Abstract
Implantable devices have been developed for continuous monitoring of heart failure. We investigated the effect of fluids and hemodynamic monitoring, using these devices, on heart failure clinical outcomes. Literature search was performed January 2000 through May 2017 of studies comparing device monitored patients with control group. Random-effects meta-analysis was used to pool outcomes across the studies. A total of 5,454 patients were included from 14 studies. There was no difference in heart failure (HF)-related admissions rate [odds ratio (OR) 1.25, 95% CI: 0.92–1.69, P=0.15], all-cause mortality (OR 1.21, 95% CI: 0.91–1.61, P=0.20) or combined admission rate and all-cause mortality (OR 1.21, 95% CI: 0.89–1.64, P=0.22) between the device monitored and the control group. In a subgroup analysis including only pressure sensors devices, there was no difference in all-cause mortality (OR 1.04, 95% CI: 0.62–1.74, P=0.89), however, there was a lower admissions rate (OR 1.63, 95% CI: 1.10–2.41, P=0.02). In a subgroup of only impedance monitoring devices, there was no difference in all-cause mortality or admissions rate. Pressure monitoring was associated with lower HF admissions rate. No improvement in these outcomes was noted with impedance monitoring.
Keywords: Heart failure (HF), device monitoring, mortality, hospital admissions rate
Introduction
In heart failure (HF) patients, hemodynamic abnormalities precede hospitalization by several weeks. The identification of these abnormalities could, in theory, allow alterations in therapy and prevention of clinical deterioration. Different non-invasive models including home monitoring of vital signs, medications management, and phone education have been tried with variable results. Large prospective studies showed no improvement in mortality or admissions rate with non-invasive home monitoring (1-4).
Invasive monitoring is a concept to evaluate hemodynamic changes that precede clinical decompensation. In general, two methods are available. One includes measurement of alterations in intrathoracic impedance, a parameter available in current defibrillators, and a second method includes alterations in recorded intracardiac pressures including left atrial (LA), right ventricular (RV) or pulmonary artery (PA) pressures.
The impedance or hemodynamic information can help in therapeutic measures to prevent readmission and minimize unneeded healthcare cost.
In this manuscript, we sought to systematically review the literature and study the impact of such strategy on HF outcome.
Methods
Data collection and analysis was performed following the recommended procedures by the Cochrane collaboration and was reported in accordance with recommendations set forth by the Preferred Reported Items for Systematic Reviews and Meta-analyses (PRISMA) statement (5).
Study endpoints
Study endpoints included: HF related admissions rate, all-cause mortality and combined HF related admissions rate and all-cause mortality.
Devices description
Internal cardioverter defibrillators (ICDs), implanted in HF patients, can monitor changes in intrathoracic impedance which correlate inversely with LV filling pressure (Medtronic Opti-Vol® Fluid Status Monitoring or St Jude Medical CorVueTM) and are referred to as impedance devices in this article. These devices sense an increase in fluids status and can alert the patient or physician using various reporting methods (6-15).
All of these studies took advantage of impedance technology in ICDs to monitor fluid-status in HF patients except one, which depended on ICD monitoring of patient activity, selected cardiac arrhythmias and amount of biventricular pacing to adjust HF treatment (11).
The devices which were used to monitor cardiac pressures are referred to as pressure sensors in this article. The LA sensors (HeartPod®) can be implanted in the atrial septum and give accurate evaluation of pressure changes (16). Sensors in the RV can directly monitor its hemodynamics (Chronicle device) (17). PA pressure can be continuously evaluated by CardioMems (18). One study used a combined ICD lead with hemodynamic monitoring properties (IHM-ICD) (19).
Information sources and literature search methods
Literature search was conducted through the electronic databases MEDLINE, EMBASE, PubMed and the Cochrane Central Register of Controlled Trials (CENTRAL) from January 2000 through May 2017 for abstracts using various combinations of terms including “invasive HF monitoring, prevention of HF readmission, CardioMems, Chronicle and heart failure, intrathoracic impedance and Optivol, Optivol and heart failure, heart failure monitoring with Optivol, CoreVue and heart failure, impedance and heart failure monitoring and implanted monitoring devices of heart failure”.
