Abstract
Background
It remains challenging to determine which individuals are likely to benefit from microsurgical correction of subclinical varicocele, as basic semen parameters often do not improve postoperatively. We aimed to develop an easily accessible tool for prognostic stratification of infertile men indicated for microsurgical correction of bilateral subclinical varicocele characterized by prolonged and clear venous reflux and no other cause for infertility.
Methods
We retrospectively analyzed the testicular biopsy, seminal analysis, and ultrasound evaluation records of 20 men managed between 2006 and 2014. Subclinical varicocele was diagnosed through bilateral testicular palpation and auscultation of venous reflux using a Doppler stethoscope, with confirmation on color Doppler sonography. We conducted receiver operating characteristic curve analysis to identify the optimal combinations of cut-offs for the Johnsen score, Copenhagen index, and testicular volume defining histological patterns with positive prognostic value for improved postoperatively reproductive capacity.
Results
Positive prognostic value was noted for the following combinations of parameters: (I) Johnsen score >8.2 in the left testicle and right testicular volume >12.8 mL predicted improved sperm concentration; (II) Johnsen score >8.2 and Copenhagen index digit II <2.5 bilaterally predicted improved total sperm motility; (III) Johnsen score >9.1 and Copenhagen index digit III <1.5 bilaterally predicted improved progressive sperm motility; (IV) Johnsen score >7.9 and right testicular volume >13.6 mL predicted improved sperm morphology.
Conclusions
Johnsen score and Copenhagen index as histopathological prognostic factors can be easily obtained upon evaluation of testicular biopsy specimens and can be simple and reliable tool to establish a more realistic prognosis for reproductive capacity in men who undergo microsurgical correction of subclinical varicocele with no other detectable cause for infertility.
Keywords: Testicular biopsy, subclinical varicocele, seminal parameters, microsurgery, male infertility
Introduction
Involuntary childlessness is becoming an increasingly prevalent issue worldwide and especially in industrialized societies, affecting 13–18% of couples of reproductive age, regardless of social, religious, or cultural background (1,2). Infertility represents the end-result of multifactorial conditions in the reproductive organs and/or gametes, as well as the interaction of such conditions with the environment and lifestyle habits. Sex-specific factors, genital infections, endocrine abnormalities, immunological factors, anatomical factors, azoospermia (obstructive or non-obstructive), and concomitant presence of varicocele or other factors such as tobacco, marijuana, and alcohol abuse are well-known stressors associated with infertility. Nevertheless, the effect of such stressors can be traced down to genetic, epigenetic, and molecular causes contributing to the biological failure of reproduction (3). Dilation of the pampiniform plexus remains the most common identifiable cause of male infertility, affecting 40–45% of infertile or subfertile men (4). The classification system currently used to evaluate abnormalities of the pampiniform plexus is over 50 years old and, although the definition of varicocele includes the presence of venous reflux, it does not take into account the type of vein dilation, reflexive time (seconds) and if the reflux is continuous or interrupted with Valsalva maneuver (5).
Subclinical varicoceles are neither palpable nor suspected on physical examination, but can be detected using accessory equipment such as a Doppler stethoscope and diagnosed using radiological techniques and/or color Doppler ultrasound. Mild vein dilation on Doppler ultrasound should not preferentially the solely criterion for diagnosing subclinical varicocele. Mild vein dilation with no detectable reflux may represent a false positive result, which creates a challenging clinical situation with no clear choice for treatment strategy and no clear parameters for post-operative follow-up. The usefulness of surgical correction of subclinical varicocele remains a matter of debate, with different approaches followed in different countries (6,7). Current guidelines established by the European Association of Urology and American Society of Reproductive Medicine do not recommend fixing subclinical varicoceles (8,9). Nevertheless, several studies reported that correcting subclinical right varicocele is beneficial in patients with clinical left varicocele (10-12).
While the exact clinical relevance of nonpalpable varicoceles is widely debated, varicocele is recognized as a cause of testicular dysfunction such as decrease in Leydig cell function, with progressive decline in serum testosterone levels and damage to the seminiferous epithelium, resulting in abnormal spermatogenesis and increased cellular apoptosis (13,14). In fact, in a series of 128 patients with subclinical varicocele followed up for 5 years without surgery, many patients demonstrated up to 44% decrease in total sperm motility and up to 21% increase in the proportion of immature sperm cells in the ejaculate; high intracellular levels of creatine-kinase activity indicated that such cells did not undergo extrusion of the cytoplasmic droplets, which typically occurs in the final steps of spermiogenesis (3,15). Therefore, the impact of subclinical varicocele may be reflected in the properties of the ejaculate.
