Abstract
Tolerance to the antinociceptive effect of mu-opioid receptor (MOPr) agonists, such as morphine and fentanyl, greatly limits their effectiveness for long-term use to treat pain. Clinical studies have shown that combination therapy and opioid rotation can be used to enhance opioid-induced antinociception once tolerance has developed. The mechanism and brain regions involved in these processes are unknown. The purpose of this study was to evaluate the contribution of the ventrolateral periaqueductal gray (vlPAG) to antinociceptive tolerance and cross-tolerance between administration and co-administration of morphine and fentanyl. Tolerance was induced by pretreating rats with morphine or fentanyl or low-dose combination of morphine and fentanyl into the vlPAG followed by assessment of cross-tolerance to the other opioid. In addition, tolerance to the combined treatment was assessed. Cross-tolerance did not develop between repeated vlPAG microinjections of morphine and fentanyl. Likewise, there was no evidence of cross-tolerance from morphine or fentanyl to co-administration of morphine and fentanyl. Co-administration did not cause cross-tolerance to fentanyl. Cross-tolerance was only evident to morphine or morphine and fentanyl combined in rats pretreated with co-administration of low-doses of morphine and fentanyl. In conclusion, cross-tolerance does not develop between morphine and fentanyl within the vlPAG. This finding is consistent with the functionally selective signaling that has been reported for antinociception and tolerance following morphine and fentanyl binding to the MOPr. This research supports the notion that combination therapy and opioid rotation may be useful clinical practices to reduce opioid tolerance and other side effects.
Keywords: opioid, functional selectivity, cross-tolerance, analgesia, mu-opioid receptor
Perspective:
This preclinical study shows that there is a reduction in cross tolerance between morphine and fentanyl within the periaqueductal gray which is key brain region in opioid antinociception and tolerance.
Introduction
Morphine and fentanyl are two of the most commonly used drugs to treat pain. Chronic use is limited by unpleasant side effects and the development of tolerance. Opioid rotation and co-administration have been used to enhance pain relief and limit these side effects28, 44. Although animal studies report additive antinociceptive effects when morphine and fentanyl are co-administered5, 39, clinical research indicates that the analgesic efficacy of co-administered morphine and fentanyl is greater than administration of either opioid alone28, 47. This effect appears to be the result of maintained fentanyl potency despite the development of tolerance to morphine42.
Many preclinical studies evaluating cross-tolerance between morphine and fentanyl show enhanced antinociception and reduced tolerance when one opioid is substituted for the other10, 35, 36, 43. Other studies show cross-tolerance with as little as a single injection, as well as with continuous administration26, 40. Route and length of administration may be key factors in the analgesic efficacy of co-administered opioids.
Opioids produce antinociception by binding to mu-opioid receptors at sites throughout the nervous system. Microinjection of either morphine or fentanyl into the ventrolateral periaqueductal gray (vlPAG) produces antinociception3 and repeated administration of either drug results in tolerance to this antinociception1. Despite these similarities, the intracellular signaling molecules appear to be distinct. Tolerance to repeated morphine injections into the vlPAG is mediated by C-Jun N –terminal kinase (JNK), whereas tolerance to repeated fentanyl microinjections is mediated by G protein–coupled receptor kinase (GRK)32. This difference suggests that within the vlPAG there should be no cross-tolerance between morphine and fentanyl microinjections. This hypothesis will be tested by microinjecting rats with morphine, fentanyl, or a combination of morphine and fentanyl directly into the vlPAG.
Methods
Subjects
Experiments were performed on male Sprague-Dawley rats (n = 93) with a mean weight of 277g (230 – 330g). Prior to surgery rats were double housed on a 12-hour light-dark cycle (lights on at 7AM). Food and water were available at all times except during testing. All procedures were approved by the Washington State University Animal Care and Use Committee and conducted in accordance with the guidelines for animal use described by the International Association for the Study of Pain.
Stereotaxic Surgery and Microinjections
Rats were anesthetized with pentobarbital (60 mg/kg, i.p.) and implanted unilaterally with a guide cannula (23 gauge; 9 mm long) aimed at the vlPAG using stereotaxic techniques (AP: +1.7 mm, ML: ±0.6 mm, DV: −4.6 mm from lambda). Following surgery, the guide cannula was occluded with a 9 mm stylet. Rats were handled daily following surgery. Morphine sulfate (a gift from the National Institute on Drug Abuse) and fentanyl citrate (Sigma-Aldrich), were dissolved in sterile saline. Drugs were administered through a 31-gauge injection cannula inserted into and extending 2 mm beyond the guide cannula. One day prior to testing, the injector was inserted into the guide cannula without drug administration to habituate them to the procedure and prevent mechanical activation of neurons on the test day.
