Abstract
Limited effectiveness of Raf and MEK inhibitors has impelled the interest to use the inhibitors of Extra-cellular Receptor Kinase (ERK) pathway in combination with Gemcitabine (GEM) in pancreatic cancer. However, off-target abundance of ERK receptors, challenging physico-chemical properties, and dose-limiting toxicity of the inhibitor has presented critical challenges towards fabricating this combination amenable for clinical translation. Herein we report a pharmaceutical nanoformulation of GEM and an ERK inhibitor (SCH 772984) co-stabilized within a pH-sensing nanocarrier (NC, with a hydrodynamic diameter of 161 ± 5.0 nm). The NCs were modularly derived from a triblock, self-assembling copolymer, and were chemically conjugated with GEM and encapsulated with SCH772984 at a loading content of 20.2% and 18.3%, respectively. Through pH-mediated unfolding of the individual blocks of the copolymer, the NCs were able to control the release of encapsulated drugs, traffic through cellular membranes, engage target receptors, suppress proliferation of pancreatic cancer cells, and accumulate at disease sites. Collectively our studies showed the feasibility of co-delivery of a combination chemotherapy consisting of GEM and an ERK inhibitor from a NC platform, which can sense and respond to tumor microenvironment of pancreatic cancer setting.
Electronic supplementary material
The online version of this article (10.1007/s12079-019-00514-w) contains supplementary material, which is available to authorized users.
Keywords: Extracellular Receptor Kinase (ERK), Pancreatic cancer, Nanocarrier, Drug delivery
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease worldwide with a grim prognosis rate of less than 8% (Siegel et al. 2018). Combination therapy has been one of the major strategies for the treatment of PDAC (Stathis and Moore 2010). This approach harnesses the non-overlapping toxicity of individual chemotherapeutic agents to minimize the emergence of drug resistance. GEM is a cytotoxic nucleoside analogue, which has been the frontline chemotherapy for PDAC since 1997 following phase III studies that showed improvement in median overall survival (OS) of approximately 3 months as compared to the best supportive care (Chen et al. 2018; Conroy et al. 2011; Zhang et al. 2017). Combination of GEM with another chemotherapeutic agents such as 5-fluorouracil, nab-paclitaxel, or cisplatin have been used and tested with limited success (Gourgou-Bourgade et al. 2013; Conroy et al. 2011). To be effective therapeutically, a combination therapy has to withstand molecular destabilization upon administration, co-localize and accumulate at pathological microenvironment, and engage cellular targets at an optimum rate and extent to exert desired therapeutic response (Moxley et al. 1967). Physico-chemical properties of the individual drug molecule used in the combination, such as water solubility, ionization state, chemical stability, molecular size, and surface dielectrics place challenges over the capacity of current delivery technologies to transport all components of the combination therapy inside the diseased cell in therapeutically relevant quantity and in bioavailable form (Dreaden et al. 2015). Hence, identifying the right combination of chemotherapeutic candidates, designing a global platform that can deliver the candidates irrespective of their molecular characteristics in the right form and function to cellular targets is an unmet challenge in achieving successful treatment paradigm for PDAC.
Extracellular receptor kinase (ERK) inhibitor(s) has been emerging as a novel target for PDAC (Hatzivassiliou et al. 2012; Hayes et al. 2016; Morris et al. 2013). This is because, mitogen activated protein kinases (MAPK) has been found to be key player in pancreatic cancer, and Ras-Raf-MEK-ERK pathway has been among the most frequently deregulated cell signaling pathways in almost all human cancers, including PDAC (Hayes et al. 2016). Since, induction of compensatory mechanisms and ERK reactivation has limited the effectiveness of Raf and MEK inhibitors in RAS-mutant cancers, ERK inhibitor (such as SCH 772984) and combination thereof, such as with GEM has been proposed to suppress the growth of PDAC cells (Neuzillet et al. 2013; Hayes et al. 2016). However, translation of such combination for clinical applications will be challenging due to multiple off-target toxicity and high hydrophobicity of ERK inhibitor and fast metabolic degradation of GEM to inactive uracil residue (Fryer et al. 2011). PDAC exhibits a set of distinct pathological hallmark, including low pH microenvironment (Cruz-Monserrate et al. 2014; Delitto et al. 2015). We hypothesized that such microenvironmental condition can be harnessed to augment the selective accumulation of prospective chemotherapeutic combinations, such as GEM and SCH 772984 when administered via nanoparticle (NP) formulations, particularly those responsive to pH shift (Lo et al. 2018; Kwon et al. 2016) (Chan et al. 2016; Dreaden et al. 2015; Kulkarni et al. 2016; Quadir et al. 2014a; Engler et al. 2011; Mi et al. 2016; Ma et al. 2014; Itaka et al. 2004). To harness the low pH microenvironment of PDAC, in this report we have designed a pH-triggered triblock copolymer that self-assembles into nano-structured particles capable of drug encapsulation at neutral pH, and activation of drug release from the assembled structures only within the acidic microenvironment of PDAC (Fig. 1a, b).
Fig. 1.
a Triblock copolymer with PEG (blue) connected to protonable blocks appended with PEA (yellow, polycarbonate block 1) and DBA (red, polycarbonate block 2) side chains. Gemcitabine is conjugated to poly (carbonate block 2). b Schematic representation of the drug delivery strategy from nanoparticles composed of sequential pH-responsive blocks. c TEM image of nanoparticles assembled from triblock copolymer: (1) NP alone (2) NP-conjugated with GEM, and (3) NP conjugated with GEM and encapsulated with SCH 772984, with corresponding histogram of particle size distribution in the inset of respective images
Physico-chemical properties of the individual drug molecule used in a combination therapy, such as water solubility, ionization state, chemical stability, molecular size and surface dielectrics pose chemical challenges over the capacity of current delivery technologies to transport all components of the combination therapy inside the diseased cell in therapeutically relevant quantity and in bioavailable form (Dreaden et al. 2015). Hence, identifying the right combination of chemotherapeutic candidates and designing a synthetic platform that can deliver the candidates irrespective of their molecular characteristics in the right form and function to cellular targets is an unmet challenge in achieving successful treatment for many disease paradigms. The significance of this work is designing a global and micro-environment selective delivery platform of a novel combination therapy consisting of drugs with widely differing physico-chemical properties by simultaneous deployment of encapsulation and chemical conjugation approach. We proved that such platform can provide effective delivery of payloads to target tissues. Broader impact of the work is relevant to the development of translational therapy for cancer and other diseases where combination therapy is required for augmented clinical outcome.
Materials and methods
All chemicals were obtained from Sigma-Aldrich and anhydrous solvents from VWR, EMD Millipore. Glassware was cleaned with a 1:3 HCl: HNO3 mixture and thoroughly dried prior to use. 1H NMR Spectra were recorded using a Bruker 400 MHz spectrometer using TMS as the internal standard. IR Spectra were recorded using an ATR diamond tip. Gel permeation chromatography (GPC) measurements were performed on a GPC machine using a RI detector and employing polystyrene as the standard and THF as the eluent. DLS measurements were carried out using a Malvern ZS 90 instrument. UV-Visible spectra were recorded using a Varian UV-Vis spectrophotometer and the fluorescence spectra were measured using a Fluoro-Log3 fluorescence spectrophotometer. TEM studies were carried out using a JEOL JEM-2100 LaB6 transmission electron microscope (JEOL USA, Peabody, Massachusetts) with an accelerating voltage of 200 kV.
