Abstract
Dehydration impairs cognitive performance in humans and rodents, although studies in animal models are limited. Estrogens have both protective effects on fluid regulation and improve performance in certain cognitive tasks. We, therefore, tested whether sex and gonadal hormones influence object recognition memory during dehydration. Because past studies used fluid deprivation to induce dehydration, which is a mixture of intracellular and extracellular fluid loss, we tested the effects of osmotic (loss of intracellular fluid) and hypovolemic (loss of extracellular fluid) dehydration on object recognition memory. After training trials consisting of exposure to two identical objects, rats were either treated with hypertonic saline to induce osmotic dehydration, furosemide to induce hypovolemic dehydration, or received a control injection and then object recognition memory was tested by presenting the original and a novel object. After osmotic dehydration, regardless of group or treatment, all rats spent significantly more time investigating the novel object. After hypovolemic dehydration, regardless of treatment, both the males and estrous females spent significantly more time investigating the novel object. While the control-treated diestrous females also spent significantly more time investigating the novel object, the furosemide-treated diestrous females spent a similar amount of time investigating the novel and original object. Follow up studies determined that loss of ovarian hormones after ovariectomy, but not loss of testicular hormones after castration, resulted in impaired memory performance in the object recognition test after hypovolemic dehydration. This series of experiments provides evidence for a protective role of ovarian hormones on dehydration-induced memory impairments.
Keywords: Hypovolemia, Ovariectomy, Castration, Osmotic Dehydration
1. Introduction
Severe dehydration negatively impacts cognition and can ultimately lead to delirium in humans (Wilson and Morley, 2003). A number of experiments in human subjects demonstrate that even mild to moderate dehydration, 1- 4% body water loss, can impact multiple domains of cognitive function (Cian et al., 2001; Patel et al., 2007; Sharma et al., 1986; Stachenfeld et al., 2018). For example, Gopinathan et al. used a combination of water restriction and exercise in heat to produce dehydration levels ranging from 1-4% and observed dose-related decreases in performance of a serial addition test, which assess both short-term memory and reasoning (Gopinathan et al., 1988). There are, however, discrepancies in the literature and a number of studies have not found any relationship between mild-moderate dehydration and cognitive function (Neave et al., 2001; Tomporowski et al., 2007; Turner et al., 2017). Methodological differences between studies, such as how dehydration was achieved, small sample sizes, population choice, and assessment of cognitive performance, likely contribute to the inconsistencies in the literature (Adan, 2012; Lieberman, 2007; Sécher and Ritz, 2012). The use of animal models to assess the effect of dehydration on cognitive function is, therefore, advantageous to circumvent the confounds inherent with human subject research. In addition, the available human subject literature lacks mechanistic studies, and animal models may provide a better opportunity to investigate how dehydration influence cognitive function.
Despite the number of studies in humans identifying a relationship between dehydration and cognition, very little work has been conducted with animal models. Using the Y maze to assess spatial memory, Faraco et al, demonstrated that after 24 or 48 h of water deprivation, male mice made fewer visits and spent less time in the novel arm of the maze, suggesting impaired spatial memory (Faraco et al., 2014). While this study provides evidence that dehydration is associated with impaired cognitive performance in rodents, a number of open questions remain. First, prolonged fluid deprivation produces a mixture of both intracellular and extracellular fluid loss (Gizowski and Bourque, 2018). It is, therefore, unclear if osmotic (loss of intracellular fluids), hypovolemic (loss of extracellular fluids), or both forms of dehydration influence cognition. Second, only male mice were used in the above experiment. Given the well reported effects of gonadal hormones on learning and memory tasks in euhydrated animals (Frick et al., 2015; Galea et al., 2008; Hamson et al., 2016), the protective effects estrogens have on fluid homeostasis (Curtis, 2009; Reckelhoff, 2001), and reports of sex differences in cognitive performance during dehydration in humans (D'anci et al., 2009; Szinnai et al., 2005), it is reasonable to hypothesize that performance in cognitive tests during dehydration are influenced by sex. Finally, in the experiment described above, the mice were dehydrated during the training, in addition, to the testing trials that assessed spatial memory. It is, therefore, unclear if dehydration influences memory consolidation or retrieval. More research, therefore, is necessary to address these issues, in addition to determining if dehydration impairs non-spatial memories, such as object memory.
In this series of experiments, we tested the hypothesis that dehydration-induced impairments in cognitive function would be influenced by sex and/or stage of the estrous cycle. We tested male and female rats, either in estrus or diestrus 2, in the novel object recognition paradigm after inducing osmotic or hypovolemic dehydration in order to determine which type of fluid loss influences memory retrieval. We predicted that females, particularly females in estrus because it follows a time of high ovarian hormone secretion (Becker et al., 2005), would be protected from dehydration-induced memory impairments due to the enhancing effects estrogens have on cognitive function (Gervais et al., 2016; Spencer et al., 2008). Follow-up studies were then used to directly determine the role of gonadal hormones during dehydration in tests of object memory.
2. Materials and Methods
2.1. Subjects and Housing
Male and female Sprague Dawley rats (Charles Rivers Laboratory) were used throughout. Animals were age-matched upon arrival into the facility and all testing occurred between 75-100 days of age. During the early part of the light phase animals were weighed, and vaginal cytology was monitored in the females as previously described (Santollo et al., 2017). Cycle stage determinations corresponded to the previous 12-h dark phase and the subsequent 12-h light phase (Becker et al., 2005). Only rats with 4-day estrous cycles were included in the experiments. Animals were pair housed in standard shoebox cages until three days before the commencement of behavioral tests. All animals had ab libitum access to standard rat chow (Teklad 2018) and water and animals in Experiments 2-4 had access to 1.5% saline, unless otherwise noted. The colony room was maintained on a 12:12 h light dark cycle (lights off at 1900 h). All experimental protocols were approved by the Animal Care and Use Committee at the University of Kentucky, and the handling and care of individual animals was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Surgery
Approximately one week after arriving into the facility animals in Experiments 3 and 4 underwent either bilateral gonadectomy or a sham surgery using an inter-abdominal approach. Rats were anesthetized with 2% isoflurane and received a subcutaneous (sc) injection of carprofen (5 mg/kg sc; Henry Schein, Dublin, OH) as an analgesic. A small skin incision was made and the abdomen wall was opened. The ovaries and testes were externalized and removed or were placed back into the abdomen (sham-operation). The muscle wall was sutured, and the skin was closed with wound clips. All animals then received a bolus injection of isotonic saline (sc, 5 ml) for hydration. All animals received a second sc injection of carprofen 24 h after surgery.