Behavioral testing
Nociception was assessed using the hot plate test in which the latency for the rat to lick the hind paw was measured when placed on a 52.5°C hotplate. The rat was removed if no response occurred within 50 s. Rats with a baseline hot plate latency greater than 25 s were not included in data analysis. Rats were randomly assigned and injected into the vlPAG with either 0.9% saline (0.4 μL), morphine (5 μg/0.4 μL), fentanyl (3 μg/0.4 μL), or a morphine/fentanyl combination (2.5 μg of morphine and 1.5 μg of fentanyl in 0.4 μl). These combination doses were chosen as half the ED50 dose for each opioid so as to result in an equiantinociceptive dose compared to each opioid alone. Nociception was assessed in a subset of rats at 5, 30, & 60 minutes after the first injection to determine optimal test time in tolerance experiments. Tolerance was established by repeated injections of either drug alone or the combination twice a day for two days1. Nociceptive testing was only conducted following the first and the last injections to prevent the development of behavioral tolerance19. Only male rats were used given that tolerance mediated by PAG is minimal in female rats20.
The presence of tolerance was assessed on Day 3 using a cumulative dosing procedure31. Increasing third log doses of morphine (cumulative doses of 1, 2.2, 4.6, 10, 22 μg/0.4 μL), fentanyl (cumulative doses of 0.46, 1, 2.2, 4.6 & 10 μg/0.4 μL), or a combination of morphine (0.5, 1.1, 2.3, 5, & 11 μg/0.4 μL) and fentanyl (0.23, 0.5, 1.1, 2.3, & 5 μg/0.4 μL) was microinjected into the vlPAG. Half of the cumulative dose of morphine and fentanyl was used at each step when co-administered. The timing for cumulative dosing for morphine and fentanyl alone have been established previously3, 31 as follows morphine was injected at 20 min intervals followed by hot plate testing 15 min after each injection. Fentanyl was injected at 4 min intervals with behavioral testing 2 min after each injection. Co-administered of morphine and fentanyl was injected at 7 min intervals to capture peak antinociception of the combination within the time course of both drugs (see Fig. 2). Rats were tested on the hot plate 5 min after each injection. Tolerance was defined as a significant rightward shift in the dose response curve by comparing ED50 values for rats pretreated with an opioid vs. the saline vehicle.
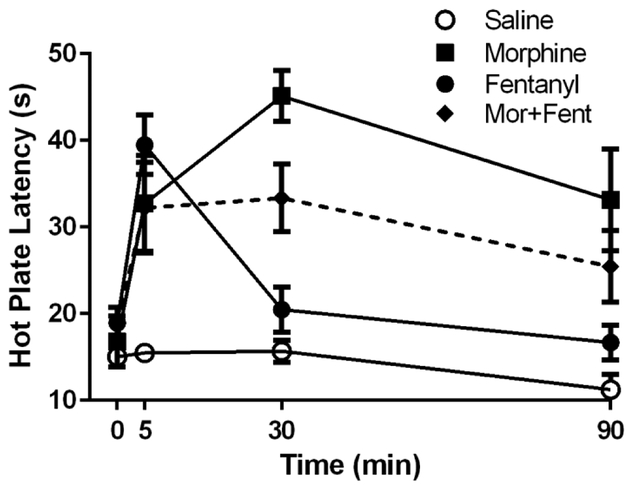
Figure 2. Time course for antinociception following vlPAG morphine, fentanyl, and co-administration of morphine and fentanyl.
Microinjection of morphine (5 μg/0.4 μL), fentanyl (3 μg/0.4 μL), and combined morphine + fentanyl (2.5 μg + 1.5 μg/0.4 μL) showed an increase in hot platency 5 min following vlPAG microinjection. Hot plate latency remained elevated for 90 min following administration of morphine (N = 8–16) or morphine and fentanyl (N = 8). In contrast, the increase in hot plate latency caused by fentanyl (N = 8–15) administration had returned to near baseline levels within 30 min. Not all rats were tested at all time points.