Synthesis of the triblock polymer (7) and GEM conjugated triblock copolymer (6-g)
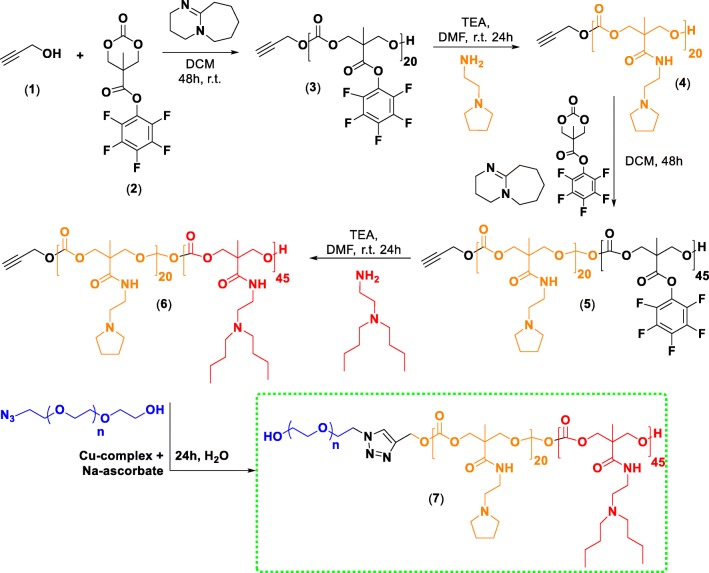
The monomer (2) constituting the pH-responsive block copolymer (7) was synthesized following the protocol established by (Sanders et al. 2010) and was isolated as pure crystals (Fig. 2). 1H NMR (400 MHz, CDCl3, TMS): δ (ppm) = 4.85 (d, 2H, CHaHb), 4.36 (d, 2H, -CHaHb), 1.55 (s, 3H, -CCH3). As shown in Fig. 2, the synthesized compound 2 (100 mg, 0.3 mmol), propargyl alcohol (1, 0.012 mmol) were dissolved in 1.5 mL anhydrous dichloromethane (DCM) under nitrogen and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 10.19 mg, 0.06 mmol) was added to the solution to facilitate polymerization. After stirring for 48 h at room temperature, the reaction was quenched by precipitating into diethyl ether and centrifuging at 7000 rpm (5094.73 g-force) for 30 min. The supernatant was decanted and an equal volume of diethyl ether was added. The solution was re-centrifuged for another 30 min at the same speed. The resulting polymer was a yellowish brown substance whose molecular weight was found to be 6722 g/mol using GPC (with THF as eluent) thereby accounting for 20 units of the monomer (2) incorporated in the first block. Compound 3 was then aminated using 2-pyrrolidin-1-yl-ethyl-amine (PEA, 2.0 M equivalent) with a catalytic amount of triethylamine using dimethylformamide (DMF) as the solvent following an earlier reported procedure to yield 4 (Engler et al. 2013). Compound 4 was then used as an initiator for further ring opening polymerization with a feed ratio of 1:50 with 2 as the monomer to yield compound 5, which was later aminated with and N, N′-dibutylethylenediamine (DBA, 2.0 M equiv.) to synthesize 6 following the same procedure. Then, 10.0 mg of N3PEG5kOH and compound 6 were separately dissolved in deionized water (DIW). For 5 mg of the polymer (6), 16.0 μL of Cu2+ complex (formed between N, N, N′, N′, N″-pentamethyldiethylenetriamine, PMDTA and CuCl2) and 16.0 μL of the sodium ascorbate (27 mg/mL in DIW) was added and the reaction was allowed to stir at room temperature for 24 h. Following this step, the content was moved to a dialysis bag (MWCO 1000) and dialyzed for 72 h with a media change every 24 h. The contents of the dialysis bag were then lyophilized and analyzed for the target triblock copolymer 7. For this copolymer 7, the yield of the product was 52%. As illustrated in supplementary figure 2, the 1H NMR (400 MHz, CDCl3, TMS) spectra for 7 showed the following δ (ppm) values = 4.3 (br m, 2H), 4–3.5 (m, CH2 PEG) 3.2 (br m, 2H), 2.6 (m, 2H), 1.88 (m, 2H), 1.44 (t, 2H), 1.28 (t, 2H), 1.01 (m, 3H). Infrared (IR) spectroscopy of the product 7 (as solid pellet) showed complete disappearance of the peak at 2154 cm−1 corresponding to azide stretching of N3PEG5kOH, indicating the complete attachment of PEG-block to the rest of the hydrophobic blocks of the copolymer (supplementary figure 3).
Fig. 2.
Synthetic route towards the triblock copolymer
For GEM conjugated triblock copolymer (6-g) as shown in supplementary figure 1, compound 5 was used as the starting material. GEM conjugation was achieved by reacting 100 mg (0.0047 mmol) of 5 dissolved in DMF. The mixture was cooled in an ice bath. A 0.5 mL DMF solution containing 0.042 mmol gemcitabine (9.0 eq.) and 0.3807 mmol DBA (81.0 equivalent) were added dropwise with constant stirring to this mixture. The solution was then brought to room temperature and allowed to stir overnight followed by precipitation in cold diethyl ether and centrifugation at 7000 rpm (5094.73 g-force) for 30 min. Compound 5-g was then attached to N3PEG5k-OH via the Cu2+ mediated click reaction. Briefly, 10 mg of N3PEG5kOH and compound 5-g were separately dissolved in deionized water (DIW). For 5 mg of drug conjugated polymer 16.0 μL of Cu2+ complex and 16.0 μL of the sodium ascorbate (27 mg/mL in DIW) were added and the reaction was allowed to stir at room temperature for 24 h. Subsequently, the contents were moved to a dialysis bag (MWCO 1000) and dialyzed for 72 h with a media change every 24 h. The contents of the dialysis bag were then lyophilized and analyzed. The 1H NMR spectra of the GEM conjugated block copolymer (6-g) is shown in supplementary figure 4.
Determination of pKa
Acid dissociation constant (pKa) values of the synthesized triblock copolymer 7 were determined by titrating the aqueous solution (5 mL) of the polymer (7) with constant stirring against 0.1 N sodium hydroxide. Two pKa values were obtained upon this titration, where the lower pKa was attributed from N, N′-dibutyl ethylenediamine while the higher one was from the 2-pyrrolidin-1-yl-ethyl-amine conjugated to the polymer backbone (Supplementary figure 5).
Determination of the critical aggregation constant (CAC) of the triblock copolymer, 7
A stock solution of 0.1 mM pyrene in DCM was prepared. An aliquot of 10 μL of this solution was taken in different vials and the DCM removed by blowing air. To each of these vials various measured amounts of the triblock copolymer, 7, was added (stock solution concentration 1 mM) so that the concentrations of the final solution varied from 0.0019 mM to 0.5 mM and the final concentration of pyrene in each vial remained at 1.0 μM. The vials were sonicated for 45 min and then allowed to stand for 3 h before recording their fluorescence spectra (Dan et al. 2011). The fluorescence emission spectra were recorded at an excitation wavelength of 337 nm with a band width of 2.5 nm for both excitation and emission. The ratio of the intensities at 373 nm and 384 nm were plotted against the concentration of the polymer and the critical concentration was determined by the inflection point of the curve (Supplementary figure 6).
Preparation of drug-free NCs from 7
To prepare drug-free NCs, 10 mg of the triblock copolymer, 7 was dissolved in 250 μL of DMSO and allowed to stir till complete dissolution. A whitish solution ensued which was added dropwise to 750 μL PBS buffer (pH 7.4) with constant stirring. The resultant solution was transferred to a Float-A-Lyzer® (MWCO 3.5–5 kDa) and allowed to dialyse against ~ 700 mL PBS buffer (pH 7.4) overnight with constant shaking at moderate speed. This dialyzed product was used for particle size analysis and other analytical experiments.
