Abstract
Background:
Human growth hormone (hGH) is best known for influencing bone and muscle growth as well as body composition but use of recombinant hGH is controversial. Amino acids are a potentially safer alternative, however, preliminary investigations of the effects of oral amino acids on hGH release have been inconclusive. Therefore, we tested the effects of a novel blend of amino acids optimized to increase hGH release.
Study Question:
Does an investigational amino acid supplement affect hGH release?
Study Design:
This was a randomized, placebo-controlled, double-blind, crossover study that included 16 (12 males, 4 females; age 32 ±14 years; body mass index 26.4 ±5.0 kg/m2) healthy participants. All participants received both placebo and the amino acid supplement after an overnight fast and completed all study visits. Treatment order was randomized and each treatment was separated by a 1-week washout period.
Measures and Outcomes:
The primary outcome were the percent change in hGH from baseline to 120 minutes and the area under the curve of hGH over baseline. Serum hGH was measured using enzyme-linked immunosorbent assay at baseline and 15, 30, 60, 90 and 120 minutes.
Results:
At 120 minutes, hGH levels increased by 682% (8-fold) from baseline and were significantly higher than placebo (p=0.01). In addition, a significantly higher mean AUC was observed for the amino acid supplement compared to placebo (20.4 [95% CI: 19.9-21.0 ng/ml] vs. 19.7 [95% CI: 18.7-20.6 ng/ml]; p=0.04).
Conclusions:
These results show that a single dose of the oral amino acid supplement was sufficient to significantly increase hGH levels in healthy adult males and females.
Keywords: amino acid, growth hormone, pituitary
INTRODUCTION
Human growth hormone (hGH) is a peptide hormone that is best known for influencing bone and muscle growth and body composition.1 The primary source of circulating hGH is the pituitary gland. hGH is secreted from the pituitary in a pulsatile manner, with the majority being released at night.2 The greatest predictor of hGH secretion is age; hGH secretion is highest during adolescence and decreases with aging.3,4 However, hGH levels are also influenced by many other external factors. Exercise, stress, hypoglycemia, and some amino acids have been shown to stimulate hGH release, while elevated glucose, free fatty acids, and other amino acids have been shown to inhibit hGH release.2
It is well established that intravenous (IV) administration of some amino acids results in significantly increased hGH secretion. In females, intravenous infusion of 183mg of arginine/kg body weight increased hGH levels >20-fold14 and 30g of arginine elevated serum hGH levels 8.6-fold in males.15 Such studies prompted testing of oral amino acids supplements (primarily arginine, lysine and orthinine) to stimulate hGH secretion; however, these studies yielded mixed results.16-20 A low-dose amino acid supplement (SeroVital®, Sanmedica International, LLC, Salt Lake City, UT) containing 2.9 g of supplemented L-lysine, L-arginine, oxo-proline, N-acetyl-l-cysteine, L-glutamine, and schizonepeta was developed based on the stimulatory effects of some amino acids on hGH release.
This was a crossover, placebo-controlled study designed to determine the acute effects of the oral amino acid supplement on secretion of hGH in healthy subjects with normal hGH.
METHODS
Study Design
This was a randomized, placebo-controlled, double-blind, crossover study conducted between October 2011 and March 2012. The study included 16 participants (12 males and 4 females) who were recruited from the Pennington Biomedical Research Center. Male and female participants between 18 and 70 years of age were eligible. Participants were excluded if they were pregnant or nursing or taking any chronic medication, including hormonal contraception. All participants received both placebo and treatment article (amino acid supplement); participants were randomized to treatment order in a 1:1 ratio via computer-generated random sequence. Blinding was maintained by study investigators and participants throughout the study.
The institutional review boards at the study site approved the protocol prior to study initiation. The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All participants provided written informed consent approved by the Pennington Biomedical Research Center Institutional Review Board (IRB Number 10036). This trial is registered with clinicaltrials.gov: NCT01540773.