Histology and data analysis
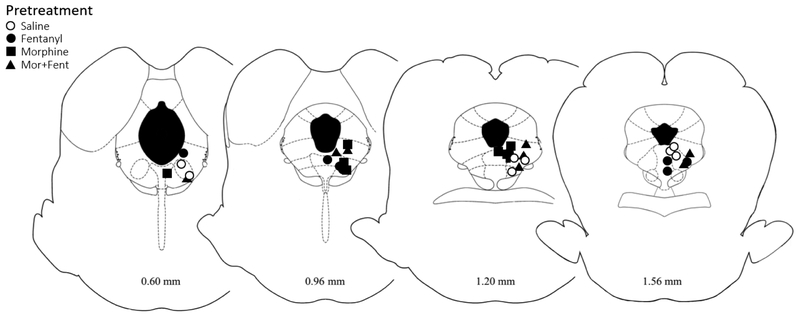
Following testing, rats received a lethal dose of Halothane. Brains were removed and stored in formalin (10%). At least 2 days later the brain was sliced coronally (100 μm) to determine the location of the injection site37. Only those injections in or bordering the vlPAG were included in data analysis (Figure 1). Dose-response curves were plotted using GraphPad (Prism 6) and the half maximal antinociceptive effect (ED50) was calculated for each group1. ANOVAs were used to determine statistically significant differences between groups (α < 0.05). Data are presented as mean ± SEM unless otherwise stated. A Bonferroni post-hoc analysis was used when necessary to compare two means.
Figure 1. Location of injection sites within the vlPAG.
Cannula placements for animals pretreated with saline, morphine, fentanyl, or morphine+fentanyl. Injection sites were similar for all groups across coronal sections of the PAG. Although the image shows the location of the cannula tip, an injection volume of 0.4 μl causes the drug to diffuse into the vlPAG. Distance from Lambda are listed below each image.
Results
Opioid-induced antinociception in vlPAG
A subset of rats used in each of the tolerance experiments were tested before and 5, 30, and 60 minutes after opioid administration to determine the time course for antinociception to co-administration of morphine and fentanyl. There were no significant differences in baseline hot plate latencies between groups prior to drug administration (F(3, 28) = 2.24 p = 0.11). Microinjection of morphine (5 μg/0.4 μL), fentanyl (3 μg/0.4 μL), and combined morphine/fentanyl (2.5 μg & 1.5 μg/0.4 μL) into the vlPAG caused a significant increase in hot plate latency compared to saline controls (Figure 2; F(3, 143) = 22.97; p < 0.05). Administration of morphine and combined morphine/fentanyl produced antinociception at 5, 30, and 90 min post injection compared to saline controls. Microinjection of fentanyl alone had a rapid onset and offset, producing a significant increase in hot plate latency compared to saline only at the 5 min time point (Bonferroni; p < 0.05).
Lack of cross-tolerance between morphine and fentanyl in vlPAG
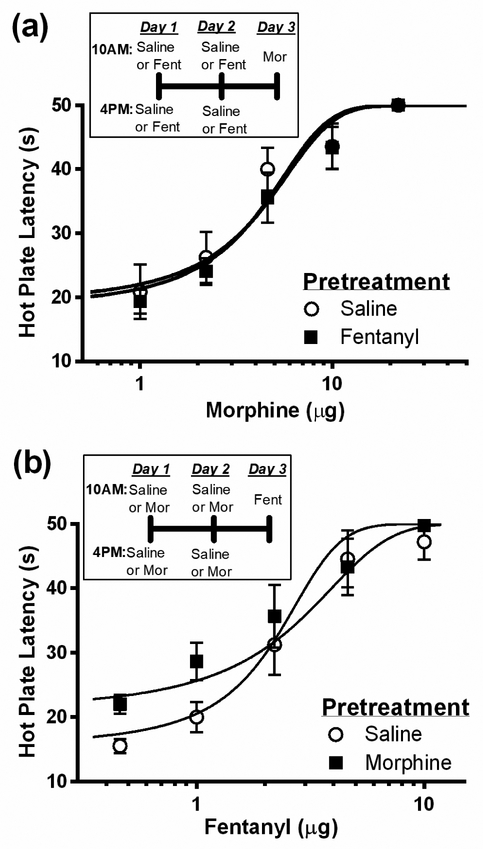
Repeated microinjections of fentanyl twice daily for 2 days did not cause a significant change in morphine potency on Day 3 compared to saline treated controls (Figure 3a; F(1, 76) = 1.66; p = 0.20). Morphine potency was 4.2 ± 1.04 μg (N = 8) and 3.2 ± 0.96 μg (N = 8) following pretreatment with fentanyl or saline, respectively. Similarly, pretreatment with morphine did not cause a significant change in fentanyl potency (Figure 3b; F(1, 71) = 1.93, p = 0.17). Fentanyl potency was 1.7 ± 0.67 μg (N = 7) and 2.4 ± 0.57 μg (N = 8) following pretreatment with morphine or saline, respectively. The lack of cross-tolerance between morphine and fentanyl is consistent with previous studies showing distinct intracellular mechanisms for tolerance to morphine and fentanyl antinociception26, 32.