Preparation of NCs from triblock copolymer conjugated with GEM (6-g)
The triblock copolymer 6-g (10.0 mg) was dissolved in 250 μL of DMSO and allowed to stir till complete dissolution. A whitish solution ensued which was added dropwise to 750 μL PBS buffer (pH 7.4) with constant stirring. The resultant solution was transferred to a Float-A-Lyzer® (MWCO 3.5–5 kDa) and allowed to dialyze against ~ 700 mL PBS buffer (pH 7.4) overnight with constant shaking at moderate speed. This dialyzed product was used for particle size analysis and other analytical experiments with drug-loaded NCs.
Preparation of SCH 772984 encapsulated NCs from the triblock copolymer (6-g)
The triblock copolymer 6-g (10.0 mg) in the presence of 5.2 mg of SCH772984 were dissolved in 250 μL of DMSO and allowed to stir till complete dissolution. A yellowish solution ensued which was added dropwise to 750 μL PBS buffer (pH 7.4) with constant stirring. The resultant solution was allowed to stir overnight and then was filtered using an ultra-centrifuge filter (MWCO 3.5–5 kDa) and centrifuged at 5000 rpm (2599.35 g-force) for 3 h. The filtrate was analysed using UV-Vis spectroscopy for analysing drug loading, and the retained suspension was re-dispersed in cold PBS to a concentration of 10 mg/mL of NCs.
Dynamic light scattering (DLS) studies
The NC suspension, composed of triblock copolymer 7, or GEM-conjugated triblock copolymer 6-g, or SCH 772984-encapsulated and GEM-conjugated 6-g, was filtered using a 0.45 μm PES filter and the size measured using DLS at a scattering angle of 90°.
TEM studies
A drop of the NC sample was placed on a 300-mesh formvar-carbon coated copper TEM grid (Electron Microscopy Sciences, Hatfield, Pennsylvania, USA) for 1 min and wicked off. Phosphotungstic acid 0.1%, pH adjusted to 7–8, was dropped onto the grid and allowed to stand for 2 min and then wicked off.
Förster-resonance energy transfer (FRET)-based spectroscopic analysis
For this experiment, two types of fluorescent dyes that form a fluorescence-resonance energy transfer (FRET) pair has been used, namely Alexa Fluor 647 (Ex: 652 nm, Em: 668 nm) and Alexa Fluor 594 (Ex: 590 nm, Em: 617 nm). The NCs containing the FRET-coupled dyes were prepared by covalently conjugating AF 647 to 7 during its synthesis and later non-covalently encapsulating AF 594 to the resulting copolymer. The product was then dialyzed against 800 mL of PBS buffer till no dye leaches out into the bulk media outside the dialysis chamber. The labeled nanoparticles were then excited at 540 nm and their emission intensities at 620 and 690 nm recorded over time at a bandwidth of 5 nm (for both emission and excitation). The procedure followed was adopted from (Alabi et al. 2013). The normalized ratio of the intensities at 620 and the sum of 620 and 690 nm were plotted versus time (Lu et al. 2011). The increase in emission intensity for the encapsulated dye was 100 times more at a lower pH compared to pH 7.4 which showed the destabilization of the assembled structures at a lower pH and a controlled release by the cleavage of the covalent bond at pH 5.0. Plasma stability of the NCs were evaluated following the similar procedure, except the NC suspension (100 μL) was mixed with equal volume of plasma isolated from a tumor-bearing mice at the bandwidth used was 2.5 nm for spectra acquisition.
LC-MS analysis
LC-MS analysis of drugs was performed on a Q-TOF Synapt G2S mass spectrometer (Waters, Milford, MA) coupled to an Acquity UPLC system (Waters). Drugs were resolved on an ACUITY UPLC HSS T3 column (1.8 μM, 100 A pore diameter, 2.1 × 150 mm, Waters) with acetonitrile/water gradient containing 0.1% formic acid. GEM was detected as [M + H]+, and SCH 772984 was detected as [M + 2H]2+ in a positive ESI mode.
Cellular uptake studies
MIA PaCa-2 cells were plated in 6-well plates at 5000 cells/well and were allowed to grow to 70% confluence. The cells were then incubated overnight with dye (Alexa Fluor 647) labelled NCs at 37 °C. After the incubation period, the cells were washed with cold PBS (3X), trypsinized, and resuspended in PBS. Subsequently, the cells were suspended in a flow cytometry buffer (PBS with 0.1% BSA), and the cell-associated fluorescence intensity of Alexa Fluor 647 was determined by BD Biosciences Accuri C6 Flow Cytometer.
Cytotoxicity and biocompatibility assay
Cytotoxicity of the drug loaded nanoparticles and the free polymer (7) were tested on pancreatic cancer cells, MIA PaCa-2. For this experiment, 5000 cells/well were seeded in 96-well plates and 24 h later, the wells were treated with different concentrations of both drug conjugated NCs and the same concentration of free drugs or the polymer alone (for the biocompatibility assay). After incubating the cells for 72 h, cell viability assay was performed by MTS assay. Cell viability was calculated using the following equation:
Confocal microscopy studies
To evaluate the uptake of dye labelled nanoparticles we used confocal imaging studies. The confocal images were taken using a Zeiss AxioObserver Z1 microscope equipped with LSM700 laser scanning module (Zeiss, Thornwood, NY), at 40X magnification with 40x/1.3 Plan-Apochromat lens. MIA PaCa-2 cells were seeded in ibidi® glass bottom dish (35 mm) at 1 × 105 cells per well and grown overnight. Then, cells were incubated with dye-labelled nanoparticles at 37 °C in DMEM high glucose medium for 24 h. At the end of this period, cells were washed with PBS and followed by addition of DAPI and analyzed using the confocal microscope. To determine the capacity of nanoparticles to penetrate pancreatic cancer cells clustered in 3D, spheroid cultures of MIA PaCa-2 cells were used. Such models are used to mimic the 3D-native environment of pancreatic cancer lacking blood vessels. Spheroid cultures have been reported to recapitulate hypoxic and acidic conditions of desmoplastic tumors (Hou et al. 2018; Tseng et al. 2016; Tseng et al. 2014; Souza et al. 2010; Haisler et al. 2013; Desai et al. 2017; Daquinag et al. 2013). To grow these cultures, cells growing in flasks were treated with the NanoShuttle™ (n3D Biosciences courtesy Greiner Bio) solution overnight, following which the cells were washed, trypsinized and seeded in a 96 well glass-bottomed plate with cell-repellent surface (n3D Biosciences courtesy Greiner Bio). Seeding density was maintained at 1 × 105 cells per spheroid, and the plate was incubated on the spheroid drive (n3D Biosciences, Greiner Bio) in the incubator overnight. After 24 h of incubation and when the spheroids were visible, they were treated with dye labelled NCs (100 μL at 10 mg/mL concentration) and incubated for another 24 h. Following this incubation period, the spheroids were washed with PBS (while placed on a holding drive so as not to disrupt the spheroids), stained with DAPI and analyzed using confocal microscope.