Procedures
Each participant reported to the Inpatient Unit in the morning on 2 occasions 1 week apart after an overnight fast. Blood was drawn from an IV line at −30, −15, 0, 15, 30, 60, 90 and 120 minutes. Study treatment (amino acid supplement or matching placebo) was administered orally at time 0. Serum samples were frozen at −80ºC until the end of study. Serum hGH (Recombinant 98/574) was measured using enzyme-linked immunosorbent assay (ELISA; Siemens Immulite 2000 Immunoassay System, Siemens Medical Solutions USA, Inc., Malvern, PA). The intra-assay coefficient of variability (CV) for the hGH assay was 3.72% and the inter-assay CV was 5.70%. The detection limit for growth hormone was 0.05 ng/ml.
Outcomes
The primary outcome measures were the percent change in hGH from baseline (0 minutes) to 120 minutes and the area under the curve (AUC) of hGH (0 to 120 minutes) over baseline.
Statistical Analysis
Based on early work showing the ability of amino acids to stimulate hGH secretion,17,19 a very conservative estimate of the mean and standard deviation for the change in hGH was 15.4 ±10 ng/mL. A sample size of 16 was employed to provide ≥80% power to detect a minimum mean change in hGH of 15.4 ±10 ng/mL using the nominal p<0.05 significance level.
Study drug was administered at 0 minutes. Baseline hGH was calculated as the average of hGH measurements −30, −15 and 0 minutes. The change from baseline to 15, 30, 60, 90 and 120 minutes was calculated as differences (ng/ml) by subtracting the baseline hGH level from the corresponding level at each time point. The area under the change curve (AUC) was calculated for each treatment using the trapezoidal rule. To avoid negative AUC’s and with invariant alterations in determining statistical significance of differential treatment effects, a constant of 20 was added to each calculated change in hGH level in the analytical sample. A mixed effects linear model was used that included terms for the effects on hGH of sequences, participants within sequence, sessions, sequence by session interaction (treatments) and residual error. Consistent with our a priori hypothesis that the study treatment would increase hGH levels, the decision was made to reject the null only if the data supported the one directional alternative consistent with a favorable response to the supplement. Statistical significance was assumed for p≤0.05. All calculations were performed using the software package SAS® Version 9.3 and data are presented as mean (95% CI) or mean ± SEM.
RESULTS
A total of 16 healthy participants (12 males and 4 females) were included in the study, received both placebo and the amino acid supplement, and completed all study visits. Of these, 56% (9/16) were Caucasian and 38% (6/16) were African American. At baseline, participant characteristics were (mean ±standard deviation [SD]): age 23 ±14 years (range: 18 to 62 years), weight 79.9 ±18.9 kg (range: 55.5 to 121.6 kg), and body mass index (BMI) 26.4 ± 5.0 kg/m2 (range: 19.1 to 36.8 kg/m2).
At baseline, the mean value for hGH was slightly higher during treatment with placebo than with the amino acid supplement, though the difference was not statistically significant (p>0.05; Table 1). There was an initial decrease in mean hGH from baseline after administration of placebo and the amino acid supplement (Figure 1). However, following treatment with the amino acid supplement, mean hGH was increased from baseline at both the 90- and 120-minute assessments while hGH remained unchanged after treatment with placebo. At t=120 minutes, hGH was 1.33 ng/ml after administration of the amino acid supplement, compared with only 0.45 ng/ml after placebo. The increase in hGH 120 minutes following administration of the amino acid supplement represented a 682% or 8-fold increase from baseline (Table 1, Figure 1). The area under the curve for hGH from time 0 to 120 minutes (AUC0-120 min) was also significantly greater following treatment with the amino acid supplement compared to placebo (Table 1).
Table 1.
Change in human growth hormone (hGH)
| Placebo | Amino Acid Supplement | |
|---|---|---|
| Baseline hGH (ng/ml) | 0.93 | 0.17 |
| hGH at t=120 minutes1 (ng/ml) | 0.45 | 1.33 |
| Change in hGH1 (ng/ml) | −0.48 | 1.15 |
| 95% Confidence interval | −1.47, 0.50 | 0.17, 2.14 |
| p-value vs placebo | 0.01 | |
| AUC0-120 min | 19.67 | 20.43 |
| 95% Confidence interval | 18.74, 20.59 | 19.90, 20.95 |
| p-value vs placebo | 0.04 |
Change from baseline (t=0) to t=120 minutes.