Figure 3. Lack of cross-tolerance between vlPAG morphine, and fentanyl.
Rats were injected twice daily for two days with saline (0.4 μL), morphine (5 μg/0.4 μL), or fentanyl (3 μg/0.4 μL) into the vlPAG. (a) The antinociceptive potency of morphine did not differ between rats pretreated with fentanyl (N = 8) or saline (N = 8). (b) Likewise, the antinociceptive potency of fentanyl did not differ between rats pretreated with morphine (N = 7) or saline (N = 8).
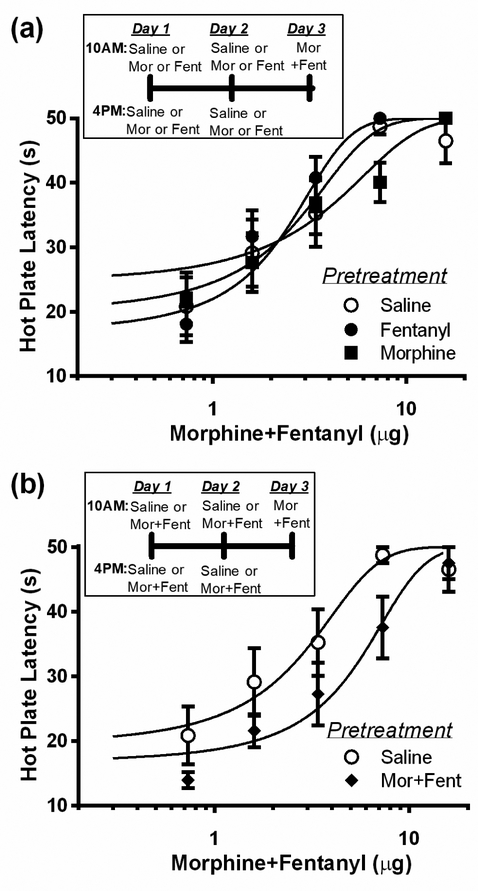
Co-administration of morphine and fentanyl
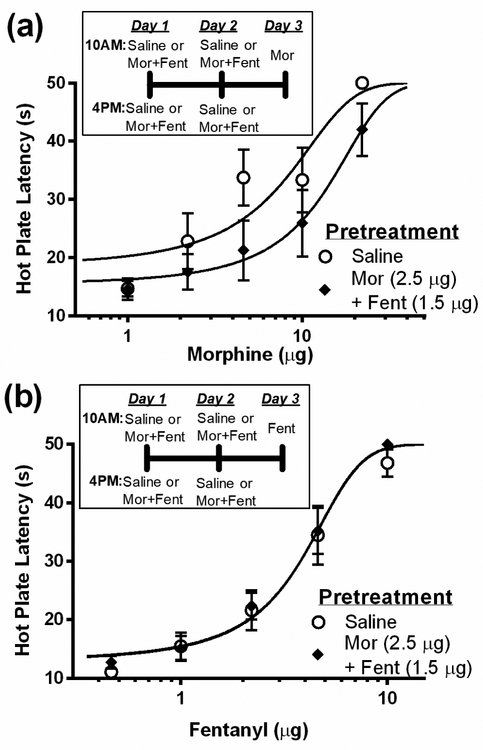
Co-administration of morphine and fentanyl for two days caused cross-tolerance to morphine, but not fentanyl antinociception. Pretreatment with morphine and fentanyl caused a significant rightward shift in the morphine dose-response curve compared to rats pretreated with saline (Figure 4a; F(1, 66) = 6.96; p < 0.05). Morphine ED50 was 12.5 ± 3.69 μg in rats pretreated with co-administered morphine/fentanyl compared to 6.2 ± 2.35 μg in rats pretreated with saline. In contrast, co-administration of morphine and fentanyl did not alter the fentanyl dose-response curve (Figure 4b). There was no significant difference in the antinociceptive potency of fentanyl (3.7 ± 0.52 vs. 3.9 ± 0.73) in rats pretreated with co-administered morphine/fentanyl or saline, respectively (F(1, 76) = 0.14; p = 0.70).