Western blotting
MIA PaCa-2 cells were treated for 0, 5, 10, 15, or 30 min with 1 nM free or encapsulated SCH 772984 or nanoparticles alone for a time response and 0.001, 0.01, 0.1, or 1 nM for 30 min for a concentration response. For drug-free nanoparticles we used the triblock copolymeric system 7, and for drug-loaded nanoparticles, we used the copolymer 6-g. Cells were directly lysed in culture plates by the addition of a lysis buffer supplemented with 1 mM PMSF and 1X protease/phosphatase inhibitor cocktail (Cell Signalling). The cell lysates were collected by scraping cells from the plate and pipetting lysates into micro-centrifuge tubes. The supernatant was separated from the cell debris after centrifugation at 13,200 rpm for 5 min at 4 °C. A protein estimation assay was carried out using a BCA kit (Thermo Scientific), and 40 μg of protein was loaded into each well of 10% SDS gels. Proteins were separated on the gel using a tris glycine buffer under reducing conditions at 100 V for 2 h. Proteins were transferred onto nitrocellulose membranes with a pore size of 0.2 μm (GE Healthcare Amersham Protran®). The blots were blocked in 5% bovine serum albumin (Calbiochem, OmniPur) dissolved in 1X tris base and tween-20. The same blocking buffer was used to dilute the antibodies. The primary antibodies (1:1000) used to detect phosphorylated ERK, and total ERK were phospho-p44/42 MAPK (T202/Y204) and p44/42 MAPK respectively (Cell Signalling, #4370S and #4695S). After detecting phosphorylated ERK, the blots were stripped using a stripping buffer (Restore PLUS Western Blot Stripping Buffer, Thermo Scientific) for total ERK detection. The secondary antibody, anti-rabbit IgG HRP, and anti-biotin HRP (Cell Signalling, #7074S and #7075P5) were used in a 1:2000 dilution and detected by the SuperSignal West Pico chemiluminescent substrate (Thermo Scientific). Immunoblots were performed in triplicate, and the figures represent one typical experiment.
In vivo experiments
Xenografts were established by injecting 0.1 mL of 2 × 106 MIA PaCa-2 Cells in saline and Matrigel® basement membrane matrix (1:1) on the hind flank of six- to eight week old athymic nude mice (Nu/Nu). Before establishing the xenograft, the mice were maintained in sterile conditions using the Innovive IVC system (Innovive), following a protocol approved by the North Dakota State University’s Institutional Animal Care and Use Committee. An acclimatization period of 1 week has been allowed before experimental manipulation. Tumors were allowed to form for 3 weeks. Fluorescently-tagged NC suspension (100 μL, composed of the triblock copolymer, 7) were injected via I.P., and the tumors were harvested after 12 h and processed by Advanced Imaging and Microscopy Laboratory at NDSU. Briefly, tumor tissues from control and nanoparticle-treated mice were collected and fixed for 24 h in formaldehyde. Paraffin-embedded 5 μm thick sections of tumor tissues were deparaffinized with Histo-Clear and ethanol. After mounting a coverslip using Hardset Mounting Medium with DAPI (Vector Labs, Burlingame, CA), slides were visualized using a Zeiss AxioObserver Z1 confocal microscope (Zeiss, Thornwood, NY).
Results and discussion
We selected SCH 772984 (XlogP3-aa = 2.5), an inhibitor of ERK1/2 for their activity against KRAS-driven pancreatic cancer (Hayes et al. 2016; Germann et al. 2015; Morris et al. 2013; Neuzillet et al. 2013). GEM (log P = −1.4) is a cytidine analog that inhibits DNA synthesis and repair, is currently a drug of choice for treating PDAC. These two drugs of opposing polarity have been encapsulated inside a pH-responsive triblock copolymer (Fig.1a) assembly in the form of nanoparticulate NCs (161.34 ± 5.0 nm, DLS) (Fig. 1c-1, Supplementary figure 7) fabricated through solvent shifting technique. The pH-responsive triblock copolymer was synthesized with a general structure of poly (ethylene glycol)-b-poly (carbonate 1)-b-poly (carbonate 2) (Fig. 1a, compound 7 in Fig. 2). As pH-responsive triggers, we used 2-pyrrolidin-1-yl-ethyl-amine (PEA, pKa = 5.4) and N, N′-dibutyl ethylenediamine (DBA pKa = 4.0), appended to block 1 and block 2 respectively to promote pH-responsivity (Zhao et al. 2012; Engler et al. 2011; Quadir et al. 2014a; Ma et al. 2014), so that, these blocks protonate sequentially between pKa values of 4.0–6.0 respectively. We synthesized the poly (carbonate 1) block first, initiated by the ring opening polymerization of a cyclic carbonate following the established procedure reported by (Sanders et al. 2010) using azido ethanol as a nucleophilic initiator. The pentafluorophenyl ester appended side chains were replaced by PEA groups by treating the resultant block with a solution of PEA in the presence of triethylamine (Fig. 2, detailed in Materials and methods section). The hydroxyl group located at the termini of poly (carbonate1) block was used for the initiation of the second poly (carbonate) block, which was subsequently conjugated to DBA by similar transamination process. While the molecular weight of poly (carbonate 1) block was found to be 6722 g/mol, the total molecular weight of the diblock copolymer was 21,247 g/mol with a PDI value of 1.2. To attach GEM covalently to the second block, a specific amount of the drug was mixed with DBA solution during the pentafluorophenyl ester deprotection step to yield drug conjugated poly (carbonate 2) block (Supplementary figure 1). To yield target triblock copolymer 7 or 6-g, the pre-formed diblock copolymer was then conjugated with the alkyne terminated PEG following Cu-assisted azide-alkyne chemistry, and the reaction was followed by IR spectroscopy, which showed complete disappearance of 2154 cm−1 signal indicating the completion of the reaction (Supplementary figure 3). 1H NMR characterization of the triblock copolymer 7 as well as that conjugated with GEM, i.e. 6-g, are shown in supplementary figure 2 and 4 respectively).
We observed that the pH-induced solubility transition of the triblock copolymer with the addition of acid/base depended strongly on the side chain amine groups located at individual blocks, which provided segmental charge repulsion and water solubility (supplementary figure 5). Hence, the block copolymer exhibited two different pKa values, at 7.09 and 4.11, as found when we increased the pH of the titrating solution from 3.0 to 10.0. These two pKa values were most likely due to the sequential protonation of PEA and DBA side chains respectively. Such a broader window of pH-shift has been designed to fit with acidic pH of cancer microenvironment.
Next, we assembled NCs from this triblock copolymer by a nanoprecipitation technique in the absence or presence of ERK inhibitor, SCH 772984. Particle sizes of drug-free and drug-loaded NPs were evaluated and visualized by transmission electron microscopy and dynamic light scattering (DLS). We observed that, nanoprecipitation of the triblock copolymer in DMSO to pH 7.4 (PBS) yielded NCs of 161 ± 5.0 nm (DLS) (Fig. 1c-1, supplementary figure 7). Copolymers conjugated with GEM also showed the formation of nanoparticles upon self-assembly (Fig. 1c-2). The mean hydrodynamic diameter of GEM and ERK inhibitor containing block copolymer assembly was found to be 207 ± 8.61 nm (Fig. 1c-3). We found out that, NCs assembled from the pH-responsive triblock copolymer were able to encapsulate 18.3 and 20.2 wt% of ERK and GEM in non-covalent and covalent form, respectively. Critical association concentration of the triblock copolymers was found to be 4.47 × 10−6 M, which was comparable to the CAC values observed with polyester and polypeptide-based block copolymers (Supplementary figure 6).