Testicular biopsy was first used clinically in the 1940s by Charny (16) and rapidly became recognized as a very valuable tool in the study of testicular abnormalities (17). For many decades, testicular biopsy was the gold-standard method to evaluate testicular function, and continues to provide valuable information in specific situations such as predicting sperm retrieval in non-obstructive azoospermia (18). Currently, preoperative testicular biopsy is avoided if possible, as this procedure may cause temporary inflammation and compromise small foci of residual spermatogenesis in patients with non-obstructive azoospermia (19). However, there are many studies concerning the safety of testicular biopsy among men with varicoceles (20-22). Marmar and Bennof reported the safety and efficacy of ultrasonically guided, single stick, percutaneous aspiration technique (21), while Cocuzza et al. described a subinguinal approach to be performed during microsurgical varicocelectomy that may be an alternative to traditional biopsy because it avoids scrotal violation (22).
Furthermore, histopathological evaluation of the testis is a difficult and time-consuming task due to the organization complexity of testicular tissue reflecting the multiple and intricate steps of spermatogenesis, particularly in eutherian mammals (23). The first step in addressing abnormal spermatogenesis is to understand the characteristics of the normal testis. Current clinical practice employs various methods for quantifying the histological features of the testis. Among all, the Johnsen and Copenhagen criteria (24,25) aims to account for morphological responses to different pathologies affecting testicular cells and can be applied to objectively and easily categorize different patterns of blood reflux to the pampiniform plexus. This type of evaluation sheds light on the role of abnormal physiology in male subfertility even when there is minimal or no immediate clear clinical alterations, such as in men presenting with subclinical varicoceles with peak retrograde flow >29 cm/s (26).
In this study, we aimed to develop an easily accessible tool for prognostic stratification of infertile men indicated for microsurgical correction of bilateral subclinical varicocele characterized by prolonged, continuous venous reflux and no other detectable cause for infertility.
Methods
Study population
In this retrospective study, we analyzed the testicular biopsy patterns of 20 infertile men aged 19–50 years who, upon exclusion of other possible causes for the inability to conceive, underwent microsurgical correction of subclinical bilateral varicocele characterized by prolonged continuous reflux upon Valsalva maneuver after standing upright for 3–5 minutes at a room temperature of 22–23 °C. The surgical technique used for the correction of varicoceles was described by Marmar and Kim (27) with a systematic use of an intraoperative Doppler ultrasound to improve identification and preservation of testicular blood supply (28). For the biopsy procedure, exposing the testis through the same subinguinal incision as for the varicocele repair using a microsurgical technique, proved to be minimally invasive, as described by Cocuzza et al. (22).
Details of physical examination of male genitalia, histological examination of testes biopsy specimens, and seminal analysis, were judiciously reviewed. Due to the nature of the study, we applied extensive exclusion criteria to minimize the influence of possible confounders factors and included: history of mumps orchitis, cryptorchidism, hypospadias, inguinal hernia, chronic sinusitis/bronchitis or allergy requiring the use of corticosteroids; obstructive or non-obstructive azoospermia; tobacco smoking; moderate/severe alcohol intake; history of drug use including anabolic steroids, marijuana, cocaine, or synthetic drugs; chronic use of any prescription medication including antidepressants, corticosteroids, non-steroidal anti-inflammatories, and testosterone replacement therapy. The study was approved by the Research Ethics Committee of the School of Medicine, University of São Paulo (approval No. 047/12). Each participant signed an informed consent form for having their data collected and analyzed for research purposes. This research was conducted in accordance with the ethical guidelines set out in the Declaration of Helsinki (29).
Systematic clinical examination of the genitalia was performed by the same expert andrologist (JH) and included physical evaluation of the testicles, vas deferens, epididymis, scrotum, and penis. Testicular volumes were measured using a Prader orchidometer (30). To exclude clinical varicocele, all patients were examined in a warm room (22–23 °C), in both standing and supine position, with and without Valsalva maneuver. Diagnosis of subclinical varicocele was carried out through bilateral testicular palpation and auscultation of venous reflux using a Doppler stethoscope, with confirmation on color Doppler sonography. In addition, testicular volume was calculated according to the ellipsoid equation (width × thickness × length × 0.52), using dedicated software running on an ultrasonographic console (GE Logic 9; General Electrics Healthcare Technologies, Milwaukee, USA) equipped with a 12-MHz linear probe.
Semen analysis
Each patient provided two semen samples (at an interval of 15 days) via masturbation after 48–72 hours of sexual abstinence. Samples were collected in sterile containers and allowed to liquefy at 37 °C for 30 minutes. Then, all macroscopic and microscopic parameters were measured manually by the same investigator (JRP), according to the 2010 World Health Organization guideline (31). Sperm concentration and motility were checked under a microscope with a 20× positive phase contrast objective and an overall magnification of 200×. The cell counts on each slide were evaluated manually to determine the number of spermatozoa per field equivalent to 1 mL, and the final result was expressed as the approximate sperm concentration (×106 spermatozoa/mL of semen). Sperm motility was determined by systematic analysis of at least five microscopic fields, so that 200 spermatozoa were classified into four categories: A, rapid progressive motility; B, slow or sluggish progressive motility; C, non-progressive motility; and D, no motility. Progressive motility was defined as the sum of categories (A + B). Sperm morphology was evaluated according to World Health Organization criteria (31).
