Abstract
Cerebral white matter injury in premature infants, known as periventricular leukomalacia (PVL), is common after hypoxia- ischemia (HI). While ionotropic glutamate receptors (iGluRs) can mediate immature white matter injury, we have previously shown that excitotoxic injury to premyelinating oligodendrocytes (preOLs) in vitro can be attenuated by group I metabotropic glutamate receptor (mGluR) agonists. Thus, we evaluated mGluR expression in developing white matter in rat and human brain, and tested the protective efficacy of a central nervous system (CNS)-penetrating mGluR agonist on injury to developing oligodendrocytes (OLs) in vivo. Group I mGluRs (mGluR1 and mGluR5) were strongly expressed on OLs in neonatal rodent cerebral white matter throughout normal development, with highest expression early in development on preOLs. Specifically at P6, mGluR1 and mGLuR5 were most highly expressed on GalC-positive OLs compared to neurons, axons, astrocytes and microglia. Systemic administration of (1S,3R) 1-aminocyclopentane-trans-1,3,-dicarboxylic acid (ACPD) significantly attenuated the loss of myelin basic protein in the white matter following HI in P6 rats. Assessment of postmortem human tissue showed both mGluR1 and mGluR5 localized on immature OLs in white matter throughout development, with mGluR5 highest in the preterm period. These data indicate group I mGluRs are highly expressed on OLs during the peak period of vulnerability to HI and modulation of mGluRs is protective in a rodent model of PVL. Group I mGluRs may represent important therapeutic targets for protection from HI-mediated white matter injury.
Keywords: Hypoxia-ischemia (HI); periventricular leukomalacia (PVL); metabotropic glutamate receptor (mGluR); oligodendrocyte (OL); excitotoxicity; (1S,3R)-1-aminocyclopentane-trans-1,3,-dicarboxylic acid (ACPD)
INTRODUCTION
Hypoxia-ischemia (HI) is a common cause of perinatal brain damage, and contributes to later life neurologic impairment including cerebral palsy, epilepsy, learning disorders and intellectual disability (Vannucci, 2000; Volpe, 2009). Both term and preterm infants are vulnerable to HI, although the pathophysiology observed is dependent on the age of the fetus and the developmental stage of the brain (Volpe, 2009). In the United States, approximately 63,000 infants are born annually with very low birth weight (VLBW ≤ 1500 g) (Martin et al., 2008; Volpe, 2009). The incidence of neurological sequelae is common, with 25–50% of VLBW infants suffering long-term cognitive, behavioral or attention deficits, while major motor deficits including cerebral palsy are present in 5–10% (Bayless and Stevenson, 2007; Allin et al., 2008; Kobaly et al., 2008; Larroque et al., 2008; Volpe, 2009). The encephalopathy of prematurity is diverse, but the most common manifestation is periventricular leukomalacia (PVL), although other neuronal/axonal deficits are also observed in the thalamus, basal ganglia, cerebral cortex, brainstem and cerebellum (Volpe, 2009).
Developmentally, white matter is most vulnerable to HI injury through midgestation and the preterm period, when premyelinating OLs (preOLs) are most prevalent (Kinney and Back, 1998; Back et al., 2001). Multiple factors have suggested that the analogous developmental period to this window of susceptibility in the human is at the end of the first postnatal week in the rodent (Silbereis et al., 2010; Jensen, 2006; Talos et al., 2006a, b). In the rodent, the subcortical white matter and specifically oligodendrocytes (OLs) appear to be highly susceptible to injury between postnatal day (P) 5–7 (Follett et al., 2004; Jantzie et al., 2005; Back et al., 2007).
The mechanisms of injury to the premature brain are multifactorial, and depend on the maturational state of antioxidant defenses, inflammatory cascades, cerebral vasculature and cell-specific receptors for the excitatory neurotransmitter glutamate (Ferriero, 2004; Folkerth et al., 2004; Jensen, 2006; Volpe, 2009). HI causes increased accumulation of extracellular glutamate and initiates excitotoxic injury and cell death (Benveniste, 1991; Follett et al., 2004; Jensen, 2006; Manning et al., 2008). Oligodendrocytes (OLs) are the primary cell type in developing white matter and express ionotropic glutamate receptors (iGluRs) including N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, although these subtypes are also expressed on white matter microglia, astrocytes and neurons (Fern and Moller, 2000; Follett et al., 2000, 2004; Manning et al., 2008).
In addition to iGLuRs, OLs also express metabotropic GluRs (mGluRs) (Luyt et al., 2003; Deng et al., 2004), which are G-protein coupled and are classified into groups I-III based on signal transduction pathways and pharmacology (Byrnes et al., 2009a). While iGluRs mediate fast glutamatergic synaptic transmission, mGluRs activate second messenger signaling cascades including those modulating glutamatergic transmission (Maiese et al., 2005; Alexander and Godwin, 2006; Byrnes et al., 2009a).
mGluR expression is developmentally regulated on OLs in vitro with upregulated expression on immature and premyelinating OLs (preOLs) compared to mature OLs (Deng et al., 2004; Luyt et al., 2006). mGluR1 and mGluR5 are variably expressed on OLs, astrocytes and endothelial cells (Maiese et al., 2005; Ferraguti and Shigemoto, 2006; Byrnes et al., 2009a). Conversely, microglia express primarily mGluR5, which can regulate their activation (Biber et al., 1999; Byrnes et al., 2009b).
Relevant to PVL, activation of group I mGluRs with the broad spectrum agonist (1S,3R) 1-aminocyclopentane-trans-1,3,-dicarboxylic acid (ACPD) and the specific group I agonist (R,S)-3,5-dihydroxyphenylglycine (DHPG) attenuated kainate and oxygen-glucose deprivation (OGD)-induced preOL death in vitro. This protective effect of ACPD and DHPG on OLs occurred independently of endocytosis of AMPA/kainate receptors from the cell surface (Deng et al., 2004), unlike what has been observed in cultured adult hippocampal neurons (Snyder et al., 2001). In contrast, the protective efficacy of group I mGluR agonists on OLs appeared to be through activation of PCKα leading to maintenance of intracellular glutathione and decreased production of reactive oxygen species (Deng et al., 2004).
While OL excitotoxicity and oxidative stress have been shown to play a major role in the pathogenesis of PVL and other cerebral white matter disorders (Haynes et al., 2003; Deng et al., 2004; Gonsette, 2008), the role of group I mGluRs located on OLs in vivo and in perinatal human tissue remains undefined. Here we evaluated the expression patterns of mGluR1 and mGluR5 on OLs in developing white matter in both rodent and human brain, and tested the protective efficacy of a CNS-penetrating mGluR agonist, ACPD, in a model of PVL.
OBJECTIVE
Based on previous results with in vitro OLs, we hypothesized that group I mGluRs were upregulated on immature OLs in situ in white matter during the preterm period, and that HI-mediated white matter injury is in part mediated by the expression of group I mGluRs on OLs. Thus, the present study investigated the spatial and temporal expression of group I mGluRs on OLs in rodent and human white matter during development. Further, we examined the protective efficacy of the CNS-penetrating mGluR agonist ACPD on white matter injury in a rodent model of PVL. These studies aimed to identify novel mechanisms of group I mGluRs in the white matter of premature brains in response to HI.