Interestingly, when the NCs were incubated in acidic pH at 5.5 mimicking pancreatic cancer microenvironment (Fryer et al. 2011; Cruz-Monserrate et al. 2014; Delitto et al. 2015), a clear reduction of particle size was observed as illustrated in Fig. 3a, which might be due to the pH-mediated unfolding of the poly (carbonate 1) block, due to protonation of appended PEA side chains (pKa value of this block is 7.09). To confirm the stability of the self-assembled nanostructure as a function of pH, we investigated Förster resonance energy transfer (FRET)-based experiments. Two fluorophores that are known to form a FRET pair, namely, AF 594 and AF 647, were encapsulated within the NCs so that the dyes are within the FRET distance. At this situation, excitation of the donor dye at 540 nm causes a fluorescent emission in the FRET channel (620 nm). As the pH was lowered, we did observe a substantial reduction in relative FRET emission intensity most likely due to the protonation of amine groups of the PEA and DBA-appended blocks leading to vesicular destabilization (Fig. 3b, supplementary figure 8). We also found out that, the NCs were stable in mouse plasma for over 8 h with minimal loss of FRET signal originating from the dyes encapsulated within the nanostructures (supplementary figure 9).
Fig. 3.
a Particle size reduces for triblock copolymeric NCs when incubated with acidic pH due protonation of the poly (carbonate 1) and poly (carbonate 2) blocks, which are appended with PEA and DBA side chains respectively. b FRET experiment shows dye loaded platform do not destabilize at pH 7.4, but FRET efficiency decreases progressively as the pH is lowered to 5.0. c Release profile of SCH 77298, showing that quantitative amount of drug was released at pH 4.5, while approximately 55% of encapsulated SCH 772984 released at pH 5.5. d Release profile of GEM from triblock self-assembled system, showing GEM release was extensively sustained at all three pH values, from 4.5 to 7.4 due to covalent conjugation of GEM with the copolymeric backbone. Each data point represents mean ± SD of 3 independent measurements
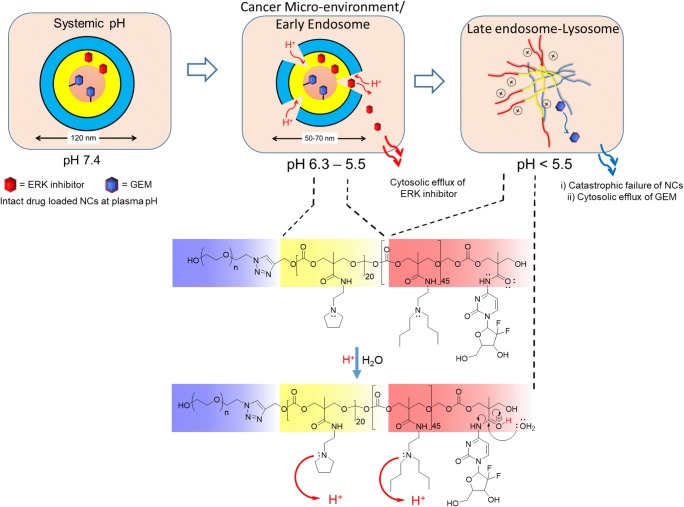
We also confirmed that, self-assembled NCs composed of triblock copolymers were able to release their encapsulated contents, i.e., Gem and ERK inhibitor (SCH 772984) in response to pH-drop from pH 7.4 (systemic circulation) via pH 5.5 (mimicking cytoplasm and early endosome of glycolytic cells) to pH 4.5 (mimicking late endosome-lysosomal conditions) over 24 h (Hu et al. 2015). The cumulative percentage release of SCH 772984 was found to be about ~75% at the end of 24 h at pH 4.5. When tested at pH 5.5, the extent of cumulative release of the drug was greater than that from pH 7.4 but substantially lower than that in pH 4.5 (Fig. 3c). For covalently conjugated GEM, only 12% and 3% of GEM was released at pH 4.5 and pH 5.5, respectively at the end of 24 h (Fig. 3d), while almost no release of the drug has occurred at systemic conditions. The differential release of GEM at pH 4.5 and 5.5 from triblock self-assembled systems indicate the capacity of these NCs to control GEM release at intracellular rather than in extracellular and systemic space over an extended period. On the other hand, the NCs triggered most of the payload release for ERK inhibitor at the micro-environmental condition of pH 5.5 available for interaction with cytosolic ERK receptors.
We observed that NC-encapsulated drugs were more effective in cell killing as compared to un-encapsulated drugs (alone or in combination) or the free polymer, which was also found to be non-toxic to cells (Fig. 4a, b). Next, we evaluated the capacity of our platform to transport therapeutic payload to disease targets. Through flow cytometry experiment, we observed substantial cellular uptake of NCs in cancer cells after 24 h (Fig. 5a). When detectors were set for channels associated with FRET fluorescence, punctated fluorescence signals were evident in cytoplasmic space indicating intact, dye loaded NCs were internalized at the time-point of the experiment (Fig. 5b, 24 h post incubation).
Fig. 4.
a Enhanced cytotoxicity of encapsulated combination therapy consisting of GEM and SCH 772984 compared to unencapsulated combination and free drugs (n = 5, P < 0.05). b Cytotoxicity of the pH-responsive triblock copolymer comparable to media (control), showing the biocompatibility of the copolymer
Fig. 5.
a Flow cytometric analysis of cellular internalization of the nanoparticles labelled with FRET dyes by MIA PaCa-2 cells show a time dependent uptake (Black: control, Blue: dye labelled nanoparticles at 24 h, Red: at 48 h and Green at 72 h) (b) Detection of FRET associated fluorescence (540 nm) in the perinuclear space of MIA PaCa-2 cancer cells, when the cells were incubated with nanoparticles conjugated with FRET coupled dyes, i.e. AF 594 and AF 647. Arrow indicates the presence of nanoparticles around the nucleus which were stained with DAPI (c) Western blot data showing the cellular availability of SCH 772984, suppressing the phosphorylation of ERK (A: Encapsulated ERK inhibitor (1 nM), B: Free ERK inhibitor (1 nM), C: Bare Nanoparticles, D: Encapsulated ERK inhibitor (varying concentrations), E: Free ERK inhibitor (varying concentrations), F: Bare NCs (varying concentrations)
To investigate if the cytotoxic effect is at least mediated by inhibition of ERK pathway mediated by SCH 772984, we studied the phosphorylation event of ERK receptors in the presence of control (empty) NPs, free SCH 772984, a combination of SCH 772984 and GEM and NP- encapsulated drug combination. To verify SCH 772984 could block the ERK signaling pathway, pancreatic cancer cells were treated with free or encapsulated SCH 772984, and cell lysates were analyzed for phosphorylated (p-ERK) and total ERK (t-ERK) expression using Western blotting (Fig. 5c). The encapsulated ERK inhibitor (EEI; 1 nM) resulted in reduced phosphorylation of ERK at the earliest time point (5 min; A); whereas the free ERK inhibitor (FEI; 1 nM) resulted in reduced phosphorylation of ERK within 15 min (B). Similarly, the EEI showed more potent inhibition of ERK as 0.1 nM EEI was sufficient to completely block ERK activation compared to >1 nM FEI at 30 min. Neither form of the inhibitor affected total ERK levels (A, B, D, E). As expected, the NCs alone as a control did not affect total or phosphorylated levels of ERK in a concentration (C) or time (F) dependent manner. The differences observed between the free and encapsulated forms of SCH 772984 could, in principle, be due to the fact that the EEI adopts the endosomal pathway as it enters the cell, whereas the FEI is most likely cleared owing to relatively immediate diffusion. Since the NC package carrying the ERK inhibitor can get disassembled only at a pH of 5 and lower, it could be said that this happens in the endosome, which has a pH < 6.0. Collectively, these studies show that, under acidic pH, ERK-inhibitor is efficiently released into the surrounding environment, and significantly inhibits the activities of ERK.