Histopathology
Testicular fragments obtained during microsurgical correction of varicocele were sent to the pathology laboratory fixed in Bouin’s solution for at least 30 minutes and then submerged in formaldehyde and embedded in paraffin. A microtome was used to obtain 4-µm thick slices, which were mounted onto appropriate glass microscope slides for analysis. Hematoxylin-eosin staining was used to facilitate adequate visualization of the spermatogenic cells. On microscopic evaluation, a sample was considered satisfactory if at least 30 seminiferous tubules were visible for cell counting.
To obtain the Johnsen score, slides were examined under an optical microscope (magnification, ×100). A score was assigned for each tubule counted. The number of tubules with a given score was multiplied by the score. The result was summed across different scores and then divided by the number of evaluated tubules, giving the final Johnsen score. The Copenhagen index was obtained by evaluating 10–30 seminiferous tubules (32) and expressed as a compound index of four digits, according to the histological pattern found in the tubules. Thorough evaluation was carried out to identify spermatogenic cells in various stages and forms, namely Sertoli cells, spermatogonia, primary and secondary spermatocytes, round and elongated spermatids, and sperm.
Receiver operating characteristic (ROC) curve analysis was conducted to identify testicular histological patterns with positive prognostic value for improved reproductive capacity after microsurgical correction of varicocele. For various cut-offs of the Johnsen score, Copenhagen index, and testicular volume, we calculated the sensitivity and specificity for predicting semen analysis parameters. The optimal cut-off was defined as the cut-off providing the highest likelihood ratio, i.e., the ratio of true positives (sensitivity) to false positives (specificity). We used the Chi-square test for contingency analysis (counting categorical variables). In the construction of frequency distribution tables, the Pearson Z-statistic was used to assess the proportions. Data are presented as mean ± standard error. Statistical analysis was conducted using GraphPad Prism 6 and Microsoft Excel 2016. We adopted a significance level of P<0.05 for all analyzes.
Johnsen and Copenhagen criteria
For testicular biopsy evaluation, we applied the criteria formulated by Johnsen (24) and McLachlan et al. (25), which are known to have good reproducibility. The Johnsen criteria are into a ten-point scoring system for quantifying spermatogenesis according to the profile of the cells encountered along the seminiferous tubules. A Johnsen score of 10 indicates maximum spermatogenesis activity, whereas a score of 1 indicates complete absence of germ cells. The method is practical and easy to perform, providing a connection between the results of seminal analyses and those of testicular biopsies (24).
The criteria formulated by McLachlan and colleagues (25), summarized into the Copenhagen index, helps quantify the characteristics of cells in the seminiferous tubules, facilitating the incidental diagnosis of carcinoma in situ. The results are expressed as a four-digit index. The first digit relates to the maturation stage of the evaluated testis (if adult or prepubertal) or characteristics associated with cancer. The second digit represents the predominant histological pattern, reflecting the appearance of seminiferous tubules. The third digit refers to the second most prevalent histological pattern, while the fourth digit describes abnormalities and additional features. The lowest values tend to represent the best reproductive prognosis. Therefore, the Copenhagen index represents a comprehensive tool for describing histological results, focusing not on the quality of spermatogenesis but on a more detailed characterization of germ cells, allowing for a more complex interpretation of the findings. For instance, the Copenhagen index reflects the intimate correlation between testicular histology and sperm recovery success in patients with non-obstructive azoospermia (25).
Results
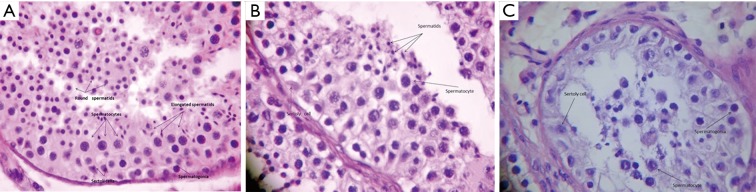
Among the 20 patients enrolled, the age was 32±7.3 years. All participants had subclinical bilateral varicocele with a mean volume of 14.3 and 14.9 mL for the right and left testicle, respectively. The frequency distribution of histological results classified according to the Copenhagen criteria (Table 1) revealed that index “1.1.1.0” had the highest prevalence (40% bilateral), followed by “1.2.2.0” (25% in the right testicle and 20% in the left testicle). Both histological results refer to a homogeneous testicular pattern, the first indicating normal spermatogenesis and the second indicating reduced number of spermatids. The frequency distribution of Johnsen scores (Table 2) revealed that scores 10, 9, and 8 had the highest prevalence among the study population, with a relative frequency of 35.0%, 30.0%, and 15.0%, respectively, in the right testis, compared to 38.9%, 33.3%, and 11.1%, respectively, in the left testis. Histological findings representative of certain combinations of Johnsen score and Copenhagen index are provided in Figure 1.
