Abstract
A number of prospective cohort studies are ongoing worldwide to investigate the impact of foetal and neonatal exposures to chemical substances on child health. To assess multiple exposure (mixture) effects and low prevalence health outcomes it is useful to pool data from several studies and conduct mega-data-analysis. To discuss a path towards data harmonization, representatives from several large-scale birth cohort studies and a biomonitoring programme formed a collaborative group, the Environment and Child Health International Birth Cohort Group (ECHIBCG). In this study, an intra-laboratory trial was performed to harmonize existing blood lead measurements within the groups’ studies. Then, decentralized analyses were conducted in individual countries’ laboratories to evaluate blood lead levels (BLL) in each study. The measurements of pooled BLL samples in French, German and three Japanese laboratories resulted in an overall mean blood lead concentration of 8.66 μg l−1 (95% confidence interval: 8.59–8.72 μg l−1) with 3.0% relative standard deviation. Except for China’s samples, BLL from each study were comparable with mean concentrations below or close to 10 μg l−1. The decentralized multivariate analyses revealed that all models had coefficients of determination below 0.1. Determinants of BLL were current smoking, age > 35 years and overweight or obese status. The three variables were associated with an increase in BLL in each of the five studies, most strongly in France by almost 80% and the weakest effect being in Norway with only 15%; for Japan, with the far largest sample (~18,000), the difference was 36%. This study successfully demonstrated that the laboratory analytical methods were sufficiently similar to allow direct comparison of data and showed that it is possible to harmonize the epidemiological data for joint analysis. This exercise showed the challenges in decentralized data analyses and reinforces the need for data harmonization among studies.
Keywords: Human Biomonitoring, HBM, Birth cohort, Lead, Harmonization, Pregnancy, Child, Environment
Introduction
Foetal and neonatal exposure to chemical substances may lead to adverse health effects such as developmental disorders, immune system dysfunction and hormone disruptions in later life (Barouki et al., 2012; Grandjean et al., 2008). Birth cohort studies are one of the major tools to identify these associations between the environment and children’s health and, when possible, to confirm causalities. In the past decades, many birth cohort studies have been conducted to investigate the impact of a variety of chemical substances (Baldacci et al., 2018; Barbone et al., 2019; Botton et al., 2016; Boucher et al., 2009; Casas et al., 2015; Clemente et al., 2016; Dzwilewski and Schantz, 2015; Hertz-Picciotto et al., 2008; Huang et al., 2016; Iszatt et al., 2015; Perera et al., 2006; Pilsner et al., 2009; Rauh et al., 2011; Shelton et al., 2014; Trasande et al., 2009). However, those individual studies often have not yielded conclusive results as regards the association between a particular exposure and health outcomes, mainly due to insufficient statistical power. To overcome this, large-scale birth cohort studies were initiated in the late 1990s in Norway and Denmark (Magnus et al., 2016; Olsen et al., 2001). The United States (US), Japan, France, UK and South Korea followed, the US and UK studies however, were terminated early (Branum et al., 2003; Dereumeaux et al., 2017; Kawamoto et al., 2014; Kishi et al., 2011; Lee et al., 2017; Vandentorren et al., 2009). These studies each involve 20,000–100,000 participants and represent major steps forward towards enabling the conduct of more conclusive studies into the relationships between the environment and common outcomes in children’s health and development. Because of practical issues and cost of sampling and chemical analyses, however, exposure data for some specific chemicals may be available for only a subset of the cohort. In addition, for studies of rare diseases such as childhood cancers, type-1 diabetes, congenital anomalies or sudden infant death syndrome (SIDS), a single study may have insufficient statistical power. The number of 100,000 also may fall short when assessing the effect of multiple environmental factors at the same time, e.g. the collective effect of lead, mercury, cadmium, persistent organic pollutants (POPs), pesticides and other compounds of emerging concern such as perfluoroalkyl and polyfluoroalkyl substances, phthalates and parabens on children’s health and development.
One way of increasing statistical power is to perform a meta-analysis of multiple study results (Huang et al., 2016). Acquiring information on associations between children’s health and environmental exposures by meta-analysis of data from different birth cohort studies poses a number of challenges: studies on environmental health employ a variety of instruments, including for health and development, questionnaires, in-person or phone interviews, physical examinations, clinical tests and for environmental exposures, interviews, environmental monitoring or sampling modelling and human biomonitoring. Especially for exposure assessment, it is often hard to compare data across studies, because of non-standardized procedures used in each study. For instance, each study may employ different sampling and analytical methods for human biomonitoring. Even if they use exactly the same methods, each laboratory performs analyses differently. To overcome such problems in meta-analysis, birth cohort consortia have been formed to aim at pooled analysis or mega-analysis instead of meta-analysis. In the mega-analysis data from individual studies are pooled or combined together in a central location and analysed as one big data set, while in the meta-analysis individual decentralised analysis results are statistically compared. In short, the mega-analysis combines data and the meta-analysis gathers results from each study. Examples are the International Childhood Cancer Cohort Consortium (I4C) (Brown et al., 2007), Environmental Health Risks in European Birth Cohorts (ENRIECO) (Gehring et al., 2013) and the Birth Cohort Consortium of Asia (BiCCA) (Kishi et al., 2017). In order to pool each study’s data, it is crucial to perform data or method harmonization that should involve standardisation of study procedures such as questionnaires, physical/developmental examinations, clinical tests and laboratory analyses before data collection or normalisation of existing data.
Our consortium, the Environment and Child Health International Birth Cohort Group (ECHIBCG) (Etzel et al., 2014), has been exercising the harmonization of study methods and aims at harmonizing data including infant health outcomes, biomarkers, environmental measurements, socioeconomic and migration status. Recently, we have focused on exposure measurements by identifying chemicals of common interest; comparing each study’s questionnaire and laboratory methodologies; and conducting inter-laboratory procedural tests. In order to make data comparable, retrospective harmonization is as important as prospective harmonization because in most cases data are already collected.
The major aims of this research were to perform ad hoc data harmonization for blood lead measurements, and to evaluate common factors associated with blood lead levels (BLL). Based on a comparison of each study’s procedures, results of a round-robin practice and findings of a joint data analysis, we present lessons learned with respect to cooperation of birth cohort and environmental health studies.