METHODS
All procedures presented were approved and in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee at Children’s Hospital Boston. Unless otherwise indicated, all chemicals and reagents were obtained from Sigma (St. Louis, MO, USA).
Animals and surgical procedures
We used a previously published model of HI cerebral injury induced by unilateral common carotid artery occlusion and systemic hypoxia in P6 Long-Evans rat pups (Follett et al., 2004; Manning et al., 2008). At P6, male pups were anesthetized with ether. A small lateral skin incision was made on the anterior ventral surface of the neck, and the left common carotid artery was exposed, isolated and permanently ligated by cauterization. Following recovery, pups were placed in a hypoxia chamber held at 6% O2 balanced N2 for 1 h. Normothermia (32–34°C) was maintained in the hypoxia chamber with a heating pad. During hypoxia, pups were monitored for behavioral seizures/convulsions (pawing, nodding, myoclonic jerks, tonic-clonic head and limb movement, loss of posture), and seizure duration was recorded for each animal, in every ACPD treatment paradigm (Rakhade et al., 2008).
Assessment of protective efficacy of systemic ACPD treatment in a rat model of PVL
Rat pups received 100 ml injections of 5, 10 or 20 mg kg−1 of the broad spectrum group I/II mGluR agonist ACPD (Tocris), or phosphate buffered saline (PBS), intraperitoneally (i.p.) 1 h prior to carotid ligation (n = 10 per group). Additional doses of ACPD were then administered immediately following hypoxia and every 12 h for the first 48 h post-HI, consistent with the reported half-life of drugs in this class (Hodge et al., 2006; Carroll, 2008). Chemical modulators of mGluRs, such as ACPD and other glycine derivatives, have half-lives between 1 and 4 h and thus pre-treatment was required to ensure adequate drug delivery and absorption by the time cerebral injury was initiated during hypoxia. Ninety-six hours post-HI, pups were euthanized via lethal injection of sodium pentobarbital (100 mg kg−1, i.p.), and transcardially perfused with PBS, followed by 4% paraformaldehyde (PFA). Brains were extracted, post-fixed in PFA overnight and then cryoprotected in 30% sucrose at 4°C. Brains were then frozen and 16 mm sections were obtained using a cryostat. Sections were mounted on slides, and stored at −20°C until immunochemical analyses were performed.
Developmental profile of group I mGluRs in the immature rodent
The normal developmental expression of group I mGluRs were assessed in naïve, male rat pups. P3, P6, P9 and P14 (n = 3 per group) pups were euthanized and transcardially perfused as described above. Brains were extracted, postfixed and cryoprotected, and then sectioned at 50 μm thickness for free-floating sections or 12 μm for slide mounted sections, and were subsequently processed for immunohistochemistry.
Developmental profile of group I mGluRs in human white matter
Human parietal-occipital lobe specimens were collected from the fetal, neonatal, pediatric and adult autopsy populations of Children’s Hospital Boston and Brigham & Women’s Hospital, Boston. Cases ranged from 19 post-conceptional weeks (PCW) to 57 years of age (n = 17; 12 males and 5 females). Additional cases were obtained from the University of Maryland Brain and Tissue Bank for Developmental Disorders (n = 4; three males and one female) (Table 1). The samples were obtained from standard diagnostic postmortem examinations, and all procedures and experiments were conducted under guidelines approved by the Clinical Research Committee at all institutions. The causes of death are listed in Table 1. We divided our study cases into those with no recognizable abnormalities on microscopic examination (n = 16), and those with minimal white matter changes (mild diffuse white matter gliosis [DWMG]) (n = 4). When possible, the postmortem interval was limited to ≤24 h.
Table 1.
Clinical characteristics of sample population*.
| Case no. | GA (weeks) | PNA (weeks) | PCA (weeks) | PMI (h) | Gender | Autopsy diagnosis | Neuropathology abnormalities |
|---|---|---|---|---|---|---|---|
| 1 | NA | 57 years | 57 years | 11 | M | Leukemia | Ø |
| 2 | NA | 43 years | 43 years | 22 | F | Hodgkin’s Disease | Ø |
| 3 | NA | 38 years | 38 years | 17 | M | Myelogenous leukemia | Ø |
| 4 | NA | 7 years | 7 years | 12 | M | Drowning | Ø |
| 5 | NA | 4 years | 4 years | 21 | F | Lymphocytic myocarditis | Ø |
| 6 | 40 | 52 | 92 | 15 | M | Drowning | Ø |
| 7 | NA | NA | 76 | 20 | M | Pediatric cocaine toxicity | Ø |
| 8 | 38 | 0 | 38 | NA | F | Hypoplastic left heart syndrome | Ø |
| 9 | 38 | 0 | 38 | 40 | F | MCA, PH | DWMG |
| 10 | 38 | 0 | 38 | 16 | M | Stillborn, alpha thalassemia | DWMG |
| 11 | 37 | 0 | 37 | 25 | M | Stillborn | Ø |
| 12 | 37 | 0 | 37 | 17 | M | ACA/pulmonary hemorrhage | DWMG |
| 13 | 32 | 0 | 32 | 51 | F | Prematurity | Ø |
| 14 | 29 | 3 | 32 | 22 | M | NEC | DWMG |
| 15 | 29 | 0 | 29 | 23 | M | Prematurity/PH | Ø |
| 16 | 27 | 0 | 27 | 60 | M | Prematurity/lung immaturity | Ø |
| 17 | 24 | 0 | 24 | 32 | M | Extreme prematurity | Ø |
| 18 | 24 | 0 | 24 | 46 | M | MCA | Ø |
| 19 | 20 | 0 | 20 | 24 | M | Severe IUGR/prematurity | Ø |
| 20 | 19 | 0 | 19 | 0 | M | Extreme prematurity | Ø |
ACA, acute chorioamnionitis; DWMG, diffuse white matter gliosis; GA, gestational age; GM, germinal matrix; IUGR, intrauterine growth restriction; MCA, multiple congenital anomalies; NEC, necrotizing enterocolitis; PCA, post-conceptional age; PH, pulmonary hypoplasia; PMI, postmortem interval; PNA, postnatal age; NA, information not available; M, male; F, female.
Immunochemical and histological analyses
Free-floating 50 or 12 mm slide mounted sections were processed and double labeled with antibodies against mGluR1 or mGluR5, in combination with cell-specific markers. Briefly, sections were rinsed and blocked for 2 h in a 5% normal goat serum solution. Primary antibodies against preOLs (anti-O4, 1:500, gift from S. Pfeiffer, University of Connecticut, Farmington, CT), immature OLs (anti-galactocerebroside C (GalC), 1:100, Millipore), neurons (anti-neuronal nuclei (NeuN), 1:500, Millipore), astrocytes (anti-glial fibrillary acidic protein (GFAP, SMI 22), 1:500, Covance), axons (anti-neurofilament 160 kDa, Millipore, 1:200), and activated microglia/macrophages (anti-CD68, 1:500, Serotec) were then applied and sections were allowed to incubate overnight at 4°C. The following day, sections were washed, and a species-appropriate fluorescent secondary antibody conjugated to Alexa-Fluor 568 (1:1000, Invitrogen) was applied for 1 h. Sections were then washed, blocked and incubated with antibodies against mGluR1 or mGluR5 (anti-mGluR1/5, 1:500, Millipore) overnight at 4°C. Subsequently, a biotinylated anti-rabbit secondary antibody was applied, followed by a fluorescent avidin conjugate. Sections were then washed and coverslipped with a Dapi containing mountant. Appropriate negative control (no primary antibody) and blocking peptide experiments were run in parallel and as per the procedure described above.
