Abstract
The aim of this narrative review was to determine effects of whole body vibration exercises (WBVE) on the pelvic floor muscle (PFM) of healthy and unhealthy individuals. Searches were performed in the databases PubMed, Scopus, Science Direct and PEDRo. The level of evidence and methodological quality of the selected papers were determined. It was included six studies with a total of 189 participants (95.76% women) with ages ranging from 18 to 68 years. It was reported that WBVE: (I) improves the PFMs strength and quality of life (QOL) in individuals with urinary incontinence; (II) does not cause (PFM) fatigue in nulliparous continent women; (III) leads to higher (PFM) activation in subjects with weakened (PFM) and achieves higher pelvic floor (PF) activation than maximum voluntary contraction alone; (IV) in an individual with postprostatectomy stress urinary incontinence (SUI), over a period of 6 weeks after starting treatment, the patient regained continence (usage of 1 safety pad) and (V) has a significant effect on the electromyographic response and additionality and the rating of perceived exertion (RPE) significantly increased with increased frequency of the mechanical vibration. Relevant findings are presented and demonstrated that the WBVE might be highly relevant to the management of clinical disorders of the (PFM). Nevertheless, this intervention must be more understood and known to be used in the management of individuals with impairment of the (PFM) and there is the necessity of more research in this area.
Keywords: Vibratory platform (VP), vibration, urinary incontinence, micturition disorder, exercise
Introduction
The pelvic floor muscles (PFM) have typical striated fibers, with specific functions and characteristic location. The composition of the PFM is about 70% of muscle fibers type I (slow contraction) and 30% of type II (rapid contraction). Among these functions, it is possible to consider the role of PFM: (I) in the reproduction; (II) in the sexuality; (III) in the urinary and fecal continences; and (IV) in holding the pelvic organs. PFM are characterized by desirable synchronous and harmonic contractions and prolonged tension, with exception for the functions of micturition and defecation (1-3).
When the PFM exhibits weakness, in general, its ability in supporting the pelvic organs decreases and, in consequence, the contraction of the anus, urethra weakens. In addition, in the women, the same occurs with the vagina (3).
Regarding to the weakness of the PFM, the pelvic organs and the PFM are relaxed and the proper control of the muscles is committed. In consequence, unpleasant and undesirable clinical disorders can be observed that are related to the pelvic floor (PF), such as pelvic organ prolapse, stress urinary incontinence (SUI), overactive bladder, overactive detrusor, micturition disorder, urinary tract infection, and fecal incontinence (3). Considering these disorders associated with the committed PFM, a decrease of the quality of life (QOL) is also verified. This would be due to emotional impairments, as depression or a sense of alienation by inducing psychological anxiety, shame, and tension. Consequently, it is verified in the individuals with these disorders a decrease of the social activities and relationship in various levels (4).
As it is expected, the clinical interventions related to the SUI and other clinical disorders related to the weakened PFM have as main target to improve the strength and power of the muscles. In consequence, the used interventions would be planned to permit that the PFM be quickly and intensively contracted when they are requested. For the enhancement of the PFM strength and power, biofeedback and electrical stimulation have been used (3).
In addition, some kinds of exercises have been used as other modality of intervention that could improve the proper biological characteristics of the PFM (5). It is also relevant to consider the anatomic location of the PFM. It is in a deep part of the body. This fact contributes to a poor direct visualization of the PFM, and leads to the lack of tactile sense and proprioception information (3). Therefore, PFM training needs alternative, feasible and suitable interventions, as the whole-body vibration exercise (WBVE) (6). There are several important biological responses due to the WBV exercise that might be considered in the interventions to the improvement of the desirable conditions involving the PFM, as the increase of the strength and power of the muscles (7). Increases in muscle activity during WBV are presumably due to tonic vibration reflex responses and increased motor unit recruitment, induced by changes in length of the muscle spindles (8).
WBV would cause a change in muscle length through vibration, and the excitement of muscle spindle recognizing this is transferred to the spinal cord through sensory nerves, and then leads to the muscle contraction by triggering the activity of α-motor neuron (9). Based on such a mechanism, WBV has been reported as an effective training method for muscle enhancement (10).