Materials and methods
Participating studies and organisations
In late 2011, the Japan Ministry of the Environment (JMOE) invited investigators associated with some large-scale 21st century birth cohort studies to discuss about how to better design the assessment of disease outcomes, biomarkers and environmental exposures. Investigators from various large-scale cohort studies discussed the benefit of data pooling among the studies and need for study harmonization (Ishitsuka et al., 2017). The JMOE suggested that it would be useful to establish working groups to define a list of core elements for inclusion in the harmonization. Such core elements could include disease outcome definitions, biomarkers and exposure measurements. A working group was therefore proposed to discuss and exchange information about ongoing and forthcoming large-scale birth cohort studies and national bio-/environmental monitoring projects. Experts from new large-scale studies of environmental influences on children’s health and development that were being planned or conducted in France, Germany, Japan, Shanghai (China) and the US constituted the Environment and Child Health International Birth Cohort Group (ECHIBCG) in 2011 (Etzel et al., 2014). Later, Denmark and Norway joined and the International Agency for Research on Cancer (IARC) became the secretariat of the group. The current ECHIBCG is jointly coordinated by Japan and Germany and consists of the Danish Birth Cohort Study (DNBC) (Olsen et al., 2001), the Etude Longitudinale Françise depuis l’Enfance (Elfe) (Dereumeaux et al., 2017; Vandentorren et al., 2009), the Japan Environment and Children’s Study (JECS) (Kawamoto et al., 2014; Nakayama et al., 2019), the Norwegian Mother and Child Study (MoBa) (Magnus et al., 2016), the Shanghai Birth Cohort Study (SBC) (Zhang et al., 2019) and experts from the German Environment Ministry, the German Environment Agency and (until September 2018) the US Environmental Protection Agency (the information of the member studies is summarised in the Supplementary Information and Table S1). Elfe, the Environmental Specimen Bank (ESB) from Germany (Kolossa-Gehring et al., 2012), JECS, SBC and MoBa provided data for this exercise (detailed information on sample selection and procedures followed is given in the Supplementary information).
Round-robin test procedures
The group undertook an inter-laboratory comparison (round-robin) project in 2014 for blood lead analysis. Participating laboratories were three from Japan, and one each from France and Germany. The group first examined each country’s custom regulations for the transportation of frozen blood samples. Second, water samples were sent from Japan on dry ice and with temperature loggers to France and Germany to evaluate the transportation process. The group used JECS in-house reference material (JECS RM) for whole blood round-robin tests among France, Germany and Japan. JECS RM was made from a pooled whole blood legally obtain from the Japan Red Cross. Aliquots of RM were undergone elemental analysis, including lead, mercury and cadmium, to confirm homogeneity and then frozen. The elemental analysis was also conducted after several cycles of freeze and thaw to verify the robustness of the RM (data not shown). Four vials of the JECS RM (2-ml polypropylene cryo-vials with 2-D barcode on the bottom) were shipped to each designated laboratory or facility in France, Germany and Japan. The vials were placed in a secondary container (polypropylene) that was packed in a plastic bag. The sample containers were then set in a Styrofoam box that contained a few kg of dry ice. A temperature logger was placed on top of the samples. Upon receipt, the participating laboratories visually observed the sample condition and filled out a shipment evaluation sheet. Each laboratory sent the completed evaluation sheet and the temperature logger back to the JECS Programme Office where the information was examined and compiled.
The JECS RM was kept in its original vials and stored frozen at or below negative 20°C until use. Freezers were temperature controlled and monitored with limited temperature fluctuation. Before use, a frozen sample was allowed to thaw at room temperature. The sample was mixed by gently rocking or mildly swirling (not shaking) the vial to remix any water that may have separated on freezing. Each laboratory analysed all 4 vials in at least 3 replicates for lead using its own methods. The laboratories were provided with an electronic reporting format, in which they reported the results of the analysis including concentrations, method summary description and method performance characteristics. The laboratory analytical procedures involved in this trial are shown in Table 1.
Table 1.
Summary of the analytical procedures employed in the laboratories that participated in the round-robin trial.
| France | Germany | Japan | |
|---|---|---|---|
| Sample preparation | Samples (300 μl) were transferred into a 13-ml polypropylene tube and mixed with 2,700 μl dilution solution. Dilution solution was made by adding 10 ml 25% NH4OH, 250 mg EDTA dipotassium salt and 250 μl Triton X-100 into a volumetric flask and filled up to 500 ml with double distilled water. |
Samples (300 μl) were transferred into a 13-ml polypropylene tube and mixed with 2,700 μl dilution solution. Dilution solution was made by adding 10 ml 25% NH4OH, 250 mg EDTA dipotassium salt and 250 μl Triton X-100 into a volumetric flask and filled up to 500 ml with double distilled water. |
Samples (200 μl) were diluted (1:19) with the dilution solution and vortex mixed. Dilution solution consisted of 2% v/v butan-1-ol, 0.1% TMAH, 0.5 g l−1 POE and 0.5 g l−1 H4EDTA |
| Instrumental analysis | ICP-MS (Agilent 7500) with ASX 500 auto-sampler | ICP-DRC-MS (Agilent 7500cx) with ASX 500 auto-sampler | ICP-MS (Agilent 7700 ICP-MS) with auto-sampler |
| Calibration and calculation | External calibration was used. The limit of quantitation was 0.15 μg l−1. | External calibration was used. The limit of quantitation was 0.15 μg l−1. | External calibration was used. The limit of quantitation was 0.14 μg l−1. |
Blood lead measurements and covariate data acquisition
BLL were determined in mothers’ whole blood during pregnancy in Japan and Norway, in whole blood in Germany (female ESB participants, non-pregnant women), and in cord blood in China and France. All measurements were above detection limits. Detection limits varied across studies, with the lowest limit in Norway (0.08 μg l−1) and the highest limit in France (0.6 μg l−1). The timing of the blood collection also varied across studies; for Norway it was 2002–08, Germany 2010–16, France 2011, Japan 2011–14 and China 2014–15. No calibrations were made for whole blood vs. cord blood or for the time period, as it was not possible to quantify the possible impact due to lack of comparison data. All BLL were harmonized to the unit of μg l-1.
Possible explanatory factors of maternal BLL as identified in a stepwise process were acquired from questionnaires developed by each study and had to be harmonized across studies. A literature search was performed first looking for factors being identified in at least one published study as influencing BLL and that had been assessed in at least the majority of our studies. Selected explanatory factors at the time of sampling were maternal smoking (current, former, not active but passive smoking in household, and never smoking including no current passive smoking); maternal age (categorized into < 25 years, 25–34 years, 35+ years); maternal body mass index (BMI) [categorized into < 18.5, 18.5–25, and 25+ kg/m2 (combining overweight and obese, as the latter group included too few subjects)]; consumption of coffee, tea, tap water, or bottled water (all in categories less than once, 1–2 times, 3+times per week); alcohol drinking [current when pregnancy was known, former (stopped before pregnancy or as soon as known), never]; chocolate consumption (categorized into less than once, 1–2 times, 3+ times per week) and whether renovation work at home took place during pregnancy. In Germany, variables referring to pregnancy referred to the sampling period, as the sampled population was non-pregnant women. Maternal education was kept in the final model for adjustment in the original categories, usually from low to high in several steps, as studies came from different parts of the world and the attempts to harmonise were not meaningful. Other harmonised variables not kept in the final analytical model were sex of offspring, pre-term or term pregnancy, or parity (first vs. later born child) (those pregnancy-related variables are not relevant in Germany as the study population was not pregnant women), as well as consumption of bread, dairy products, seafood, or vegetables, and year of construction of the house where the woman lived. Slight compromises had to be made in variables related to food consumption, as dietary questions were not identical. In China, Japan and Norway the item “tea” consisted of only green tea while in France and Germany it included different types of tea. In Japan, coffee consumption was specified as only from beans and green tea as only from leaves. In France, units of coffee and tea consumption were measured in less than once, once, or 2+ times per week.
