Abstract
This commentary briefly summarizes the extraordinary resurgence of hormesis within the biological, biomedical, toxicological and risk assessment domains over the past two decades. It places this resurgence within the context of challenging the scientific validity of the threshold and linear dose responses. It argues that conducting research on mechanisms that actuate and regulate the stimulatory response features of hormesis will provide the knowledge needed to develop potentially transformational applications aimed at protecting and enhancing biological resiliency as well as treating/curing a multitude of diverse medical conditions.
Keywords: Hormesis, Hormetic, Threshold dose response, Linear non-threshold dose response, LNT, Biphasic dose response, Risk assessment
Commentary
Since 1998 our research has challenged the authenticity of the two prevailing dose-response models, the linear non-threshold (LNT) and the threshold models, which are used to determine the safety and health risks of chemicals and ionizing radiation. In the past 20 years, overwhelming evidence has been produced to verify the authenticity and ubiquity of a once forgotten, nonlinear dose-response model known as hormesis (Calabrese 2008; Calabrese 2016a, b; Calabrese and Baldwin 2001b, 2003a).
To investigate the existence, prevalence and significance of hormesis, several databases of experimental dose-response studies were constructed nearly 20 years ago to serve as a reliable tool for investigating many hormesis-related phenomena (Calabrese and Baldwin 1997, 2001a, 2003b; Calabrese et al. 1999, 2006; Nascarella et al. 2009). The fidelity of the databases was established by the requirement that experimental dose-response studies in these databases must satisfy strict entry and evaluation criteria. Such requirements were meant to assure that ample data would be available to permit rigorous statistical analyses of all dose responses and therefore could determine, with statistical significance, if a particular dose-response study complied with the biphasic stimulatory features indicative of a hormetic response. It is worth noting that the selection/evaluation process is sufficiently stringent so as to underestimate the occurrence of hormesis in the dose-response literature, giving the process a conservative bias.
Applying the statistics-based approach, as just described, our efforts in mining the scientific archives have thus far resulted in over 15,000 experimental studies demonstrating a varied array of hormetic dose responses (Calabrese and Blain 2011; Nascarella et al. 2009). Furthermore, these responses were found to be independent of both the type of stimulating agent and the species of responding organism. The quantitative features that now help define and characterize a typical hormetic dose response have been elaborated (Calabrese et al. 2019) and represent another example of the research utility afforded by the process of mining data from over a century of archived scientific literature (Calabrese and Blain 2011). More recently, the hormetic database offered convincing evidence that two nominally distinct and scientifically valid responses (known as “adaptive responses” by the biological research community and the “pre- and post-conditioning responses” by the medical research community) display features, including dosing ranges, response profiles and mediating pathways, that are identical to those characterizing the hormetic response (Calabrese 2016a, b). This revelation is especially important because it further authenticates hormesis and, at the same time, unifies under the rubric of hormesis what were once considered disparate phenomena. The fact that different terms (e.g., hormetic, adaptive, conditioning, among others) have been used by different researchers from different biological research communities (e.g., toxicology, biology, medicine) to refer to the same stimulatory response indicates that each research community assumed it was investigating a unique phenomenon of central importance to its own special research area. However, the increasing realization that this stimulatory response is of common rather than of unique importance to many different research communities should inspire the formation of widespread collaborations to further enhance understanding of the hormetic response.
In addition to our ongoing research on hormesis, a parallel multi-year effort was also undertaken to clarify the somewhat obscure historical origins and scientific foundations central to the LNT model. The result of this detailed and comprehensive endeavor was both surprising and unsettling. It revealed, for the first time, a litany of scientific transgressions, ranging from flawed research to the deceptive actions of preeminent scientists, aimed at preserving the LNT model at any cost, regardless of its dogmatic origins (Calabrese 2015, 2017, 2018; Calabrese et al. 2019). Put simply, the scientific foundations of LNT were found to be shaky, fraudulent and scientifically invalid. In the past 20 years, an abundant amount of evidence has been generated that strongly (1) supports the authenticity of hormesis as a valid and viable alternative to the LNT dose-response model and, at the same time, (2) invalidates the scientific foundations upon which the prevailing LNT model resides, thereby invalidating LNT itself.