We included randomized clinical trials, observational studies and double armed studies. Inclusion criteria were published data that showed the clinical outcomes we chose. Study population had to be 18 years or older. We included studies that compared outcomes between two groups: a group with invasive implanted devices as a guidance of therapy and the other group used conventional medical therapy (controls). So, the control group received standard HF therapy and did not utilize the device information even if the device was implanted before.
Exclusion criteria included unpublished data, single armed studies, studies performed on patients during hospitalization only or if the patient’s age is younger than 18 years. We excluded all studies without clinical outcome or without the outcome of our interest (mortality and HF-related hospitalization).
Two reviewers (A Halawa and T Enezate) identified articles eligible for further review. If a study met the inclusion criteria, the manuscript was obtained and reviewed. In addition, bibliographic references, of identified studies and review articles, were reviewed to identify randomized clinical trials that did not show on electronic search.
Reviewers focused on demographics/baseline characteristics, study design, device used, sample size, duration and aim of each study and type of endpoint measures including HF related admissions, all-cause mortality and combined HF related admissions and all-cause mortality.
The second author verified all the extracted data. The number of events in each trial was obtained when available. Table 1 depicts study designs and patients’ characteristics.
Table 1. Summary of studies design and patients’ baseline characteristics.
| Author/study | Year | Design | Duration | Device used | Age (years) | Male, % | NYHA class | EF, % | Group | Number |
|---|---|---|---|---|---|---|---|---|---|---|
| Abraham (18) | 2001 | RCT | 6 months | Cardio MEMS | 62 | 73 | I–III | Mixed | Control | 280 |
| Device | 270 | |||||||||
| Adamson (19) | 2011 | RCT | 12 months | RVP sensor | 55 | 69 | II–III | <35 | Control | 198 |
| Device | 202 | |||||||||
| Bourge (17) | 2008 | RCT | 6 months | Chronicle | 58 | 65 | III–IV | Mixed | Control | 140 |
| Device | 134 | |||||||||
| Jermyn (20) | 2017 | Case-series | 15 months | Cardio MEMS | N/A | 68 | III | Mixed | Control | 32 |
| Device | 34 | |||||||||
| Böhm (8) | 2016 | RCT | 23 months | ICD-OptiVol | 66 | 80 | II–III | <35 | Control | 497 |
| Device | 505 | |||||||||
| Boriani (9) | 2017 | RCT | 24 months | ICD-OptiVol | 66 | 76 | III–IV | <35 | Control | 428 |
| Device | 437 | |||||||||
| Catanzariti (6) | 2009 | Prospective observation | 12 months | InSync Sentry | 66 | 84 | II–III | <35 | Control | 102 |
| Device | 430 | |||||||||
| Domenichini (10) | 2016 | RCT | 12 months | ICD-OptiVol | 68 | 94 | II–III | <35 | Control | 39 |
| Device | 41 | |||||||||
| Hindricks (11) | 2014 | RCT | 12 months | ICD-OptiVol | 66 | 81 | II–III | <35 | Control | 331 |
| Device | 333 | |||||||||
| Landolina (12) | 2012 | RCT | 16 months | ICD-OptiVol | 68 | 79 | I–III | <35 | Control | 101 |
| Device | 99 | |||||||||
| Lüthje (13) | 2015 | RCT | 15 months | ICD-OptiVol | 66 | 77 | II–III | <40 | Control | 89 |
| Device | 87 | |||||||||
| Maines (7) | 2007 | Case control | 12 months | InSync Sentry | 70 | 85 | II–III | <35 | Control | 27 |
| Device | 27 | |||||||||
| Shochat (15) | 2016 | RCT (in press) | 12 months | lung impedance | N/A | N/A | N/A | <35 | Control | 128 |
| Device | 128 | |||||||||
| van Veldhuisen (14) | 2011 | RCT | 15 months | InSync Sentry | 64 | 86 | II–III | <35 | Control | 167 |
| Device | 168 |
EF, ejection fraction; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association; RCT, randomized controlled trial; RVP, right ventricular pressure; N/A, not available; Mixed, preserved and reduced ejection fraction.