Statistical analysis
Because all of the studies had to follow their own countries/regions’ data protection rules/legislation, most of the studies were not able to share the data with others. Thus, we employed de-centralized data analysis using different statistical packages. The first step was a descriptive analysis, obtaining univariate statistics by study, e.g. means, medians and percentiles. For visualisation, we created a boxplot-like figure in which the 10th and 90th percentiles were used as whiskers, the 1st (25th percentile) and 3rd quartile (75th percentile) as the box range, the median (50th percentile) as box separator, and the 99th percentile as external extra asterisk, rather than the common boxplot definition, to avoid distraction by outliers because we aimed at a visual comparison of the majority of measured values across studies. After identifying common factors influencing BLL, studies analysed their own data and selected factors having influence on the BLL, irrespective of the magnitude. From this list, a first stage linear model was developed including all factors on the combined list. From this run, all variables that did not result in at least a 20% change in BLL in at least one of the studies were removed. This final model was applied independently to each study to obtain the coefficients of the individual explanatory factors and their uncertainty, as well as the unadjusted and adjusted R2 of the model, indicating the percentage variation in BLL explained by the model (final selection of variables described in the previous section). The 20% change criterion for choosing variables was preferred over the p-value due to the highly varying sample sizes across studies; while for Japan even tiny changes became statistically significant, this was only the case for major changes in China, for instance. Finally, we applied to the data the coefficients for smoking, maternal age and BMI, which were the relevant explanatory factors with highest comparability across studies, to compare predictions of how much BLL increase on average with exposure to current smoking, high maternal age, and high BMI in the individual studies. Instructions for analyses were developed at the IARC together with the study principal investigators, to make sure the approach was identical; afterwards analyses were carried out de-centrally, as mentioned above. All studies had ethics approval at the national level.
Results
Inter-laboratory comparison
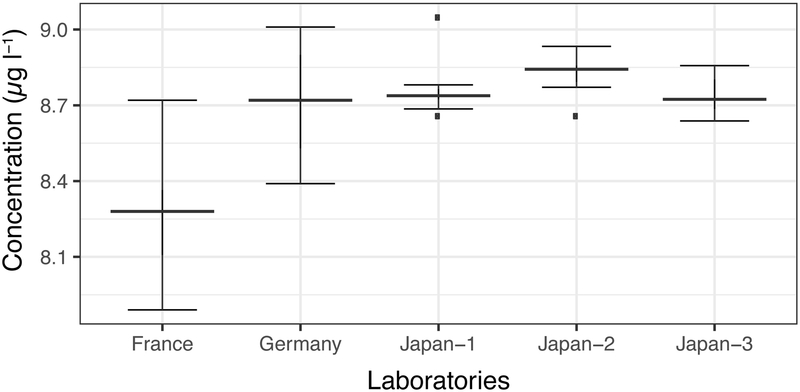
Germany used the inductively-coupled plasma mass spectrometry with dynamic reaction cell technology (ICP-DRC-MS) method, while the rest of laboratories used the inductivity coupled plasma mass spectrometry (ICP-MS), with slightly different sample pre-treatment procedures. The result of the round-robin trial is illustrated in Figure 1. French and German laboratories reported mean lead concentrations of 8.71 and 8.27 μg l−1 with relative standard deviations (RSDs) of 2.9% and 2.5%, respectively. Three Japanese laboratories showed mean concentrations of 8.76, 8.83 and 8.75 μg l−1 with RSDs of 1.1%, 0.8% and 0.8%, respectively. Overall mean concentration was 8.66 μg l−1 (95% confidence interval: 8.59–8.72 μg l−1) with 3.0% RSD.
Figure 1.
Concentrations of lead in the whole blood reference material as determined in the round-robin exercise (μg l−1).
Boxplots were depicted using 1.5 times the interquartile range as whiskers, interquartile range as the box ranges and the median as the box separators.
Current blood lead levels
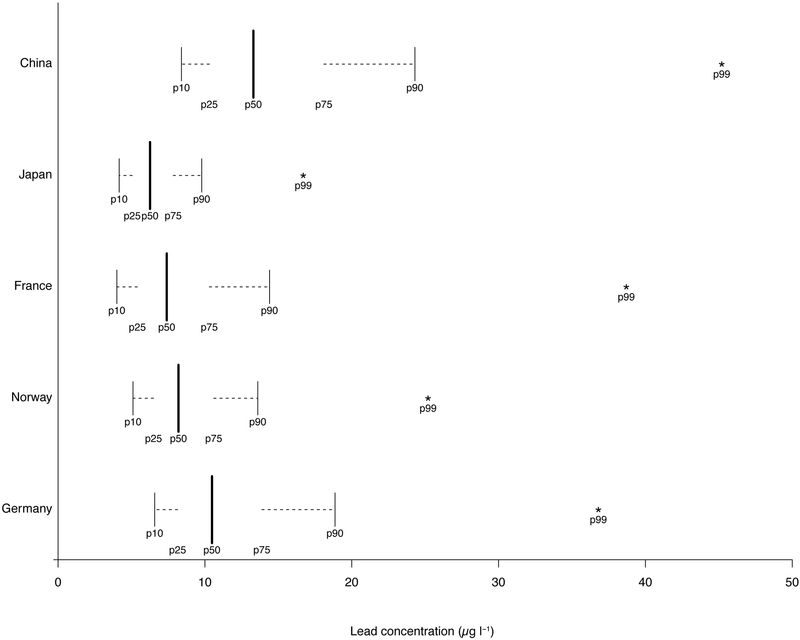
Each study using its own method reported lead concentrations in whole blood samples. Numbers of available samples differed greatly by study, with 17,998 samples from Japan, 2,982 from Norway, 1,842 from Germany, 1,670 from France, and 423 from China. Distributions of BLL by study are displayed in Figure 2. With the exception of China, median values were close to or lower than 10 μg l−1 and 90th percentiles close to or lower than 20 μg l−1. None of the 99th percentiles exceeded 50 μg l−1. Maximum levels were 212 μg l−1 in Norway, 107 μg l−1 in France, 103 μg l−1 in Germany, 80.5μg l−1 in China, and 74.5 μg l−1 in Japan.
Figure 2.
Illustrative representation of summary statistics of blood lead level in each study.
Summary statistics of blood lead levels in each study are illustrated in boxplot-like shapes. P10, P25, P50, P75, P90 and P99 represents the 10th, 25th, 50th, 75th, 90th and 99th percentile, respectively.