As a viable alternative to the two prevailing models, hormesis has been shown to apply universally to all chemical and physical agents, including ionizing and non-ionizing forms of radiation. The LNT and threshold models together dictate that all doses of any agent will only elicit biological effects ranging somewhere on a spectrum from toxic to innocuous, but never stimulatory or beneficial. By contrast, the hormetic model is unique in that certain low doses of almost any agent will stimulate rather than inhibit biological organisms. The hormetic stimulation of cells is generally characterized by low dose-induced profiles of gene expressions and metabolic pathways that are markedly different, quantitatively and qualitatively, from those profiles depicting the inhibitory or toxic effects of high doses of the same agent. The low-dose stimulatory profiles reflect significant increases in specific basic cellular functions (e.g., bioenergetics, anabolic and catabolic reactions, and defense and repair systems) that boost the overall biological resiliency of an organism (Leak et al. 2018). Since increased biological resiliency is intrinsically linked to improvements in fitness, healing and survival, hormesis offers extraordinary opportunity for the creative minds of scientists and bioengineers to exploit its stimulatory feature for the development of novel applications beneficial to society (Calabrese and Agathokleous 2018). The translation of hormetic applications to society, however, will not serendipitously transpire. It will require research to produce a deeper and broader fundamental understanding of hormesis and to develop biotechnological strategies that can modulate and control important features of the stimulatory response (i.e., its initiation, termination, magnitude and duration). Significant potential benefits may be derived in the future from the research and development of hormesis-based applications. For example, such applications may be used to (1) protect/prevent humans from the toxic effects of exposures to chemical, radiological and biological agents; (2) enhance human performance and cognition; (3) develop new therapeutic strategies, devices and drugs for the treatment of numerous medical conditions, ranging from inflammatory, infectious and chronic diseases to body wounds and traumatic brain injuries; and, finally, (4) develop new science-based health risk assessment guidelines for exposure to toxic agents that could not only reduce environmental clean-up costs but also improve general health via the hormetic induction of biological defense mechanisms.
Prior to about 1998, the scientific community was barely aware of hormesis and hence did not recognize its potential as a viable alternative to the LNT and threshold dose-response models. In practical terms, this lack of scientific recognition meant that a stimulatory response induced by any agent at low dose (i.e., a hormetic response) would be categorically defined either as toxic by LNT dogma (and to be banned) or as innocuous by the threshold model (and to be ignored). Thus, decades of strict adherence to the two prevailing (and compromised) dose-response models ensured not just that stimulatory responses to low doses would be completely missed but also that any opportunity to exploit these hormetic responses for the development of prospective technological applications would be lost. By 2018 however, the scientific recognition of hormesis as a legitimate and alternative dose-response model has improved considerably and is frankly at an all-time high. This enhanced recognition by the scientific community may best be exemplified by the fact that “hormesis” had been cited in the scientific research literature (i.e., Web of Science) only around 200 times in 1998, but more than 10,000 times in 2018, about a 50-fold increase after 20 years (Fig. 1).
Fig. 1.
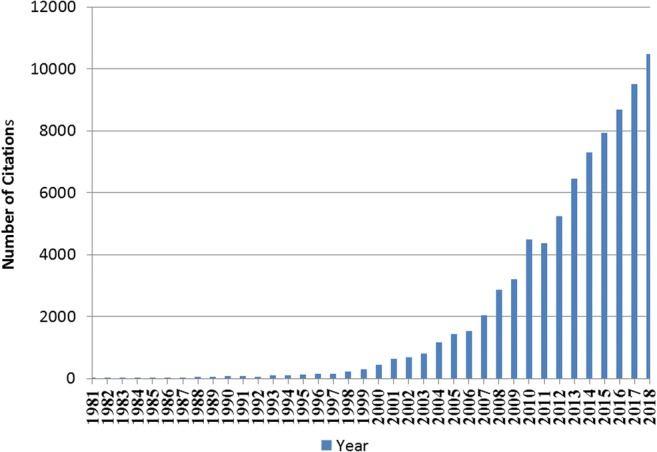
Hormesis/hormetic citations in the Web of Science by year
While much progress has been made, many questions remain and need to be answered in order to accelerate both unqualified acceptance of the hormesis model and the rate at which beneficial hormetic applications may be safely translated to society. Identifying hormetic biomarkers and acquiring knowledge of common mechanisms and pathways that mediate the hormetic response and inform its likely association with epigenetic phenomena (Bernal et al. 2013) will be of critical importance to ensuring the development of future hormesis-based applications. One intriguing but largely ignored area of special research interest involves characterizing hormetic responses to various forms of non-ionizing radiation, such as light and radio frequency radiation (Huang et al. 2011; Sannino et al. 2014; Sun et al. 2016), and then exploiting these responses to develop alternative transformational technologies that may, for example, be used to enhance human performance and safely treat a broad spectrum of diverse medical conditions (Crocetti et al. 2013; Pilla et al. 2011; Salehpour et al. 2018). Failing to explore the scientifically valid foundations of hormesis means sacrificing the long-term benefits of potentially transformational advancements for the immediate convenience of preserving familiar but flawed LNT dogma.
Acknowledgements
EJC acknowledges longtime support from the US Air Force (AFOSR FA9550-13-1-0047) and ExxonMobil Foundation (S18200000000256). The views and conclusions contained herein are those of the author and should not be interpreted as necessarily representing policies or endorsement, either expressed or implied. Sponsors had no involvement in study design, collection, analysis, interpretation, writing and decision to and where to submit for publication consideration.