We then set out to understand if NCs can penetrate inside dense cell clusters of pancreatic microenvironment devoid of the vasculature, we grew spheroid cultures of pancreatic cancer cells. Spheroid cultures have been reported to recapitulate disease microenvironment more accurately and can provide an estimation of depth profile that such pH-dissociable NCs can shuttle through a 3D cellular matrix that is devoid of any vasculature. A spheroid culture mimics native cellular environments (Haisler et al. 2013) and has been extensively used as a model system by (Tseng et al. 2014; Daquinag et al. 2013) and other research groups (Leonard and Godin 2016; Molina et al. 2010) to study various cancer types (Souza et al. 2010), drug screening (Hou et al. 2018), the effect of metabolic inhibitors (Noel et al. 2017; Desai et al. 2017) and as in vitro assay procedures as well as for 3D bioprinting (Tseng et al. 2016). Hence, we treated the 3-D spheroidal cultures of MIA PaCa-2 cells with fluorescent dye labeled pH-dissociable NCs. Using the detector for Alexa Fluor 647 emission intensity, we observed the treated spheroid across the Z-axis at every 10-μm thickness for colocalization of dye fluorescence with DAPI treated nuclei of pancreatic cancer cells (Fig. 6) 24 h post-treatment. We observed the presence of fluorescent signals from Alexa Fluor 647 down to 30 μm depths from the spheroid surface, indicating the capacity of NCs to penetrate inside dense cellular microenvironments that is usually acidic. Lastly, when NCs labelled with FRET coupled dyes (i.e., AF 594 and 647), injected to tumor bearing nude mice (PDAC cells xenografted on hind flank) through i.p., we observed substantial accumulation of fluorescently labeled NCs within the tumor after 12 h post injection (tumor histology in Fig. 6-1, untreated mice in Fig. 6-2), most likely guided by enhanced permeation and retention (EPR) effect originating from the altered tumor vasculature.
Fig. 6.
a Penetration depth of nanoparticles through Z- stacks of MIA PaCa-2 spheroid culture showing the fluorescence of Alexa Fluor 647 originating from NCs (red, indicated by arrows) co-localizes with DAPI stained nucleus (blue) down to 30 μm from spheroid surface. b NC fluorescence (red, indicated by arrows) recovered from histology cross-section of tumors harvested from mice treated with fluorescently labelled NCs: (1) Treated mice (2) untreated mice
To elucidate the mechanism of activation and release of GEM and SCH 772984 from pH-responsive NCs as illustrated in Fig. 7, we first chemically characterized the released drug molecules from NCs using LC-MS method. LC-MS data showed that the mass spectra of released GEM and SCH 772984 match with that of the corresponding pure drugs in terms of molecular weight (supplementary figure 10). This observation indicates the release of unaltered GEM and ERK upon NC destabilization by pH. Secondly, we measured the hydrodynamic diameter of NCs after incubating the system at pH 5.0. At this condition, hydrodynamic diameters of NCs were reduced at least by two-fold (supplementary figure 11). Hence, we hypothesize that, either during the in vitro release study (where the pH was decreased gradually), or for in vitro cell culture experiments (where intrinsic pH-drop is taking place along endosomal-lysosomal pathway), protonation of PEA (pKa = 5.4) side chains appended to poly (carbonate) block 1 occurs first. The charged residues of PEAs facilitate the unfolding of this block from the NCs, leading to the reduction of overall NC hydrodynamic diameter. Most likely, the polymer chains constituting the NCs also become thermodynamically relaxed due to entropy gain at this stage. Further reduction on pH to <5.0, protonates the DBA residues (pKa = 4.0) in block 2. In addition, at this pH, the unstable amide bond between GEM and the polymer chain is also cleaved by increasing proton concentration, resulting in release of the drug in its pure form. When pH value approaches near the pKa of block 2, catastrophic destabilization of NCs take place due to electrostatic charge repulsion, resulting in complete loss of nanoparticle structure. Protonation associated with unfolding of polypeptide, and subsequent dissociation of nanoparticulate structures have been reported earlier by Hammond et al. (Quadir et al. 2014b; Engler et al. 2009, 2011; Poon et al. 2010) and Kataoka et al. (Yan et al. 2009; Bae et al. 2007; Lee et al. 2007). On-demand, pH-activable supramolecular self-assembly of micelles have been reported by Gao et al. using ionizable block copolymers with adjustable hydrophobicity. (Wang et al. 2017; Ma et al. 2014; Zhou et al. 2011; Gao et al. 2010). Colocalization experiment in H2009 cells in vitro revealed that these pH-sensing polymeric nanoprobes were activated in early or late endosome, or in lysosome. Such activation depended on the pKa value of the tertiary amines located on the hydrophobic segments of the nanoprobe-forming block copolymers. A homologous set of these pH-activable nanoparticles was also found to respond differentially to different pH conditions existing within cancer microenvironment in in vivo settings (Zhao et al. 2017; Wang et al. 2014).
Fig. 7.
Mechanism of activation of nanocarriers (NCs) in response to intracellular pH in vitro. The NCs are stable at plasma pH of 7.4. Upon cellular internalization, sequential protonation of different domains of NCs along the endosomal-lysosomal pathway is responsible for the diffusion of drugs out of the NC matrix into cytosolic space
Conclusions
In summary, we showed that triblock copolymers composed of sequentially ionizable blocks form a pH-responsive nanoscale self-assembly, which can deliver a novel combination chemotherapy with a small-molecule inhibitor of ERK for enhanced suppression of cancer cells. Such nanoscale assembly could provide a safer and effective platform to deliver combination chemotherapy of diverse physico-chemical properties for the treatment and translational research of pancreatic and other forms of cancer.
Electronic supplementary material
(DOCX 2874 kb)
Acknowledgments
This research was supported by NIH grant number 1P20 GM109024 from the National Institute of General Medical Sciences (NIGMS). TEM material is based upon work supported by the NSF under Grant No. 0923354. Funding for the Core Biology Facility used in this publication was made possible by NIH Grant Number 2P20 RR015566 from the National Center for Research Resources. MS analysis was supported through NIH funded COBRE Mass Spec Core Facility Grant 5P30GM103329-05. SM acknowledges the support through the NIH grant 1R01 GM 114080 (NIGMS). Partial support for this work was received from NSF Grant No. IIA-1355466 from the ‘North Dakota Established Program to Stimulate Competitive Research (EPSCoR)’ through the Center for Sustainable Materials Science. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Any opinions, findings, and conclusions or recommendations expressed are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. The work is partially supported by Merit review grant from Department of Veterans Affairs (Sushanta K. Banerjee, 5I01BX001989-04 and Snigdha Banerjee, I01BX001002-05), KUMC Lied Basic Science Grant Program (SKB), and Grace Hortense Greenley Trust, directed by The Research Foundation in memory of Eva Lee Caldwell (SKB). We thank S. Golovko for her excellent assistance with MS analysis.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sushanta K. Banerjee, Email: sushanta.banerjee@va.gov, Email: sbanerjee2@kumc.edu, Email: cancerresearchunit@icloud.com
Mohiuddin Quadir, Phone: (701) 231-6283, Email: mohiuddin.quadir@ndsu.edu.
