Abstract
Race is a social construct that involves a person’s self-assigned, and externally-perceived, group membership. Group membership can determine much about Americans’ lives and health. Complex health disorders such as cardiovascular disease, asthma, and obesity disproportionately affect Non-Hispanic black Americans. An individual’s risk of any of these disorders encompasses both genetic predisposition and environmental stimuli. We propose that environmental stressors may be large contributors to differences in preterm birth rates in the United States between racial groups. Environmental exposures differ by race due to ongoing residential, educational and economic racial segregation as well as discrimination. Characterizing and mitigating environmental factors that contribute to differential preterm risk could identify women at risk, prevent some preterm births, and reduce perinatal health disparities.
Keywords: Race, pregnancy, preterm birth, disparities, environment
Introduction
The infant mortality rate in the United States exceeds that of most other developed nations, ranking 26th among Organisation for Economic Co-operation and Development countries.1 Non-Hispanic black infants in the United States die more than twice as often as non-Hispanic white infants (11.4 versus 4.9 per 1,000 live births).2 This disparity reflects disparities in preterm birth (PTB) rates, since two-thirds of infant mortality occurs in preterm infants.3 The PTB rate is 52 percent higher for black (13.8%) than white (9.0%) women. Efforts to reduce PTB and its disparities have failed (Figure 1). We propose that racial disparities in PTB are a cumulative biosensor of exposures that vary by race, arising from longstanding inequities.
Figure 1.
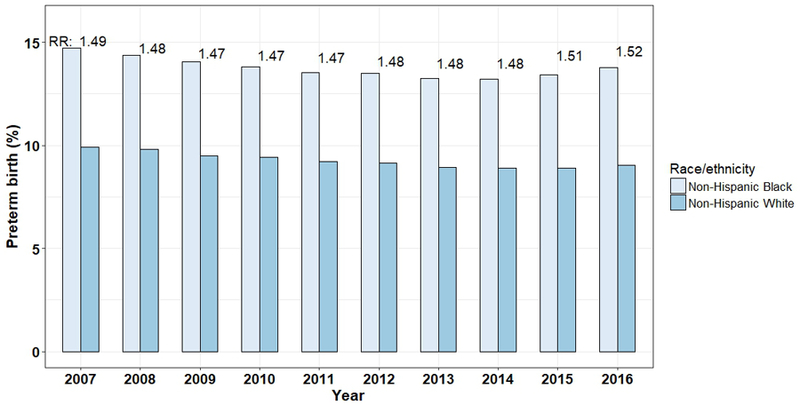
Black-white disparities in preterm birth over 10 years in the US64,65; RR=relative risk
PTB disparities are not due to genetic sequence variation between racial groups
While some monogenic diseases track (incompletely) with race, such as sickle cell anemia and cystic fibrosis, the vast majority of health conditions cannot be mapped to genetic variation between racial groups. Most human genetic variation is found within ancestral groups with only 5–10% of gene frequencies differing between ancestral groups.4 Nonetheless, different frequencies of single nucleotide polymorphisms (SNPs) by race have led some investigators to search for genetic differences that cause racial disparities in PTB. However, SNPs explain an exceedingly small portion of PTB risk, and are often not replicated.5,6 Some strong evidence supports that disparities in birth outcomes are largely attributable to environmental, as opposed to genetic variation. One example is the phenomenon of erosion of immigrant health over generations. Birth weight (BWT) distributions of infants born to African-born black women and US-born white women nearly overlap, whereas infants born to US-born black women were substantially smaller.7 Indeed in a study of 27 states’ births in 2008, foreign-born black women had significantly lower odds of PTB than US-born black women even after adjustment for sociodemographic, health behavioral and medical risk factors (adj. OR 0.727; CI 0.726–0.727).8 Others have used twin and kinship studies to analyze the genetic versus the environmental contributions to PTB. These find that the pooled genetic contribution (<35%) is dwarfed by non-genetic or broadly-defined environmental influences, particularly when self-identified African Americans are compared to self-identified European Americans.9,10 Further, PTB is a heterogeneous phenotype comprised of distinct pathophysiologic pathways. Spontaneous PTB results from premature cervical remodeling and/or myometrial contractility, whereas medically-indicated PTB occurs when providers elect to deliver a fetus due to maternal or fetal conditions such as preeclampsia or intrauterine growth restriction. Black women are at higher risk of both spontaneous and medically-indicated PTB,11 and it is unlikely that a set of genetic sequences that track with race would lead to such different phenotypes. Given the small contribution of genetics to PTB, coupled with the very small differences in genetic sequences between racial groups in the US, broadly-defined environmental factors must be responsible for racial disparities in PTB.