2.3. Novel Object Recognition (NOR) Paradigm
A five day NOR paradigm was used with an open field measuring 75 cm (w) X 75 cm (l) X 45 cm (h). Grid lines on the open field divided it into 16 squares and were used to assess activity. On day one all rats were habituated to the empty open field for 10 minutes. Training days 2-4 consisted of exposure to two identical original objects for one 5 min trial. A testing trial occurred on day 5 and consisted of exposure to one original and one novel object for 5 min. All habituation, training, and testing occurred during the first half of the light cycle. The objects used for Experiment 1 were a small toy traffic cone as the original object and a vase as the novel object. The objects used for Experiments 2-4 were green bulb shaped spray bottles as the original objects and a blue cylindrical spray bottle as the novel object. The original and novel objects were similar in height (~20 cm) and width (~8.5 cm) and pilot studies indicated there were no differences in exploratory behavior when rats were exposed to these objects. Objects were filled with sand to provide weight (except for the cone) and were secured to the floor of the open field with magnets to prevent them from being knocked over or moved. Although a single training trial in the NOR paradigm is most common, there are many studies that use multiple training days and/or trials (Antunes and Biala, 2012). We used this extended training protocol to enhance performance because we predicted that dehydration would impair memory but wanted to avoid a floor effect which could mask any hormone-related effects.
2.4. NOR Scoring
All habituation, training, and testing trials were video recorded for subsequent scoring of activity (to rule out lethargy as a confounding variable) and object exploration (memory test). Activity was quantified as the number of grid lines crossed during the 5 min test trial. A line was considered “crossed” when both front paws passed over the grid line. Object exploration was quantified as time spent sniffing, licking or touching the objects with the rat’s head or front paws during the 5 min test trial (Antunes and Biala, 2012). Touching the object with their sides or rear did not count as object exploration. Videos were scored by at least two individuals blinded to the experimental conditions with an inter-scorer reliability of at least 90%.
2.5. Experiment 1: Does osmotic dehydration impair object recognition memory in male and female rats?
Intact male (n = 21), diestrous 2 (n = 20), and estrous (n = 19) female rats were habituated and trained in the NOR paradigm as described above. Two groups of females were used, one group was habituated and tested during diestrus (D2) and the second group was habituated and tested during estrus (E). On test day (day 5) animals were injected sc with 0.1 ml lidocaine (Henry Schein, Dublin, OH) and then immediately injected sc either 1 ml of 0.15 M NaCl (Sigma, St. Louis, MO) or 2 M NaCl to produce osmotic dehydration. Animals were returned to their cages without access to water. Fifteen minutes later animals were placed in the open field with the original and novel object for 5 min. Animals were then returned to their cage, and given preweighed water bottles that were measured 30 min later to confirm dehydration. The doses of NaCl and time interval used were based on previous studies demonstrating that osmotic dehydration occurs within 15 −30 min of treatment (Krause et al., 2003).
2.6. Experiment 2: Does hypovolemic dehydration impair object recognition memory in male and female rats?
Intact male (n = 19), D2 (n = 19), and E (n = 18) female rats were habituated and trained in the NOR paradigm as described above. Again, the females were divided into groups that were habituated and tested during D2 and E. On test day (day 5) animals were injected sc with either 0.9% saline or 20 μg/kg furosemide (Salix; Merck Animal Health, Madison, NJ). Animals were returned to their cages without access to water, saline, or food. Three h later animals were placed in the open field with the original and novel object for 5 min. Animals were then returned to their cages and given preweighed water and saline bottles that were measured 30 min later to confirm dehydration. The dose of furosemide and time interval chosen was based on previous research demonstrating that hypovolemic dehydration occurs within 3 h of treatment (Begg et al., 2012).
2.7. Experiment 3: Does hypovolemic dehydration impair object recognition memory in castrated male rats?
This protocol was identical to that described in Experiment 2 except 16 sham-operated males and 18 castrated (CAST) males were used in this experiment. The study commenced approximately 2 weeks after surgery.
2.8. Experiment 4: Does hypovolemic dehydration impair object recognition memory in ovariectomized female rats?
This protocol was identical to that described in Experiment 2 except 15 sham-operated females and 16 ovariectomized (OVX) females were used in this experiment. The study commenced approximately 2 weeks after surgery. The sham females were habituated (Day 1) and tested (Day 5) during estrus.
2.9. Serum Osmolarity Measures
Male (n = 7), D2 female (n = 8), and E female (n = 7) rats were used to determine if the dose of NaCl used in Experiment 1 produced a comparable change in serum osmolarity between groups. Animals were injected sc with 0.1 ml lidocaine, then immediately injected sc either 1 ml of 0.15 M NaCl (control) or 2 M NaCl, and then placed back in their cages without access to water. Fifteen minutes later animals were lightly anesthetized with 2% isoflurane and 500 μl of tail blood was collected. Blood clotted at RT for 1 h, spun at 4°C for 15 min at 12,000 rpm, the serum was collected, and stored at −80°C until processing. This was repeated the following week with animals receiving the opposite NaCl treatment to achieve a crossover design. Aliquots containing 50 μl serum were analyzed for osmolarity using an automatic osmometer (μOSMETT E, Precisions Systems Inc., Natick, MA) using a two-point calibration curve.
2.10. Data Analysis
Data are presented as means ± SEM throughout. The software package Statistica was used to analyze all data. Activity, fluid measures, and serum osmolarity were analyzed with two factor (group X treatment) ANOVAs. Object exploration time was analyzed with three factor (group X treatment X object) ANOVAs. Fisher’s post hoc tests were used as a priori tests and to follow up significant ANOVA results. A significance value of p < 0.05 was used throughout. Cohen’s d effect size was calculated (pair-wise comparisons) using the Campbell Collaboration Effect Size Calculator and eta squared was calculated for ANOVA analysis as the SSeffect/(SStotal).