The muscular activation due to the WBVE should vary according to the biomechanical parameters, such as, the frequency, amplitude, peak-to-peak displacement and acceleration peak. Moreover, the loading, the posture, the work time and rest time involving the exposition to the mechanical vibration generated in the vibratory platform (VP) must be also considered (11) in a WBV intervention.
The type of VP that produces the mechanical vibration would be also discussed, and it was described that side-alternating mechanical vibration (side alternating VP) caused greater muscle activation than vertical mechanical vibration. A synchronous and a triplanar VP produce vertical mechanical vibration (12). Authors have reported that augmented amplitude and loading increase muscle activation (3). It was shown that augmented frequency increase muscle activation, whereas another study reported that muscle activation was increased by resonance when the resonance frequency was close to the natural frequency of muscle, regardless of the augmentation of frequency (13). It was shown that the neuromuscular activation increased with flexing of the knee (14).
Clinical interventions with WBV have been proven effective in the enhancement of muscle strength in individuals with various diseases, such as chronic back pain, Parkinson’s disease, multiple sclerosis, stroke, cerebral palsy and SUI. Moreover, improvement of the leg muscle strength of postmenopausal women or in several populations of elderlies has been also reported (15).
The rating of perceived exertion (RPE) has been improved due to the WBVE in population with chronic stroke (16), in healthy individuals during applied simultaneously squats (7), in breast cancer survivors (17) and in older adults (18). Individuals with PFM have also presented an increase of the RPE (3).
Some authors (19) reported that the WBVE improves the QOL in individuals with metabolic syndrome. Other authors (6) have also reported that WBVE improve QOL of individuals with SUI or impairment of some functions of the PFM.
WBV intervention, as a possible mode of exercise training, to be used to the management of the clinical disorders of the PF, is based in important findings that have been reported. In addition, there is scientific basis, although scarce, for hypothesizing about beneficial effects of WBVE also on the PFM in continent as well as incontinent subjects (1,6).
Although it might be suggested that WBV could have beneficial effects on individuals with PF disorders, there is not a revision about this subject; therefore, the aim of this narrative review was to determine effects of WBV on the symptoms related to the PF disorders and to provide information on the more suitable vibration exercise regimens that may improve health in this population group.
Methods
Search methods for identification of studies
Searches were performed using the keywords: (I) “Whole Body vibration” and “Urinary incontinence”; (II) “Whole Body vibration” and “Pelvic floor”; (III) “Whole Body Vibration”; (IV) “Urinary Incontinence”; (V) “Whole Body vibration” and “exercise”; (VI) “Urinary incontinence” and “physiotherapy”; (VII) “Urinary incontinence” and “treatment”. PubMed, Scopus and PEDRo online databases were accessed for research on July 9th 2018. Each identified abstract was independently evaluated by three authors. If at least one of the authors considered one reference eligible, the full text was obtained for complete assessment. All studies were independently abstracted by three of the authors and disagreements were resolved by consensus.
Data collection and analysis
The authors extracted descriptive and outcome data from the included studies. The search strategy was based on the Population, Interventions, Comparator, Outcomes, and Study Designs (PICOS) (20) that was used to frame inclusion and exclusion criteria for studies. Aspects of the population (sample size, age and sex, control and intervention groups) and of the intervention (type of the VP, number of bouts, amplitude, peak-to-peak displacement, presence of supervisor, frequency, and duration of each session) were considered. The follow-up (if the patients included were analyzed) and loss to follow-up (if there was a loss in the sample); the outcome measures; and the design of the study were also pointed out.
Inclusion criteria (eligibility criteria)
Articles, independent of the year of the publication, were included for review if they met the search criteria and described a study using WBV generated by an VP in individuals with disorders of the PF or to promote improvement of the PF in healthy individuals.