Studies identification
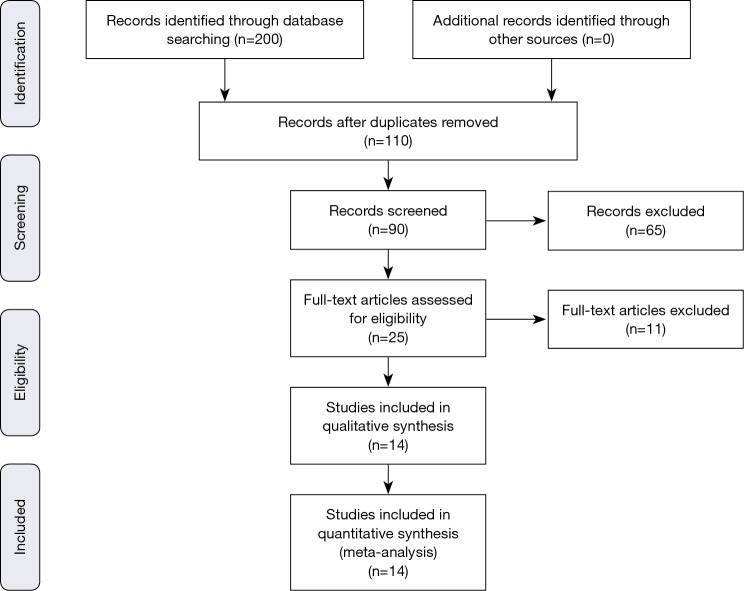
The previously described sources were searched for potential studies. The search was limited to English-language literature. The initial search identified 200 citations. One hundred and ten citations were excluded by identifying abstract/title. The final search identified 14 original papers that fulfilled the inclusion criteria (Figure S1) (6-15,17-20).
Figure S1.
PRISMA data flow chart depicting the results of the primary search and selection process of the studies that met inclusion criteria.
Risk of bias assessment and data quality
Methodological quality was defined as the control of bias assessed through reported methods in each study using Cochrane risk of bias tool (21) and Newcastle-Ottawa Scale (NOS) (22) to evaluate the quality of randomized and observational cohort trials.
This tool tests for bias and classifies its risk to low, intermediate and high (21). The reviewers (A Halawa and T Enezate) independently assessed each study quality using the risk of bias tool components. Most of the randomized trials were single or non-blinded, two studies had lost follow-up in >20% of its population, otherwise, there was no evidence of high risk bias in regards to population selection, randomization, concealment allocation, groups comparability, adequate follow up or attrition biases (Tables S1,S2). Overall, a Funnel plot test showed a symmetrical distribution of the studies indicating low risk of publication bias (Figure S2).
Table S1. Risk bias assessment for randomized controlled trials.
| Study ID | Study design | Adequate randomization | Allocation concealment | Blinding | Baseline characteristics balanced | Lost to follow-up <20% | Incomplete data (attrition bias) |
|---|---|---|---|---|---|---|---|
| Abraham (18) | RCT | Yes | Yes | Single | Yes | Yes | No |
| Bourge (17) | RCT | Yes | Yes | Single | Yes | Yes | No |
| van Veldhuisen (14) | RCT | Yes | Yes | Single | Yes | Yes | No |
| Adamson (19) | RCT | Yes | Yes | Single | Yes | Yes | No |
| Böhm (8) | RCT | Yes | Yes | None | Yes | Yes | No |
| Boriani (9) | RCT | Yes | Yes | Double | Yes | No | No |
| Domenichini (10) | RCT | Yes | Yes | None | Yes | Yes | No |
| Hindricks (11) | RCT | Yes | Yes | Single | Yes | Yes | No |
| Landolina (12) | RCT | Yes | Yes | Double | Yes | Yes | No |
| Lüthje (13) | RCT | Yes | Yes | – | Yes | Yes | No |
| Shochat (15) | RCT | Yes | Yes | Yes | Yes | No | No |
RCT, randomized controlled trial.