3. Results
3.1. Experiment 1: Does osmotic dehydration impair object recognition memory in male and female rats?
Although 1 ml of 2 M NaCl has previously been used to induce osmotic dehydration in females (Krause et al., 2003), we were unaware of any reports that compared males and female using this dose. Therefore, we first measured serum samples to ensure that 2 M NaCl-treatment induced a similar increase in osmolarity between groups. As expected, serum osmolarity was higher after treatment with 2 M NaCl, compared to control, F(1,19) = 74.5, p < 0.001, η2 = 0.55 (Table 1). There was no effect of group, F(2,19) = 1.7, p = 0.21, η2 = 0.005, or an interaction between group and treatment, F(2,19) = 0.5, p = 0.59, η2 = 0.01. In addition, analysis of the percent change revealed no effect of group, F(2,19) = 0.58, p = 0.57, η2 = 0.06.
Table 1.
Serum Osmolarity
| Group | 0.15 M NaCl (mOsm/L) |
2 M NaCl (mOsm/L) |
Percent Increase |
|---|---|---|---|
| D2 Females | 301.1 ± 1.9 | 311.9 ± 1.9 | 3.6% |
| E Females | 296.9 ± 1.3 | 308.9 ± 2.5 | 4.0% |
| Males | 301.1 ± 1.2 | 310.0 ± 1.2 | 3.0% |
Next, we asked whether dehydration resulting from loss of intracellular fluids would impair performance in the novel object recognition paradigm in males and females. To confirm that our treatment induced dehydration, water intake was measured for 30 min after the testing trial. As expected, animals treated with 2 M NaCl consumed significantly more water after the test trial than control-treated rats, F(1,53) = 52.551, p < 0.001, η2 = 0.48 (Fig. 1A). There was no effect of group, F(2,53) = 1.94, p = 0.15, η2 = 0.35 nor an interaction between group and treatment, F(2,53) = 0.51, p = 0.60, η2 = 0.01. One bottle had a water spillage during weighing and, therefore, the data was excluded from the analysis.
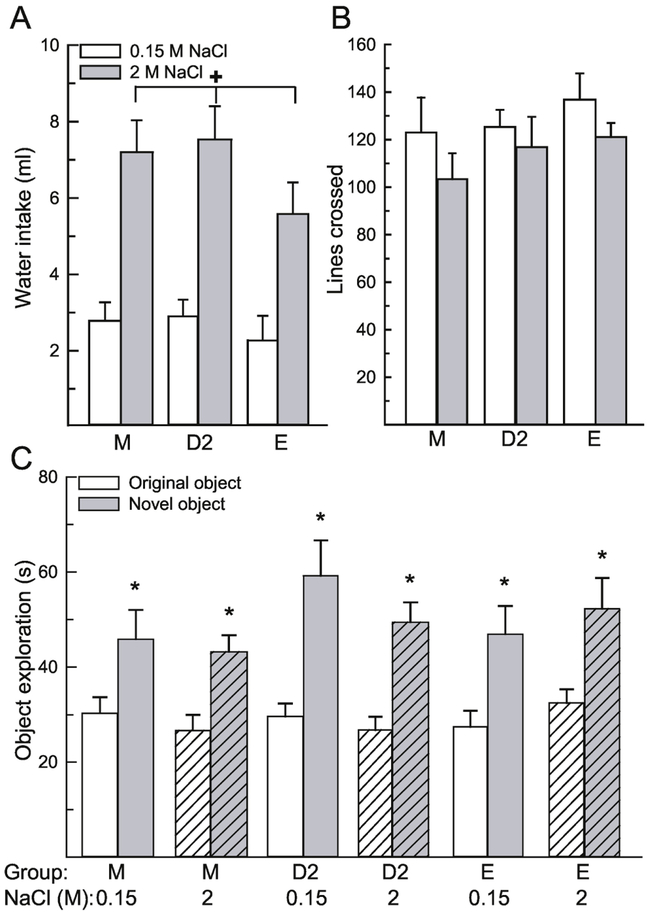
Figure 1.
Osmotic dehydration did not influence performance in the novel object recognition task. (A) Rats treated with 2 M NaCl consumed significantly more water compared to rats treated with 0.15 M NaCl, which confirmed that the treatment induced dehydration. (B) General activity, measured by the number of grid lines crossed in the open field, did not differ between any group. (C) All groups, regardless of treatment and sex/hormone status, spent significantly more time investigating the novel object, compared to the original object. Abbreviations: males (M), diestrous 2 females (D2), estrous females (E). Hashed bars denote the treatment groups. +Greater than control, p < 0.001. *Greater than time investigating original object, p < 0.001.
To ensure that impaired activity after 2 M NaCl treatment did not influence object exploration, we examined activity during the test period by analyzing the number of grid lines crossed in the open field during the test trial. Activity was not influenced by a main effect of treatment, F(1,54) = 2.62, p = 0.11, η2 = 0.04, group, F(2,54) = 1.01, p = 0.37, η2 = 0.03, or an interaction between treatment and group, F(2,54) = 0.13, p = 0.88, η2 = 0.005 (Fig 1B).
Time spent exploring the objects in the open field was influenced by the object, F(1,54) = 79.32, p < 0.001, η2 = 0.58 (Figure 1C). More time was spent investigating the novel, compared to the original object, p < 0.001. All other main or interactive effects were not significant, F(1,54) = 0.16-1.65, p = 0.20-0.74. A priori we were interested in sex and stage of estrous cycle differences in object recognition performance during dehydration. Post hoc analysis revealed that each group, regardless of sex/hormone group or treatment, spent significantly more time investigating the novel object, compared to the original object, p = 0.005- 0.0003, d = 0.41-.83.
3.2. Experiment 2: Does hypovolemic dehydration impair object recognition memory in male and female rats?
Next, we asked whether dehydration resulting from loss of extracellular fluids would impair performance in the novel object recognition paradigm in males and females. As expected, animals treated with furosemide consumed significantly more fluids after the test trial than control-treated rats, F(1,50) = 37.36, p < 0.001, η2 = 0.42 (Fig. 2A), which confirmed dehydration. There was no effect of group, F(2,50) = 0.07, p = 0.93, η2 = 0.002 nor an interaction between group and treatment, F(2,50) = 0.95, p = 0.39, η2 = 0.02. Separate analysis of water and saline revealed intake patterns similar to the total intake (data not shown). Both water and saline intake were significantly greater after treatment with furosemide, compared to control treatment F(1,50) = 53.09 and 5.62, p < 0.001 and = 0.03, η2 = 0.47 and 0.09 (water and saline, respectively). There was no effect of group on water or saline intake, F(1,50) = 1.07 and 0.18, p = 0.35 and 0.83, η2 = 0.0006 and 0.007. There was no interaction between treatment and group for saline intake, F(2,50) = 0.14, p = 0.87, η2 = 0.005 but water intake was influenced by an interaction between treatment and group, F(2,50) = 3.87, p = 0.03, η2 = 0.07. Post hoc analysis revealed that dehydrated male rats consumed more water than all other groups, p < 0.01.