Figure 4. Co-administration of morphine and fentanyl cause cross-tolerance to morphine but not fentanyl.
(a) Repeated microinjections of morphine (2.5 μg/0.4 μL) and fentanyl (1.5 μg/0.4 μL) into the vlPAG (N = 7) for two days caused a rightward shift in the morphine dose response curve compared to saline pretreated rats (N = 7) as would be expected with the development of tolerance. (b) In contrast, co-administration of morphine and fentanyl (N = 8) had no effect on the fentanyl dose-response curve compared to rats pretreated with saline (N = 8).
Cross-tolerance was not evident when the experiment was conducted in the opposite direction. That is, pretreatment with morphine or fentanyl for two days did not cause a shift in the combined morphine/fentanyl dose-response curve (Figure 5a; F(2, 114) = 1.03; p = 0.36). Pretreatment with morphine or fentanyl alone caused log shifts to co-administered morphine/fentanyl of only 0.07 and −1.0, respectively. However, combined pretreatment with morphine and fentanyl caused a rightward shift in the combined dose-response curve on Day 3 (Figure 5b; F(1, 76) = 9.91; p < 0.05). This tolerance was evident by a full one-third log shift in the combined morphine/fentanyl ED50. This was the largest rightward shift in the dose response curve for any of the drug combinations (Table 1).
Figure 5. Lack of tolerance to morphine and fentanyl combined following pretreatment with morphine or fentanyl alone.
(a) Twice daily microinjections of morphine (5 μg/0.4 μL) or fentanyl (5 μg/0.4 μL) for two days did not cause tolerance to the combination of morphine+fentanyl. (b) Twice daily microinjections of morphine+fentanyl for two days caused a rightward shift in the combined dose-response. (N = 8/group)
Table 1:
Cross-tolerance measures as a log shift relative to saline controls
| Cross-tolerance | Log shift |
|---|---|
| Fentanyl to Morphine | 0.12 |
| Morphine to Fentanyl | −0.15 |
| Mor/Fent to Morphine | 0.30* |
| Mor/Fent to Fentanyl | 0.02 |
| Morphine to Mor/Fent | 0.07 |
| Fentanyl to Mor/Fent | −0.10 |
| Mor/Fent to Mor/Fent | 0.33* |
p < .05
Discussion
The current study found that cross-tolerance did not develop between morphine and fentanyl when microinjected into the vlPAG using the same paradigm that produces tolerance to each drug alone1, 31, 45. In addition, rats treated with either opioid alone did not show tolerance to the co-administration of morphine and fentanyl. Only two conditions resulted in antinociceptive tolerance; pretreatment with low dose combination of both opioids followed by testing with the same combination or with morphine alone (Table 1).
A lack of cross-tolerance between morphine and fentanyl has also been reported following systemic administration10, 35, 36, 43. The clinical use of fentanyl to treat breakthrough pain in patients undergoing chronic opioid treatment also suggests a lack of cross-tolerance between fentanyl and other opioids9, 18, 33. Co-administration of fentanyl is frequently used to reestablish pain relief when tolerance has developed to a particular opioid27, 47. In addition to enhancing analgesia, co-administration of opioids has been reported to reduce side effects such as nausea, vomiting, and sedation24, 28, 38, 41.
The lack of cross-tolerance between morphine and fentanyl suggests that these two opioids act at different sites and/or via different mechanisms. Our studies showing a lack of cross-tolerance between morphine and fentanyl when injected into the vlPAG supports the hypothesis that different mechanisms are engaged. The vlPAG plays an important role in opioid antinociception and tolerance1, 29, 45. In addition, the lack of cross-tolerance when rats are pretreated with a single opioid(morphine or fentanyl) and then given the co-administration also suggests distinct neural mechanisms underlie tolerance to each drug. The important implication of this experiment is that lower doses of the opioids can be used for effective antinociception after tolerance has developed to a single opioid. However, when both morphine and fentanyl are combined during pretreatment and tolerance assessment, we find tolerance does develop, likely because both morphine and fentanyl tolerance mechanisms are being activated. An interesting finding is that rats pretreated with repeated co-administration of morphine and fentanyl produces cross-tolerance to morphine alone, but not fentanyl alone. This may be attributed to the half doses that were used in the co-administration pretreatment compared to when the drugs were administered alone. It is possible that the dose of fentanyl used in the pretreatment is inadequate to induce tolerance, whereas the dose used for morphine is sufficient to induce tolerance.