Table 1. Prevalence of testicular histological findings according to the Copenhagen criteria.
| Index | Absolute frequency | Relative frequency (%) | Absolute difference (%) | P value | |||
|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | ||||
| 1.1.1.0 | 8 | 8 | 40.0 | 40.0 | 0.0 | 1.0000 | |
| 1.2.2.0 | 5 | 4 | 25.0 | 20.0 | 5.0 | 0.7044 | |
| 1.3.3.0 | 1 | 2 | 5.0 | 10.0 | 5.0 | 0.5465 | |
| 1.5.5.0 | 1 | 1 | 5.0 | 5.0 | 0.0 | 1.0000 | |
| 1.6.3.0 | 4 | 4 | 20.0 | 20.0 | 0.0 | 1.0000 | |
| 1.7.2.0 | 1 | 1 | 5.0 | 5.0 | 0.0 | 1.0000 | |
| Total | 20 | 20 | 100.0 | 100.0 | |||
To compare the frequencies for each index, we used the Z-statistic for independent proportions (Pearson). No significant differences were noted (P<0.05). 1.1.1.0, adult testis with homogeneous aspect, normal spermatogenesis, and no abnormalities. 1.2.2.0, adult testis with homogeneous aspect, reduced number of spermatids, and no abnormalities. 1.3.3.0, adult testis with homogeneous aspect, maturation arrest at the primary spermatocyte stage, and no abnormalities. 1.5.5.0, adult testis with homogeneous aspect and characteristics indicative of Sertoli-cell-only syndrome. 1.6.3.0, adult testis with heterogeneous aspect and normal spermatogenesis or reduced number of spermatids. 1.7.2.0, adult testis with heterogeneous aspect and germ cell arrest at primary spermatocyte stage.
Table 2. Prevalence of testicular histological according to the Johnsen score.
| Score | Absolute frequency | Relative frequency (%) | Absolute difference (%) | P value | |||
|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | ||||
| 10 | 7 | 7 | 35.0 | 38.9 | 3.9 | 0.8041 | |
| 9 | 6 | 6 | 30.0 | 33.3 | 3.3 | 0.8255 | |
| 8 | 3 | 2 | 15.0 | 11.1 | 3.9 | 0.7210 | |
| 7 | 1 | 0 | 5.0 | 0.0 | 5.0 | 0.3049 | |
| 6 | 1 | 1 | 5.0 | 5.6 | 0.6 | 0.9391 | |
| 5 | 1 | 1 | 5.0 | 5.6 | 0.6 | 0.9391 | |
| 4 | 0 | 0 | 0.0 | 0.0 | 0.0 | – | |
| 3 | 0 | 0 | 0.0 | 0.0 | 0.0 | – | |
| 2 | 1 | 1 | 5.0 | 5.6 | 0.6 | 0.9391 | |
| 1 | 0 | 0 | 0.0 | 0.0 | 0.0 | – | |
| Total | 20 | 18 | 100.0 | 100.0 | |||
To compare the frequencies for each score, we used the Z-statistic for independent proportions (Pearson). No significant differences were noted (P<0.05). Score 10, complete spermatogenesis, numerous sperm cells with organized germinal epithelium. Score 9, presence of many sperm cells with disorganized germinal epithelium. Score 8, presence of few sperm cells. Score 7, no sperm cells, but numerous spermatids. Score 6, no sperm cells, with few spermatids. Score 5, no sperm cells or spermatids, but numerous spermatocytes. Score 4, few spermatocytes. Score 3, only spermatogonia. Score 2, only Sertoli cells, no germ cells. Score 1, acellular seminiferous tubules.
Figure 1.
Photomicrograph of testicular biopsy specimen exhibiting findings characteristic of (A) Copenhagen index 1.1.1.0 and Johnsen score 10 (HE, ×250), (B) Copenhagen index 1.2.2.0 and Johnsen score 9 (HE, ×400), and (C) Copenhagen index 1.3.3.0 and Johnsen score 4 (HE, ×400).