Table S2. Risk bias assessment for observational studies.
| Study ID | Study design | Selection | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort | Comparability | Ascertainment of exposure | Demonstration that outcome of interest was Not present at start of study | Assessment of outcome | Enough follow-up length | |||
| Catanzariti (6) | Prospective | Truly representative | Multi-center | Secured records/office visits | Yes | Independent assessment | Yes | |
| Maines (7) | Case-control | Truly representative | Single center | Secured records | Yes | Independent assessment | Yes | |
| Jermyn (20) | Case-series | Truly representative | Single center | Secured records/phone calls | Yes | Independent assessment | Yes | |
Figure S2.
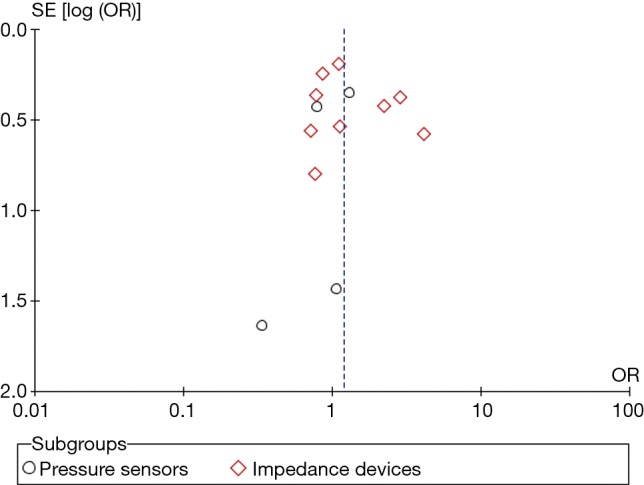
Funnel plot showing symmetrical distribution of the studies and low risk of publication bias. SE, standard error; OR, odds ratio.
Statistical analysis and data synthesis
From the abstracted data, we calculated the odds ratio (OR) using the inverse variance method for each study outcome to allow for pooling of similar outcomes. The average effects for outcomes and 95% confidence intervals (CIs) were obtained using a random effects model, as described by DerSimonian (21,23).
To assess heterogeneity of treatment effect among trials, we used the I2 statistic. The I2 statistic represents the proportion of heterogeneity of treatment effect across trials that were not attributable to chance or random error. A value of 50% or more reflects significant heterogeneity due to real differences in study populations, protocols, interventions and outcomes (23).
The P value threshold for statistical significance was set at 0.05 for effect sizes. Analyses were conducted using features on RevMan version 5.3.5 (The Nordic Cochrane Center, Copenhagen, Denmark).
Methods for including both-armed zero events
In the case of zero events for an outcome in both groups simultaneously, we utilized a continuity factor of 1. We added this to each arm in order to avoid computational errors. Studies reporting no outcomes were not included in the analysis (24).
Results
A total of 5,454 patients were included from 14 studies, 3 observational (1 case-control, 1 case-series and 1 prospective study) and 11 randomized controlled trials. The device group included a total of 2,895 patients (640 in pressure sensor subgroup and 2,255 in the impedance monitoring group) while the control group included a total of 2,559 patients (650 in pressure sensor subgroup and 1,909 in the impedance monitoring subgroup).
Mean age was 64.6 years and 78% of studied patients were male. The majority of patients in the impedance monitoring group had HF with reduced ejection fraction (HFrEF). The pressure sensor group included HFrEF and preserved ejection fraction (HFpEF) except one study where the majority of patients has HFrEF (20).
The control group received standard HF therapy and were matched with the device monitored patients. Both device and control groups were comparable in terms of demographics, cause and type of HF (ischemic vs. non-ischemic, HFrEF vs. HFpEF), comorbidities, and New York Heart Association functional class which was II–III. Difference in HF medications regimen were highlighted too. Only one study had higher diuretic use in the control group (17). Follow-up period varied between 6–24 months (Table 1) (6-15,17-20).