Exclusion criteria
The papers were removed due to: (I) be replies, editorials, letters or abstracts; (II) be conducted with animal, or with other techniques than WBVE, or with combined techniques, or with occupational approach; and (III) be based on a different disease or short communications; and (IV) be in a language different of English.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) presentation
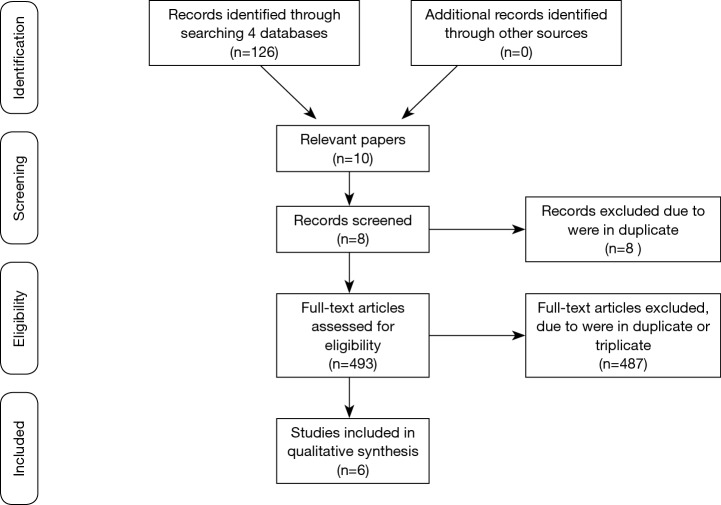
A flowchart following the PRISMA recommendations (21) (http://prisma-statement.org/) was done to show the steps in the selection of the publications analyzed in this narrative review (Figure 1). In view of the methodological quality of the selected publications, it was decided to consider this study a narrative review.
Figure 1.
Flowchart.
Level of evidence and methodological quality of the selected papers
The level of evidence of each selected publication was individually assessed by using the National Health and Medical Research Council hierarchy of evidence (22). The level of evidence was defined as follows: (I) I, the systematic review of level II studies; (II) II, the RCT; (III) III-1, the pseudo-randomized controlled trial (alternate allocation, as a crossover study or some other similar method); (IV) III-2, the comparative study with concurrent controls (non-randomized experimental trial, cohort study, case-control study, interrupted time series with a control group); (V) III-3, the comparative study without concurrent control (historical control, two or more single arm study, interrupted time series without a parallel control group; and (VI) IV, the case series with either post-test or pre-test/post-test outcomes. Each manuscript was assigned to a one reviewer, cross-checked by a second reviewer and if there were disagreement a third party was consulted and the issue discussed until consensus was reached. Moreover, methodological quality of the selected clinical trials studies was determined by the PEDRo scale (http://www.pedro.org.au/english/downloads/pedro-scale/). PEDRo scale consists of eleven items. One item on the PEDRo scale (eligibility criteria) is related external validity and is generally not used to calculate the method score, leaving a score ranging of 0 to 10. Those selected articles with a score of seven or greater in the PEDRo scale were considered of ‘high’ methodological quality, those with a score of five to six would be of ‘fair’ quality and a score of four or below were classified as ‘poor’ quality.
Results
Our searches identified 501 potentially relevant studies (Figure 1) in the various databanks that were searched. Eight were removed because they were in duplicate. Considering the exclusion criteria 487 were removed. This resulted in six studies with a total of 189 participants (95.76% women) and the age ranging between 18 and 68 years to be analyzed. Two studies with healthy individuals and four with individuals with some PF dysfunction.
Table 1 shows the characteristics of the studies and participants, the main outcomes, the level of evidence and the methodological quality of the selected publications. Following the level of evidence (22), of the six articles included, two (1,6) were in the level II (RCT), one (23) at the level III-1, two (3,24) in the level III-2 and one (25) at level IV. Following the PEDRo scale of the clinical trials, one (6) work was considered of “high” quality, one (1) was “fair” and one (23) was “poor” quality.
Table 1. Selected studies with the sample size, study design, the age of the participants, the aim, assessment, findings, the level of evidence and the methodological quality.