References
- Alabi CA, Love KT, Sahay G, Yin H, Luly KM, Langer R, Anderson DG. Multiparametric approach for the evaluation of lipid nanoparticles for siRNA delivery. Proc Natl Acad Sci. 2013;110:12881–12886. doi: 10.1073/pnas.1306529110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y, Nishiyama N, Kataoka K. In vivo antitumor activity of the folate-conjugated pH-sensitive polymeric micelle selectively releasing adriamycin in the intracellular acidic compartments. Bioconjug Chem. 2007;18:1131–1139. doi: 10.1021/bc060401p. [DOI] [PubMed] [Google Scholar]
- Chan WW, Chhowalla M, Glotzer S, Gogotsi Y, Hafner JH, Hammond PT, Hersam MC, Javey A, Kagan CR, Khademhosseini A, Kotov NA, Lee ST, Li Y, Mohwald H, Mulvaney PA, Nel AE, Nordlander PJ, Parak WJ, Penner RM, Rogach AL, Schaak RE, Stevens MM, Wee AT, Willson CG, Fernandez LE, Weiss PS. Nanoscience and nanotechnology impacting diverse fields of science, engineering, and medicine. ACS Nano. 2016;10:10615–10617. doi: 10.1021/acsnano.6b08335. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhou D, Liu Z, Huang X, Liu Q, Kang Y, Chen Z, Guo Y, Zhu H, Sun C. Combination of gemcitabine and erlotinib inhibits recurrent pancreatic cancer growth in mice via the JAK-STAT pathway. Oncol Rep. 2018;39:1081–1089. doi: 10.3892/or.2018.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Unicancer, G. T. D. o. and Intergroup, P FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- Cruz-Monserrate Z, Roland CL, Deng D, Arumugam T, Moshnikova A, Andreev OA, Reshetnyak YK, Logsdon CD. Targeting pancreatic ductal adenocarcinoma acidic microenvironment. Sci Rep. 2014;4:4410. doi: 10.1038/srep04410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan K, Bose N, Ghosh S. Vesicular assembly and thermo-responsive vesicle-to-micelle transition from an amphiphilic random copolymer. Chem Commun. 2011;47:12491–12493. doi: 10.1039/c1cc15663b. [DOI] [PubMed] [Google Scholar]
- Daquinag AC, Souza GR, Kolonin MG. Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng Part C Methods. 2013;19:336–344. doi: 10.1089/ten.tec.2012.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delitto D, Black BS, Sorenson HL, Knowlton AE, Thomas RM, Sarosi GA, Moldawer LL, Behrns KE, Liu C, George TJ, Trevino JG, Wallet SM, Hughes SJ. The inflammatory milieu within the pancreatic cancer microenvironment correlates with clinicopathologic parameters, chemoresistance and survival. BMC Cancer. 2015;15:783. doi: 10.1186/s12885-015-1820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PK, Tseng H, Souza GR (2017) Assembly of hepatocyte spheroids using magnetic 3D cell culture for CYP450 inhibition/induction. Int J Mol Sci 18 [DOI] [PMC free article] [PubMed]
- Dreaden EC, Kong YW, Morton SW, Correa S, Choi KY, Shopsowitz KE, Renggli K, Drapkin R, Yaffe MB, Hammond PT. Tumor-targeted synergistic blockade of MAPK and PI3K from a layer-by-layer nanoparticle. Clin Cancer Res. 2015;21:4410–4419. doi: 10.1158/1078-0432.CCR-15-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A, Bonner D, Buss H, Cheung E, Hammond P. The synthetic tuning of clickable pH responsive cationic polypeptides and block copolypeptides. Soft Matter. 2011;7:5627–5637. doi: 10.1039/c1sm05064h. [DOI] [Google Scholar]
- Engler A, Chan J, Coady D, O'Brien J, Sardon H, Nelson A, Sanders D, Yang Y, Hedrick J. Accessing new materials through polymerization and modification of a polycarbonate with a pendant activated Ester. Macromolecules. 2013;46:1283–1290. doi: 10.1021/ma4001258. [DOI] [Google Scholar]
- Engler AC, Lee HI, Hammond PT. Highly efficient "grafting onto" a polypeptide backbone using click chemistry. Angew Chem Int Ed Eng. 2009;48:9334–9338. doi: 10.1002/anie.200904070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer RA, Barlett B, Galustian C, Dalgleish AG. Mechanisms underlying gemcitabine resistance in pancreatic cancer and sensitisation by the iMiD™ lenalidomide. Anticancer Res. 2011;31:3747–3756. [PubMed] [Google Scholar]
- Gao W, Chan JM, Farokhzad OC. pH-responsive nanoparticles for drug delivery. Mol Pharm. 2010;7:1913–1920. doi: 10.1021/mp100253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann U, Furey B, Roix J, Marklan W, Hoover R, Aronov A, Hale M, Chen G, Martinez-Botella G, Alargova R. The selective ERK inhibitor BVD-523 is active models of MAPK pathway-dependent cancers, including those with intrinsic and acquired drug resistance. In 106th annual meeting of the American Association for Cancer Research. Cancer Res. 2015;75:4693. [Google Scholar]
- Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Boige V, Bérille J, Conroy T. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23–29. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- Haisler WL, Timm DM, Gage JA, Tseng H, Killian TC, Souza GR. Three-dimensional cell culturing by magnetic levitation. Nat Protoc. 2013;8:1940–1949. doi: 10.1038/nprot.2013.125. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Liu B, O'Brien C, Spoerke JM, Hoeflich KP, Haverty PM, Soriano R, Forrest WF, Heldens S, Chen H, Toy K, Ha C, Zhou W, Song K, Friedman LS, Amler LC, Hampton GM, Moffat J, Belvin M, Lackner MR. ERK inhibition overcomes acquired resistance to MEK inhibitors. Mol Cancer Ther. 2012;11:1143–1154. doi: 10.1158/1535-7163.MCT-11-1010. [DOI] [PubMed] [Google Scholar]
- Hayes TK, Neel NF, Hu C, Gautam P, Chenard M, Long B, Aziz M, Kassner M, Bryant KL, Pierobon M, Marayati R, Kher S, George SD, Xu M, Wang-Gillam A, Samatar AA, Maitra A, Wennerberg K, Petricoin EF, Yin HH, Nelkin B, Cox AD, Yeh JJ, Der CJ. Long-term ERK inhibition in KRAS-mutant pancreatic Cancer is associated with MYC degradation and senescence-like growth suppression. Cancer Cell. 2016;29:75–89. doi: 10.1016/j.ccell.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Tiriac H, Sridharan BP, Scampavia L, Madoux F, Seldin J, Souza GR, Watson D, Tuveson D, Spicer TP. Advanced development of primary pancreatic organoid tumor models for high-throughput phenotypic drug screening. SLAS Discov. 2018;23:574–584. doi: 10.1177/2472555218766842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YB, Dammer EB, Ren RJ, Wang G. The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl Neurodegener. 2015;4:18. doi: 10.1186/s40035-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaka K, Kanayama N, Nishiyama N, Jang W, Yamasaki Y, Nakamura K, Kawaguchi H, Kataoka K. Supramolecular nanocarrier of siRNA from PEG-based block catiomer carrying diamine side chain with distinctive pK(a) directed to enhance intracellular gene silencing. J Am Chem Soc. 2004;126:13612–13613. doi: 10.1021/ja047174r. [DOI] [PubMed] [Google Scholar]