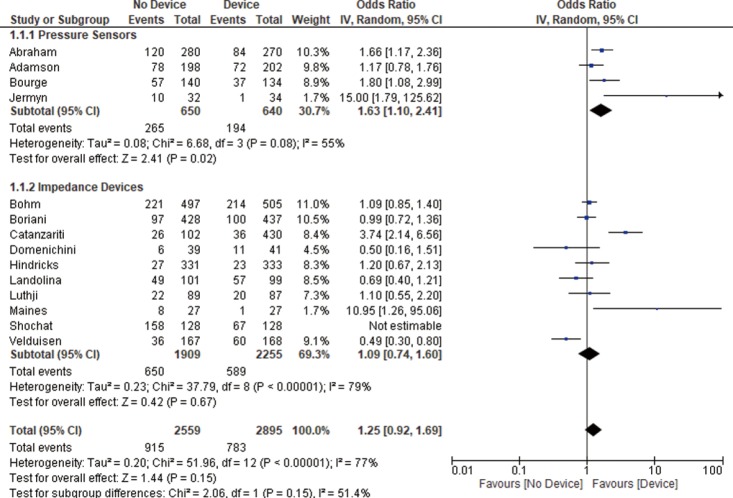
All studies reported HF related admissions rate. We found no significant difference between the device and the control groups in terms of admissions rate (OR 1.25, 95% CI: 0.92–1.69, P=0.15, Figure 1).
Figure 1.
HF related readmission rate. The measure of effect of device versus control group on readmission rate of each study was plotted using OR and 95% CI on a forest plot. The overall results showing no statistically significant difference between both groups. However, pressure sensors group was associated with lower HF related readmission rates. HF, heart failure; OR, odds ratio; CI, confidence interval.
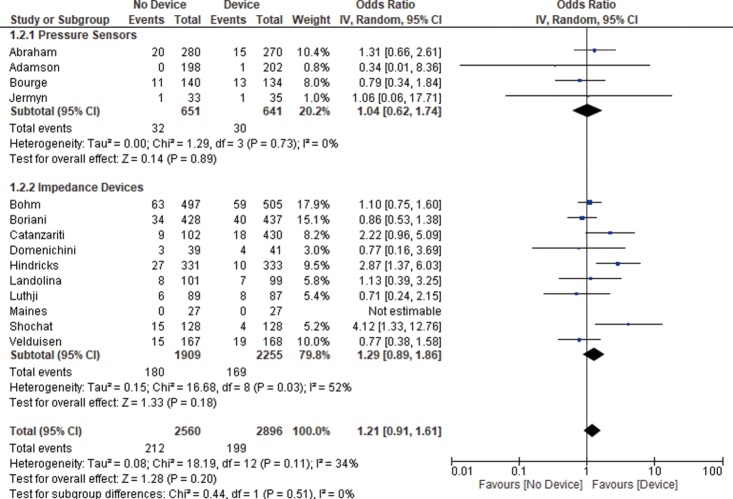
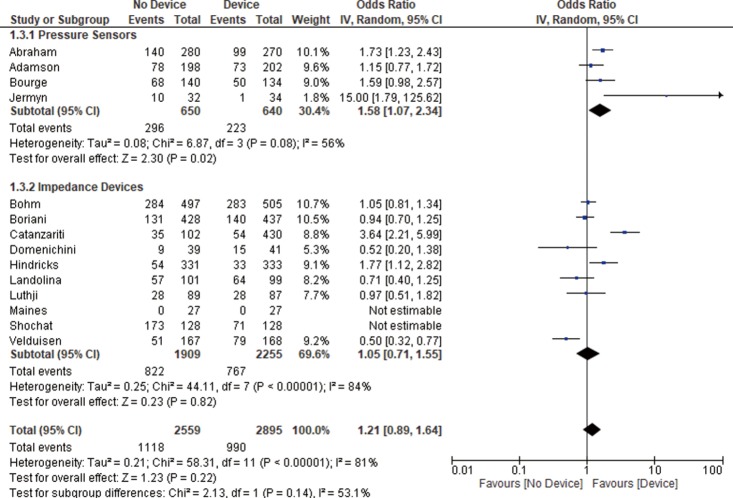
Thirteen studies reported all-cause mortality and combined HF related admission and all-cause mortality. We found no significant difference between the device group and the control group in terms of all-cause mortality (OR 1.21, 95% CI: 0.91–1.61, P=0.20, Figure 2) nor the combined admissions rate and all-cause mortality (OR 1.21, 95% CI: 0.89–1.64, P=0.22, Figure 3) at the end of the follow up period.