| Studies | Sample size and population | Study design | Age (years old) | Aims | Assessment | Findings | LE | MQ |
|---|---|---|---|---|---|---|---|---|
| Crevenna et al., 2016 | n=1 (male) with SUI after radical prostatectomy | Case report | 55 | To present a new form of WBV therapy on a therapeutic bed to treat the disabling and isolating symptom “incontinence” | The urine loss with pads/day | The patient regained continence within a time of 6 weeks after starting WBV therapy with an Evocell® device. The urine loss almost stopped completely | IV | Not applicable |
| Lee et al., 2016 | N=13 (7 males, 6 females healthy adults) | Cross-sectional study | Unclear | To investigate the EMG response of PFM to WBV while using different body posture and vibration frequencies | EMG activity PFM was recorded using an anal probe and the RPE was assessed with a modified Borg scale | The vibration frequency, body posture, and muscle stimulated had a significant effect on the EMG response. The PFM had high activation at 12 and 26 Hz (P<0.05) | III-2 | Not applicable |
| Farzinmehr et al., 2015 | 43 women with SUI | Randomized clinical trial | 36–68 | To determine whether WBV training is effective at improving PFM strength | PFM strength was assessed based on the Oxford Scale; quality of life by the I-QOL and the severity of incontinence by VAS | WBV training was effective in PFM strength similar to PFMT, reduced the severity of incontinence and increased I-QOL score. Significant differences were found in each group pre and post intervention (P=0.0001) | II | High |
| Stania et al., 2015 | 33 nulliparous continent women | Randomized clinical trial | 20–24 | To evaluate bioelectrical activity of the PFM during synchronous low and high-intensity WBV | Pelvic floor sEMG activity was recorded using a small diameter vaginal probe | A comparison of mean normalized amplitudes between 30, 60 and 90 s trials did not reveal significant differences in any on the groups | II | Fair |
| Luginbuehl et al., 2012 | 27 women (8 weeks to 1-year postpartum) and 23 women nulliparas or >1-year postpartum) | Randomized cross-over trial | 18–45 | To determine the optimal SR-WBV load modality regarding PFM activity in order to complete the SR-WBV training methodology for future PFMT with SR-WBV | The PFM activity were calculated for both SR-WBV modalities together (time effect) and for both SR-WBV modalities separately (modality-time interaction) | As there is no SR-WBV modality dependent difference regarding PFM activity, the continuous modality is recommended in clinical practice as it is easier to apply and less time consuming | III-1 | Poor |
| Lauper et al., 2009 | 23 healthy controls and 26 post-partum women with PFM weakness | Cross- sectional study |
18–40 | To determine if two different WBV, SV and SRV, using various intensities lead to a reactive activation of PFM | The PFM activity was measured by the EMG | Both WBV procedures were able to activate PFM significantly depending on vibration intensity. The SRV achieved a significantly higher activation than maximum voluntary contraction, especially in women post partum (6–12 Hz) | III-2 | Not applicable |
LE, level of evidence; MQ, methodological quality; SUI, stress urinary incontinence; WBV, whole body vibration; EMG, electromyogram; PFM, pelvic floor muscle; RPE, rating of perceived exertion; I-QOL, incontinence quality of life questionnaire; VAS, visual analog scale; PFMT, pelvic floor muscle training; sEMG, surface EMG; SR-WBV, stochastic whole body vibration; SV, sinusoidal vibration; SRV, stochastic resonance vibration.
Several outcomes were assessed: (I) RPE (3); (II) PF surface electromyography (EMG) activity (1,3,23,24); (III) PFM strength based on modified Oxford grading (MOG), by digital palpation of a physical therapist (6); (IV) the severity of their SUI using visual analog scale (VAS) (6); (V) the QOL by the incontinence QOL (I-QOL) (6); (VI) SUI post-prostatectomy by the use of safety pad (25).
Regarding the main findings, based on the results, the Table 1 showed: single whole-body vibrations in nulliparous continent women does not cause PFM fatigue (1); improving PFMs strength and QOL in individuals with urinary incontinence (6); sinusoidal vibration (SV) compared to stochastic resonance (SR) vibration, leads to higher PFM activation in subjects with weakened PFMs and achieves higher PF activation than maximum voluntary contraction alone (24); no SR-WBV modality dependent difference regarding PFM activity, the continuous modality is recommended in clinical practice as it is easier to apply and less time consuming (23); in an individual with postprostatectomy SUI, over a period of 6 weeks after starting treatment, the patient regained continence (usage of 1 safety pad) (25) and that vibration frequency, body posture, and muscle stimulated had a significant effect on the EMG response and additionality, the RPE significantly increased with increased frequency of the mechanical vibration (19).
The WBV protocols (biomechanical parameters) are shown in Table 2, as the frequency and amplitude or peak to peak displacement; the position of the individual in the base of the platform, and a description of the protocols used (working time, number of the sessions or rest time). The type of the platform used was clearly defined in the articles, synchronous (1,23,25), triplanar (6) and side-alternating (24,26), VP.