We defined testicular histological patterns as associations of cut-off values for Johnsen score, Copenhagen index, and testicular volume with postoperative improvements in semen parameters (Table 3). ROC curve analysis indicated postoperative improvement in sperm concentration was associated with the combination of Johnsen score >8.2 in the left testicle and right testicular volume >12.8 mL (P<0.05). Postoperative improvement in total sperm motility was associated with the combination of Johnsen score >8.2 (bilateral) and Copenhagen index digit II <2.5 in both testicles (P<0.05). Postoperative improvement in total progressive sperm motility was associated with the combination of Johnsen score >9.1 (bilateral) and Copenhagen index digit III <1.5 in the right testicle (P<0.05). Finally, postoperative improvement in sperm morphology was associated with the combination of Johnsen score >7.9 in the right testicle, Copenhagen index digit II <4.0 in the left one and right testicular volume >13.6 mL (P<0.05).
Table 3. Performance of the Johnsen score, Copenhagen index, and testicular volume as predictors of sperm concentration, motility, and morphology.
| Parameter | Johnsen score (right/left) | Copenhagen index, digit II (right/left) |
Copenhagen index, digit III (right/left) |
Testicular volume (right/left) |
|---|---|---|---|---|
| Sperm concentration | >7.4/>8.2 | 0/0 | 0/0 | >12.8/0 |
| Sensitivity (%) | 37.5/50.0 | 0/0 | 0/0 | 75.0/0 |
| Specificity (%) | 91.6/91.6 | 0/0 | 0/0 | 90.0/0 |
| P value | 0.05/0.04* | 0/0 | 0/0 | 0.04*/0 |
| Total sperm motility | >8.2/>8.2 | <2.5/<2.5 | <2.5/0 | >12.0/0 |
| Sensitivity (%) | 55.5/44.4 | 55.5/66.6 | 33.3/0 | 55.0/0 |
| Specificity (%) | 90.9/90.9 | 81.8/81.8 | 92.3/0 | 90.0/0 |
| P value | 0.01*/0.02* | 0.04*/0.03* | 0.06/0 | 0.07/0 |
| Progressive motility | >9.1/>9.1 | <2.5/<2.5 | <1.5/0 | >13.6/0 |
| Sensitivity (%) | 76.9/76.9 | 46.1/53.8 | 73.3/0 | 66.6/0 |
| Specificity (%) | 71.4/71.7 | 85.7/85.7 | 66.6/0 | 85.7/0 |
| P value | 0.02*/0.03* | 0.07/0.06 | 0.03*/0 | 0.06/0 |
| Morphology | >7.9/>7.0 | 0/<4.0 | 0/0 | >13.6/>11.3 |
| Sensitivity (%) | 50.0/37.0 | 0/50.0 | 0/0 | 62.5/50.0 |
| Specificity (%) | 91.6/91.6 | 0/85.7 | 0/0 | 90.9/90.9 |
| P value | 0.03*/0.08 | 0/0.03* | 0/0 | 0.03*/0.06 |
*, statistically significant (P<0.05).
Discussion
The World Health Organization estimates that approximately 800 million people have problems related to infertility (33). Among the causes of male infertility, varicocele is the most common, affecting 11.7% of fertile men and about 40% of men who fail to conceive (34). While the exact pathophysiology of varicocele remains unclear, it is suggested to develop as a mechanism to clear blood reflux and increased scrotal temperature (±2.5 °C) caused by imbalance in the counter-current heat exchange in the scrotum due to venous stasis, alterations of the hypothalamic-pituitary-testicular axis, increased levels of oxidative stress leading to peroxidation of membrane lipids and damage to sperm DNA, or reflux of kidney and adrenal metabolites (2,9,35-38).
Varicocele often causes testicular hypotrophy or atrophy and abnormalities in spermatogenesis (33,35,37). Such findings were also noted in patients enrolled in this study, all of whom had bilateral subclinical varicoceles with prolonged reflux time (>2 s) and no other possible cause for infertility. Bryniarski et al. (39) reported the incidence of varicocele at 80–95% on the left side only, at 25–45% bilaterally, and rarely on the right side. Physiologically, the left gonad is more affected due to insertion of the gonadal vein into the left renal vein at an angle of 90°, causing venous blood reflux into the plexus pampiniform in individuals with valvular defect. Nevertheless, the deleterious effects of varicocele laterality on spermatogenesis remain unclear (35,36).
While testicular biopsy remains a valuable tool for investigating male infertility in obstructive and non-obstructive azoospermia, its usefulness in cases involving infertility associated with subclinical varicocele remains largely controversial (26). In our study, testicular histology was compatible with seminal analysis because no azoospermic participant were included. Several discrepancies between clinical and histological aspects have been reported (34). Varicocele negatively affects the physiology and fertilization potential of sperm, and correction of subclinical right varicocele can improve semen analysis parameters in 56% of infertile men with palpable varicocele on the left side (10,12). While histological findings are generally nonspecific, hypoplasia of germ cells and maturation arrest are often noted (40). According to Silber and Rodriguez-Rigau (41), histological findings of maturation arrest and decreased number of spermatids are often found in testicles with normal volume and early stages of the spermatogenic process.