Figure 2.
All-cause mortality. The measure of effect of device versus control group on all-cause mortality rate of each study was plotted using OR and 95% CI on a forest plot. The overall results and subgroup analysis showing no statistically significant difference between both groups. OR, odds ratio; CI, confidence interval.
Figure 3.
Combined HF related readmission and all-cause death. The measure of effect of device versus control group on combined HF related readmission and all-cause mortality rate of each study was plotted using OR and 95% CI on a forest plot. The overall results showing no statistically significant difference between both groups. However, pressure sensors group associated with lower combined HF related readmission rates and all-cause mortality. HF, heart failure; OR, odds ratio; CI, confidence interval.
Subgroup analysis
Pressure sensor devices were used in four studies (total of 1,290 patients: 640 patients in the device group and 650 patients in the control group). Subgroup analysis showed that monitoring with pressure sensor was associated with lower HF admissions rate (OR 1.63, 95% CI: 1.10–2.41, P=0.02, Figure 1) and lower combined HF admissions rate and all-cause mortality (OR 1.58, 95% CI: 1.07–2.34, P=0.02, Figure 3). However, there was no difference in all-cause mortality (OR 1.04, 95% CI: 0.62–1.74, P=0.89, Figure 2).
Impedance monitoring devices were used in eleven studies (total of 4,164 patients: 2,255 in the device group and 1,909 in the control group). Subgroup analysis showed that monitoring with these devices was not associated with different HF admissions rate (OR 1.09, 95% CI: 0.74–1.60, P=0.67, Figure 1) nor all-cause mortality (OR 1.29, 95% CI: 0.89–1.86, P=0.18, Figure 2) or combined HF admissions rate and all-cause mortality (OR 1.05, 95% CI: 0.71–1.55, P=0.82, Figure 3).
Discussion
Our goal in this paper is to shed a light on new approach in monitoring heart failure treatment. The complexity of HF comes not only from the multiple non-cardiac comorbidities but also from the physiological and anatomical changes that proceed and continue during heart failure exacerbations. All of these factors make monitoring HF a challenging field that require further advancements.
In general, our paper shows that usage of intra-cardiac devices isn’t linked to improving rates of HF admission or all-cause mortality. The failure could be blamed on the progressive nature of this disease despite optimal medical management and maximum monitoring. it is interesting that pressure monitoring was associated with lower HF related admissions rate. In our opinion, these are very important findings despite the lack of mortality benefits.
When we combined HF related admissions rate and all-cause mortality together, pressure sensing monitoring was associated with better outcome than the control group. We think the combined outcome improvement is mostly related to the significant improvement in re-hospitalizations rate. This observation was not noted in the impedance monitoring group. As matter of fact, impedance monitoring was not associated with any improvement in mortality nor HF hospitalizations rate in our analysis.
Our results could be explained by the fact that intra-cardiac filling pressure values are part of the mechanism of decompensated HF and they precede the symptoms sometimes by weeks (4,25). This time gap between the hemodynamic changes and the development of clinically recognizable symptoms, if identified correctly, may serve as an opportunity for physicians to intervene and prevent clinical decompensation.
Decreased intrathoracic impedance is fairly sensitive (in about 76% of cases) in detecting fluids overload (26). Thoracic impedance changes can be recognized later in the course of heart failure exacerbation which makes it a less effective tool in predicting this clinical event.