A potential contributing factor to the lack of cross-tolerance between opioids is that the affinity and efficacy at the MOPr differs between agonists. It has been shown that MOPr agonists bind and activate different splice variants of the MOPr, which may be linked to the ligand-biased effects seen in this study. Fentanyl, but not morphine antinociception is blocked following deletion of a particular exon on the MOPr, although the MOPr isoforms in the vlPAG have not been identified34. In addition, the formation of heterodimers (e.g., MOPr/DOPr) could contribute to downstream signaling involved in tolerance for the different opioids8.
Morphine and fentanyl also differ in efficacy. Morphine efficacy is lower than that of fentanyl whether assessed with [35S]GTPγS23, 25, 46 or when assessing the antinociceptive effects following systemic or intrathecal administration22, 30. The relationship between efficacy and antinociceptive tolerance is not clear because efficacy correlates with MOPr internalization12. Efficacy is unlikely to have an effect on the lack of cross-tolerance reported here because we have found that morphine and fentanyl have equal antinociceptive efficacies when microinjected into the vlPAG1.
These initial differences in receptor coupling and regulation may lead to differences in activation of signaling cascades and tolerance development. Ligand-biased signaling at the MOPr is the most likely explanation for the lack of cross-tolerance between morphine and fentanyl25. Morphine is typically inferior to fentanyl in inducing MOPr phosphorylation, desensitization, and internalization. Fentanyl causes phosphorylation of the MOPr via GRK, whereas morphine uses a PKC mediated mechanism17. In many tissue preparations morphine is very weak at inducing MOPr internalization compared to other agonists such as fentanyl6, 7, 25, 26, 48. This functionally selective difference in signaling has been shown to alter morphine and fentanyl antinociception. Blockade of MOPr internalization with dyn-DN had no effect on morphine antinociception, but enhanced fentanyl antinociception2. In contrast, inhibition of Gαi/o-proteins by pertussis toxin (PTX) caused a reduction in morphine, but not fentanyl-induced antinociception4, 14, 15.
Blockade of a component of β-arrestin signaling (i.e. G-protein receptor kinase or extracellular signal regulated kinase) has been shown to prevent tolerance to agonists, such as fentanyl, and have no effect on tolerance to morphine2, 16, 21, 26, 32. In contrast, inhibition of proteins downstream of G-protein signaling (i.e. protein kinase C or c-Jun n-terminal kinase) causes a reduction in morphine, but not fentanyl tolerance16, 26, 32. Activation of different signaling cascades would limit the development of cross-tolerance between morphine (G-protein-dependent pathway) and fentanyl (β-arrestin-dependent pathway).
The impact of differences in the duration of action between morphine and fentanyl is less clear. Fentanyl produces a rapid (3 min) and short-lived (< 30 min) antinociceptive effect compared to morphine microinjection into the vlPAG (peak effects of 15–30 min and duration of 1–2 hours)3. The short antinociceptive effect of fentanyl may be caused by rapid internalization, which would limit signaling through G proteins. This could explain the lack of cross-tolerance from fentanyl to morphine, but not from morphine to fentanyl because prolonged G protein signaling by morphine should cause adaptations that affect any MOPr bound ligand.
A final difference between the two drugs is how they are metabolized. Morphine is metabolized into morphine-6-glucurunide or morphine-3-glucurunide, whereas there are no known active metabolites of fentanyl11, 13. The combined MOPr activation of morphine and morphine-6-glucurunide may contribute to the development of tolerance. Furthermore, morphine-3-glucurunide activation of TLR4 has been recently shown to contribute to morphine tolerance within the PAG11. Once again, this difference may contribute to differences in tolerance between morphine and fentanyl, but is unlikely to prevent cross-tolerance between these drugs.
In conclusion, the current study shows a clear lack of cross-tolerance between morphine and fentanyl when microinjected into the vlPAG. Although tolerance occurs with co-administration of morphine and fentanyl into the vlPAG, cross-tolerance was only evident to morphine not fentanyl. The implication of this research is that once tolerance develops to a single opioid, co-administration of lower doses of two different opioid can be co-administered to achieve antinociception. These data support clinical findings suggesting that co-administration of opioids is more effective than administration of a single opioid whether it is morphine or fentanyl. The presence of distinct tolerance mechanisms provides new targets for drug development to improve pain treatment by limiting the development of tolerance.