Determinants of blood lead levels
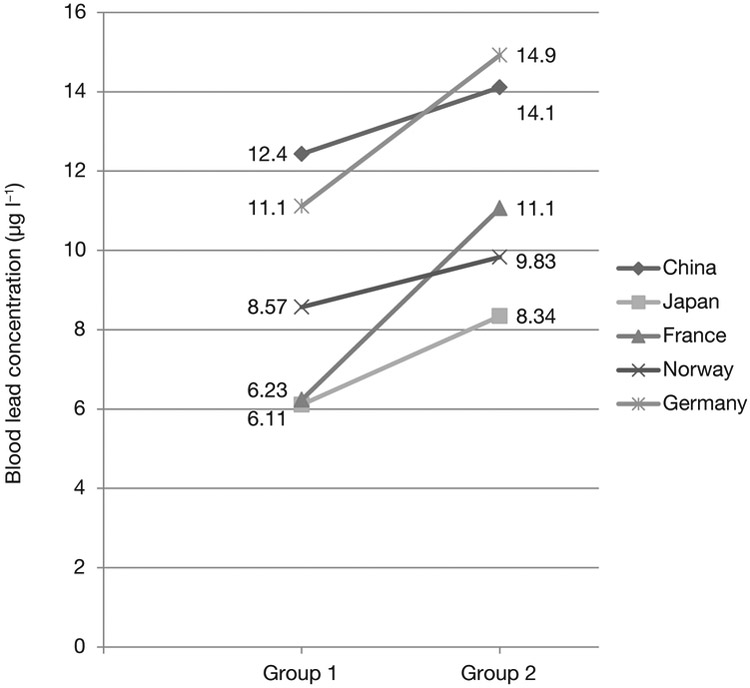
Each group analysed their own data to examine determinants of BLL according to the same instructions as described in the previous section. Table 2 shows the results of the final model with explanatory factors having an impact of at least a 20% change in average BLL in at least one study, and subsequently applied to all studies. Items shown in Table 2 include the number of subjects in each category, the coefficient of change and its standard error compared to the reference category, as well as the intercept of the model (reflecting the BLL by country sample with all explanatory factors in their reference category; bottom of the table) and the R2 values. In line with Figure 2, the intercept shown in Table 2 confirms the lowest BLL in Japan and France and the highest in China and in Germany. The R2 was below 0.1 in each study; the highest R2 was seen in France. Current smoking was associated with increased BLL in all studies in a similar magnitude (including the very low number of subjects in China), except for Germany, where the increase was more pronounced. A positive association also was seen in all studies with increasing maternal age, whereas most studies showed an increase in overweight and obese women, with the exception of Norway. Patterns of coffee, tea or tap water consumption showed weaker associations, not always entirely consistent across studies, and even lesser so for alcohol, chocolate and renovation work at home (Table 2). Figure 3 shows the change in BLL by of women who were never active smokers and were not passive smokers, were aged <25 years, and were underweight compared to those who were current smokers, aged 35 years or older, and were overweight or obese. The combination of those 3 variables was associated with an increase in BLL in each of the 5 studies; most strongly in France by almost 80% and the weakest effect in Norway with only 15%, for Japan, with the far largest sample, the difference was 36%.
Table 2.
Analyses of factors influencing lead concentration in blood by study, showing the numbers in categories (N), the regression coefficient (β) and its standard error (S.E.), and the intercept and unadjusted and adjusted R2 at the bottom of the table.
| French National Birth Cohort Study (Elfe) |
German Environmental Specimen Bank (ESB) |
Japan Environment and Children’s Study (JECS) |
Norwegian Mother and Child Cohort Study (MoBa) |
Shanghai Birth Cohort Study |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size for the lead analysis | 1,670 | 1,842 | 17,998 | 2,982 | 423 | |||||||||||
| Timing and type of sampling for lead |
Cord blood 2011 |
Whole blood from women aged 20–29 2010–2016 |
Maternal blood (mid–late trimester) 2011–2014 |
Maternal blood (week 18 of pregnancy) 2002–2008 |
Cord blood 2014–2015 |
|||||||||||
| Variable | N | β | S.E. | N | β | S.E. | N | β | S.E. | N | β | S.E. | N | β | S.E. | |
| Smoking | Never | 701 | 1.00 | - | 1081 | 1.00 | - | 7032 | 1.00 | - | 1552 | 1.00 | - | 335 | 1.00 | - |
| Passive | 20 | 0.08 | 1.87 | 332 | 0.84 | 0.30 | 2894 | 0.17 | 0.07 | 100 | 0.35 | 0.46 | 74 | 1.43 | 0.88 | |
| Former | 399 | −0.09 | 0.42 | 200 | 1.65 | 0.36 | 6729 | 0.34 | 0.05 | 1157 | 0.20 | 0.19 | 12 | 3.98 | 2.60 | |
| Current | 380 | 1.13 | 0.49 | 213 | 3.33 | 0.35 | 884 | 1.27 | 0.11 | 173 | 1.19 | 0.39 | 2 | NA | NA | |
| Maternal age& | <25 years | 255 | 1.00 | - | 1224 | 1.00 | - | 1403 | 1.00 | - | 241 | 1.00 | - | 25 | 1.00 | - |
| 25-34 years | 1144 | 0.81 | 0.56 | 618 | 0.66 | 0.24 | 9149 | 0.21 | 0.08 | 2257 | −0.003 | 0.32 | 352 | 0.69 | 1.67 | |
| 35+ years | 261 | 1.98 | 0.68 | 0 | NA | NA | 4000 | 0.60 | 0.09 | 484 | 0.53 | 0.38 | 46 | 0.58 | 2.10 | |
| BMI | <18.5 kg/m2 (underweight) | 123 | 1.00 | - | 112 | 1.00 | - | 2573 | 1.00 | - | 94 | 1.00 | - | 56 | 1.00 | - |
| ≥18.5 and 25 kg/m2 (healthy) | 1032 | 0.80 | 0.69 | 1494 | 0.43 | 0.56 | 11579 | 0.07 | 0.06 | 1913 | −0.22 | 0.47 | 320 | 1.68 | 1.20 | |
| ≥25 kg/m2 (overweight and obese) | 494 | 1.72 | 0.73 | 228 | 0.48 | 0.61 | 1691 | 0.36 | 0.09 | 918 | −0.46 | 0.49 | 47 | 1.10 | 1.75 | |
| Coffee consumption* | Less than 1 time per week | 790 | 1.00 | - | 653 | 1.00 | - | 13116 | 1.00 | - | 1489 | 1.00 | - | 0 | 1.00 | - |
| 1-2 times per week$ | 225 | 0.72 | 0.64 | 85 | 1.00 | 0.54 | 1309 | 0.27 | 0.08 | 410 | 0.60 | 0.26 | 0 | NA | NA | |
| More than 3 times per week$$ | 482 | 1.52 | 0.41 | 1101 | 0.27 | 0.25 | 1287 | 0.20 | 0.08 | 1083 | 0.91 | 0.20 | 0 | NA | NA | |
| Tea consumption** | Less than 1 time per week | 937 | 1.00 | - | 501 | 1.00 | - | 8822 | 1.00 | - | 2091 | 1.00 | - | 358 | 1.00 | - |
| 1-2 times per week$ | 233 | −0.07 | 0.60 | 160 | −0.14 | 0.39 | 2335 | 0.07 | 0.07 | 380 | 0.04 | 0.26 | 0 | NA | NA | |
| More than 3 times per week$$ | 205 | 1.95 | 0.52 | 1181 | 0.21 | 0.24 | 4686 | 0.31 | 0.05 | 511 | 1.07 | 0.23 | 65 | 0.32 | 1.16 | |
| Tap water consumption# | Less than 1 time per week | 640 | 1.00 | - | 37 | 1.00 | - | 10380 | 1.00 | - | 87 | 1.00 | - | 101 | 1.00 | - |
| 1-2 times per week$ | 80 | 0.56 | 0.83 | 12 | 0.69 | 1.60 | 1390 | 0.10 | 0.08 | 30 | −0.20 | 0.96 | 0 | NA | NA | |
| More than 3 times per week$$ | 781 | 0.68 | 0.44 | 1788 | 0.39 | 0.78 | 4073 | 0.25 | 0.05 | 2829 | −0.48 | 0.50 | 322 | 0.79 | 1.06 | |
| Bottle water consumption | Less than 1 time per week | 344 | 1.00 | - | NA | 1.00 | - | 7379 | 1.00 | - | 1800 | 1.00 | - | 165 | 1.00 | - |
| 1-2 times per week$ | 152 | 0.76 | 0.66 | NA | NA | NA | 1834 | −0.13 | 0.08 | 377 | −0.20 | 0.25 | 0 | NA | NA | |
| More than 3 times per week$$ | 999 | −0.89 | 0.48 | NA | NA | NA | 6630 | −0.21 | 0.05 | 640 | −0.26 | 0.21 | 258 | 2.28 | 1.58 | |
| Alcohol (when pregnancy was known) | Never | 1269 | 1.00 | - | NA | 1.00 | - | 8018 | 1.00 | - | 278 | 1.00 | - | 347 | 1.00 | - |