Lack of specificity is a problem in impedance devices and it could alter its effect on HF outcome. For example, respiratory infections (viral or bacterial) can increase the intra-thoracic content of fluids and drop impedance similar to decompensated HF.
And while data from intracardiac pressures are recorded on daily basis, impedance monitors are recorded periodically or with incidence of symptoms. This fact gives the daily recorded pressure sensors a significant clinical advantage over impedance sensors.
Unfortunately, HF is a systemic disease usually associated with multiple comorbidities. Lack of adherence to medications, poor discharge planning, inadequate follow up and absence of social and financial support are major factors that might have effects on overall admission rate and negative overall results in dissociation with cardiac pathophysiology. These factors are outcome changers and they should also be considered in any future evaluation of the intra-cardiac devices (2,27).
Finally, the lower readmission rate observed in pressure sensors group are very attractive and can help to decrease the substantial cost burden and morbidity in this sick population. Direct left atrium sensors, a different style of pressure sensors, have been developed. These pressures sensors showed promising results in advanced heart failure patients (16). At least one prospective, randomized, controlled trial will hopefully address this method of heart failure monitoring (28).
Limitations
Our analysis included a mixture of observational and randomized controlled single blinded studies. Double blinding was not feasible as patients in the experimental arm had to download data or report an alarm to the health provider. We included a variety of implantable devices with different acting mechanisms and wide range of monitoring parameter targets. Impedance monitoring is an additional manufacturing property of ICDs. In these patients, the devices were implanted for prevention of sudden cardiac death not for impedance monitoring purpose. This could bias the data collection and interpretation.
Follow up varied from one study to another (6–15 months). Endpoints were not the same in all studies and the definition of events varied from one to another.
Finally, the pressure sensor devices were tested in HFpEF and HFrEF patients, while the impedance devices were mostly used in HFrEF patients (i.e., those who required ICD).
Conclusions
Implantable cardiac monitoring devices were not associated with significant effect on readmission or mortality rates in heart failure patients. However, intra-cardiac pressure monitoring was associated with lower re-admissions rate. No benefits were observed with impedance monitoring. Further clinical trials are required to further define the benefits and roles of these devices in HF management and to improve the treatment guiding algorithms and performance.
Acknowledgments
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jaarsma T, van der Wal MH, Lesman-Leegte I, et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch Intern Med 2008;168:316-24. 10.1001/archinternmed.2007.83 [DOI] [PubMed] [Google Scholar]
- 2.Desai AS. Home Monitoring Heart Failure Care Does Not Improve Patient Outcomes: Looking Beyond Telephone-Based Disease Management. Circulation 2012;125:828-36. 10.1161/CIRCULATIONAHA.111.031179 [DOI] [PubMed] [Google Scholar]
- 3.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med 2010;363:2301-9. 10.1056/NEJMoa1010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong MK, Romano PS, Edgington S, et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients With Heart Failure: The Better Effectiveness After Transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med 2016;176:310-8. 10.1001/jamainternmed.2015.7712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. 2011. Available online: https://training.cochrane.org/handbook
- 6.Catanzariti D, Lunati M, Landolina M, et al. Monitoring intrathoracic impedance with an implantable defibrillator reduces hospitalizations in patients with heart failure. Pacing Clin Electrophysiol 2009;32:363-70. 10.1111/j.1540-8159.2008.02245.x [DOI] [PubMed] [Google Scholar]
- 7.Maines M, Catanzariti D, Cemin C, et al. Usefulness of intrathoracic fluids accumulation monitoring with an implantable biventricular defibrillator in reducing hospitalizations in patients with heart failure: A case-control study. J Interv Card Electrophysiol 2007;19:201-7. 10.1007/s10840-007-9155-4 [DOI] [PubMed] [Google Scholar]