AUC0-120 min = area under the curve from 0 to 120 minutes.
Figure 1. Percent change in serum hGH concentrations over 120 minutes.
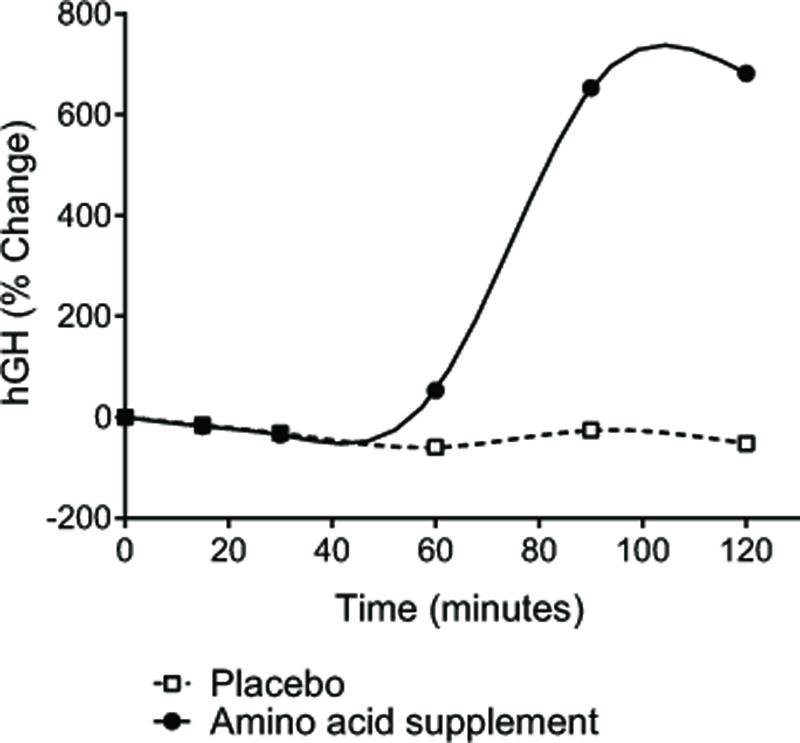
Data are the % change from baseline in serum hGH levels following ingestion of SeroVital® or matching placebo (N=16). After an overnight fast, baseline blood samples were collected at −30, −15, and 0 minutes. Treatment was administered at 0 minutes and blood was collected at 15, 30, 60, 90, and 120 minutes.
The adverse events reported during the study were nausea and lightheadedness that occurred in a participant treated with the amino acid supplement. Both events were deemed unlikely related to treatment (they were attributed to study procedures, eg, blood draw) by the investigator.
DISCUSSION
In this placebo-controlled, crossover design study, we report 682% or 8-fold increase in mean hGH from baseline following oral administration of an amino acid supplement in healthy fasted volunteers. In contrast, mean hGH remained similar to baseline after oral administration of placebo. Similarly, the AUC of hGH during the 120 minutes after treatment was significantly increased after administration of the amino acid supplement compared to placebo.
Prior to this study, only intravenous administration of amino acids was shown to reliably increase endogenous hGH14, 15 while studies of the effects of oral administration of amino acids on hGH release yielded conflicting results.16-20 Potential reasons for these variable outcomes may be the dose and composition of amino acids used, fasting vs fed conditions,21 controlling for timing and study procedures (eg, venipuncture),22,23 or small sample size.18,24 In addition, growth hormone binding proteins may interfere with available hGH.25 Moreover, multiple limitations with current methodologies for measuring hGH can result in inter-assay variability of as much as 2- to 3-fold.24,26 For example, assays may employ different antibodies to target specific hGH epitopes (monoclonal or polyclonal).24 Thus, each assay is associated with variability that limits quantitative comparisons across assays; the magnitude of the change within a single assay (ie, change from baseline) is the most appropriate comparison.