Compliance with ethical standards
Conflict of interests
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Walter J. Kozumbo, Email: kozumbo@gmail.com
Edward J. Calabrese, Phone: 413-545-3164, Email: edwardc@schoolph.umass.edu
References
- Bernal AJ, Dolinoy DC, Huang D, Skaar DA, Weinhouse C, Jirtle RL. Adaptive radiation-induced epigenetic alterations mitigated by antioxidants. FASEB J. 2013;27:665–671. doi: 10.1096/fj.12-220350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008;27:1451–1474. doi: 10.1897/07-541.1. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. On the origins of the linear no-threshold (LNT) dogma by means of untruths, artful dodges and blind faith. Environ Res. 2015;142:432–442. doi: 10.1016/j.envres.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Preconditioning is hormesis part I: documentation, dose-response features and mechanistic foundations. Pharmacol Res. 2016;110:242–264. doi: 10.1016/j.phrs.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Preconditioning is hormesis part II: how the conditioning dose mediates protection: dose optimization within temporal and mechanistic frameworks. Pharmacol Res. 2016;110:265–275. doi: 10.1016/j.phrs.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Flaws in the LNT single-hit model for cancer risk: an historical assessment. Environ Res. 2017;158:773–788. doi: 10.1016/j.envres.2017.07.030. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. From Muller to mechanism: how LNT became the default model for cancer risk assessment. Environ Pollut. 2018;241:289–302. doi: 10.1016/j.envpol.2018.05.051. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Agathokleous E. Building biological shields via Hormeis. Trends Pharmacol Sci. 2018;40(1):8–10. doi: 10.1016/j.tips.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. A quantitatively-based methodology for the evaluation of chemical hormesis. Hum Ecol Risk Assess. 1997;3:545–554. doi: 10.1080/10807039709383710. [DOI] [Google Scholar]
- Calabrese EJ, Baldwin LA. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci. 2001;62:330–338. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22:285–291. doi: 10.1016/S0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol Sci. 2003;71:246–250. doi: 10.1093/toxsci/71.2.246. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Blain RB. The hormesis database: the occurrence of hormetic dose responses in the toxicological literature. Regul Toxicol Pharmacol. 2011;61:73–81. doi: 10.1016/j.yrtph.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA, Holland CD. Hormesis: a highly generalizable and reproducible phenomenon with important implications for risk assessment. Risk Anal. 1999;19:261–281. doi: 10.1023/a:1006977728215. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Staudenmayer JW, Stanek EJ, Hoffmann GR. Hormesis outperforms threshold model in National Cancer Institute antitumor drug screening database. Toxicol Sci. 2006;94:368–378. doi: 10.1093/toxsci/kfl098. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Agathokleous E, Kozumbo WJ, Stanek EJ, 3rd, Leonard D. Estimating the range of the maximum hormetic stimulatory response. Environ Res. 2019;170:337–343. doi: 10.1016/j.envres.2018.12.020. [DOI] [PubMed] [Google Scholar]
- Crocetti S, Beyer C, Schade G, Egli M, Frohlich J, Franco-Obrego A. Low intensity and frequency pulsed electromagnetic fields selectively impair breast Cancer cell viability. PLoS One. 2013;8(9):e72944. doi: 10.1371/journal.pone.0072944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose-Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Calabrese EJ, Kozumbo WJ, Gidday JM, Johnson TJ, Mitchell JR, Ozaki CK, Wetzker R, Bast A, Belz RG, Bøtker HE, Koch S, Mattson MP, Simon RP, Jirtle RL, Melvin E, Andersen ME (2018) Enhancing and extending biological performance and resilience. Dose-Response 16(3). 10.1177/1559325818784501 [DOI] [PMC free article] [PubMed]
- Nascarella MA, Stanek EJ, III, Hoffmann GR, Calabrese EJ. Quantification of hormesis in anticancer-agent dose responses. Dose-Response. 2009;7:160–171. doi: 10.2203/dose-response.08-025.Nascarella. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla A, Fitzsimmons R, Muehsam D, Wu J, Rohde C, Casper D. Electromagnetic fields as first messenger in biological signaling: application to calmodulin-dependent signaling in tissue repair. Biochim Biophys Acta. 2011;1810:1236–1245. doi: 10.1016/j.bbagen.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR. Brain Photobiomodulation therapy: a narrative review. Mol Neurobiol. 2018;55:6601–6636. doi: 10.1007/s12035-017-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannino A, Zeni O, Romeo S, Massa R, Gialanella G, Grossi G, Manti L, Vijayalaxmi, Scarfi MR. Adaptive response in human blood lymphocytes exposed to non-ionizing radiofrequency fields: resistance to ionizing radiation-induced damage. J Radiat Res. 2014;55:210–217. doi: 10.1093/jrr/rrt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Wei XX, Fei Y, Su LL, Zhao XY, Chen GD, Xu ZP (2016) Mobile phone signal exposure triggers a hormesis-like effect in Atm(+/+) and Atm(−/−) mouse embryonic fibroblasts. Sci Rep 6 [DOI] [PMC free article] [PubMed]