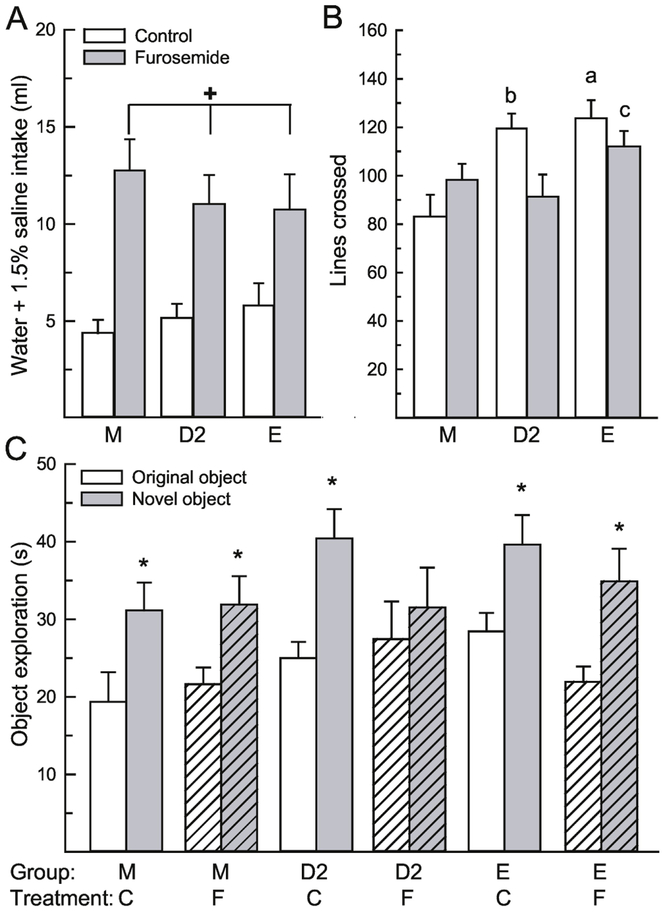
Figure 2.
Hypovolemic dehydration impaired performance in the novel object recognition task in a sex/hormone dependent manner. (A) Rats treated with furosemide consumed significantly more water and 1.5% saline compared to rats treated with saline control, which confirmed that the treatment induced dehydration. (B) General activity was influenced by group and treatment. E and saline-treated D2 rats were more active than male and furosemide-treated D2 rats. (C) Regardless of treatment, both groups of males and E rats spent significantly more time investigating the novel, compared to the original object. In D2 rats, however, only the control-treated group spent more time investigating the novel object. Abbreviations: males (M), diestrous 2 females (D2), estrous females (E), saline control (C), furosemide (F). Hashed bars denote the treatment groups. +Greater than control-treated, p < 0.001. aGreater than control and furosemide-treated males and furosemide-treated D2 females, p < 0.05. bGreater than control males and furosemide-treated D2 females, p < 0.05. cGreater than control males, p < 0.01. *Greater than time investigating original object, p < 0.001.
To ensure that impaired activity after furosemide treatment did not influence object exploration, we examined activity during the test period by analyzing the number of grid lines crossed in the open field during the test trial. Activity was not influenced by a main effect of treatment, F(1,50) = 1.74, p = 0.19, η2 = 0.02, but was influenced by group, F(2,50) = 6.37, p = 0.003, η2 = 0.17, and an interaction between treatment and group, F(2,50) = 4.21, p = 0.02, η2 = 0.12 (Fig 2B). Males were significantly less active than both groups of females, p < 0.05. In addition, control-treated E females were significantly more active than both groups of males and furosemide-treated D2 rats, p < 0.05. Control-treated D2 females were significantly more active than control treated males and furosemide-treated D2 females, p < 0.05. Finally, furosemide-treated E females were more active than control-treated males, p < 0.001.
Time spent exploring the objects in the open field was influenced by the object, F(1,50) = 38.78, p < 0.001, η2 = 0.42 (Figure 2C); as more time was spent investigating the novel, compared to the original object. All other main or interactive effects were not significant, F(1,50) = 0.14-2.16, p = 0.12-0.87. A priori we were interested in sex and stage of estrous cycle differences in object recognition performance during dehydration. Post hoc analysis revealed that both control and furosemide treated male and E rats spent significantly more time investigating the novel object, compared to the control object, p = 0.014-0.02, d = 0.41-0.52. In addition, the control-treated D2 rats spent significantly more time investigating the novel object, compared to the original object, p < 0.001, d = 0.67, however, the furosemide-treated D2 rats spent similar amounts of time investigating the novel and original object, p = 0.36, d = 0.10.
3.3. Experiment 3: Does hypovolemic dehydration impair object recognition memory in castrated male rats?
To directly test for a role of gonadal hormones on dehydration-induced memory impairments, we first tested whether loss of testicular hormones influenced performance during dehydration. As expected, animals treated with furosemide consumed significantly more fluids after the test trial than control-treated rats, F(1,29) = 40.67, p < 0.001, η2 = 0.54 (Fig. 3A), again confirming dehydration. There was no effect of surgery, F(1,29) = 4.10, p = 0.05, η2 = 0.05, nor an interaction between treatment and surgery, F(1,29) = 1.08, p = 0.31, η2 = 0.01. Separate analysis of water and saline intakes revealed similar patterns (data not shown). Both water and saline intakes were significantly greater after treatment with furosemide, compared to control treatment, F(1,29) = 16.85 and 9.90, p = 0.0003 and 0.003, η2 = 0.37 and 0.23 (water and saline, respectively). Neither surgery or an interaction between surgery and treatment influenced water or saline intake, F(1,29) = 0.14- 3.60, p = 0.07-0.71, η2 = 0.00- 0.08. One bottle had a water spillage during weighing and, therefore, the data was excluded from the analysis.