HIGHLIGHTS.
The periaqueductal gray is site of action for reduced opioid cross-tolerance
Co-administration of low-dose opioids can enhance antinociception
Lack of cross-tolerance to opioids supports the clinical use of opioid rotation
Acknowledgments:
This study was supported in part by the National Institute of Drug Abuse (DA015498). The authors would like to thank Rachel Reid and Davina Fitzgibbon for technical assistance. The authors declare no competing financial interests.
Disclosures:
This research was funded by NIH DA015498. There are no other financial relationships or conflicts of interest associated with this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bobeck EN, Haseman RA, Hong D, Ingram SL, Morgan MM. Differential development of antinociceptive tolerance to morphine and fentanyl is not linked to efficacy in the ventrolateral periaqueductal gray of the rat. J Pain. 13:799–807, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobeck EN, Ingram SL, Hermes SM, Aicher SA, Morgan MM. Ligand-biased activation of extracellular signal-regulated kinase 1/2 leads to differences in opioid induced antinociception and tolerance. Behav Brain Res. 298:17–24, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobeck EN, McNeal AL, Morgan MM. Drug dependent sex-differences in periaqueducatal gray mediated antinociception in the rat. Pain. 147:210–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodnar RJ, Paul D, Rosenblum M, Liu L, Pasternak GW. Blockade of morphine analgesia by both pertussis and cholera toxins in the periaqueductal gray and locus coeruleus. Brain Res. 529:324–328, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Bolan EA, Tallarida RJ, Pasternak GW. Synergy between mu opioid ligands: evidence for functional interactions among mu opioid receptor subtypes. J Pharmacol Exp Ther. 303:557–562, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 278:18776–18784, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Celver J, Xu M, Jin W, Lowe J, Chavkin C. Distinct domains of the mu-opioid receptor control uncoupling and internalization. Mol Pharmacol. 65:528–537, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia. Expert Rev Mol Med. 14:e9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Leon-Casasola OA. Current developments in opioid therapy for management of cancer pain. Clin J Pain. 24 Suppl 10:S3–7, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Duttaroy A, Yoburn BC. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 82:1226–1236, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ. Toll-like Receptor 4 Mediates Morphine-Induced Neuroinflammation and Tolerance via Soluble Tumor Necrosis Factor Signaling. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 42:661–670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 32:829–839, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Golembiewski J, Torrecer S, Katke J. The use of opioids in the postoperative setting: focus on morphine, hydromorphone, and fentanyl. J Perianesth Nurs. 20:141–143, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Gomes BA, Shen J, Stafford K, Patel M, Yoburn BC. Mu-opioid receptor down-regulation and tolerance are not equally dependent upon G-protein signaling. Pharmacol Biochem Behav. 72:273–278, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Goode TL, Raffa RB. An examination of the relationship between mu-opioid antinociceptive efficacy and G-protein coupling using pertussis and cholera toxins. Life Sci. 60:PL107–113, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, Henderson G, Dewey WL. The effect of protein kinase C and G protein-coupled receptor kinase inhibition on tolerance induced by muopioid agonists of different efficacy. J Pharmacol Exp Ther. 332:1127–1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol. 153 Suppl 1:S379–388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korkmazsky M, Ghandehari J, Sanchez A, Lin HM, Pappagallo M. Feasibility study of rapid opioid rotation and titration. Pain Physician. 14:71–82, 2011 [PMC free article] [PubMed] [Google Scholar]
- 19.Lane DA, Morgan MM. Antinociceptive tolerance to morphine from repeated nociceptive testing in the rat. Brain Res. 1047:65–71, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci. 27:1517–1524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macey TA, Bobeck EN, Hegarty DM, Aicher SA, Ingram SL, Morgan MM. ERK1/2 activation counteracts morphine tolerance in the periaqueductal gray of the rat. J Pharmacol Exp Ther. 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madia PA, Dighe SV, Sirohi S, Walker EA, Yoburn BC. Dosing protocol and analgesic efficacy determine opioid tolerance in the mouse. Psychopharmacology (Berl). 2009 [DOI] [PubMed] [Google Scholar]