We propose several histological patterns positively predictive of improved sperm concentration following the correction of unquestionable subclinical varicoceles with long or very long venous blood reflux. These patterns are defined using Johnsen score and testicular volume cut-offs. Varicocele triggers testicular atrophy proportional to the severity and degree of involvement (26,40,42), which is in agreement with our present findings. While testicular volume was previously found to be a poor predictor of reproductive capacity, testicular atrophy remains the most commonly agreed upon indication for varicocele repair (43). In our study, the combination of testicular volume and histopathological parameter cut-offs was a predictor of sperm motility (total and progressive) and morphology, suggesting that taking into consideration histological characteristics may help establish a more accurate prognosis of seminal quality after surgical repair of a varicocele.
Subclinical varicocele is not palpable on physical examination, requiring ultrasound for diagnosis. Its clinical relevance remains widely debated, though several studies have reported that surgical treatment of subclinical varicocele can be beneficial. Pasqualotto and colleagues analyzed the outcomes of varicocelectomy in a group of patients with varicocele grade II or III on the left side but without contralateral varicocele, and in another group of patients with varicocele of the same degree on the left side but subclinical varicocele on the right side. Varicocelectomy effectively improved semen parameters in men with palpable and non-palpable varicocele (compared to the outcomes noted in patients with varicocele only on the left side) with increase testicular volume in both groups, though the increase was greater in the group were both sides were corrected (right subclinical varicocele) (14). Other studies have reported remarkable improvement in seminal parameters after surgical correction of subclinical varicocele and there is large evidence demonstrating significant benefits in testicular histology, regardless of the degree of varicocele (6,44). Thus, our study reinforces the hypothesis that well-selected patients presenting with subclinical varicoceles provided they have long and continuous refluxes, does have a negative impact upon male fertility potential.
Clinical varicocele can induce a stressful event that affects several testicular parameters simultaneously, as reflected on seminal analysis and sperm functional tests, in special creatine-kinase activity and reactive oxygen species (15,45). While studies focus on the abnormalities in sperm concentration, motility, and morphology, the increase in oxidative stress and DNA damage in sperm cells are clearly observed and may be associated with subclinical varicocele (45). Considering the good prognostic performance of the histopathological predictors evaluated in our study, we believe that varicocelectomy can be successful in treating male subfertility associated with subclinical varicocele in adequately selected patients for whom no other cause could be found to justify the status of male infertility (including known risk factors).
In this study, we provided cut-off values for the Johnsen score, Copenhagen index, and testicular volume defining testicular biopsy patterns predictive of improvement in the reproductive capacity of infertile men following microsurgical correction of subclinical varicoceles. Our findings should not be interpreted as promoting unrestricted indication for microsurgical correction of subclinical varicocele or the use of testicular biopsy as a standard procedure prior to surgical correction. Instead, our findings represent proof that it is possible to develop an accurate and easily accessible tool suitable in a subset of patients with no other identifiable cause of male infertility, whose diagnosis would otherwise be ruled out as idiopathic infertility. Once the decision for microsurgery has already been taken by the treating physician and the patient, such a tool can really help predict whether the patient might actually benefit from the varicocele repair. Furthermore, our findings demonstrate that widely used testicular histopathology techniques can help obtain parameters with positive predictive value for improved postoperative semen quality.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Research Ethics Committee of the School of Medicine, University of São Paulo (approval No. 047/12). Each participant signed an informed consent form for having their data collected and analyzed for research purposes. This research was conducted in accordance with the ethical guidelines set out in the Declaration of Helsinki.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324. 10.1016/j.fertnstert.2012.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athayde KS, Cocuzza M, Agarwal A, et al. Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J Androl 2007;28:613-20. 10.2164/jandrol.106.001966 [DOI] [PubMed] [Google Scholar]