- 8.Böhm M, Drexler H, Oswald H, et al. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J 2016;37:3154-63. 10.1093/eurheartj/ehw099 [DOI] [PubMed] [Google Scholar]
- 9.Boriani G, Da Costa A, Quesada A, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail 2017;19:416-25. 10.1002/ejhf.626 [DOI] [PubMed] [Google Scholar]
- 10.Domenichini G, Rahneva T, Diab IG, et al. The lung impedance monitoring in treatment of chronic heart failure (the LIMIT-CHF study). Europace 2016;18:428-35. 10.1093/europace/euv293 [DOI] [PubMed] [Google Scholar]
- 11.Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): A randomised controlled trial. Lancet 2014;384:583-90. 10.1016/S0140-6736(14)61176-4 [DOI] [PubMed] [Google Scholar]
- 12.Landolina M, Perego GB, Lunati M, et al. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: The evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012;125:2985-92. 10.1161/CIRCULATIONAHA.111.088971 [DOI] [PubMed] [Google Scholar]
- 13.Lüthje L, Vollmann D, Seegers J, et al. A randomized study of remote monitoring and fluid monitoring for the management of patients with implanted cardiac arrhythmia devices. Europace 2015;17:1276-81. 10.1093/europace/euv039 [DOI] [PubMed] [Google Scholar]
- 14.van Veldhuisen DJ, Braunschweig F, Conraads V, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 2011;124:1719-26. 10.1161/CIRCULATIONAHA.111.043042 [DOI] [PubMed] [Google Scholar]
- 15.Shochat MK, Shotan A, Blondheim DS, et al. Non-Invasive Lung IMPEDANCE-Guided Preemptive Treatment in Chronic Heart Failure Patients: A Randomized Controlled Trial (IMPEDANCE-HF Trial). J Card Fail 2016;22:713-22. 10.1016/j.cardfail.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 16.Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation 2010;121:1086-95. 10.1161/CIRCULATIONAHA.108.800490 [DOI] [PubMed] [Google Scholar]
- 17.Bourge RC, Abraham WT, Adamson PB, et al. Randomized Controlled Trial of an Implantable Continuous Hemodynamic Monitor in Patients With Advanced Heart Failure. The COMPASS-HF Study. J Am Coll Cardiol 2008;51:1073-9. 10.1016/j.jacc.2007.10.061 [DOI] [PubMed] [Google Scholar]
- 18.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011;377:658-66. 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 19.Adamson PB, Gold MR, Bennett T, et al. Continuous Hemodynamic Monitoring in Patients With Mild to Moderate Heart Failure: Results of the Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients With Chronic Heart Failure (REDUCEhf) Trial. Congest Heart Fail 2011;17:248-54. 10.1111/j.1751-7133.2011.00247.x [DOI] [PubMed] [Google Scholar]
- 20.Jermyn R, Alam A, Kvasic J, et al. Hemodynamic-guided heart-failure management using a wireless implantable sensor: Infrastructure, methods, and results in a community heart failure disease-management program. Clin Cardiol 2017;40:170-6. 10.1002/clc.22643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells G, Shea B, O’Connell D, et al. The Newcasete-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. 2012. Available online: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf
- 23.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2012. doi: 10.2307/632432 Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [DOI]
- 24.Cheng J, Pullenayegum E, Marshall JK, et al. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: a simulation study. BMJ Open 2016;6:e010983. 10.1136/bmjopen-2015-010983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soga Y, Ando K, Arita T, et al. Efficacy of fluid assessment based on intrathoracic impedance monitoring in patients with systolic heart failure. Circ J 2011;75:129-34. 10.1253/circj.CJ-10-0730 [DOI] [PubMed] [Google Scholar]
- 26.Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005;112:841-8. 10.1161/CIRCULATIONAHA.104.492207 [DOI] [PubMed] [Google Scholar]
- 27.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations After Heart Failure Diagnosis. A Community Perspective. J Am Coll Cardiol 2009;54:1695-702. 10.1016/j.jacc.2009.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurer MS, Adamson PB, Costanzo MR, et al. Rationale and design of the left atrial pressure monitoring to optimize heart failure therapy study (LAPTOP-HF). J Card Fail 2015;21:479-88. 10.1016/j.cardfail.2015.04.012 [DOI] [PubMed] [Google Scholar]