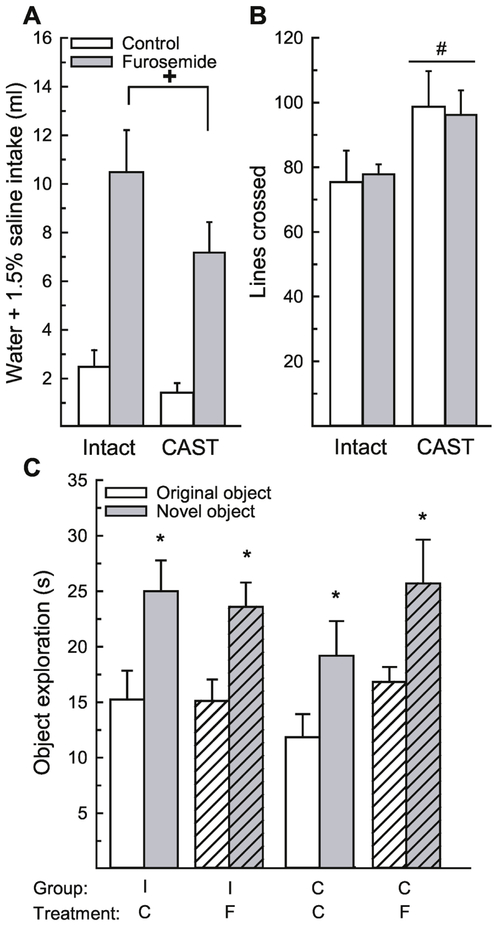
Figure 3.
Loss of testicular hormones did not influence performance in the novel object recognition paradigm during dehydration. (A) Rats treated with furosemide consumed significantly more water and 1.5% saline compared to rats treated with saline control, which confirmed that the treatment induced dehydration. (B) Castrated rats were significantly more active in the open field compared to intact rats. (C) Regardless of surgery status or treatment, all groups spent significantly more time investigating the novel object, compared to the control object. Abbreviations: intact (I), castration (C), saline control (C), furosemide (F). Hashed bars denote the treatment groups. +Greater than control-treated rats, p < 0.001. #Greater than intact rats, p = 0.02. *Greater than time investigating original object, p < 0.001.
Again, to ensure that impaired activity after furosemide treatment did not influence object exploration, we examined activity during the test trial. Activity was influenced by a main effect of surgery, F(1,30) = 5.88, p = 0.02, η2 = 0.16 (Figure 3B). Castrated rats were significantly more active than intact rats. There was no main effect of treatment, F(1,30) = 0.00, p = 0.99, η2 = 0.00, nor an interaction between treatment and surgery, F(1,30) = 0.08, p = 0.77, η2 = 0.00.
Time spent exploring the objects in the open field was influenced by the object, F(1,30) = 33.90, p < 0.001, η2 = 0.53, with no other main or interactive effects, F(1,30) = 0.11-2.20, p = 0.15-0.97, η2 = 0.00 – 0.04 (Figure 3C). Rats spent significantly more time investigating the novel, compared to the original, object. Again, a priori we wanted to determine whether gonadal hormones influenced object recognition during dehydration. Post hoc analysis revealed that regardless of treatment, both intact and CAST animals spent significantly more time investigating the novel, compared to the original object, p = 0.016- 0.003, d = 0.37-0.55.
3.4. Experiment 4: Does hypovolemic dehydration impair object recognition memory in ovariectomized female rats?
Next, we asked whether ovarian hormone loss influences performance in the novel object recognition paradigm after hypovolemic dehydration. Again, as expected, animals treated with furosemide consumed significantly more fluids after the test trial than control-treated rats, F(1,27) = 41.86, p < 0.001, η2 = 0.53 (Fig. 4A). There was also a main effect of surgery, F(1,27) = 7.82, p = 0.009, η2 = 0.10; surprisingly, intact animals consumed more fluids than OVX rats. There was no interaction between surgery and treatment, F(1,27) = 2.27, p = 0.14, η2 = 0.03. Separate analysis of water and saline intakes revealed similar patterns (data not shown). Both water and saline intakes were significantly greater after treatment with furosemide, compared to control treatment, F(1,27) = 39.08 and 9.60, p < 0.001 and = 0.005, η2 = 0.58 and 0.18 (water and saline, respectively). Neither surgery or an interaction between surgery and treatment influenced water intake, F(1,27) = 0.41 and 0.77, p = 0.53 and 0.38, η2 = 0.006 and 0.01. Saline intake, however, was influenced by surgery and an interaction between surgery and treatment, F(1,27) = 9.28 and 7.03, p = 0.005 and 0.01, η2 = 0.18 and 0.13. Intact, but not OVX, rats consumed more saline after furosemide-treatment, p < 0.001.
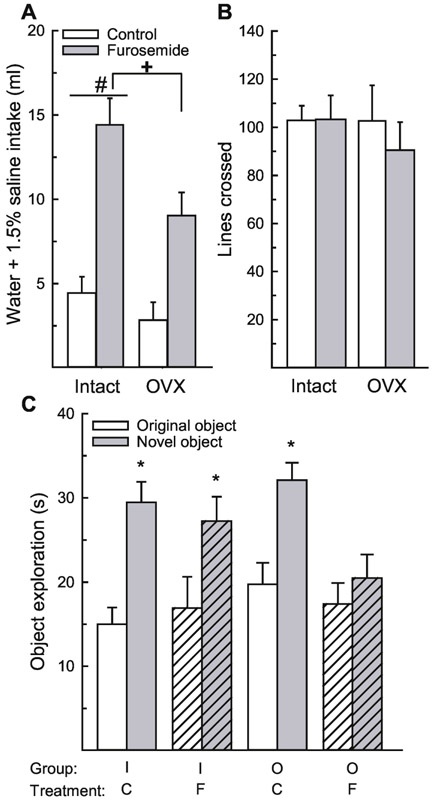
Figure 4.