- 3.Hallak J. Asymptomatic male currently not desiring fertility with bilateral subclinical varicocele found on ultrasound evaluation and borderline semen analysis results. Asian J Androl 2016;18:315-6. 10.4103/1008-682X.172645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre M, Hsieh TC, Lipshultz L. Varicocele repair in the era of modern assisted reproductive techniques. Curr Opin Urol 2012;22:517-20. 10.1097/MOU.0b013e328358e191 [DOI] [PubMed] [Google Scholar]
- 5.Dubin L, Amelar RF. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril 1970;21:606. 10.1016/S0015-0282(16)37684-1 [DOI] [PubMed] [Google Scholar]
- 6.Cantoro U, Polito M, Muzzonigro G. Reassessing the Role of Subclinical Varicocele in Infertile Men With Impaired Semen Quality: A Prospective Study. Urology 2015;85:826-30. 10.1016/j.urology.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 7.Seo JT, Kim KT, Moon MH, et al. The significance of microsurgical varicocelectomy in the treatment of subclinical varicocele. Fertil Steril 2010;93:1907-10. 10.1016/j.fertnstert.2008.12.118 [DOI] [PubMed] [Google Scholar]
- 8.Jungwirth A, Giwercman A, Tournaye H, et al. European Association of Urology Guidelines on Male Infertility: The 2012 Update. Eur Urol 2012;62:324-32. 10.1016/j.eururo.2012.04.048 [DOI] [PubMed] [Google Scholar]
- 9.Practice Committee of the American Society for Reproductive Medicine. Society for Male Reproduction and Urology Report on varicocele and infertility: a committee opinion. Fertil Steril 2014;102:1556-60. 10.1016/j.fertnstert.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 10.Pasqualotto FF, Lucon AM, de Goes PM, et al. Is it worthwhile to operate on subclinical right varicocele in patients with grade II-III varicocele in the left testicle? J Assist Reprod Genet 2005;22:227-31. 10.1007/s10815-005-4926-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbendary MA, Elbadry AM. Right subclinical varicocele: how to manage in infertile patients with clinical left varicocele? Fertil Steril 2009;92:2050-3. 10.1016/j.fertnstert.2009.05.069 [DOI] [PubMed] [Google Scholar]
- 12.Sun XL, Wang JL, Peng YP, et al. Bilateral is superior to unilateral varicocelectomy in infertile males with left clinical and right subclinical varicocele: a prospective randomized controlled study. Int Urol Nephrol 2018;50:205-10. 10.1007/s11255-017-1749-x [DOI] [PubMed] [Google Scholar]
- 13.Zhu SM, Rao T, Yang X, et al. Autophagy may play an important role in varicocele. Mol Med Rep 2017;16:5471-9. 10.3892/mmr.2017.7253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden RP, Tanrikut C. Testosterone and Varicole. Urol Clin North Am 2016;43:223. 10.1016/j.ucl.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 15.Hallak J, Sharma RK, Pasqualotto FF, et al. Creatine kinase as an indicator of sperm quality and maturity in men with oligospermia. Urology 2001;58:446-51. 10.1016/S0090-4295(01)01224-9 [DOI] [PubMed] [Google Scholar]
- 16.Gordon DL, Barr AB, Herrigel JE, et al. Testicular biopsy in man. I. Effect upon sperm concentration. Fertil Steril 1965;16:522-30. 10.1016/S0015-0282(16)35651-5 [DOI] [PubMed] [Google Scholar]
- 17.Levin HS. Testicular biopsy in the study of male infertility: its current usefulness, histologic techniques, and prospects for the future. Hum Pathol 1979;10:569-84. 10.1016/S0046-8177(79)80100-8 [DOI] [PubMed] [Google Scholar]
- 18.Cito G, Coccia ME, Dabizzi S, et al. Relevance of testicular histopathology on prediction of sperm retrieval rates in case of non-obstructive and obstructive azoospermia. Urologia 2018;85:60-7. 10.1177/0391560318758940 [DOI] [PubMed] [Google Scholar]
- 19.Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod 1997;12:1688-92. 10.1093/humrep/12.8.1688 [DOI] [PubMed] [Google Scholar]
- 20.Uygur MC, Arik AI, Erol D, et al. Quantitative evaluation of biopty gun testis needle biopsy - Correlation between biopsy score of varicocele-bearing testis and sperm count. J Reprod Med 1999;44:445-9. [PubMed] [Google Scholar]
- 21.Marmar JL, Benoff S. The safety of ultrasonically guided testis aspiration biopsies and efficacy of use to predict varicocelectomy outcome. Hum Reprod 2005;20:2279-88. 10.1093/humrep/dei027 [DOI] [PubMed] [Google Scholar]
- 22.Cocuzza M, Pagani R, Lopes RI, et al. Use of subinguinal incision for microsurgical testicular biopsy during varicocelectomy in men with nonobstructive azoospermia. Fertil Steril 2009;91:925-8. 10.1016/j.fertnstert.2007.12.065 [DOI] [PubMed] [Google Scholar]