| Former | 105 | 0.16 | 0.70 | NA | NA | NA | 7320 | 0.15 | 0.05 | 2599 | 0.57 | 0.30 | 43 | −1.04 | 1.38 | |
| Current | 283 | −0.13 | 0.46 | NA | NA | NA | 505 | 0.81 | 0.13 | 79 | 1.91 | 0.61 | 2 | NA | NA | |
| Chocolate consumption## | Less than 1 time per week | 212 | 1.00 | - | 196 | 1.00 | - | 2773 | 1.00 | - | 880 | 1.00 | - | 0 | 1.00 | - |
| 1-2 times per week | 174 | 0.39 | 0.71 | 173 | 0.20 | 0.43 | 4797 | −0.12 | 0.05 | 1244 | −0.19 | 0.20 | 0 | NA | NA | |
| More than 3 times per week | 1087 | −0.70 | 0.52 | 1472 | −0.52 | 0.32 | 8358 | −0.23 | 0.05 | 794 | −0.25 | 0.22 | 0 | NA | NA | |
| Works at home | No renovation of residential home+ | 887 | 1.00 | - | NA | 1.00 | - | 17044 | 1.00 | - | NA | 1.00 | - | 356 | 1.00 | - |
| Renovation of residential home++ | 616 | 0.33 | 0.35 | NA | NA | NA | 564 | 0.28 | 0.06 | NA | NA | NA | 16 | −3.22 | 2.20 | |
| Value | Value | Value | Value | Value | ||||||||||||
| Intercept (α) | 6.23 | 11.11 | 6.11 | 8.57 | 12.43 | |||||||||||
| R2 | 0.09 | 0.04 | 0.03 | 0.03 | 0.05 | |||||||||||
| R2 (adjusted) | 0.08 | 0.04 | 0.03 | 0.03 | 0.00 | |||||||||||
NA: not applicable
age (Germany)
from leaf (Japan)
green tea (China, Japan and Norway)
all types of water (Germany)
sweets including chocolate (Germany)
weekly (France)
daily (France and China, except for coffee)
after becoming pregnant (Japan)
after becoming pregnant (Japan)
Figure 3.
Change in blood lead concentrations according to the influence of the variables age, BMI and smoking patterns for each study.
Women who never smoked and had no passive smoke exposure, were aged <25 years and were underweight (Group 1) in comparison to current smoking, age 35+ years and overweight or obese women (Group 2).
Discussion
Round-robin trial
The United States National Institute of Standards and Technology (NIST) standard reference material (SRM) 995c (toxic elements in caprine blood), which was certified for lead at four concentration levels: a base level and three progressively elevated levels, was initially considered suitable for use in the round-robin trial. It appeared, however, that in most countries a health certificate was required to import the SRM 955c (made of goat blood). Considering that NIST was not ready to issue the certificate, the use of the SRM 955c did not seem practical. The group decided to use a JECS in-house RM made of human blood officially acquired from the Japanese Red Cross. The shipment took from 3 days and went well with the temperature kept below negative 60°C on dry ice. China found that it could not import human materials easily. Sending samples on dry ice to China was also extremely difficult and expensive. There are existing mechanisms of international laboratory accreditation such as the German Environmental Quality Assurance Scheme (G-EQUAS) and the Canadian Programme run by the Centre de Toxicologie du Quebec (CTQ). Since lead is a well-studied contaminant, all the laboratories performed very well on the blood lead analysis. Repeatability of the measurements was satisfactory with RSD of replicate analyses being < 3% for all the participating laboratories. With this precision, a common statistical practice resulted in detecting statistically significant differences (p < 0.05) among individual laboratories’ results. However, such small differences were considered irrelevant for the anticipated data use. After pooling data from all the laboratories, the overall RSD was still 3.0%.
Determinants of blood lead levels
The major aim of this part of the study was to evaluate common factors associated with BLL, and some observed consistencies (see Table 2). Some inconsistencies across the five studies from five different countries are of interest. The R2 was below 10% in each study, suggesting that, albeit being associated with the BLL, the explanatory factors have little predictive power to estimate individual BLL. This was common across studies, although we used a very inclusive approach of investigating potential explanatory factors. This confirms that not all relevant information that may explain variation in BLL can be captured by questionnaires. For example, on dietary-related factors, individual dietary patterns (e.g., how many vegetables one eats on average) have less influence on BLL than the food location or the processing method of the vegetables the person consumes. This is the likely reason why all dietary factors, including type of water consumption or beverages like coffee, tea or alcohol, did not emerge as strong associations. It is an important finding however, as this observation applies commonly to all countries. The main modifiable factor at the individual level to lower BLL appears to be quitting smoking, again consistent across countries. Other consistent factors are either not modifiable, like age, or not recommendable to attain for health reasons, e.g. BLL were lower in underweight persons. Additionally, the cause-and-effect relationship is not always clear. For instance, there are partly opposite findings in the literature for the relation between BLL and BMI. Although some authors find increased BLL being related to lower BMI and obesity in children, adolescents, and adults (Cassidy-Bushrow et al., 2016; Scinicariello et al., 2013; Shao et al., 2017), there also is sex-specific evidence for the opposite (Wang et al., 2015). Additionally, the effect of chronic lead exposure, as expressed e.g. by dentin lead levels (Kim et al., 1995), might differ from the effect of short-term lead exposure, which in our data cannot be disentangled. And finally, the actual cause-and-effect relationship could possibly be both ways—either the increased food intake of obese individuals subsequently results in increased lead uptake, or alterations in metabolism or the epigenome causing obesity are the effect of lead exposure, e.g. Wang et al. (2015) conclude that oxidative stress caused by lead exposure may be one factor inducing obesity.