A notable strength of the current study is the randomized, placebo-controlled, double-blind, crossover design in which each participant received the treatment article (amino acid supplement) and matching placebo in a randomized order and both investigators and participants were blinded to the treatment. Thus, each participant served as their own control, reducing the need for a large number of participants by minimizing the influence of confounding variables. This study design is appropriate for assessing the effects of acute treatment with a test article. Additionally, the study was powered to detect changes in hGH based on estimates obtained from previous studies.17,19 Finally, the inclusion of male and female participants across a broad range of ages and BMIs strengthens the external validity of the results.
In conclusion, these results demonstrate that a single dose of the amino acid supplement is sufficient to produce a statistically significant increase in hGH without evidence of a waning effect during the testing period. Our findings strengthen the evidence that oral administration of a specifically formulated combination of amino acids can increase serum hGH levels.
Acknowledgements
We thank the study volunteers for participating in the study and Alok Gupta, MD (Permanente Medical Group, Pinole, CA) for clinical assistance. This was an investigator-led study funded by Sierra Research Group, LLC, Salt Lake City, UT. Medical writing assistance was provided by Sonja K Billes, PhD (August Scientific) and funding was provided by the Sponsor. CST is also supported by a National Health and Medical Research Centre Early Career Fellowship from Australia (#1037275). WDJ was also funded in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: This was an investigator-led study funded by Sierra Research Group, LLC, Salt Lake City, UT.
Footnotes
Conflicts of interest:
CST, WDJ, and JR have no conflict of interest. ALH provides consultant services for Sierra Research Group, LLC. and Basic Research, LLC. FLG is a consultant for Beachbody, Basic Research, LLC., Eisai, Inc., General Nutrition Corporation, Melior Discoveries, and Techenterprises, is on advisory boards for Baronova, Inc., Curves-Jenny Craig, Gelesis, Microbiome Therapeutics, Novo Nordisk, Novartis, Plensat, and Zafgen, holds stock or stock options in Microbiome Therapeutics, Plensat, and Zafgen, and holds patents in Neuroquest.
References
- 1.Murray PG, Higham CE Clayton PE 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-GH axis: the past 60 years. J Endocrinol 226, T123–140, doi: 10.1530/JOE-15-0120 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Giustina AVeldhuis, JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19, 717–797, doi: 10.1210/edrv.19.6.0353 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Hersch ECMerriam, GR. Growth hormone (GH)-releasing hormone and GH secretagogues in normal aging: Fountain of Youth or Pool of Tantalus? Clin Interv Aging 3, 121–129 (2008). [PMC free article] [PubMed] [Google Scholar]
- 4.Ho KY, Evans WS, Blizzard RM et al. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 64, 51–58, doi: 10.1210/jcem-64-1-51 (1987). [DOI] [PubMed] [Google Scholar]
- 5.Chikani V, Cuneo RC, Hickman I et al. Impairment of anaerobic capacity in adults with growth hormone deficiency. J Clin Endocrinol Metab 100, 1811–1818, doi: 10.1210/jc.2015-1006 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Amato G, Carella C, Fazio S et al. Body composition, bone metabolism, and heart structure and function in growth hormone (GH)-deficient adults before and after GH replacement therapy at low doses. J Clin Endocrinol Metab 77, 1671–1676, doi: 10.1210/jcem.77.6.8263158 (1993). [DOI] [PubMed] [Google Scholar]