Environmental factors must play a role in black-white disparities in PTB
We propose a framework wherein micro- and macro-environmental factors shape the likelihood of a healthy, term delivery (Figure 2). Micro-environmental factors include behavioral factors where an element of individual choice can be made, such as smoking and diet. Macro-environmental factors include social and physical environmental exposures such as neighborhood violence, air and water pollution, heavy metals, and other exposures that are not directly due to an individual’s decisions, but are present in the environment. Both micro- and macro-environmental exposures can affect an individual woman’s physiology, and alter the likelihood of delivering preterm. We will argue that the macro-environment plays a significant and under-appreciated role in population-level disparities.
Figure 2.
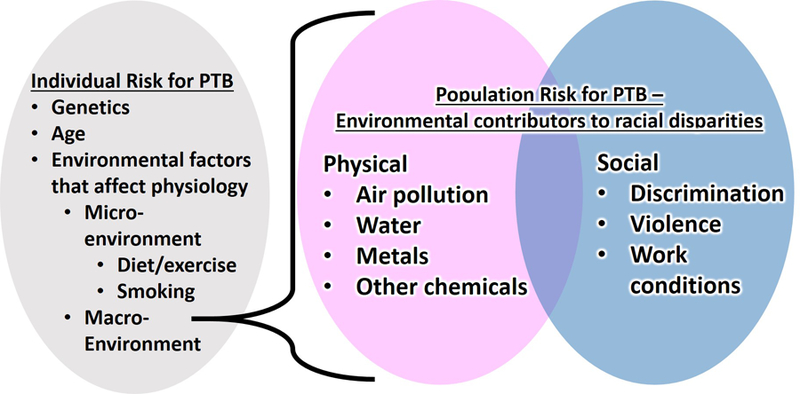
Expanded framework for environmental exposures that shape racial disparities in preterm birth (PTB)
Micro-environmental incompletely explain black-white differences in PTB
To implicate an exposure as a contributing factor to black-white disparities, it must be more highly prevalent among black women. Smoking is one of the strongest risk factors for PTB.12 Differences in smoking rates do not explain differences in PTB risk. Only 5.6% of Non-Hispanic black women report smoking in pregnancy, compared with 10.1% of Non-Hispanic white women.13 White women who smoke have similar PTB rates (12.9%) to black women who do not smoke (13.5%), suggesting that while smoking increased individuals’ risk of PTB, differential PTB rates between races is not due to smoking.
Diets are culturally patterned practices that can differ by race/ethnicity.14 No single dietary practice has been implicated in PTB. A key micronutrient during pregnancy hypothesized to be involved in racial disparities in health is vitamin D. Unlike smoking, vitamin D deficiency is more common among black women; lighter pigmented skin is more efficient at synthesizing vitamin D from sunlight.15 While some observational studies have shown associations between vitamin D deficiency and PTB,16 intervention trials have not. In a trial in South Carolina, 257 women were randomized to three different doses of vitamin D supplementation and while 25(OH)D levels increased according to dose, there was no difference in PTB.17 In a recent, large trial of 1164 women randomized to high doses of monthly vitamin D vs. placebo in Bangladesh, there were no differences in birth outcomes.18 These results suggest that supplementing vitamin D for black women is unlikely to mitigate disparities in PTB.
Iron deficiency and anemia, which are more common among African Americans,19,20 has likewise been associated with PTB in observational studies,21 but randomized controlled trials of iron supplementation have been disappointing.22 In a recent study, over 15,000 women were randomized to one of three interventions: folic acid alone, folic acid with iron, and multiple micronutrients.23 While the last group had a significant decrease in spontaneous PTB, there was no difference in the folic acid with iron group compared with the folic acid only group. Simply treating iron deficiency is unlikely to mitigate racial disparities in PTB.
