 second
- FEV1%
FEV1 as a percentage of predictive value
- FVC
Forced Vital Capacity
- HC
Healthy Controls
- OCT
Optical Coherence Tomography
- RML
Right Middle Lobe
- RUL
Right Upper Lobe
- SAC
Segmental Allergen Challenge
REFERENCES:
- 1.Pawankar R, Canonica GW, Holgate ST, Lockey RF. Allergic diseases and asthma: a major global health concern. Current opinion in allergy and clinical immunology. 2012; 12: 39–41. [DOI] [PubMed] [Google Scholar]
- 2.Vignola AM, Chiappara G, Siena L, Bruno A, Gagliardo R, Merendino AM, Polla BS, Arrigo AP, Bonsignore G, Bousquet J, Chanez P. Proliferation and activation of bronchial epithelial cells in corticosteroid-dependent asthma. The Journal of allergy and clinical immunology. 2001; 108: 738–46. [DOI] [PubMed] [Google Scholar]
- 3.Cohen L, E X, Tarsi J, Ramkumar T, Horiuchi TK, Cochran R, DeMartino S, Schechtman KB, Hussain I, Holtzman MJ, Castro M, Program NSAR. Epithelial cell proliferation contributes to airway remodeling in severe asthma. American journal of respiratory and critical care medicine. 2007; 176: 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awadh N, Muller NL, Park CS, Abboud RT, FitzGerald JM. Airway wall thickness in patients with near fatal asthma and control groups: assessment with high resolution computed tomographic scanning. Thorax. 1998; 53: 248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. The American review of respiratory disease. 1993; 147: 405–10. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins HA, Cool C, Szefler SJ, Covar R, Brugman S, Gelfand EW, Spahn JD. Histopathology of severe childhood asthma: a case series. Chest. 2003; 124: 32–41. [DOI] [PubMed] [Google Scholar]
- 7.Little SA, Sproule MW, Cowan MD, Macleod KJ, Robertson M, Love JG, Chalmers GW, McSharry CP, Thomson NC. High resolution computed tomographic assessment of airway wall thickness in chronic asthma: reproducibility and relationship with lung function and severity. Thorax. 2002; 57: 247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, Nishimura K, Itoh H, Izumi T. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. American journal of respiratory and critical care medicine. 2000; 162: 1518–23. [DOI] [PubMed] [Google Scholar]
- 9.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, Fahy JV. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. American journal of respiratory and critical care medicine. 2001; 163: 517–23. [DOI] [PubMed] [Google Scholar]
- 10.Tanizaki Y, Kitani H, Okazaki M, Mifune T, Mitsunobu F, Kimura I. Mucus hypersecretion and eosinophils in bronchoalveolar lavage fluid in adult patients with bronchial asthma. J Asthma. 1993; 30: 257–62. [DOI] [PubMed] [Google Scholar]
- 11.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. Optical coherence tomography. Science (New York, NY). 1991; 254: 1178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun SH, Tearney GJ, Vakoc BJ, Shishkov M, Oh WY, Desjardins AE, Suter MJ, Chan RC, Evans JA, Jang IK, Nishioka NS, de Boer JF, Bouma BE. Comprehensive volumetric optical microscopy in vivo. Nature medicine. 2006; 12: 1429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lilly CM, Nakamura H, Belostotsky OI, Haley KJ, Garcia-Zepeda EA, Luster AD, Israel E. Eotaxin expression after segmental allergen challenge in subjects with atopic asthma. American journal of respiratory and critical care medicine. 2001; 163: 1669–75. [DOI] [PubMed] [Google Scholar]
- 14.Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest. 2005; 127: 1999–2006. [DOI] [PubMed] [Google Scholar]
- 15.Cho JL, Ling MF, Adams DC, Faustino L, Islam SA, Afshar R, Griffith JW, Harris RS, Ng A, Radicioni G, Ford AA, Han AK, Xavier R, Kwok WW, Boucher R, Moon JJ, Hamilos DL, Kesimer M, Suter MJ, Medoff BD, Luster AD. Allergic asthma is distinguished by sensitivity of allergen-specific CD4+ T cells and airway structural cells to type 2 inflammation. Science translational medicine. 2016; 8: 359ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afshar R, Strassner JP, Seung E, Causton B, Cho JL, Harris RS, Hamilos DL, Medoff BD, Luster AD. Compartmentalized chemokine-dependent regulatory T-cell inhibition of allergic pulmonary inflammation. The Journal of allergy and clinical immunology. 2013; 131: 1644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas SY, Banerji A, Medoff BD, Lilly CM, Luster AD. Multiple chemokine receptors, including CCR6 and CXCR3, regulate antigen-induced T cell homing to the human asthmatic airway. Journal of immunology. 2007; 179: 1901–12. [DOI] [PubMed] [Google Scholar]
- 18.Thomas SY, Lilly CM, Luster AD. Invariant natural killer T cells in bronchial asthma. The New England journal of medicine. 2006; 354: 2613–6; author reply −6. [DOI] [PubMed] [Google Scholar]
- 19.Adams DC, Hariri LP, Miller AJ, Wang Y, Cho JL, Villiger M, Holz JA, Szabari MV, Hamilos DL, Scott Harris R, Griffith JW, Bouma BE, Luster AD, Medoff BD, Suter MJ. Birefringence microscopy platform for assessing airway smooth muscle structure and function in vivo. Science translational medicine. 2016; 8: 359ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park B, Pierce M, Cense B, de Boer J. Real-time multi-functional optical coherence tomography. Optics express. 2003; 11: 782–93. [DOI] [PubMed] [Google Scholar]
- 21.Adams DC, Pahlevaninezhad H, Szabari MV, Cho JL, Hamilos DL, Kesimer M, Boucher RC, Luster AD, Medoff BD, Suter MJ. Automated segmentation and quantification of airway mucus with endobronchial optical coherence tomography. Biomed Opt Express. 2017; 8: 4729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hariri LP, Mino-Kenudson M, Mark EJ, Suter MJ. In vivo optical coherence tomography: the role of the pathologist. Archives of pathology & laboratory medicine. 2012; 136: 1492–501. [DOI] [PubMed] [Google Scholar]
- 23.Chae EJ, Kim TB, Cho YS, Park CS, Seo JB, Kim N, Moon HB. Airway Measurement for Airway Remodeling Defined by Post-Bronchodilator FEV1/FVC in Asthma: Investigation Using Inspiration-Expiration Computed Tomography. Allergy, asthma & immunology research. 2011; 3: 111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coxson HO, Lam S. Quantitative assessment of the airway wall using computed tomography and optical coherence tomography. Proceedings of the American Thoracic Society. 2009; 6: 439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiggs BR, Hrousis CA, Drazen JM, Kamm RD. On the mechanism of mucosal folding in normal and asthmatic airways. J Appl Physiol (1985). 1997; 83: 1814–21. [DOI] [PubMed] [Google Scholar]
- 26.Doeing DC, Solway J. Airway smooth muscle in the pathophysiology and treatment of asthma. J Appl Physiol (1985). 2013; 114: 834–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, Davies DE, Howarth PH. Effect of bronchoconstriction on airway remodeling in asthma. The New England journal of medicine. 2011; 364: 2006–15. [DOI] [PubMed] [Google Scholar]
- 28.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008; 372: 1107–19. [DOI] [PubMed] [Google Scholar]
- 29.Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology (Bethesda). 2005; 20: 28–35. [DOI] [PubMed] [Google Scholar]
- 30.Shifren A, Witt C, Christie C, Castro M. Mechanisms of remodeling in asthmatic airways. J Allergy (Cairo). 2012; 2012: 316049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.