Table 2. Description of the WBV protocols (biomechanical parameters).
| Studies | Type of the VP | F (Hz) | A/PPD (mm) | Position | Sessions/sets | Sets/time series/rest between series |
|---|---|---|---|---|---|---|
| Crevenna et al., 2016 | Synchronous (Evocell) | 20–26 | – | Supine and changed from passive to active (performing pelvic floor exercises) treatment | 2×/week–6 week | 2 times a week for a period of 6 weeks |
| Lee et al., 2016 | Side-alternating (Galileo® Advanced Plus, Novotec Medical GmbH, Germany) | 6, 12, 18, and 26 | 3 (A) | Knee flexion angles of 20°, 30°, and 40° | 3× of 15 sec | 1 min of rest between each 15 sec |
| Farzinmehr et al., 2015 | Triplanar (Power plate, USA) | 30–50 | 2.5–5 | The PFMT: static contractions of PFM associated with static contraction of hip adductors, gluteal and abdominal muscles in supine, sitting, standing, crook lying, bridging, mini-squatting, squatting positions | 3 days/week–4 weeks | 3–4 set with 15–20 repetitions and a 60 sec pause between set |
| Stania et al., 2015 | Synchronous (Fitvibe 600, Gyma Uniphy N.V.) | Group I: 20; group II: 40 | Group I: 2; group II: 4 | Standing with their knees and hips joints flexed to 35° | Three exercises static (30, 60 and 90 s) were performed in randomized order | 3 set of 30, 60 and 90 s with 60 sec pause between set |
| Luginbuehl et al., 2012 | Synchronous (SRT-Zeptor, Frei Swiss, Zurich) | 10–12 | 4 | Slightly bent knees and neutral hip position | A single set and over three sets | Continuous load: 3 sets of 60 and 60 s rest between the three sets. Intermittent load: 3 sets of 12×5 s and 10 sec rest in between and an additional rest of 60 sec between the 3 sets |
| Lauper et al., 2009 | Galileo 9001 (REMEDA GmbH, Horgen, Switzerland) (SV) and Zeptor med1 (Idiag AG, Fehraltorf, Switzerland) (SRV) | SR-WBV: 2, 4, 6, 8, 10, 12; SV: 5, 15, 25 | 2–4 | Standing on the floor or on the OVP with slightly bent knees and neutral hip position | Two tests in a week | SRV and SV at two test occasions within 1 week |
VP, vibratory platform; f, frequency; A, amplitude; PPD, peak-to-peak displacement; WBV, whole body vibration; PFMT, pelvic floor muscle training; SR WBV, stochastic whole body vibration; SV, sinusoidal vibration; SRV, stochastic resonance vibration.
Considering the biomechanical parameters, the range of the frequency of the mechanical vibrations varied from 12 up to 35 Hz; and the amplitude from 2 up to 4 mm. In general, the patients stood standing at the base of the VP, but the flexion of the knees varied from 20 up 40 degrees. Nevertheless, the study performed by Crevenna added a novel form of synchronous high-intensity whole body vibration therapy by means of an Evocell® device. Vibrations of 20–26 Hz were applied by a specific treatment couch (170 cm × 82 cm × 66 cm, Evocell®) with an adjustable frequency ranging from 15 to 30 Hz.
Discussion
The PFM are associated with the maintenance of important biological responses (27). However, the weakness of these muscles leads to unpleasant and undesirable clinical disorders that committed a person QOL (28). Some interventions have been used to the management of these disorders of the PFM (2,10,29) but these techniques are not simple and need a convenient and private place to be carried out (6). It is important to consider that, in our knowledge, this is the first review about the use of this suitable, non-invasive and simple intervention involving the use of the WBVE to the management of the disorders of PFM. In general and considering the clinical practice, the current revision highlights the relevance of a non-invasive intervention: (I) to the management of the PFM disorders; and (II) as prevention strategy targeting modifiable risk factors to the PFM disorders that should be incorporated into clinical practice before the absence of symptomatology.