Macro-environmental factors that may help to explain racial disparities in PTB
A large body of evidence has connected neighborhoods to health.24 This is particularly relevant due to residential racial segregation and environmental inequity between black and white neighborhoods.25,26 The extent to which segregation and disproportionate exposure to toxic environments among black families contributes to racial disparities in PTB is unknown. Yet, evidence is mounting to connect physical and social environmental exposures to PTB risk. Most implicated exposures are concentrated in black neighborhoods, and black women living in racially-segregated black neighborhoods have higher risk of PTB than black women living in integrated neighborhoods.27
Physical environmental exposures
Several physical environmental exposures have well-documented associations with PTB risk and disproportionately affect black families. Air pollution exposure increases the risk of PTB28,29 and black Americans are more highly exposed due to residential proximity to industrial facilities,30 traffic,31 and other sources. One recent study of over 37,000 births in China demonstrated that as interquartile range of short-term exposure to gases (SO2, and NO2) and particulate matter (<2.5 (PM2.5) and <10 (PM10) microns in diameter) increased, the odds of PTB were 3.7–6.5 times higher.32 Lead exposures also disproportionately affect black families.33 According to the Environmental Protection Agency’s biomonitoring program, regardless of poverty level, black children (1.1 µg/dL) have higher median lead levels than white children (0.8 µg/dL) and are twice as likely to have elevated levels (>5 ug/dL).34,35 Lead has been shown to be associated with increased risk of PTB.36 A recent case-control study (n=102 preterm cases and 306 term controls) also in China found that infants in the highest tertile of lead levels, versus the lowest, had higher odds of being born preterm (adj. OR 2.96, CI: 1.49–5.87).37
Phthalates have also been shown to increase the risk of PTB.38 These ubiquitous chemicals are used as plasticizers, and are components of personal care products, fast food packaging, and building materials (e.g., flooring). Phthalate metabolite levels can vary by race/ethnicity due micro-environmental 39 as well as macro-environmental exposures.40 In a prospective, nested case-control study (n=130 cases, 352 controls) in Boston, women who delivered preterm had higher geometric means of several phthalate metabolites and higher odds of spontaneous PTB (ORs = 1.22–1.67 per ln-unit increase). While evidence that physical environmental exposures contribute to PTB risk is accumulating, it is not yet clear what proportion of racial disparities in PTB is attributable to differences in the physical environment.
Social human environmental exposures
Social environments (human interactions) also strongly affect PTB risk. Exposure to racism and discrimination is associated with increased risk of PTB.41,42 In a case-control study of 312 black women (104 cases and 208 controls) in Chicago, women who reported high levels of exposure to lifetime interpersonal racism were more likely to have delivered a very low BWT (<1,500g) infant than an infant >2,500g (adj. OR 2.6, CI: 1.2–5.3).41
Violent crime, a severe chronic urban stressor, is often concentrated in lower-income and minority neighborhoods,43 and has been shown to affect birth outcomes. A study in North Carolina found that among over 30,000 live births, neighborhood exposure to violent crime was associated with PTB;44 notably, the range of violent crime counts near black women was higher than for white women with so little overlap, that race-specific tertiles were necessary for analysis. Recently, investigators used a natural experiment to perform a population-based longitudinal analysis of BWT in Mexico before and after the rise of the Mexican Drug War. Increase in violent crime was associated with lower BWT after adjustment for maternal and community confounding variables.45 Like physical environmental exposures, the extent to which differential social exposures by race may explain racial disparities in PTB is unknown.
Interaction of physical and social environments
In 1992, Geronimus proposed an analytic framework to explain why black women had increased risk of adverse perinatal outcomes.46 She postulated that black-white infant mortality differential among infants born to older (versus younger) women was due to the cumulative effects of socioeconomic disadvantage. One potential mechanism transmitting socioeconomic disadvantage to racial disparities is through increasing susceptibility to physical exposures through chronic stress and related physiologic impacts on immune and metabolic function (allostatic load).47 Indeed there is evidence that social and physical environments interact to result in differential birth outcomes.48 Ponce et al analyzed birth certificate data for 37,347 deliveries in California, and found that air pollution was associated with odds of PTB only among women in the lowest socioeconomic stratum.49 A recent analysis of 53,843 mother-infant pairs in California, used an exposure index to include air, land, and water pollution sources.50 Women in the highest quintile of exposure had twice the odds of PTB versus women in the lowest quintile, and, dichotomizing the index, only women in the low-socioeconomic stratum had elevated odds of PTB with higher pollution exposures.50 These data suggest that socioeconomic disadvantage changes susceptibility to physical environments.