Brain sections were also evaluated for myelin basic protein (MBP) via immunohistochemistry. Serial coronal sections were blocked and incubated overnight with anti-MBP (1:1000, SMI-99, Sternberger Monoclonals). The following day, an anti-mouse Alexa-Fluor 488/568 secondary antibody was applied for 1 h. Slides were then rinsed and coverslipped. An investigator blinded to all treatment conditions assessed lesion size. Using a semiquantitative scale as previously described (Follett et al., 2000, 2004; Manning et al., 2008), MBP immunostaining/white matter injury following HI was rated on a scale of 0 (no injury) to 5 (severe injury). These scores were assigned and compared with the white matter in the hemisphere contralateral to carotid ligation in three stereotactically similar sections per animal.
Western blot analyses
Western blot analyses were performed on tissue homogenates from regions of dissected human parietal-occipital white matter and cortex (Table 1). Adult cases were used as indices of maturity (n = 3) and as the standard for protein expression. After membrane protein extraction, a Bradford protein assay was performed to determine total protein amount, and 30 mg of protein/sample was loaded on precast 7.5% Tris glycine gels. Following transfer to polyvinyldifluoride membranes, membranes were blocked for 1 h in 5% non-fat dry milk in Tris buffered saline solution containing 0.1% Tween-20. Membranes were then incubated in mGluR5 primary antibody solution (1:500, Millipore) overnight at 4°C. An HRP conjugated anti-rabbit IgG secondary antibody was then applied for 1 h and after appropriate washes protein bands were visualized using SuperSignal West Femto Maximum Sensitivity ECL substrate. Relative optical density was measured for each band using FujiFilm software.
Statistical analyses
All data presented are expressed as mean ± standard error of the mean (SEM). ANOVA analysis was used for multiple comparisons. Normally distributed data between two independent groups were analyzed with Student’s t-test. The general convention of a probability value of P < 0.05 was used to establish statistical significance.
RESULTS
Group I mGluRs are developmentally expressed on immature OLs in the neonatal rodent brain
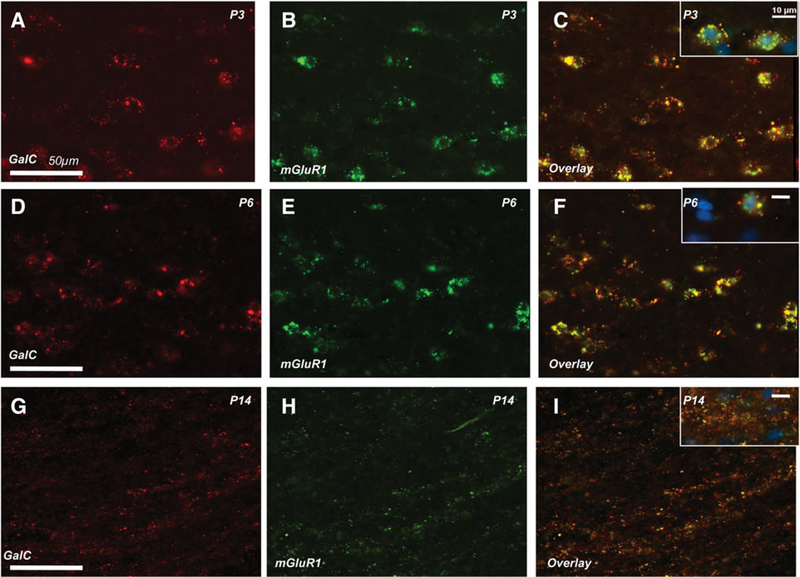
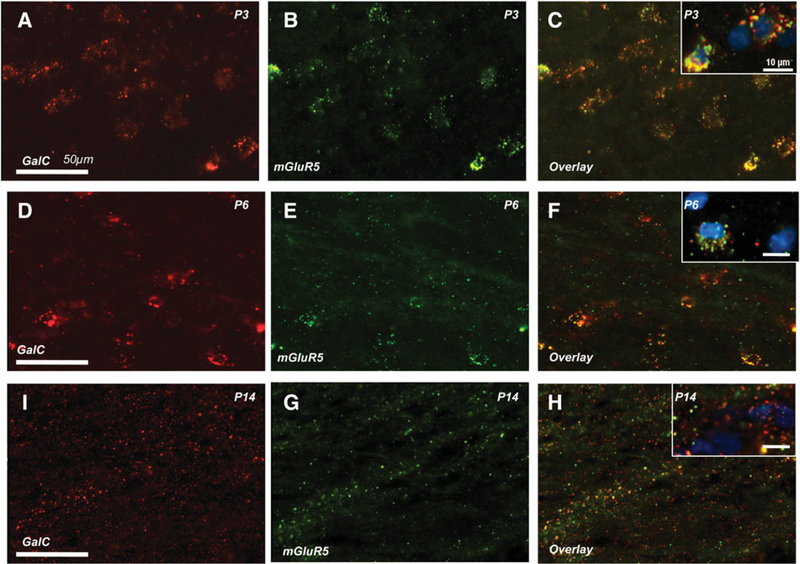
Given our previous data indicating that mGluR1 and mGluR5 are expressed on developing OLs in vitro (Deng et al., 2004), we first assessed cell specific expression of mGluR1 and mGluR5 on OLs, neurons, astrocytes and activated microglia in immature rats from P3 to P14. In OLs, the regional and subcellular localization varied with age. In white matter, mGluR1 was prominently localized on GalC-positive OL cell somas at earlier ages (P3-P6, Fig. 1A–F), while in later postnatal development (P14) mGluR1 was almost exclusively localized to cell processes (Fig. 1G–I). At P14, there appeared to be a ventral-dorsal gradient with the highest density of process staining localized to the gray:white junction, and on white matter fibers radiating through the lower layers of the cortex and subplate (Fig. 1G–I). mGluR5 was also maximally expressed on GalC-positive OL cell bodies from P3 to P6 in white matter (Fig. 2A–F). By P14, mGluR5/GalC colocalization was minimal, and limited to restricted patches of OL processes (Fig. 2G–I). Overall at P14, mGLuR5 labeling of OL processes was of lower density than that of mGluR1. Co-labeling with the O4 OL marker revealed similar patterns at all ages (data not shown).
Fig. 1. Developmental expression of mGluR1 in rodent callosal white matter.
At postnatal day 6 (P6), mGluR1 (E, green) is highly expressed on GalC-positive OL cell bodies and processes (red) in callosal white matter (B-F). mGluR1 (green) is also strongly co-expressed on GalC-positive OL cell bodies and processes (red) from P3 (A-C) through P14 (G-I).
Fig. 2. Developmental expression of mGluR5 in rodent callosal white matter.