The search of alternative and suitable procedures to the muscle strengthening and to convenient management without impairment of the PFM is desirable, as the WBVE. Following these consideration, it was reported that WBV: (I) in nulliparous continent women does not cause PFM fatigue (1); (II) in individuals with urinary incontinence occurs improvement of the PFM (6); (III) in subjects with weakened PFM occurs muscle activation in the PFM (24); (IV) in an individual with postprostatectomy SUI, over 6 weeks after starting treatment, the patient regained continence (25); and (V) in healthy individuals occurs a significant effect on the EMG response (3) of WBV suitable for PFM. Moreover, Lauper, in healthy women and post-partum women, reported that stochastic resonance vibration caused greater muscle activation than SV. Putting together these findings, it is relevant to consider the approach of authors that suggest the organization of the PF as a myofascial unit with synergic and antagonistic activity of muscular bundles. Furthermore, it would be necessary to point out that, they are more or less interlaced, with multiple functions and not only the function of pelvic cup closure. Probably, as described by Cardinale and Bosco, the transmission of mechanical vibrations to the whole body stimulates the primary endings of the muscle spindles, which in turn activates α-motor neurons leading to muscle contractions. WBV also would increase the synchronization of motor units (8) and this increased synchronization of motor units signifies that more muscle fibers are contracted at once and hence more force can be produced. Probably, these phenomena would occur in the PFM and aid to understand the findings reported by several authors (1,3,25) in the different studied populations. Moreover, the reduction of the severity of incontinence due to the WBVE (6) would be also justified.
These considerations are also in agreement with authors (30) that evaluated the resting and functional activity of the PFM depending on the orientation of pelvis. They reported that posterior pelvic tilt position determines higher resting and functional bioelectric activity of PFM.
Considering the effect of the WBVE in the QOL, authors (6) also showed the beneficial effects of WBV in improving the QOL in individuals with urinary incontinence in four-week treatment period and after three months follow up. This finding is in agreement with authors that used a conservative treatment in the form of muscle training in the PF to the management of SUI and they observed an improvement of the QOL of the individuals. An improvement in the QOL due to the exposition to the WBVE was also observed in populations with metabolic syndrome (19) and COPD (31).
The improvement of the RPE in individuals with disorder of the PFM was described by Lee et al. This finding could be related with the increase of the synchronization of motor units due to the WBVE (3). Moreover, enhanced muscular performance could improve also the risk factors of fall, has been shown among older adults (18).
According to the position to perform the WBV, only one (25) of the six studies described the supine position. Generally, the authors reported the standing position on the floor or on the base of the VP. However, authors (32) described a supine position seems to be the most suitable for assessing the function and mechanism of PFM, as the most conducive to PFM relaxation, when they compared the resting and functional bioelectrical activities of PFM depending on three different positions of the lower limbs in the supine position. For theses authors, the higher EMG activity of the PFM occurred in the horizontal position and when standing with the ankles in the dorsiflexion position than when standing with the ankles in the plantar flexion position. This consideration about the position of the individual is very relevant and more studies are necessary to try to identify the best condition to the WBVE to be performed in individuals with PFM disorders.
In the selected papers about the effects of the WBVE in the PFM, the types of the VP used in the studies were synchronous (1,23,25) triplanar (6) and side-alternating (3,24). The frequency of the mechanical vibrations varied from 12 up to 35 Hz; and the amplitude from 2 up to 4 mm. In general, the patients stood standing at the base of the platform, but the flexion of the knees varied from 20 up 40 degrees. Nevertheless, a study (28) added a novel form of synchronous high-intensity whole body vibration therapy by means of an Evocell® device. Vibrations of 20–26 Hz were applied by a specific treatment couch (170 cm × 82 cm × 66 cm, Evocell®) with an adjustable frequency ranging from 15 to 30 Hz.
Naturally, this narrative review has some limitations that must be considered. One of the limitations is that is not possible to draw certain strong conclusion based on a small number of studies (only six publications) that evaluated the intervention with WBVE, in two types of populations, healthy and unhealthy. Moreover, the results are very hard to summarize, since these few studies have several types of design (one case study with one patient, two randomized clinical trials, two non-randomized studies, and one cross over study). Considering the LE, two studies have the level II (RCT), one at the level III-1 and two in the level III-2. About the methodological quality, only one was considered of “high” quality. Another aspect is the variability of outcomes (EMG, QOL, symptoms of urinary incontinence, PFM strength). Further, it is difficult to prove the effects of whole body vibration on outcomes, because differences exist in the WBV parameters and clinical conditions of the individuals in the studies. In addition, relevant studies published in other languages other than English may be missed; and there may be publication bias, due to the greater possibility of publication of studies with favorable intervention results. In view of the methodological quality of almost all the selected publications, it was decided to consider this study a narrative review.