Coffee and tea consumption were associated with higher BLL, of somewhat differing magnitude by country, and it was not possible to disentangle whether the underlying factor was the coffee, the tea or the water in which it was prepared. A number of dietary factors (e.g. chocolate intake) were associated with BLL in France, albeit not strongly, but were not confirmed in the other countries. Interestingly, the association was reversed for chocolate intake in several countries. We did not find an association between gestational age, sex of the offspring or parity and BLL in any country.
In this study, we aimed to identify possible potential problems of decentralised data analyses. Evaluation of BLL determinants was performed in a decentralised manner. It indicated the analytical part could be harmonised using an appropriate reference material. On the other hand, it proved difficulty in decentralised analysis due to the difference in sample types (cord blood for France and China, maternal blood for Japan and Norway and non-pregnant women’s blood for Germany) and questionnaire data. Our practice showed the importance of harmonisation of study protocols for future mega-analysis of biomonitoring and cohort studies.
Comparison of blood lead levels in each study
Measured BLL were lowest in Japan, followed by France and Norway, somewhat higher in Germany and highest in China. This applies however to our study sub-samples and is not necessarily representative for a cross-country comparison, due to different sampling strategies of study participants and study methodologies including selection of sub-sample for lead measurement. First, the impact of whole blood vs. cord blood and sampling in slightly different time periods, as well as some analytical uncertainty as shown by the round-robin test, cannot be quantified to calibrate the measured values; in addition, differences across most countries were not very large. Per this exercise, we had to use BLL data from difference sample matrices across the studies. Even though maternal to cord blood lead concentration ratio have been found to be close to 1 (Iwai-Shimada et al., 2019), we cannot compare the results directly. This also indicates the importance of pre-harmonisation for pooled data analysis. Second, the sample from Japan is by far the largest and therefore had considerably less random variation, possibly being the most representative. Third, direct comparison with Germany and China is not straightforward. In Germany, sampled women were not pregnant and different lifestyles, living conditions and behaviours make them less comparable to the pregnant women in the other studies. In China, the study was only carried out in Shanghai, a metropolitan area, while the other studies included fewer urban environments. Nevertheless, all studies have in common that in general measured BLL were low, with very few levels exceeding 50 μg l−1.
Implication of the harmonization exercise
We demonstrated that BLL data can be harmonised by using a reference material. The data can be compared and pooled in reference to the measurement results of the reference material, even though each study employs its own analytical protocols and own laboratory and performs the analysis in different time and places. Our practice also showed the usefulness of a round-robin exercise to illustrate the performance of each study analysis. We knew that each study used laboratories having accreditations for the particular analysis or having participated in an external quality assurance programme such as the German External Quality Assessment Scheme (G-EQUAS). However, that was not sufficient to evaluate the comparability of the analysis data among the group. The round-robin results demonstrated the importance of the use of reference materials in each study in order to pool the data afterwards.
There have been efforts to pool cohort study data with environmental measurements, e.g. the Environmental Health Risks in European Birth Cohorts (ENRIECO) project (Casas et al., 2013; Gehring et al., 2013). The ENRIECO proved a possibility of pooling data from different cohort studies, while identifying some difficulties in calibrating the results from different laboratories and different measurement timings. The practice also revealed the importance of harmonizing questionnaire harmonization. The data harmonization in ECHIBCG also confirms such experiences, namely that some precision in items needs to be sacrificed for the creation of joint variables. This applies to some extent already to variables having exactly the same meaning in each study, e.g. maternal age, when assessed categorically and those categories differ across countries. It becomes an even bigger challenge for variables seemingly the same but reflecting slightly different habits in different countries; e.g. fish and seafood consumption across diverse countries such as France, Norway, Germany, China and Japan do not necessarily mean exactly the same type of fish or seafood. The likely effect of collapsing categories, broadening definitions or lumping precise definitions into broader groups is a potential dilution of associations, if they exist; in our case, the association between BLL and their possible explanatory factors. Evidently, this is inherent in the design, namely the conduct of independent national studies and their multinational pooling, and the overall gain in statistical power as well as the opportunity to identify study-specific associations outweighs the limitations from compromises made at the harmonization stage.
Conclusions
In this project of the ECHIBCG, a consortium of cohort studies, supported by Germany and Japan, compared BLL across five countries and evaluated potential explanatory factors of increased levels. The first purpose was to demonstrate that the laboratory analytical methods were sufficiently similar to allow direct comparison of data, as well as demonstrating that it is possible to harmonize the epidemiological data for joint analysis. From this perspective, the exercise was successful and encourages further joint projects. The second purpose—comparing BLL—revealed three associations that were consistent across countries, namely, smoking, age and, to a lesser extent, BMI. Other factors appeared to be more modestly related or differed by country. This exercise showed the challenges in decentralized data analyses and reinforces the need for data harmonization among studies.
Supplementary Material
Highlights.
Blood lead levels were compared across five countries and potential determinants were evaluated.
Intra-laboratory trial on lead analysis showed variation was small enough for joint analysis.
Current smoking, age and BMI were the most prominent explanatory factors of blood lead levels.
The challenges in decentralized analysis reinforce the need for data harmonization and pooling.
Acknowledgements
The US National Children’s Study (NCS) was initially a member of the group and played a significant role: The Environment and Child Health International Birth Cohort Group (ECHIBCG) acknowledges the NCS for sharing various methods and procedures and providing many in-kind contributions to the group including a teleconference facility and translation capacity. The ECHIBCG would also like to thank Prof Thomas Göen from the Institute and Outpatient Clinic of Occupational, Social and Environmental Medicine, Friedrich-Alexander-University, Germany, for carrying out the blood lead measurements in German samples; Dr C. Dereumeaux from the French Biomonitoring Agency, for performing the lead measurement and producing the first results on lead determinants in the Elfe study; for Norwegian samples, blood lead measurements were performed at Department of Occupational and Environmental Medicine at Lund University, Sweden (this work was partly supported by the Research Council of Norway through its Centres of Excellence funding scheme, project number 262700); and Dr Chonghuai Yan for having measured the lead blood levels in the Shanghai Birth Cohort study.
The ECHIBCG scientific secretariat at the International Agency for Research on Cancer (IARC) is supported by funding from the Ministry of the Environment, Japan, and from the Ministry for Environment, Nature Conservation and Nuclear Safety, Germany. Original studies were funded by the German Federal Ministry for the Environment, Nature Conservation and Nuclear Safety (for the German Environmental Specimen Bank); the French Ministry of Health and the French Ministry of Environment and Public Health; the Ministry of the Environment of Japan; the Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01 and grant no.2 UO1 NS 047537-06A1); the Danish National Research Foundation, Danish Ministry of Health, Danish Research Councils, EU, NIH, March of Dimes Foundation; and the Shanghai Bureau of Health.
Footnotes
Chemical compounds studied in this article: Lead (PubChem CID: 5352425)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
There are no conflicts of interest to declare.