At postnatal day 6 (P6), mGluR5 (E, green) is highly expressed on the cell bodies of GalC-positive OLs (red) in callosal white matter (D-F). In contrast to the consistent GluR1 expression pattern throughout development (Fig. 1), the peak of mGluR5 (green) on GalC-positive OLs (red) is between P3 (C) and P6 (F). By P14 (H), mGLuR5-GalC co-expression is reduced and restricted to patches of OL processes throughout the corpus callosum and is similar to that observed in older animals.
Both mGluR1 and mGluR5 were also developmentally regulated on non-OL components of developing white matter. At P6, in contrast to OLs that highly expressed both mGluR1 and mGluR5, group I mGluRs were minimally present on CD68-positive activated microglia and GFAP-positive cell bodies (see supplementary Fig. 1A–D online). However, with advancing age (P9-P14) both group I mGluR subunits were increasingly expressed on astrocytic processes, while mGluR5 expression increased on activated microglia. Interestingly, this increased expression of mGluR5 on astrocytes and microglia in the white matter in the second postnatal week is occurring during a time that this receptor is diminishing in expression on GalC-positive OLs.
Neuronal expression followed a different pattern with both mGluR1 and mGluR5 present in the cortex prior to P9, although at lower levels compared to OLs. Consistent with previous reports, group I mGluRs then gradually increased during the second postnatal week on neuropil and dendrites (data not shown) (Boer et al., 2010). In addition, both mGluR1 and mGluR5 were minimally present on neurofilament 160 kDa-positive axons in white matter and cortex at P6 (see supplementary Fig. 1E,F online), corroborating prior evidence of very low levels of group I mGluRs on axons and consistent with the literature reporting that the majority of mGluR1 and mGluR5 receptors (i.e. >90%) are located postsynaptically on dendritic spines (Romano et al., 1995; Lujan et al., 1997; Olive, 2009).
Administration of the group I/II mGluR agonist, ACPD, attenuates white matter injury in a rodent model of PVL
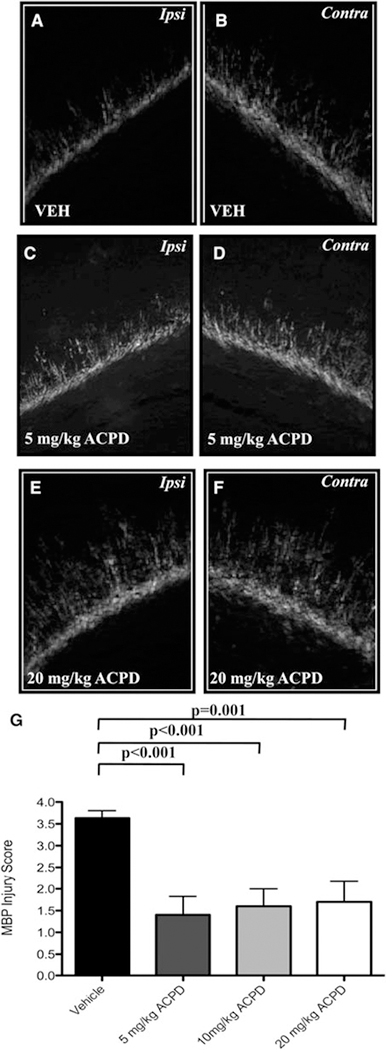
Given the high expression of mGluR1 and mGluR5 on developing OLs in rodent white matter throughout the first postnatal week, and the protective effects of mGluR agonists against OL injury in vitro (Deng et al., 2004), we next examined the protective efficacy of ACPD against HI-mediated white matter injury in our P6 rat model of PVL. Administration of ACPD in four divided doses during P6-P8 significantly attenuated callosal and periventricular white matter loss as assessed by MBP protein expression. HI in vehicle-treated rats resulted in significant loss of MBP and unilateral decreases in white matter in the hemisphere ipsilateral to carotid ligation compared to MBP expression in the contralateral hemisphere (Fig. 3A,B; 3.6 ± 0.17 versus 2.2 ± 0.20, P < 0.0001). In contrast, the administration of ACPD at each of the three doses examined (5, 10 or 20 mg kg−1), significantly attenuated the loss of MBP and protected white matter when MBP injury was assessed 96 h post-HI. ACPD treatment preserved myelin processes radiating into the cortex and pericallosal process bundles compared with vehicle-treated controls (Fig. 3C–F). Semiquantitative scoring by a blinded investigator confirmed a significant protection of ACPD on white matter injury (5 mg kg−1: 1.4 ± 0.43 versus 0.5 ± 0.17, P < 0.001; 10 mg kg−1: 1.6 ± 0.4 versus 1.1 ± 0.31, P < 0.001; 20 mg kg−1: 1.7 ± 0.47 versus 1.1 ± 0.31, P = 0.001, Fig. 3G).
Fig. 3. Administration of ACPD attenuates white matter loss after HI in postnatal day 6 (P6) Long-Evans rats.
HI results in the significant loss of MBP in the hemisphere ipsilateral to carotid ligation (A), compared to MBP expression in the uninjured, contralateral hemisphere (B). Systemic administration of ACPD for 48 h significantly attenuates callosal and periventricular white matter loss 96 h following HI when administered at 5 mg kg−1 (C,D) or 2o mg kg−1 (E,F). Treatment with ACPD preserves myelin processes radiating into the cortex and pericallosal process bundles at each dose examined (C,E) compared to vehicle-treated controls, and significantly decreases overall MBP injury scores (G).
While ACPD appeared to be protective in developing white matter, this agent has been shown to be proconvulsant at older ages, when neuronal expression of group I mGluRs is higher in cortex. The convulsant effect of group I mGluR agonists (McDonald et al., 1993) has been attributed to presynaptic actions of glutamate (Zhong et al., 2000; Ure et al., 2006). We thus evaluated whether administration of ACPD was proconvulsant in our model of PVL in P6 Long-Evans rats. Importantly, there were no significant differences in either percentage of animal seized per treatment group (77% vehicle-treated HI animals versus 50% 5 mg kg−1 ACPD versus 70% 10 mg kg−1 ACPD versus 80% 20 mg kg−1 ACPD, P > 0.05) or cumulative seizure duration (19.7 min vehicle-treated HI animals versus 18.3 min 5 mg kg−1 ACPD versus 19.1 min 10 mg kg−1 ACPD versus 20.8 min 20 mg kg−1, P > 0.05). Consistent with our results showing low mGluR expression on neurons at this age, these data suggest that mGluRs do not significantly contribute to HI-induced seizure activity in this model.