Conclusions
Relevant findings are presented and demonstrated that the WBVE could be appropriated to the management of PFM. Moreover, the insight to use WBVE for treatment of PFM disorders is highly relevant. The portability, safety, and effectiveness suggest that WBVE could be an alternative to traditional exercise-based training. Nevertheless, this intervention must be more understood and known to be used in the management of individuals with impairment of the PFM and there is a need of more research in this area.
Acknowledgments
The authors thank the Brazilian Agencies (CNPq and FAPERJ) for the support.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Stania M, Chmielewska D, Kwaśna K, et al. Bioelectrical activity of the pelvic floor muscles during synchronous whole-body vibration – a randomized controlled study. BMC Urol 2015;24;15:107. [DOI] [PMC free article] [PubMed]
- 2.Bourcier AP, Bonde B, Haab F. Functional assessment of pelvic floor muscles. In: Appell RA, Bourcier AP, La Torre F. Pelvic floor dysfunction - Investigations & Conservative Treatment. Rome: Casa Editrice Scientifica Internazionale, 1991:97-106. [Google Scholar]
- 3.Lee J, Lee K, Song C. Determining the Posture and Vibration Frequency that Maximize Pelvic Floor Muscle Activity During Whole-Body Vibration. Med Sci Monit 2016;22:4030-6. 10.12659/MSM.898011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ptak M, Brodowska A, Ciećwież S, et al. Quality of Life in Women with Stage 1 Stress Urinary Incontinence after Application of Conservative Treatment-A Randomized Trial. Int J Environ Res Public Health 2017;14;14-6. 10.3390/ijerph14060577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akulenko LV, Kasyan GR, Kozlova YO, et al. Female pelvic floor dysfunction from the perspectives of genetic studies. Urologiia 2017;1:76-81. 10.18565/urol.2017.1.76-81 [DOI] [PubMed] [Google Scholar]
- 6.Farzinmehr A, Moezy A, Koohpayehzadeh J, et al. A Comparative Study of Whole Body Vibration Training and Pelvic Floor Muscle Training on Women's Stress Urinary Incontinence: Three- Month Follow- Up. J Family Reprod Health 2015;9:147. [PMC free article] [PubMed] [Google Scholar]
- 7.Marín PJ, García Rioja J, Bernardo-Filho M, et al. Effects of Different Magnitudes of Whole-Body Vibration on Dynamic Squatting Performance. J Strength Cond Res 2015;29:2881-7. 10.1519/JSC.0000000000000940 [DOI] [PubMed] [Google Scholar]
- 8.Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev 2003;31:3-7. 10.1097/00003677-200301000-00002 [DOI] [PubMed] [Google Scholar]
- 9.Pollock RD, Woledge RC, Martin FC, et al. Effects of whole body vibration on motor unit recruitment and threshold. J Appl Physiol 2012;112:388-95. 10.1152/japplphysiol.01223.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SY, Son WM, Kwon OS. Effects of whole body vibration training on body composition, skeletal muscle strength, and cardiovascular health. J Exerc Rehabil 2015;11:289-95. 10.12965/jer.150254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rauch F, Sievanen H, Boonen S, et al. International Society of Musculoskeletal and Neuronal Interactions. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact 2010;10:193-8. [PubMed] [Google Scholar]
- 12.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol 2010;108:877-904. 10.1007/s00421-009-1303-3 [DOI] [PubMed] [Google Scholar]
- 13.Di Giminiani R, Masedu F, Tihanyi J, et al. The interaction between body position and vibration frequency on acute response to whole body vibration. J Electromyogr Kinesiol 2013;23:245-51. 10.1016/j.jelekin.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 14.Abercromby AF, Amonette WE, Layne CS. Variation in neuromuscular responses during acute whole-body vibration exercise. Med Sci Sports Exerc 2007;39:1642-50. 10.1249/mss.0b013e318093f551 [DOI] [PubMed] [Google Scholar]
- 15.Sharififar S, Coronado RA, Romero S, et al. The Effects of Whole Body Vibration on Mobility and Balance in Parkinson Disease: a Systematic Review. Iran J Med Sci 2014;39:318-26. [PMC free article] [PubMed] [Google Scholar]