- 23.Madia PA, Navani DM, Yoburn BC. [(35)S]GTPgammaS binding and opioid tolerance and efficacy in mouse spinal cord. Pharmacol Biochem Behav. 101:155–165, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Marinangeli F, Ciccozzi A, Aloisio L, Colangeli A, Paladini A, Bajocco C, Coaccioli S, Varrassi G. Improved cancer pain treatment using combined fentanyl-TTS and tramadol. Pain Pract. 7:307–312, 2007 [DOI] [PubMed] [Google Scholar]
- 25.McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, Henderson G, Kelly E. mu-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol. 78:756–766, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci U S A. 107:11608–11613, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercadante S. Opioid combination: rationale and possible clinical applications. Ann Palliat Med. 2:189–196, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Mercadante S, Villari P, Ferrera P, Casuccio A. Addition of a second opioid may improve opioid response in cancer pain: preliminary data. Support Care Cancer. 12:762–766, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Meyer PJ, Fossum EN, Ingram SL, Morgan MM. Analgesic tolerance to microinjection of the muopioid agonist DAMGO into the ventrolateral periaqueductal gray. Neuropharmacology. 52:1580–1585, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mjanger E, Yaksh TL. Characteristics of dose-dependent antagonism by beta-funaltrexamine of the antinociceptive effects of intrathecal mu agonists. J Pharmacol Exp Ther. 258:544–550, 1991 [PubMed] [Google Scholar]
- 31.Morgan MM, Fossum EN, Levine CS, Ingram SL. Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav. 85:214–219, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Morgan MM, Reid RA, Saville KA. Functionally selective signaling for morphine and fentanyl antinociception and tolerance mediated by the rat periaqueductal gray. PloS one. 9:e114269, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morita T, Takigawa C, Onishi H, Tajima T, Tani K, Matsubara T, Miyoshi I, Ikenaga M, Akechi T, Uchitomi Y, Japan Pain RPM, Psycho-Oncology Study G. Opioid rotation from morphine to fentanyl in delirious cancer patients: an open-label trial. J Pain Symptom Manage. 30:96–103, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci U S A. 106:4917–4922, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paronis CA, Holtzman SG. Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperidine or fentanyl in rats. J Pharmacol Exp Ther. 262:1–9, 1992 [PubMed] [Google Scholar]
- 36.Pawar M, Kumar P, Sunkaraneni S, Sirohi S, Walker EA, Yoburn BC. Opioid agonist efficacy predicts the magnitude of tolerance and the regulation of mu-opioid receptors and dynamin-2. Eur J Pharmacol. 563:92–101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson SJ: The rat brain, in stereotaxic coordinates. 2nd edition, Academic Press, Sydney, 2005. [Google Scholar]
- 38.Richards P, Riff D, Kelen R, Stern W, MoxDuo Study T. Analgesic and adverse effects of a fixed-ratio morphine-oxycodone combination (MoxDuo) in the treatment of postoperative pain. J Opioid Manag. 7:217–228, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Romero A, Miranda HF, Puig MM. Analysis of the opioid-opioid combinations according to the nociceptive stimulus in mice. Pharmacol Res. 61:511–518, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Romero A, Miranda HF, Puig MM. Antinociceptive effects of morphine, fentanyl, tramadol and their combination, in morphine-tolerant mice. Pharmacol Biochem Behav. 97:363–369, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Ross FB, Wallis SC, Smith MT. Co-administration of sub-antinociceptive doses of oxycodone and morphine produces marked antinociceptive synergy with reduced CNS side-effects in rats. Pain. 84:421–428, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Simpson DM, Messina J, Xie F, Hale M. Fentanyl buccal tablet for the relief of breakthrough pain in opioid-tolerant adult patients with chronic neuropathic pain: a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 29:588–601, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Sirohi S, Dighe SV, Madia PA, Yoburn BC. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J Pharmacol Exp Ther. 330:513–519, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slatkin NE. Opioid switching and rotation in primary care: implementation and clinical utility. Curr Med Res Opin. 25:2133–2150, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Tortorici V, Robbins CS, Morgan MM. Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behav Neurosci. 113:833–839, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Traynor JR, Nahorski SR. Modulation by mu-opioid agonists of guanosine-5’-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 47:848–854, 1995 [PubMed] [Google Scholar]
- 47.Vasudevan A, Snowman CE, Sundar S, Sarge TW, Hess PE. Intrathecal morphine reduces breakthrough pain during labour epidural analgesia. Br J Anaesth. 98:241–245, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 23:737–746, 1999 [DOI] [PubMed] [Google Scholar]