Group I mGluRs are localized on immature OLs in human white matter
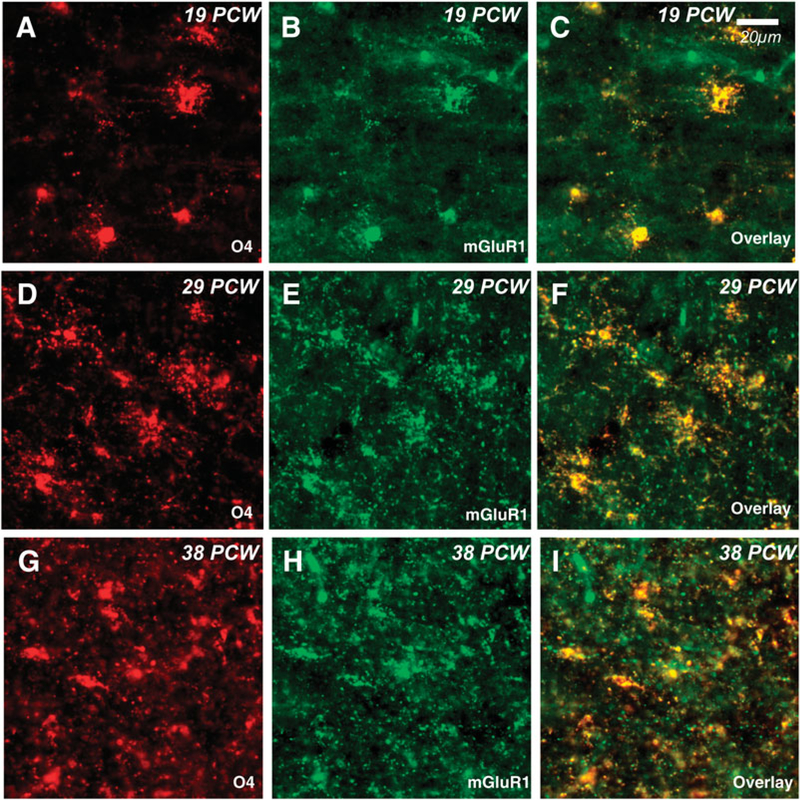
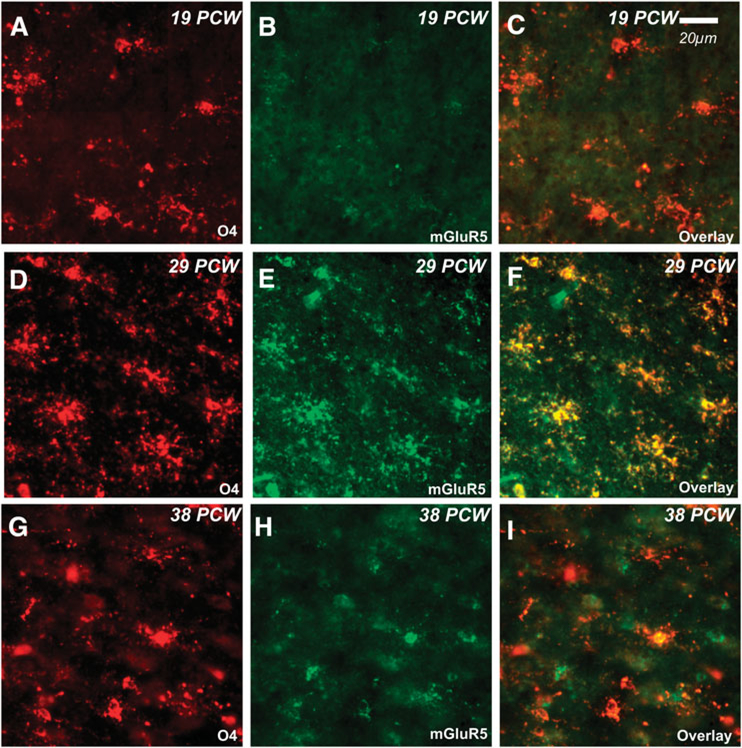
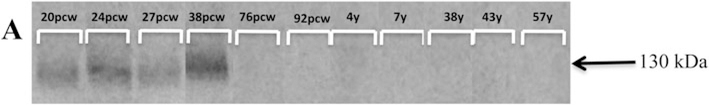
We next determined whether group I mGluRs weredevelopmentally regulated in human brain tissue in the premature period. Using the O4 marker for immature OLs, we evaluated the expression of mGluRs in postmortem parietal-occipital lobe tissue from cases 19 to 38 PCW (Table 1). Throughout the investigated time course, mGluR1 was highly expressed on O4-positive OLs (Fig. 4). In contrast, mGluR5 was minimally expressed on OLs at 19 PCW (Fig. 5A–C), and then increased by 29 PCW (Fig. 5D–F), but returned to lower levels at 38 PCW (Fig. 5G–I). The transient upregulation of mGluR5 was similar to that observed in the rodent. Also corroborating rodent staining patterns, mGluR5 was expressed at very low levels on GFAP-positive astrocytes prior to and at 36 PCW, but then increased beginning at 38 PCW (see supplementary Fig. 2 online). In addition, neuronal expression of mGluR5 gradually increased with age from low levels in scattered cortical neurons during the preterm period to widespread expression in most neuronal cell bodies and dendrites at term birth (data not shown), consistent with published data (Byrnes et al., 2009a; Boer et al., 2010) and similar in pattern to that seen in the rodent. Western blot analyses performed on human parietal-occipital white matter confirmed the transient increase in white matter mGluR5 expression (Fig. 6), whereby mGluR5 was strongly expressed in white matter from 20 to 38 PCW, compared to a marked postnatal decrease (76 PCW-7 years) and very low levels sustained through adulthood.
Fig. 4. Developmental expression of mGluR1 on human OLs.
Expression of mGluR1 (green) on O4-positive preOLs (red) in parietal-occipital white matter is consistent throughout gestation. O4-mGluR1 co-expression (yellow) is high through 19 post-conceptional weeks (PCW, A-C), 29 PCW (D-F) and 38 PCW (G-I).
Fig. 5. Developmental expression of mGluR5 on human OLs.
A biphasic expression pattern is observed with mGluR5 (green) on human O4-positive OLs (red) whereby co-expression (yellow) is low at 19 post-conceptional weeks (PCW, A-C) and 38 PCW (G-I). The peak of mGluR5 expression on O4-positive preOLs is observed at 29 PCW.
Fig. 6. Developmental expression of mGluR5 in human subcortical white matter.
Western blot analyses reveal that mGluR5 protein is strongly expressed in human subcortical, parietal-occipital white matter from 20 to 38 PCW, compared to a marked postnatal decrease (76 PCW-7 years) and very low levels sustained through adulthood.
CONCLUSIONS
The expression of mGluR1 and mGluR5 in the white matter of neonatal rodents is developmentally regulated. mGluR1 is strongly expressed on GalC-positive OLs from P3 to P14, whereas mGLuR5 is maximally expressed on GalC-positive cell bodies from P3 to P6. In contrast, both mGluR1 and mGluR5 are minimally expressed on astrocytes, microglia, neurons and axons at this early stage of development, but increase during the second postnatal week. Hence, group I mGluRs are present primarily on OLs during the peak period of vulnerability to Hi-mediated white matter injury.
Patterns of mGluR1 and mGluR5 in human white matter appear to follow the same developmental trends as the rodent. While mGluR1 is expressed on O4-positive OLs throughout gestation (19–38 PCW), mGluR5 is maximally expressed on O4-positive OLs through the preterm period. With advancing gestational age, mGluR5 is increasingly expressed on cortical neurons, dendrites and GFAP-positive astrocytes in the white matter. This expression pattern validates group I mGluRs as targets on premyelinating OLs, and confirms group I mGluR expression during the peak period of vulnerability to HI-mediated white matter injury.