- Kulkarni P, Haldar M, Katti P, Dawes C, You S, Choi Y, Mallik S. Hypoxia responsive, tumor penetrating lipid nanoparticles for delivery of chemotherapeutics to pancreatic Cancer cell spheroids. Bioconjug Chem. 2016;27:1830–1838. doi: 10.1021/acs.bioconjchem.6b00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon EJ, Skalak M, Lo Bu R, Bhatia SN. Neuron-targeted nanoparticle for siRNA delivery to traumatic brain injuries. ACS Nano. 2016;10:7926–7933. doi: 10.1021/acsnano.6b03858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Fukushima S, Bae Y, Hiki S, Ishii T, Kataoka K. A protein nanocarrier from charge-conversion polymer in response to endosomal pH. J Am Chem Soc. 2007;129:5362−+. doi: 10.1021/ja071090b. [DOI] [PubMed] [Google Scholar]
- Leonard F, Godin B. 3D in vitro model for breast Cancer research using magnetic levitation and bioprinting method. Methods Mol Biol. 2016;1406:239–251. doi: 10.1007/978-1-4939-3444-7_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JH, Hao L, Muzumdar MD, Raghavan S, Kwon EJ, Pulver EM, Hsu F, Aguirre AJ, Wolpin BM, Fuchs CS, Hahn WC, Jacks T, Bhatia SN. iRGD-guided tumor-penetrating Nanocomplexes for therapeutic siRNA delivery to pancreatic Cancer. Mol Cancer Ther. 2018;17:2377–2388. doi: 10.1158/1535-7163.MCT-17-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Owen SC, Shoichet MS. Stability of self-assembled polymeric micelles in serum. Macromolecules. 2011;44:6002–6008. doi: 10.1021/ma200675w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wang Y, Zhao T, Li Y, Su L, Wang Z, Huang G, Sumer B, Gao J. Ultra-pH-sensitive Nanoprobe library with broad pH Tunability and fluorescence emissions. J Am Chem Soc. 2014;136:11085–11092. doi: 10.1021/ja5053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi P, Kokuryo D, Cabral H, Wu H, Terada Y, Saga T, Aoki I, Nishiyama N, Kataoka K. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat Nanotechnol. 2016;11:724–730. doi: 10.1038/nnano.2016.72. [DOI] [PubMed] [Google Scholar]
- Molina JR, Hayashi Y, Stephens C, Georgescu MM. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia. 2010;12:453–463. doi: 10.1593/neo.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, Carr D, Deng Y, Jin W, Black S, Long B, Liu J, Dinunzio E, Windsor W, Zhang R, Zhao S, Angagaw MH, Pinheiro EM, Desai J, Xiao L, Shipps G, Hruza A, Wang J, Kelly J, Paliwal S, Gao X, Babu BS, Zhu L, Daublain P, Zhang L, Lutterbach BA, Pelletier MR, Philippar U, Siliphaivanh P, Witter D, Kirschmeier P, Bishop WR, Hicklin D, Gilliland DG, Jayaraman L, Zawel L, Fawell S, Samatar AA. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013;3:742–750. doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- Moxley JH, De Vita VT, Brace K, Frei E. Intensive combination chemotherapy and X-irradiation in Hodgkin's disease. Cancer Res. 1967;27:1258–1263. [PubMed] [Google Scholar]
- Neuzillet C, Hammel P, Tijeras-Raballand A, Couvelard A, Raymond E. Targeting the Ras-ERK pathway in pancreatic adenocarcinoma. Cancer Metastasis Rev. 2013;32:147–162. doi: 10.1007/s10555-012-9396-2. [DOI] [PubMed] [Google Scholar]
- Noel P, Munoz R, Rogers GW, Neilson A, Von Hoff DD, Han H (2017) Preparation and metabolic assay of 3-dimensional spheroid co-cultures of pancreatic cancer cells and fibroblasts. J Vis Exp 126(e56081):1-8 [DOI] [PMC free article] [PubMed]
- Poon Z, Chen S, Engler AC, Lee HI, Atas E, von Maltzahn G, Bhatia SN, Hammond PT. Ligand-clustered "patchy" nanoparticles for modulated cellular uptake and in vivo tumor targeting. Angew Chem Int Ed Eng. 2010;49:7266–7270. doi: 10.1002/anie.201003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir MA, Morton SW, Deng ZJ, Shopsowitz KE, Murphy RP, Epps TH, Hammond PT. PEG-polypeptide block copolymers as pH-responsive endosome-solubilizing drug nanocarriers. Mol Pharm. 2014;11:2420–2430. doi: 10.1021/mp500162w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir MA, Morton SW, Deng ZJ, Shopsowitz KE, Murphy RP, Thomas H, Epps I, Hammond PT. PEG-polypeptide block copolymers as pH-responsive endosome solubilizing drug nanocarriers. Mol Pharm. 2014;11:2420–2430. doi: 10.1021/mp500162w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Fukushima K, Coady D, Nelson A, Fujiwara M, Yasumoto M, Hedrick J. A simple and efficient synthesis of functionalized cyclic carbonate monomers using a versatile Pentafluorophenyl Ester intermediate. J Am Chem Soc. 2010;132:14724–14726. doi: 10.1021/ja105332k. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS, Bronk LF, Ananta JS, Mandelin J, Georgescu M-M, Bankson JA, Gelovani JG, Killian TC, Arap W, Pasqualini R. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010;5:291–296. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- Tseng H, Balaoing LR, Grigoryan B, Raphael RM, Killian TC, Souza GR, Grande-Allen KJ. A three-dimensional co-culture model of the aortic valve using magnetic levitation. Acta Biomater. 2014;10:173–182. doi: 10.1016/j.actbio.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng H, Gage JA, Haisler WL, Neeley SK, Shen T, Hebel C, Barthlow HG, Wagoner M, Souza GR. A high-throughput in vitro ring assay for vasoactivity using magnetic 3D bioprinting. Sci Rep. 2016;6:30640. doi: 10.1038/srep30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang C, Li Y, Huang G, Zhao T, Ma X, Wang Z, Sumer B, White M, Gao J (2017) Digitization of endocytic pH by hybrid ultra-pH-Sensitive nanoprobes at single-organelle resolution. Adv Mater 29:1-9 [DOI] [PMC free article] [PubMed]
- Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X, Zhao T, Sumer B, DeBerardinis R, Gao J. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater. 2014;13:204–212. doi: 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Takehiko I, Horacio C, Jin KH, Ji-Hun S, Nobuhiro N, Hiroki O, Kensuke O, Kazunori K. Charge-conversional Polyionic complex micelles—efficient Nanocarriers for protein delivery into cytoplasm. Angew Chem. 2009;121:5413–5416. doi: 10.1002/ange.200900064. [DOI] [PubMed] [Google Scholar]
- Zhang XW, Ma YX, Sun Y, Cao YB, Li Q, Xu CA. Gemcitabine in combination with a second cytotoxic agent in the first-line treatment of locally advanced or metastatic pancreatic cancer: a systematic review and meta-analysis. Target Oncol. 2017;12:309–321. doi: 10.1007/s11523-017-0486-5. [DOI] [PubMed] [Google Scholar]
- Zhao T, Huang G, Li Y, Yang S, Ramezani S, Lin Z, Wang Y, Ma X, Zeng Z, Luo M, de Boer E, Xie X, Thibodeaux J, Brekken R, Sun X, Sumer B, Gao J (2017) A transistor-like pH nanoprobe for tumour detection and image-guided surgery. Nat Biomed Eng 1:1-20 [DOI] [PMC free article] [PubMed]
- Zhao X, Poon Z, Engler AC, Bonner DK, Hammond PT. Enhanced stability of polymeric micelles based on postfunctionalized poly (ethylene glycol)-b-poly (γ-propargyl L-glutamate): the substituent effect. Biomacromolecules. 2012;13:1315–1322. doi: 10.1021/bm201873u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Wang Y, Huang X, Luby-Phelps K, Sumer B, Gao J. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew Chem Int Ed. 2011;50:6109–6114. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 2874 kb)