Ovarian hormones protect against hypovolemic dehydration-induced memory impairments in the novel object recognition paradigm. (A) Rats treated with furosemide consumed significantly more water and 1.5% saline compared to rats treated with saline control, which confirmed that the treatment induced dehydration. In addition, intact rats consumed more than OVX rats. (B) There was no difference in activity between any group. (C) Both groups of intact rats and control-treated OVX rats spent significantly more time investigating the novel, compared to the original object. Furosemide-treated OVX rats spent a similar amount of time investigating the novel and original object. Abbreviations: intact (I), ovariectomized (O), saline control (C), furosemide (F). Hashed bars denote the treatment groups. +Greater than control-treated rats, p < 0.001. #Greater than OVX rats, p = 0.009. *Greater than time investigating original object, p < 0.001.
Again, to ensure that impaired activity after furosemide treatment did not influence object exploration, we examined activity during the test trial. Activity was not influenced by a main or interactive effects of surgery and/or treatment, F(1,27) = 0.28-0.34, p = 0.56-0.60, η2 = 0.01 (Figure 4B).
Time spent exploring the objects in the open field was influenced by the object, F(1,27) = 65.56, p < 0.001, η2 = 0.3 and an interaction between object and treatment, F(1,27) = 7.27, p = 0.01, η2 = 0.03 (Figure 4C), with no other main or interactive effects, F(1,27) = 0.01-3.54, p = 0.07-0.91, η2 = 0.00- 0.08. Rats treated with furosemide spent less time exploring the novel object, compared to all other groups, p = 0.009. Again, a priori we wanted to determine whether ovarian hormones influenced object recognition during dehydration. Post hoc analysis revealed that regardless of treatment, intact animals spent significantly more time investigating the novel, compared to the original object, p < 0.001, d = 0.42 and 0.87 (control- vs. furosemide-treated, respectively). In OVX rats, the control-treated group also spent significantly more time exploring the novel, compared to the original object, p < 0.001, d = 0.71, however, furosemide-treated rats spent similar amounts of time exploring the novel and original objects, p = 0.22, d = 0.16.
4. Discussion
Despite decades of research on the impact of dehydration on cognitive function in humans, there are scant reports in animal models (Adan, 2012; Wilson and Morley, 2003). Testing the relationship between dehydration and cognitive function in animal models is advantageous because it can circumvent the confounding variables associated with human subject research that have likely contributed to inconsistencies in the literature. For example, numerous studies in humans induce dehydration by combining physical exercise in a hot and humid environment (thermal stress) with water restriction (Sécher and Ritz, 2012). Fatigue and/or any beneficial effect of exercise, therefore, is a confounding variable in many studies. These confounds can be easily circumvented in animal model because dehydration can be induced via a simple drug injection. Animal models also provide a better opportunity to identify underlying neuronal mechanisms. Here, we demonstrated for the first time that object memory retrieval is impaired after hypovolemic dehydration in a sex and hormone specific manner. This study expands upon findings from previous reports of impaired spatial memory in dehydrated mice by demonstrating that dehydration can affect memory retrieval in non-spatial tasks and can be influenced by gonadal hormone status (Faraco et al., 2014).
Prolonged water deprivation induces dehydration that is a mixture of both intracellular and extracellular water loss (Gizowski and Bourque, 2018). Because a previous report demonstrated impaired spatial memory after 24 and 48 h fluid restriction, one goal here was to determine the effects of osmotic and hypovolemic dehydration on memory performance (Faraco et al., 2014). In our first experiment, we induced osmotic dehydration which was confirmed after the test trial by examining water intake. As expected, rats treated with hypertonic saline drank more water than control-treated rats. During the novel object recognition test trial, all groups, regardless of hydration status, spent significantly more time investigating the novel object, compared to the original object. This suggests that osmotic dehydration does not impair retrieval of object memory. Importantly, the 15 min interval between NaCl-treatment and testing is consistent with previous studies using disruptors of memory retrieval, which typically use a 10-30 min delay (Mohammadkhani et al., 2015; Pezze et al., 2017; Stackman et al., 2016; Tian et al., 2019). Therefore, the lack of effect of osmotic dehydration was likely not the result of insufficient time needed for impairments in memory retrieval. This, however, does not rule out the possibility that osmotic dehydration can influence other domains of cognitive function. Indeed, hypertonic saline infusions are associated with changes in blood flow to brain areas important for cognitive function, such as the prefrontal cortex, in human subjects (Farrell et al., 2008). Future studies are necessary to determine whether osmotic dehydration influences learning, memory consolidation, or spatial memory.
Next, we tested the hypothesis that hypovolemic dehydration impairs object memory in a sex and hormone dependent manner. We predicted that females, or females in estrus, would be protected from memory impairments due to the reported effects estrogens have on cognitive function (Spencer et al., 2008). In partial support of our hypothesis, dehydrated D2 females did not discriminate between the novel and original object during the test trial, although males and females in estrus were not affected by dehydration. This suggests that during times of low ovarian hormone exposure, hypovolemic dehydration can inhibit object memory. To confirm the role of ovarian hormones, our follow up study demonstrated that removal of ovarian hormones was also associated with impaired object memory retrieval after hypovolemic dehydration. Because males are exposed to relatively constant levels of testicular hormones, we also asked whether loss of gonadal hormones could impair memory performance after hypovolemic dehydration in males. Unlike females, however, castration in male rats had no impact on object memory performance during hypovolemic dehydration. This was surprising given the previously reported impairments in spatial location memory in dehydrated male mice (Faraco et al., 2014). The difference between our findings and those from Faraco et al. could be the result of the different brain areas engaged during spatial and object memory tasks (Antunes and Biala, 2012; Warburton and Brown, 2015; Zimmermann and Eschen, 2017). Another factor which could underlie the different findings is that in our study, rats were only dehydrated during the test trial, and not during both the training and test trial. This could suggest that dehydration in spatial memory tasks influences memory consolidation. Future studies are needed to dissociate the effects of dehydration on different aspects of learning, memory consolidation, and memory retrieval.
One factor that could impact performance in the object memory test is low activity levels. In the intact study (Expt. 2), the dehydrated D2 females were less active than the control D2 females. Their activity, however, was not different from either group of males, both of which spent more time interacting with the novel object compared to the original object. Furthermore, activity levels in the follow up OVX study (Expt. 4) showed no difference between groups. Therefore, we do not believe that the inability to discriminate between the novel and original object was secondary to lethargy. This could suggest that ovarian hormones provide protection against multiple dehydration-related impairments in behavior and physiology. Or it could suggest that this specific group of rats had lower basal activity levels, which is more likely because, again, there were no difference in activity in the follow-up OVX experiment. Dehydration-induced changes in anxiety or neophobia could also have influenced performance during the test trials in a sex or hormone specific manner. These potential confounds will need to be explored in future studies.