The systemically administrable mGluR agonist ACPD significantly attenuated white matter injury in a rat model of PVL, during a period of high mGluR expression on preOLs. These data are consistent with prior evidence of protective efficacy of selective group I agonists in in vitro models of preOL injury. Given the primary expression pattern of group I mGluRs on OLs described above in the immature brain, these data suggest that this protective efficacy is at least in part related to direct modulation of mGluRs on OLs.
DISCUSSION
There is substantial literature regarding the developmental regulation of group I mGluRs on neurons and their role in synaptic plasticity, synaptogenesis and neuronal excitability (Di Giorgi Gerevini et al., 2004; Bear, 2005; Catania et al., 2007), as well as their role in the pathogenesis of glutamatemediated cell death in disorders of the adult brain, including epilepsy, stroke, chronic pain, schizophrenia and Alzheimer’s disease (Catania et al., 2007; Luscher and Huber, 2010; Ribeiro et al., 2010). Their relevance as a therapeutic target has been suggested as group I mGluR agonists have been shown to be protective in models of stroke in adult rodent brain (Bao et al., 2001; Kelland and Toms, 2001).
However, the role of mGluRs in developmental disorders in the immature brain or their expression on non-neuronal cell populations has not been completely described in vivo. Here we report for the first time that the systemically administrable group I/II mGluR agonist, ACPD, protects against white matter injury in an animal model of PVL. Importantly, both mGluR1 and mGluR5 are prominently expressed on immature OLs in rodent and human subcortical developing white matter. The protection in vivo is consistent with our prior findings of a direct protective effect of mGluR agonists on OLs in vitro. Given the presence of group I mGluRs on human OLs, this protective strategy may have future clinical potential in the treatment of perinatal HI-mediated white matter injury.
Similar patterns of expression of group I mGluRs on OLs in developing rodent and human subcortical white matter
It is well established that iGluRs are expressed on all cell populations in the developing white matter of both rodents and humans (Jensen, 2006; Talos et al., 2006a, b). The developmental expression of iGluRs critically regulates neuronal, synaptic and glial differentiation (Butt, 2006). It has been postulated that glutamate also modulates myelination by OLs, although the mechanism whereby iGluRs might regulate this process is unclear (Karadottir and Attwell, 2007; Wake et al., 2011). However, the transient upregulation of these receptors during development render cells vulnerable to excitotoxicity in diseases characterized by excessive glutamate release (McDonald and Johnston, 1990; Jensen, 2006).
Group I mGluRs are also of interest in HI injury, because they are present on many cell types in the brain and their activation may influence outcome, as related to modulation of downstream protein kinases involved in cell growth, differentiation and survival, and their direct effects on endothelium, neuroinflammation and apoptosis (Luyt et al., 2006; Catania et al., 2007; Byrnes et al., 2009a; Ribeiro et al., 2010). Indeed, we found both mGluR1 and mGluR5 to be developmentally regulated on glial cells in rodent and human white matter. mGluR1 was persistently present on immature rodent and human OLs, while mGluR5 was transiently increased throughout this time course of selective vulnerability to injury. The transient overexpression of mGluR5 on OLs in human and rodent white matter in the preterm period may promote OL survival, as mGluR5 has been shown to be important for OL proliferation, migration, neuron-glia signaling and the prevention of apoptosis (Luyt et al., 2006). We observed mGluR1 and mGluR5 on OL processes and cell bodies throughout the first two postnatal weeks and in the premature human brain. While mGluRs on OLs may be the primary target of the ACPD effects observed here, we document expression of mGluR1 and mGluR5 on astrocytes and microglia, although at lower levels, throughout this period of susceptibility. mGluR5 receptors are upregulated on reactive astrocytes in ALS, MS and epilepsy, and it is hypothesized that the interaction between OLs, astrocytes and microglia influence the response mediated by mGluRs under pathologic conditions characterized by neuroinflammation (D’Antoni et al., 2008; Drouin-Ouellet et al., 2011). In culture, mGluR activation modulates microglial reactivity and decreases the production of proinflammatory mediators, enhancing OL progenitor survival (Farso et al., 2009; Taylor et al., 2010). Specific to the developing rodent brain, neuroinflammation induces mGluR5 expression on astrocytes and microglia, and administration of minocycline significantly protects against ibotenic acid-induced excitotoxicity (Drouin-Ouellet et al., 2011). Interestingly, we have also shown that doxycycline and minocycline inhibit microglial activation and protect neurons and OLs from HI in neonatal rodents (Lechpammer et al., 2008; Jantzie and Todd, 2010). Hence, while this report focused on the actions of group I mGluRs on OLs, there is likely to be other important mechanisms involving microglia and astrocytes.
Protective efficacy of ACPD in an animal model of PVL
A novel finding in this study is the protective effect of group I mGluR agonists in models of white matter injury in vivo. Prior studies using premature HI injury models have shown that glutamate excitotoxicity is mediated by iGluRs present on preOLs in immature white matter, and blockade of the AMPA or NMDA subtypes of iGluRs protects the white matter when administered after HI to neonatal rats (Follett et al., 2000, 2004; Manning et al., 2008; Liu et al., 2009). Given the prominent role glutamate appears to play in this pathophysiology, we reasoned that mGluRs may also modulate preOL injury. Here, importantly, ACPD was protective and significantly attenuated callosal and periventricular white matter loss after preterm HI. The in vivo protection observed here is consistent with our previous finding that the specific mGluR1 agonist DHPG was protective against OGD and kainate-induced excitotoxicity, and that developing OLs in vitro express both mGluR1 and mGluR5 (Deng et al., 2004). In that study, no protection was seen with DCG-IV or L-AP4, group II or III specific mGluR agonists, indicating the protective effects on OL injury were primarily related to actions on group I mGluRs. Specifically, ACPD and DHPG significantly protected preOLs, and the protective effects were reversed by (S)-methyl-4-carboxy-phenylglycine (MCPG, a broad-spectrum mGluR antagonist) (Deng et al., 2004). In addition, the protective effects of DHPG were partially prevented by 7-hydroxyiminocyclo-propanchromen-1a-carboxylic acid ethyl ester (CPCCOEt, selective mGluR1 antagonist) or 2-methyl-6-(phenylethynyl)-pyridine (MPEP, selective mGluR5 antagonist) and fully abolished by the combination of the two agents, whereas the antagonists per se (MCPG, CPCCOEt, MPEP or CPCCOEt plus MPEP) had no effect (Deng et al., 2004). These data in combination with our in vivo study here indicate that ACPD was effective via modulation of group I mGluRs with agonists. While mGluR agonists are protective in our model, there have been no reports of the opposite effects of mGluR antagonists. MPEP administration to preOLs in vitro or to naïve prepubertal rodents has not been reported to induce cell death or white matter injury (Luyt et al., 2003; Deng et al., 2004; Lojkova and Mares, 2005).