- 16.Liao LR, Ng GY, Jones AY, et al. Cardiovascular stress induced by whole-body vibration exercise in individuals with chronic stroke. Phys Ther 2015;95:966. 10.2522/ptj.20140295 [DOI] [PubMed] [Google Scholar]
- 17.Van Ruymbeke B, Boone J, Coorevits P, et al. Whole-body vibration in breast cancer survivors: a pilot study exploring its effects on muscle activity and subjectively perceived exertion. Int J Rehabil Res 2014;37:371. 10.1097/MRR.0000000000000072 [DOI] [PubMed] [Google Scholar]
- 18.Marín PJ, Santos-Lozano A, Santin-Medeiros F, et al. Whole-body vibration increases upper and lower body muscle activity in older adults: potential use of vibration accessories. J Electromyogr Kinesiol 2012;22:456. 10.1016/j.jelekin.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 19.Carvalho-Lima RP, Sá-Caputo DC, Moreira-Marconi E, et al. Quality of life of patients with metabolic syndrome is improved after whole body vibration exercises. Afr J Tradit Complement Altern Med 2017;14:59. 10.21010/ajtcam.v14i4S.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costantino G, Montano N, Casazza G. When should we change our clinical practice based on the results of a clinical study? Searching for evidence: PICOS and PubMed. Intern Emerg Med 2015;10:525. 10.1007/s11739-015-1225-5 [DOI] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med 2009;6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Health and Medical Research Council (NHMRC) additional levels of evidence and grades for recommendations for developers of guidelines. Available online: http://www.nhmrc.gov.au/file. Accessed in 02/16/2017.
- 23.Luginbuehl H, Lehmann C, Gerber R, et al. Continuous versus intermittent stochastic resonance whole body vibration and its effect on pelvic floor muscle activity. Neurourol Urodyn 2012;31:683. 10.1002/nau.21251 [DOI] [PubMed] [Google Scholar]
- 24.Lauper M, Kuhn A, Gerber R, et al. Pelvic floor stimulation: what are the good vibrations? Neurourol Urodyn 2009;28:405-10. 10.1002/nau.20669 [DOI] [PubMed] [Google Scholar]
- 25.Crevenna R, Cenik F, Margreiter M, et al. Whole body vibration therapy on a treatment bed as additional means to treat postprostatectomy urinary incontinence. Wien Med Wochenschr 2017;167:139-41. 10.1007/s10354-016-0469-7 [DOI] [PubMed] [Google Scholar]
- 26.Soligo M, Livio S, De Ponti E, et al. Pelvic floor assessment after delivery: how should women be selected? Eur J Obstet Gynecol Reprod Biol 2016;206:153-7. 10.1016/j.ejogrb.2016.09.021 [DOI] [PubMed] [Google Scholar]
- 27.Thor KB, de Groat WC. Neural control of the female urethral and anal rhabdosphincters and pelvic floor muscles. Am J Physiol Regul Integr Comp Physiol 2010;299:R416. 10.1152/ajpregu.00111.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Özdemır ÖÇ, Bakar Y, Özengın N, et al. The effect of parity on pelvic floor muscle strength and quality of life in women with urinary incontinence: a cross sectional study. J Phys Ther Sci 2015;27:2133. 10.1589/jpts.27.2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazi T, Takahashi S, Ismail S, et al. Prevention of pelvic floor disorders: international urogynecological association research and development committee opinion. Int Urogynecol J 2016;27:1785. 10.1007/s00192-016-2993-9 [DOI] [PubMed] [Google Scholar]
- 30.Ptaszkowski K, Zdrojowy R, Slupska L, et al. Assessment of bioelectrical activity of pelvic floor muscles depending on the orientation of the pelvis in menopausal women with symptoms of stress urinary incontinence: continued observational study. Eur J Phys Rehabil Med 2017;53:564-74. [DOI] [PubMed] [Google Scholar]
- 31.Furness T, Joseph C, Naughton G, et al. Benefits of whole-body vibration to people with COPD: a community-based efficacy trial. BMC Pulm Med 2014;14;38. 10.1186/1471-2466-14-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro Brazález B, Torres Lacomba M, de la Villa P, et al. The evaluation of pelvic floor muscle strength in women with pelvic floor dysfunction: A reliability and correlation study. Neurourol Urodyn 2018;37:269-77. 10.1002/nau.23287 [DOI] [PubMed] [Google Scholar]