It is well established that gonadal hormones, particularly estrogens, enhance cognitive function. It is, therefore, not surprising that our results supported our prediction that ovarian hormones would protect against dehydration-induced impairments in memory retrieval. Indeed, circulating estrogens have been implicated in protecting against glucocorticoid-induced impairments in memory retrieval (Mohammadkhani et al., 2015). Our intact studies (Expt. 1 and 2) support previous research showing no sex differences in object recognition memory performance (Bisagno et al., 2003), however, our OVX and CAST results are inconsistent with prior reports. Specifically, both ovariectomy and castration have been reported to impair performance in the object recognition task (Aubele et al., 2008; Jacome et al., 2010; Luine, 2015; Scharfman et al., 2007), yet we did not find impaired performance in the control-treated OVX and CAST groups. These inconsistencies are possibly related to task difficulty. Our testing protocol used repeated training trials separated by 24 h, while the previous reports used a single training trial with a 4 h inter-trial-interval before the test trial. Due to the repeated exposure to the original objects, the task difficulty was likely easier with our protocol and could have allowed the OVX and CAST groups to overcome the deficit associated with gonadal hormone loss. Furthermore, gonadal hormone effects on cognitive function are often small and not always observed (Luine, 2015), therefore it is reasonable to hypothesize that interactions between task difficulty and hormonal status can influence performance in the object recognition test. Because we expected dehydration to impair object recognition performance (Faraco et al., 2014), we needed a protocol where dehydration would not cause a floor effect. We, therefore, used a protocol designed to enhance performance. The ease of this task (3 training trials), however, could also have made it difficult to observe any dehydration-related impairments in the males and E females. Additional studies designed to alter the task difficulty are necessary to determine if there are conditions where dehydration can cause object memory impairments in males and estrous females.
While this series of experiments demonstrates a protective role of ovarian hormones on dehydration-induced memory retrieval impairments, many additional questions need to be addressed before more general conclusions can be drawn. As discussed above, it will be important to determine if dehydration impairs memory performance in males and estrous females when the task difficulty is increased. Tests examining the effect of dehydration on spatial memory are also necessary, particularly because spatial memory tasks engage different areas of the brain than object memory (Antunes and Biala, 2012; Warburton and Brown, 2015; Zimmermann and Eschen, 2017). While the report from Faraco and colleagues suggests that dehydration impairs spatial memory, the subjects were dehydrated during both the training and testing trial (Faraco et al., 2014). This highlights another important area of investigation, determining whether dehydration during training, learning trials or memory consolidation impacts performance. Finally, because elderly human populations are most commonly dehydrated (Sfera et al., 2016), and are at risk for cognitive decline (Park et al., 2003) it will be important to determine if dehydration-induced memory impairments are enhanced in aged animals.
This series of experiments demonstrated for the first time that ovarian hormones provide protection against dehydration-induced object memory retrieval impairments in female rats. In addition, the impairments in memory retrieval were limited to hypovolemic dehydration, with performance being unaffected after osmotic dehydration. As discussed above, this is a good starting point for studies investigating the effect of dehydration on cognitive function, but many more behavioral studies are necessary before general conclusions can be drawn. In addition, these animal studies are necessary, not only to help reconcile inconsistencies in the human literature, but also because they provide the opportunity to investigate the mechanisms by which dehydration influences cognition. It has been suggested that changes glucocorticoids, the neurotransmitters GABA and glutamate, and hindbrain catecholamines maybe underly dehydration-induced impairments in cognitive function (Adan, 2012; Masento et al., 2014; Wilson and Morley, 2003), however there are no empirical studies to support these claims. The role these systems play in mediating interactions between dehydration and cognition is an important area for future research. Ultimately, this research has the potential to help identify interventions useful to populations at risk for both dehydration and diminished cognitive function, such as athletes and the elderly (Gatterer et al., 2011; Park et al., 2003; Sfera et al., 2016).
Highlights.
Osmotic dehydration did not impair novel object recognition memory performance.
Hypovolemic dehydration impaired memory retrieval only in diestrous female rats.
Loss of ovarian hormones impaired memory retrieval after hypovolemic dehydration.
Loss of testicular hormones had no effect on memory after hypovolemic dehydration.
Acknowledgments
This work was supported by NIH grant DA035150 and University of Kentucky, College of Arts and Sciences Start-Up Funds. We thank Calista Whorf, Mariah Montgomery, Evelyn Perler-Tomboly, Alexander Schneider, Sahana Holla, and Daniel Abul-Khoudoud for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adan A, 2012. Cognitive performance and dehydration. J Am Coll Nutr 31, 71–78. [DOI] [PubMed] [Google Scholar]
- Antunes M, Biala G, 2012. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13, 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer MF, 2008. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav 54, 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E, 2005. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146, 1650–1673. [DOI] [PubMed] [Google Scholar]
- Begg DP, Sinclair AJ, Weisinger RS, 2012. Reductions in water and sodium intake by aged male and female rats. Nutr Res 32, 865–872. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN, 2003. Chronic D-amphetamine induces sexually dimorphic effects on locomotion, recognition memory, and brain monoamines. Pharmacol Biochem Behav 74, 859–867. [DOI] [PubMed] [Google Scholar]
- Cian C, Barraud PA, Melin B, Raphel C, 2001. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol 42, 243–251. [DOI] [PubMed] [Google Scholar]
- Curtis KS, 2009. Estrogen and the central control of body fluid balance. Physiol Behav 97, 180–192. [DOI] [PubMed] [Google Scholar]
- D'anci KE, Vibhakar A, Kanter JH, Mahoney CR, Taylor HA, 2009. Voluntary dehydration and cognitive performance in trained college athletes. Percept Mot Skills 109, 251–269. [DOI] [PubMed] [Google Scholar]