References
- Baldacci S, Gorini F, Santoro M, Pierini A, Minichilli F, Bianchi F, 2018. Environmental and individual exposure and the risk of congenital anomalies: A review of recent epidemiological evidence. Epidemiol. Prev 42, 1–34. 10.19191/EP18.3-4.S1.P001.057 [DOI] [PubMed] [Google Scholar]
- Barbone F, Rosolen V, Mariuz M, Parpinel M, Casetta A, Sammartano F, Ronfani L, Vecchi Brumatti L, Bin M, Castriotta L, Valent F, Little DL, Mazej D, Snoj Tratnik J, Miklavčič Višnjevec A, Sofianou K, špirić Z, Krsnik M, Osredkar J, Neubauer D, Kodrič J, Stropnik S, Prpić I, Petrović O, Vlašić-Cicvarić I, Horvat M, 2019. Prenatal mercury exposure and child neurodevelopment outcomes at 18 months: Results from the Mediterranean PHIME cohort. Int. J. Hyg. Environ. Health 222, 9–21. 10.1016/j.ijheh.2018.07.011 [DOI] [PubMed] [Google Scholar]
- Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ, 2012. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Heal. A Glob. Access Sci. Source 11, 42 10.1186/1476-069X-11-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botton J, Philippat C, Calafat AM, Carles S, Charles MA, Slama R, the EDEN mother-child cohort study group, 2016. Phthalate pregnancy exposure and male offspring growth from the intra-uterine period to five years of age. Environ. Res 151, 601–609. 10.1016/j.envres.2016.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Muckle G, Bastien CH, 2009. Prenatal exposure to polychlorinated biphenyls: A neuropsychologic analysis. Environ. Health Perspect 117, 7–16. 10.1289/ehp.11294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branum AM, Collman GW, Correa A, Keim SA, Kessel W, Kimmel CA, Klebanoff MA, Longnecker MP, Mendola P, Rigas M, Selevan SG, Scheidt PC, Schoendorf K, Smith-Khuri E, Yeargin-Allsopp M, 2003. The National Children’s Study of environmental effects on child health and development. Environ. Health Perspect 111, 642–646. 10.1289/ehp.5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Dwyer T, Kasten C, Krotoski D, Li Z, Linet MS, Olsen J, Scheidt P, Winn DM, 2007. Cohort profile: The International Childhood Cancer Cohort Consortium (I4C). Int. J. Epidemiol 36, 724–730. 10.1093/ije/dyl299 [DOI] [PubMed] [Google Scholar]
- Casas M, Chevrier C, Hond E. Den, Fernandez MF, Pierik F, Philippat C, Slama R, Toft G, Vandentorren S, Wilhelm M, Vrijheid M, 2013. Exposure to brominated flame retardants, perfluorinated compounds, phthalates and phenols in European birth cohorts: ENRIECO evaluation, first human biomonitoring results, and recommendations. Int. J. Hyg. Environ. Health 216, 230–42. 10.1016/j.ijheh.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Casas M, Nieuwenhuijsen M, Martínez D, Ballester F, Basagaña X, Basterrechea M, Chatzi L, Chevrier C, Eggesbø M, Fernandez MF, Govarts E, Guxens M, Grimalt JO, Hertz-Picciotto I, Iszatt N, Kasper-Sonnenberg M, Kiviranta H, Kogevinas M, Palkovicova L, Ranft U, Schoeters G, Patelarou E, Petersen MS, Torrent M, Trnovec T, Valvi D, Toft GV, Weihe P, Weisglas-Kuperus N, Wilhelm M, Wittsiepe J, Vrijheid M, Bonde JP, 2015. Prenatal exposure to PCB-153, p,p’-DDE and birth outcomes in 9000 mother-child pairs: Exposure-response relationship and effect modifiers. Environ. Int 74, 23–31. 10.1016/j.envint.2014.09.013 [DOI] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, Havstad S, Basu N, Ownby David R, Park SK, Ownby Dennis R, Johnson CC, Wegienka G, 2016. Detectable Blood Lead Level and Body Size in Early Childhood. Biol. Trace Elem. Res 171, 41–7. 10.1007/s12011-015-0500-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente DBP, Casas M, Vilahur N, Begiristain H, Bustamante M, Carsin AE, Fernández MF, Fierens F, Gyselaers W, Iñiguez C, Janssen BG, Lefebvre W, Llop S, Olea N, Pedersen M, Pieters N, Marina LS, Souto A, Tardón A, Vanpoucke C, Vrijheid M, Sunyer J, Nawrot TS, 2016. Prenatal ambient air pollution, placental mitochondrial DNA content, and birth weight in the INMA (Spain) and ENVIRONAGE (Belgium) birth Cohorts. Environ. Health Perspect 124, 659–665. 10.1289/ehp.1408981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereumeaux C, Fillol C, Charles MA, Denys S, 2017. The French human biomonitoring program: First lessons from the perinatal component and future needs. Int. J. Hyg. Environ. Health 220, 64–70. 10.1016/j.ijheh.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Dzwilewski KLC, Schantz SL, 2015. Prenatal chemical exposures and child language development. J. Commun. Disord 57, 41–65. 10.1016/j.jcomdis.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzel R, Charles M-A, Dellarco M, Gajeski K, Jöckel K-H, Hirschfeld S, Kamijima M, Kawamoto T, Kolossa-Gehring M, Nakayama S, Schmidt B, Tian Y, Wolz B, Zaros C, Zhang J, 2014. Harmonizing Biomarker Measurements in Longitudinal Studies of Children’s Health and the Environment. Biomonitoring 1, 50–62. 10.2478/bimo-2014-0006 [DOI] [Google Scholar]
- Gehring U, Casas M, Brunekreef B, Bergström A, Bonde JP, Botton J, Chévrier C, Cordier S, Heinrich J, Hohmann C, Keil T, Sunyer J, Tischer CG, Toft G, Wickman M, Vrijheid M, Nieuwenhuijsen M, 2013. Environmental exposure assessment in European birth cohorts: results from the ENRIECO project. Environ. Health 12, 8 10.1186/1476-069X-12-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Bellinger D, Bergman Å, Cordier S, Davey-Smith G, Eskenazi B, Gee D, Gray K, Hanson M, Van Den Hazel P, Heindel JJ, Heinzow B, Hertz-Picciotto I, Hu H, Huang TTK, Jensen TK, Landrigan PJ, McMillen IC, Murata K, Ritz B, Schoeters G, Skakkebæk NE, Skerfving S, Weihe P, 2008. The Faroes statement: Human Health effects of developmental exposure to chemicals in our environment. Basic Clin. Pharmacol. Toxicol 102, 73–75. 10.1111/j.1742-7843.2007.00114.x [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Park HY, Dostal M, Kocan A, Trnovec T, Sram R, 2008. Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin. Pharmacol. Toxicol 102, 146–154. 10.1111/j.1742-7843.2007.00190.x [DOI] [PubMed] [Google Scholar]