- 23.Lüpold S. Ejaculate quality and constraints in relation to sperm competition levels among eutherian mammals. Evolution 2013;67:3052-60. [DOI] [PubMed] [Google Scholar]
- 24.Johnsen SG. Testicular biopsy score count - a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1970;1:2-25. [DOI] [PubMed] [Google Scholar]
- 25.McLachlan RI, Rajpert-De Meyts E, Hoei-Hansen CE, et al. Histological evaluation of the human testis - approaches to optimizing the clinical value of the assessment: Mini Review. Hum Reprod 2007;22:2-16. 10.1093/humrep/del279 [DOI] [PubMed] [Google Scholar]
- 26.Chen SS. Significant predictive factors for subfertility in patients with subclinical varicocele. Andrologia 2017;49:5. 10.1111/and.12781 [DOI] [PubMed] [Google Scholar]
- 27.Marmar JL, Kim Y. Subinguinal microsurgical varicocelectomy: a technical critique and statistical analysis of semen and pregnancy data. J Urol 1994;152:1127-32. 10.1016/S0022-5347(17)32521-1 [DOI] [PubMed] [Google Scholar]
- 28.Cocuzza M, Pagani R, Coelho R, et al. The systematic use of intraoperative vascular Doppler ultrasound during microsurgical subinguinal varicocelectomy improves precise identification and preservation of testicular blood supply. Fertil Steril 2010;93:2396-9. 10.1016/j.fertnstert.2009.01.088 [DOI] [PubMed] [Google Scholar]
- 29.World Medical Association World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013;310:2191-4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 30.Prader A. Testicular size: assessment and clinical importance. Triangle 1966;7:240-3. [PubMed] [Google Scholar]
- 31.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: Geneva World Health Organization; 2010. [Google Scholar]
- 32.Skakkebaek NE, Hulten M, Jacobsen P, et al. Quantification of human seminiferous epithelium. II. Histological studies in eight 47, XYY men. J Reprod Fertil 1973;32:391. 10.1530/jrf.0.0320391 [DOI] [PubMed] [Google Scholar]
- 33.Masson P, Brannigan RE. The Varicocele. Urol Clin North Am 2014;41:129. 10.1016/j.ucl.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 34.Barazani Y, Nagler HM. Other work has highlighted the limitations of using histopathology to predict success after varicocelectomy. Fertil Steril 2011;95:487. 10.1016/j.fertnstert.2010.11.058 [DOI] [PubMed] [Google Scholar]
- 35.Shiraishi K, Matsuyama H, Takihara H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int J Urol 2012;19:538-50. 10.1111/j.1442-2042.2012.02982.x [DOI] [PubMed] [Google Scholar]
- 36.Anawalt BD. Approach to Male Infertility and Induction of Spermatogenesis. J Clin Endocrinol Metab 2013;98:3532-42. 10.1210/jc.2012-2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naughton CK, Nangia AK, Agarwal A. Varicocele and male infertility: Part II - Pathophysiology of varicoceles in male infertility. Hum Reprod Update 2001;7:473-81. 10.1093/humupd/7.5.473 [DOI] [PubMed] [Google Scholar]
- 38.Hallak J. Utility of sperm DNA fragmentation testing in different clinical scenarios of male reproductive abnormalities and its influence in natural and assisted reproduction. Transl Androl Urol 2017;6:S509-12. 10.21037/tau.2017.06.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bryniarski P, Kaletka Z, Huk J, et al. Testicular volume and fertility potential in men operated due to varicocele and testicular hypotrophy in adolescence. Cent European J Urol 2013;66:56-9. 10.5173/ceju.2013.01.art18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Condorelli R, Calogero AE, La Vignera S. Relationship between Testicular Volume and Conventional or Nonconventional Sperm Parameters. Int J Endocrinol. 2013;2013:145792. 10.1155/2013/145792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silber SJ, Rodriguez-Rigau LJ. Quantitative analysis of testicle biopsy: determination of partial obstruction and prediction of sperm count after surgery for obstruction. Fertil Steril 1981;36:480-5. 10.1016/S0015-0282(16)45798-5 [DOI] [PubMed] [Google Scholar]
- 42.Zini A, Buckspan M, Berardinucci D, et al. The influence of clinical and subclinical varicocele on testicular volume. Fertil Steril 1997;68:671-4. 10.1016/S0015-0282(97)00311-7 [DOI] [PubMed] [Google Scholar]
- 43.Pinto KJ, Kroovand RL, Jarow JP. Varicocele related testicular atrophy and its predictive effect upon fertility. J Urol 1994;152:788-90. 10.1016/S0022-5347(17)32710-6 [DOI] [PubMed] [Google Scholar]
- 44.Dhabuwala CB, Kumar AB, Kerkar PD, et al. Patterns of Doppler recordings and its relationship to varicocele in infertile men. Int J Androl 1989;12:430-8. 10.1111/j.1365-2605.1989.tb01333.x [DOI] [PubMed] [Google Scholar]
- 45.Cocuzza M, Athayde KS, Agarwal A, et al. Impact of clinical varicocele and testis size on seminal reactive oxygen species levels in a fertile population: a prospective controlled study. Fertil Steril 2008;90:1103-8. 10.1016/j.fertnstert.2007.07.1377 [DOI] [PubMed] [Google Scholar]