- Faraco G, Wijasa TS, Park L, Moore J, Anrather J, Iadecola C, 2014. Water deprivation induces neurovascular and cognitive dysfunction through vasopressin-induced oxidative stress. J Cereb Blood Flow Metab 34, 852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Zamarripa F, Shade R, Phillips PA, McKinley M, Fox PT, Blair-West J, Denton DA, Egan GF, 2008. Effect of aging on regional cerebral blood flow responses associated with osmotic thirst and its satiation by water drinking: a PET study. Proc Natl Acad Sci U S A 105, 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM, 2015. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem 22, 472–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Uban KA, Epp JR, Brummelte S, Barha CK, Wilson WL, Lieblich SE, Pawluski JL, 2008. Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol 62, 247–260. [DOI] [PubMed] [Google Scholar]
- Gatterer H, Schenk K, Ferrari P, Faulhaber M, Schopp E, Burtscher M, 2011. Changes in hydration status of soccer players competing in the 2008 European Championship. J Sports Med Phys Fitness 51, 89–94. [PubMed] [Google Scholar]
- Gervais NJ, Hamel LM, Brake WG, Mumby DG, 2016. Intra-perirhinal cortex administration of estradiol, but not an ERβ agonist, modulates object-recognition memory in ovariectomized rats. Neurobiol Learn Mem 133, 89–99. [DOI] [PubMed] [Google Scholar]
- Gizowski C, Bourque CW, 2018. The neural basis of homeostatic and anticipatory thirst. Nat Rev Nephrol 14, 11–25. [DOI] [PubMed] [Google Scholar]
- Gopinathan PM, Pichan G, Sharma VM, 1988. Role of dehydration in heat stress-induced variations in mental performance. Arch Environ Health 43, 15–17. [DOI] [PubMed] [Google Scholar]
- Hamson DK, Roes MM, Galea LA, 2016. Sex Hormones and Cognition: Neuroendocrine Influences on Memory and Learning. Compr Physiol 6, 1295–1337. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V, 2010. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem 94, 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ, 2003. Estrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav 79, 267–274. [DOI] [PubMed] [Google Scholar]
- Lieberman HR, 2007. Hydration and cognition: a critical review and recommendations for future research. J Am Coll Nutr 26, 555S–561S. [DOI] [PubMed] [Google Scholar]
- Luine V, 2015. Recognition memory tasks in neuroendocrine research. Behav Brain Res 285, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masento NA, Golightly M, Field DT, Butler LT, van Reekum CM, 2014. Effects of hydration status on cognitive performance and mood. Br J Nutr 111, 1841–1852. [DOI] [PubMed] [Google Scholar]
- Mohammadkhani R, Darbandi N, Vafaei AA, Ahmadalipour A, Rashidy-Pour A, 2015. Glucocorticoid-induced impairment of long-term memory retrieval in female rats: influences of estrous cycle and estrogen. Neurobiol Learn Mem 118, 209–215. [DOI] [PubMed] [Google Scholar]
- Neave N, Scholey AB, Emmett JR, Moss M, Kennedy DO, Wesnes KA, 2001. Water ingestion improves subjective alertness, but has no effect on cognitive performance in dehydrated healthy young volunteers. Appetite 37, 255–256. [DOI] [PubMed] [Google Scholar]
- Park HL, O'Connell JE, Thomson RG, 2003. A systematic review of cognitive decline in the general elderly population. Int J Geriatr Psychiatry 18, 1121–1134. [DOI] [PubMed] [Google Scholar]
- Patel AV, Mihalik JP, Notebaert AJ, Guskiewicz KM, Prentice WE, 2007. Neuropsychological performance, postural stability, and symptoms after dehydration. J Athl Train 42, 66–75. [PMC free article] [PubMed] [Google Scholar]
- Pezze MA, Marshall HJ, Fone KC, Cassaday HJ, 2017. Role of the anterior cingulate cortex in the retrieval of novel object recognition memory after a long delay. Learn Mem 24, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckelhoff JF, 2001. Gender differences in the regulation of blood pressure. Hypertension 37, 1199–1208. [DOI] [PubMed] [Google Scholar]
- Santollo J, Torregrossa AM, Daniels D, 2017. Sex differences in the drinking response to angiotensin II (AngII): Effect of body weight. Horm Behav 93, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, Maclusky NJ, 2007. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci 26, 2595–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfera A, Cummings M, Osorio C, 2016. Dehydration and Cognition in Geriatrics: A Hydromolecular Hypothesis. Front Mol Biosci 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VM, Sridharan K, Pichan G, Panwar MR, 1986. Influence of heat-stress induced dehydration on mental functions. Ergonomics 29, 791–799. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS, 2008. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 29, 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachenfeld NS, Leone CA, Mitchell ES, Freese E, Harkness L, 2018. Water intake reverses dehydration associated impaired executive function in healthy young women. Physiol Behav 185, 103–111. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Cohen SJ, Lora JC, Rios LM, 2016. Temporary inactivation reveals that the CA1 region of the mouse dorsal hippocampus plays an equivalent role in the retrieval of long-term object memory and spatial memory. Neurobiol Learn Mem 133, 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinnai G, Schachinger H, Arnaud MJ, Linder L, Keller U, 2005. Effect of water deprivation on cognitive-motor performance in healthy men and women. Am J Physiol Regul Integr Comp Physiol 289, R275–280. [DOI] [PubMed] [Google Scholar]
- Sécher M, Ritz P, 2012. Hydration and cognitive performance. J Nutr Health Aging 16, 325–329. [DOI] [PubMed] [Google Scholar]
- Tian SW, Yu XD, Cen L, Xiao ZY, 2019. Glutamate transporter GLT1 inhibitor dihydrokainic acid impairs novel object recognition memory performance in mice. Physiol Behav 199, 28–32. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD, Beasman K, Ganio MS, Cureton K, 2007. Effects of dehydration and fluid ingestion on cognition. Int J Sports Med 28, 891–896. [DOI] [PubMed] [Google Scholar]
- Turner JM, Marsteller DA, Luxkaranayagam AT, Fletcher JM, Stachenfeld NS, 2017. Mild exercise in female subjects impairs complex learning independent of hydration status and emotion. Physiol Behav 180, 113–119. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Brown MW, 2015. Neural circuitry for rat recognition memory. Behav Brain Res 285, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MM, Morley JE, 2003. Impaired cognitive function and mental performance in mild dehydration. Eur J Clin Nutr 57 Suppl 2, S24–29. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Eschen A, 2017. Brain regions involved in subprocesses of small-space episodic object-location memory: a systematic review of lesion and functional neuroimaging studies. Memory 25, 487–519. [DOI] [PubMed] [Google Scholar]