- Huang J, Zhu T, Qu Y, Mu D, 2016. Prenatal, perinatal and neonatal risk factors for intellectual disability: A systemic review and meta- Analysis. PLoS One 11, e0153655 10.1371/journal.pone.0153655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitsuka K, Nakayama SF, Kishi R, Mori C, Yamagata Z, Ohya Y, Kawamoto T, Kamijima M, 2017. Japan Environment and Children’s Study: backgrounds, activities, and future directions in global perspectives. Environ. Health Prev. Med 22, 61 10.1186/s12199-017-0667-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iszatt N, Stigum H, Verner MA, White RA, Govarts E, Murinova LP, Schoeters G, Trnovec T, Legler J, Pelé F, Botton J, Chevrier C, Wittsiepe J, Ranft U, Vandentorren S, Kasper-Sonnenberg M, Klümper C, Weisglas-Kuperus N, Polder A, Eggesbø M, 2015. Prenatal and postnatal exposure to persistent organic pollutants and infant growth: A pooled analysis of seven European birth cohorts. Environ. Health Perspect 123, 730–736. 10.1289/ehp.1308005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai-Shimada M, Kameo S, Nakai K, Yaginuma-Sakurai K, Tatsuta N, Kurokawa N, Nakayama SF, Satoh H, 2019. Exposure profile of mercury, lead, cadmium, arsenic, antimony, copper, selenium and zinc in maternal blood, cord blood and placenta: the Tohoku Study of Child Development in Japan. Environ. Health Prev. Med 24, 35 10.1186/s12199-019-0783-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, Yamagata Z, Kayama F, Kishi R, Ohya Y, Saito H, Sago H, Okuyama M, Ogata T, Yokoya S, Koresawa Y, Shibata Y, Nakayama S, Michikawa T, Takeuchi A, Satoh H, 2014. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 14, 25 10.1186/1471-2458-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Hu H, Rotnitzky A, Bellinger D, Needleman H, 1995. A longitudinal study of chronic lead exposure and physical growth in Boston children. Environ. Health Perspect 103, 952–7. 10.1289/ehp.95103952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi R, Sasaki S, Yoshioka E, Yuasa M, Sata F, Saijo Y, Kurahashi N, Tamaki J, Endo T, Sengoku K, Nonomura K, Minakami H, Cho K, Yamada T, Ban S, Kanazawa A, Washino N, Konishi K, Katoh S, Uno A, Baba T, Yila T, Miyashita C, Braimoh T, Kashino I, Okada E, Kobayashi S, Otake Y, Limpar M, Nakajima S, Baba T, Miyamoto T, Maeda N, Haruyama H, Okuyama K, 2011. Cohort profile: The hokkaido study on environment and Children’s Health in Japan. Int. J. Epidemiol 40, 611–618. 10.1093/ije/dyq071 [DOI] [PubMed] [Google Scholar]
- Kishi R, Zhang JJ, Ha EH, Chen PC, Tian Y, Xia Y, Tsuchiya KJ, Nakai K, Kim S, Hong SJ, Hong YC, Lee JR, Jan Mohamed HJB, Parajuli RP, Adair LS, Chong YS, Guo YL, Wang SL, Nishijo M, Kido T, Tai PT, Nandasena S, 2017. Birth Cohort Consortium of Asia: Current and Future Perspectives. Epidemiology 28, S19–S34. 10.1097/EDE.0000000000000698 [DOI] [PubMed] [Google Scholar]
- Kolossa-Gehring M, Becker K, Conrad A, Schröter-Kermani C, Schulz C, Seiwert M, 2012. Environmental surveys, specimen bank and health related environmental monitoring in Germany. Int. J. Hyg. Environ. Health 215, 120–126. 10.1016/j.ijheh.2011.10.013 [DOI] [PubMed] [Google Scholar]
- Lee E, Baik D, Park Y, Ki M, 2017. The current status of health data on Korean children and adolescents. Epidemiol. Health 39, e2017059 10.4178/epih.e2017059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, Handal M, Haugen M, Høiseth G, Knudsen GP, Paltiel L, Schreuder P, Tambs K, Vold L, Stoltenberg C, 2016. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int. J. Epidemiol 45, 382–388. 10.1093/ije/dyw029 [DOI] [PubMed] [Google Scholar]
- Nakayama SF, Iwai-Shimada M, Oguri T, Isobe T, Takeuchi A, Kobayashi Y, Michikawa T, Yamazaki S, Nitta H, Kawamoto T, 2019. Blood mercury, lead, cadmium, manganese and selenium levels in pregnant women and their determinants: the Japan Environment and Children’s Study (JECS). J. Expo. Sci. Environ. Epidemiol In press. 10.1038/s41370-019-0139-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J, Melbye M, Olsen SF, Sørensen T.I. a., Aaby P, Nybo Andersen AM, Taxbøl D, Hansen KD, Juhl M, Schow TB, Sørensen HT, Andresen J, Mortensen EL, Wind Olesen A, Søndergaard C, 2001. The Danish National Birth Cohort - its background, structure and aim. Scand. J. Public Health 29, 300–307. 10.1177/14034948010290040201 [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, Hoepner L, Barr D, Tu YH, Camann D, Kinney P, 2006. Effect of prenatal exposure to airborne polycyclic aromatic hydocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ. Health Perspect 114, 1287–1292. 10.1289/ehp.9084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Hu H, Ettinger A, Sánchez BN, Wright RO, Cantonwine D, Lazarus A, Lamadrid-Figueroa H, Mercado García A, Téllez-Rojo MM, Hernández-Avila M, 2009. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ. Health Perspect 117, 1466–1471. 10.1289/ehp.0800497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R, 2011. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ. Health Perspect 119, 1196–201. 10.1289/ehp.1003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scinicariello F, Buser MC, Mevissen M, Portier CJ, 2013. Blood lead level association with lower body weight in NHANES 1999-2006. Toxicol. Appl. Pharmacol 273, 516–23. 10.1016/j.taap.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W, Liu Q, He X, Liu H, Gu A, Jiang Z, 2017. Association between level of urinary trace heavy metals and obesity among children aged 6-19 years: NHANES 1999-2011. Environ. Sci. Pollut. Res. Int 24, 11573–11581. 10.1007/s11356-017-8803-1 [DOI] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, Hansen RL, Hertz-Picciotto I, 2014. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ. Health Perspect 122, 1103–9. 10.1289/ehp.1307044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Cronk C, Durkin M, Weiss M, Schoeller DA, Gall EA, Hewitt JB, Carrel AL, Landrigen PJ, Gillman MW, 2009. Environment and obesity in the national children’s study. Environ. Health Perspect 117, 159–166. 10.1289/ehp.11839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandentorren S, Bois C, Pirus C, Sarter H, Salines G, Leridon H, 2009. Rationales, design and recruitment for the Elfe longitudinal study. BMC Pediatr. 9, 58 10.1186/1471-2431-9-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Chen C, Nie X, Han B, Li Q, Chen Yi, Zhu C, Chen Yingchao, Xia F, Cang Z, Lu M, Meng Y, Zhai H, Lin D, Cui S, Jensen MD, Lu Y, 2015. Blood lead level and its association with body mass index and obesity in China - Results from SPECT-China study. Sci. Rep 5, 18299 10.1038/srep18299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tian Y, Wang W, Ouyang F, Xu J, Yu X, Luo Z, Jiang F, Huang H, Shen X, Shanghai Birth Cohort, 2019. Cohort profile: the Shanghai Birth Cohort. Int. J. Epidemiol 48, 21–21g. 10.1093/ije/dyy277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.