The protective effects of ACPD were maximal at the lowest dose examined, without additional benefit at higher doses, and the timing of ACPD administration corresponded with peak levels of mGluR1 and mGluR5 expression on OLs during the preterm period, the peak period of OL cell death following HI, and the pharmacokinetics of glycine-derived mGluR modulators (Hodge et al., 2006; Carroll, 2008). This developmental expression afforded the ability to preferentially target OLs during their peak vulnerability to HI, prior to the window of maximal neuronal mGluR expression, and likely avoiding proconvulsant effects (McDonald et al., 1993). Given the prior literature in P7 Sprague Dawley rats (McDonald et al., 1993), it was prudent to examine behavioral seizure activity in our model, as it is well established that P6 Long-Evans rats have seizures during hypoxia. However, as reported, seizure activity was not altered by ACPD. Of note, Sprague Dawley rats are 2 days more mature than Long-Evans rats, due to a longer gestational period (Talos et al., 2006a, b; Dean et al., 2011), and hence were significantly older than those in our study (approximately analogous to a P9-P11 Long-Evans rat). Further, McDonald and colleagues tested ACPD at doses outside our range of 5–10 mg kg−1. Given the primary expression of group I mGluRs on OLs at P6 and markedly lower expression levels on neurons, astrocytes and microglia at this time point, our results indicate that ACPD’s protective effect is in part related to direct modulation of mGluRs on OLs.
In conclusion, the selective expression of mGluRs on OLs inpremature white matter may represent an age-specific therapeutic target. Their developmental expression on both rodent and human OLs may also be a subject for further studies of neuron-glia and glia-glia interactions (Maiese et al., 2005).
Supplementary Material
ACKNOWLEDGEMENTS
The authors are very grateful for the assistance provided by Bela Kosaras M.D., Jocelyn Lippman Bell Ph.D., Mirna Lechpammer M.D., Ph.D. and the support contributed by the Hearst Foundation (L.L.J.), the Heart and Stroke Foundation of Canada (L.L.J.), the Alberta Heritage Foundation for Medical Research (L.L.J.), PO1-NS038475 (F.E.J.), RO1-NS31718 (F.E.J.) and DP1-OD003347 (F.E.J.). The authors are also very grateful for the human tissue obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD.
Footnotes
Statement of interest
None.
Supplementary material
The supplementary material referred to in this article can be found online at journals.cambridge.org/ngb.
REFERENCES
- Alexander GM and Godwin DW (2006) Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Research 71, 1–22. [DOI] [PubMed] [Google Scholar]
- Allin M, Walshe M, Fern A, Nosarti C, Cuddy M, Rifkin L et al. (2008) Cognitive maturation in preterm and term born adolescents. Journal of Neurology, Neurosurgery, and Psychiatry 79, 381–386. [DOI] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ and Kinney HC (2001) Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. Journal of Neuroscience 21, 1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Riddle A and McClure MM (2007) Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke 38, 724–730. [DOI] [PubMed] [Google Scholar]
- Bao WL, Williams AJ, Faden AI and Tortella FC (2001) Selective mGluR5 receptor antagonist or agonist provides neuroprotection in a rat model of focal cerebral ischemia. Brain Research 922, 173–179. [DOI] [PubMed] [Google Scholar]
- Bayless S and Stevenson J (2007) Executive functions in school-age children born very prematurely. Early Human Development 83, 247–254. [DOI] [PubMed] [Google Scholar]
- Bear MF (2005) Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes, Brain and Behaviour 4, 393–398. [DOI] [PubMed] [Google Scholar]
- Benveniste H (1991) The excitotoxin hypothesis in relation to cerebral ischemia. Cerebrovascular & Brain Metabolism Reviews 3, 213–245. [PubMed] [Google Scholar]
- Biber K, Laurie DJ, Berthele A, Sommer B, Tolle TR, Gebicke-Harter PJ et al. (1999) Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. Journal of Neurochemistry 72, 1671–1680. [DOI] [PubMed] [Google Scholar]
- Boer K, Encha-Razavi F, Sinico M and Aronica E (2010) Differential distribution of group I metabotropic glutamate receptors in developing human cortex. Brain Research 1324, 24–33. [DOI] [PubMed] [Google Scholar]
- Butt AM (2006) Neurotransmitter-mediated calcium signaling in oligodendrocyte physiology and pathology. Glia 54, 666–675. [DOI] [PubMed] [Google Scholar]
- Byrnes KR, Loane DJ and Faden AI (2009a) Metabotropic glutamate receptors as targets for multipotential treatment of neurological disorders. Neurotherapeutics 6, 94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes KR, Stoica B, Loane DJ, Riccio A, Davis MI and Faden AI (2009b) Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 57, 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI (2008) Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic potential for addiction. Annals of the New York Academy of Sciences 1141, 221–232. [DOI] [PubMed] [Google Scholar]
- Catania MV, D’Antoni S, Bonaccorso CM, Aronica E, Bear MF and Nicoletti F (2007) Group I metabotropic glutamate receptors: a role in neurodevelopmental disorders? Molecular Neurobiology 35, 298–307. [DOI] [PubMed] [Google Scholar]
- Dean JM, Moravec MD, Grafe M, Abend N, Ren J, Gong X et al. (2011) Strain-specific differences in perinatal rodent oligodendrocytes lineage progression and its correlation with human. Developmental Neuroscience, doi: 10.1159/000327242. Epub ahead of Print, August 24, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Wang H, Rosenberg PA, Volpe JJ and Jensen FE (2004) Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proceedings of the National Academy of Sciences of the U.S.A. 101, 7751–7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antoni S, Berretta A, Bonaccorso CM, Bruno V, Aronica E, Nicoletti F et al. (2008) Metabotropic glutamate receptors in glial cells. Neurochemistry Research 33, 2436–2443. [DOI] [PubMed] [Google Scholar]
- Di Giorgi Gerevini V.D., Caruso A, Cappuccio I, Ricci Vitiani L., Romeo S, Della Rocca C. et al. (2004) The mGlu5 metabotropic glutamate receptor is expressed in zones of active neurogenesis of the embryonic and postnatal brain. Brain Research. Developmental Brain Research 150, 17–22. [DOI] [PubMed] [Google Scholar]
- Drouin-Ouellet J, Bownell A-L, Saint-Pierre M, Fasano C, Emond V, Trudeau L-E et al. (2011) Neuroinflammation is associated with changes in glial mGluR5 expression and the development of neonatal excitotoxic lesions. Glia 59, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farso MC, O’Shea RD and Beart PM (2009) Evidence group I mGluR drugs modulate the activation profile of lipopolysaccharide-exposed microglia in culture. Neurochemical Research 34, 1721–1728. [DOI] [PubMed] [Google Scholar]
- Fern R and Moller T (2000) Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. Journal of Neuroscience 20, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F and Shigemoto R (2006) Metabotropic glutamate receptors. Cell and Tissue Research 326, 483–504. [DOI] [PubMed] [Google Scholar]
- Ferriero D (2004) Neonatal brain injury. New England Journal of Medicine 351, 1985–1995. [DOI] [PubMed] [Google Scholar]
- Folkerth RD, Haynes RL, Borenstein NS, Belliveau RA, Trachtenberg F, Rosenberg PA et al. (2004) Developmental lag in superoxide dismutases relative to other anti-oxidant enzymes in premyelinated human telencephalic white matter. Journal of Neuropathology and Experimental Neurology 63, 990–999. [DOI] [PubMed] [Google Scholar]
- Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA et al. (2004) Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. Journal of Neuroscience 24, 4412–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Rosenberg PA, Volpe JJ and Jensen FE (2000) NBQX attenuates excitotoxic injury in developing white matter. Journal of Neuroscience 20, 9235–9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsette RE (2008) Oxidative stress and excitotoxicity: a therapeutic issue in multiple sclerosis? Multiple Sclerosis 14, 22–34. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA et al. (2003) Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. Journal of Neuropathology and Experimental Neurology 62, 441–450. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V et al. (2006) The mGluR antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-admnistration in C57BL/6J mice. Psychopharmacology 183, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Cheung PY and Todd KG (2005) Doxycycline reduces cleaved caspase-3 and microglial activation in an animal model of neonatal hypoxia-ischemia. Journal of Cerebral Blood Flow Metabolism 25, 314–324. [DOI] [PubMed] [Google Scholar]
- Jantzie LL and Todd KG (2010) Doxycycline inhibits proinflammatory cytokines but not acute cerebral cytogenesis after hypoxia-ischemia in neonatal rats. Journal of Psychiatry and Neuroscience 35, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen F (2006) Developmental factors regulating susceptibility to perinatal brain injury and seizures. Current Opinion in Pediatrics 18, 628–633. [DOI] [PubMed] [Google Scholar]
- Karadottir R and Attwell D (2007) Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience 145, 1426–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland EE and Toms NJ (2001) Group I metabotropic glutamate receptors limit AMPA receptor-mediated oligodendrocyte progenitor cell death. European Journal of Pharmacology 424, R3–R4. [DOI] [PubMed] [Google Scholar]
- Kinney HC and Back SA (1998) Human oligodendroglial development: relationship to periventricular leukomalacia. Seminars in Pediatric Neurology 5, 180–189. [DOI] [PubMed] [Google Scholar]
- Kobaly K, Schluchter M, Minich N, Friedman H, Taylor HG, Wilson-Costello D et al. (2008) Outcomes of extremely low birth weight (<1 kg) and extremely low gestational age (<28 weeks) infants with bronchopulmonary dysplasia: effects of practice changes in 2000 to 2003. Pediatrics 121, 73–81. [DOI] [PubMed] [Google Scholar]
- Larroque B, Ancel PY, Marret S, Marchand L, Andre M, Arnaud C et al. (2008) Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet 371, 813–820. [DOI] [PubMed] [Google Scholar]
- Lechpammer M, Manning SM, Samonte F, Nelligan J, Sabo E, Talos DM et al. (2008) Minocycline treatment following hypoxic/ischaemic injury attenuates white matter injury in a rodent model of periventricular leukcomalacia. Neuropathology and Applied Neurobiology 34, 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Lin N, Wu B and Qiu Y (2009) Neuroprotective effect of memantine combined with topiramate in hypoxic-ischemic brain injury. Brain Research 1282, 173–182. [DOI] [PubMed] [Google Scholar]
- Lojkova D and Mares P (2005) Anticonvulsant action of an antagonist of metabotropic glutamate receptor mGluR5 MPEP in immature rats. Neuropharmacology 49, 219–229. [DOI] [PubMed] [Google Scholar]
- Lujan R, Roberts JD, Shigemoto R, Ohishi H and Somogyi P (1997) Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1a, mGluR2, mGluR5, relative to neurotransmitter release sites. Journal of Chemical Neuroanatomy 13, 219–241. [DOI] [PubMed] [Google Scholar]
- Luscher C and Huber KM (2010) Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyt K, Varadi A, Durant CF and Molnar E (2006) Oligodendroglial metabotropic glutamate receptors are developmentally regulated and involved in the prevention of apoptosis. Journal of Neurochemistry 99, 641–656. [DOI] [PubMed] [Google Scholar]
- Luyt K, Varadi A and Molnar E (2003) Functional metabotropic glutamate receptors are expressed in oligodendrocyte progenitor cells. Journal of Neurochemistry 84, 1452–1464. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ and Li F (2005) Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Current Neurovascular Research 2, 425–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning SM, Talos DM, Zhou C, Selip DB, Park HK, Park CJ et al. (2008) NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. Journal of Neuroscience 28, 6670–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B et al. (2008) Annual summary of vital statistics: 2006. Pediatrics 121, 788–801. [DOI] [PubMed] [Google Scholar]
- McDonald JW and Johnston MV (1990) Physiological and pathological roles of excitatory amino acids during central nervous system development. Brain Research. Brain Research Reviews 15, 41–70. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Fix AS, Tizzano JP and Schoepp DD (1993) Seizures and brain injury in neonatal rats induced by 1S,3R-ACPD, a metabotropic glutamate receptor agonist. Journal of Neuroscience 13, 4445–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF (2009) Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Current Drug Abuse Reviews 2, 83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhade SN, Zhou C, Aujla PK, Fishman R, Sucher NJ and Jensen FE (2008) Early alterations of AMPA receptors mediate synaptic potentiation induced by neonatal seizures. Journal of Neuroscience 28, 7979–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro FM, Paquet M, Cregan SP and Ferguson SS (2010) Group I metabotropic glutamate receptor signaling and its implication in neurological disease. CNS & Neurological Disorders - Drug Targets 9, 574–595. [DOI] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van Den Pol AN and Olney JW (1995) Distribution of metabotropic glutamate receptor mGluR immunoreactivity in rat brain. Journal of Comparative Neurology 355, 455–469. [DOI] [PubMed] [Google Scholar]
- Silbereis JC, Huang EJ, Back SA and Rowitch DH (2010) Towards improved animal models of neonatal white matter injury associated with cerebral palsy. Disease Models & Mechanisms 3, 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR and Bear MF (2001) Internalization of ionotropic glutamate receptors in response to mGluR activation. Nature Neuroscience 4, 1079–1085. [DOI] [PubMed] [Google Scholar]
- Talos D, Fishman R, Park H, Folkerth R, Follett P, Volpe JJ et al. (2006a) Developmental regulation of alpha-amino-3-hydroxy- 5-methyl-4-isoxazole-proprionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. Journal of Comparative Neurology 497, 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos D, Follett P, Folkerth R, Fishman R, Trachtenberg F, Volpe JJ et al. (2006b) Developmental regulation of alpha-amino- 3-hydroxy-5-methyl-4-isoxazole-proprionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. Journal of Comparative Neurology 497, 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DL, Pirianov G, Holland S, McGinnity CJ, Norman AL, Reali C et al. (2010) Attenuation of proliferation in oligodendroctye precursor cells by activated microglia. Journal of Neuroscience Research 88, 1632–1644. [DOI] [PubMed] [Google Scholar]
- Ure J, Baudry M and Perassolo M (2006) Metabotropic glutamate receptors and epilepsy. Journal of the Neurological Sciences 247, 1–9. [DOI] [PubMed] [Google Scholar]
- Vannucci RC (2000) Hypoxic-ischemic encephalopathy. American Journal of Perinatology 17, 113–119. [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurology 8, 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Lee PR and Fields RD (2011) Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Gerber G, Kojic L and Randic M (2000) Dual modulation of excitatory synaptic transmission by agonists at group I metabotropic glutamate receptors in the rat spinal dorsal horn. Brain Research 887, 359–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.