- 7.Boot AM, Engels MA, Boerma GJ et al. Changes in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiency. J Clin Endocrinol Metab 82, 2423–2428, doi: 10.1210/jcem.82.8.4149 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Allen DBCuttler, L. Clinical practice. Short stature in childhood--challenges and choices. N Engl J Med 368, 1220–1228, doi: 10.1056/NEJMcp1213178 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chikani V, Cuneo RC, Hickman I et al. Growth hormone (GH) enhances anaerobic capacity: impact on physical function and quality of life in adults with GH deficiency. Clin Endocrinol (Oxf), doi: 10.1111/cen.13147 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Cuneo RC, Salomon F, Wiles CM et al. Growth hormone treatment in growth hormone-deficient adults. I. Effects on muscle mass and strength. J Appl Physiol (1985) 70, 688–694 (1991). [DOI] [PubMed] [Google Scholar]
- 11.Salomon F, Cuneo RC, Hesp R et al. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med 321, 1797–1803, doi: 10.1056/NEJM198912283212605 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Barake M, Klibanski ATritos, NA. Effects of recombinant human growth hormone therapy on bone mineral density in adults with growth hormone deficiency: a meta-analysis. J Clin Endocrinol Metab 99, 852–860, doi: 10.1210/jc.2013-3921 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Molitch ME, Clemmons DR, Malozowski S et al. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96, 1587–1609, doi: 10.1210/jc.2011-0179 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Merimee TJ, Rabinowtitz D Fineberg SE Arginine-initiated release of human growth hormone. Factors modifying the response in normal man. N Engl J Med 280, 1434–1438, doi: 10.1056/NEJM196906262802603 (1969). [DOI] [PubMed] [Google Scholar]
- 15.Alba-Roth J, Muller OA, Schopohl J et al. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J Clin Endocrinol Metab 67, 1186–1189, doi: 10.1210/jcem-67-6-1186 (1988). [DOI] [PubMed] [Google Scholar]
- 16.Corpas E, Blackman MR, Roberson R et al. Oral arginine-lysine does not increase growth hormone or insulin-like growth factor-I in old men. J Gerontol 48, M128–133 (1993). [DOI] [PubMed] [Google Scholar]
- 17.Suminski RR, Robertson RJ, Goss FL et al. Acute effect of amino acid ingestion and resistance exercise on plasma growth hormone concentration in young men. Int J Sport Nutr 7, 48–60 (1997). [DOI] [PubMed] [Google Scholar]
- 18.Lambert MI, Hefer JA, Millar RP et al. Failure of commercial oral amino acid supplements to increase serum growth hormone concentrations in male body-builders. Int J Sport Nutr 3, 298–305 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Isidori A, Lo Monaco, ACappa M A study of growth hormone release in man after oral administration of amino acids. Curr Med Res Opin 7, 475–481, doi: 10.1185/03007998109114287 (1981). [DOI] [PubMed] [Google Scholar]
- 20.Fogelholm GM, Naveri HK, Kiilavuori KT et al. Low-dose amino acid supplementation: no effects on serum human growth hormone and insulin in male weightlifters. Int J Sport Nutr 3, 290–297 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Ho KY, Veldhuis JD, Johnson ML et al. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest 81, 968–975, doi: 10.1172/JCI113450 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White HK, Petrie CD, Landschulz W et al. Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab 94, 1198–1206, doi: 10.1210/jc.2008-0632 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Janssen YJ, Frolich M Roelfsema F The absorption profile and availability of a physiological subcutaneously administered dose of recombinant human growth hormone (GH) in adults with GH deficiency. Br J Clin Pharmacol 47, 273–278 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bidlingmaier M Strasburger CJ Growth hormone assays: current methodologies and their limitations. Pituitary 10, 115–119, doi: 10.1007/s11102-007-0030-1 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Blum WF, Hall K, Ranke MB et al. Growth hormone insensitivity syndromes: a preliminary report on changes in insulin-like growth factors and their binding proteins during treatment with recombinant insulin-like growth factor I. Kabi Pharmacia Study Group on Insulin-like Growth Factor I Treatment in Growth Hormone Insensitivity Syndromes. Acta Paediatr Suppl 82 Suppl 391, 15–19 (1993). [DOI] [PubMed] [Google Scholar]
- 26.Nindl BC, Hymer WC, Deaver DR et al. Growth hormone pulsatility profile characteristics following acute heavy resistance exercise. J Appl Physiol (1985) 91, 163–172 (2001). [DOI] [PubMed] [Google Scholar]