Social and physical inequity is not limited to residential life. Employment opportunities vary by race in the United States resulting in differential physical occupational exposures51 and higher levels of job strain defined as high-demand and low-control in the workplace,52 among black workers.53 In a case-control (n=1,242 preterm cases, 4,413 term controls) study in Quebec, Croteau and colleagues found that women with high levels of job strain including physically demanding postures and lack of control at work had higher odds of PTB.54 Further in a study of black and white women in North Carolina, job strain was associated with higher odds of PTB, specifically among black women (OR 1.8, 95% CI 1.1–3.1) compared with white women (OR 1.0, 95% CI: 0.5–2.0).55 Taken together, given that race often tracks with socioeconomic position that determines where women live and work, interactions of physical and social environments likely explain much of the racial disparity in PTB.
Improving education alone will not solve disparities
One way to improve socioeconomic position is through education. However, education alone does not eliminate birth outcome disparities. In 1992, Schoendorf demonstrated that racial disparities in infant mortality existed even among infants born to college-educated women and that the disparity was entirely explained by differential risk of LBW (7% and 3% among black and white infants, respectively).56 This phenomenon persists today. Disparities widen as education levels rise, as shown in Figure 3, which displays PTB rates by education level in 2016 in the US.13 These data suggest that education alone does not mitigate exposures that differ by race over a lifetime. Whether racial disparities among well-educated women are due to earlier life exposures (or even exposure to prior generations that could be passed along through epigenetic phenomena) or due to ongoing exposure to racism or other environmental stressors, which are not eliminated with education, remains unknown.
Figure 3.
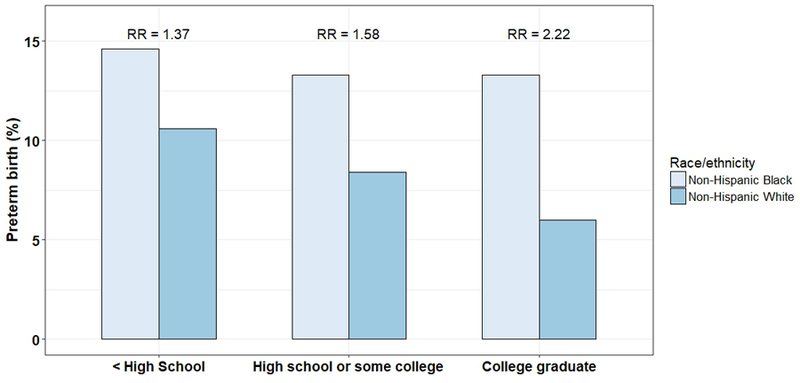
Black-white disparities in preterm birth according to maternal education in the US. Data from CDC Wonder, singleton births from 2016;13 RR=relative risk
The study complexity needed to address racial disparities in PTB
Tackling racial disparities in PTB will require rigorously quantifying the relative contribution of adverse environmental factors that are disproportionately concentrated in black communities. One way this could be studied is by analyzing interracial couples with one black parent and one white parent. A recent population-based study of over 1.6 million live-births in California demonstrated that couples where one parent is black and the other is white have lower risk of spontaneous PTB than when both parents are black; the risk reduction was greater when the woman was white (adjusted OR: 0.7, 95% CI: 0.7–0.8) than when the man was white (adjusted OR: 0.9, 95% CI: 0.8–1.0).57 While the risk reduction may be due to unmeasured socioeconomic position differences between couples who have interracial marriages compared to black couples, understanding which exposures differ among these families may shed light on environmental causes of PTB. Investigation into how the environment may shape racial disparities in preterm birth requires interdisciplinary collaboration between perinatal and environmental scientists, sociologists and other social scientists, biostatisticians, epidemiologists, and public health professionals. Identifying priorities for environmental exposure reduction is a critical step to motivate policy efforts to reduce racial disparities in PTB.
The importance of phenotyping PTBs
Due to the small impact of any individual environmental exposure on PTB, it is critical to clarify which subtype of PTB is affected, and to have large study populations. Otherwise associations can be missed (Type II error). Theoretically, if a toxic chemical affects the risk of preterm labor (one presentation of spontaneous PTB) with a relative risk of 1.4, but not preterm preeclampsia (one presentation of medically-indicated PTB) with a relative risk of 0.9, the overall relative risk of PTB from the chemical might be 1.2 with a confidence interval that crosses 1. In this case, the chemical might be deemed falsely benign with respect to PTB. Phenotyping PTBs is resource-intensive, but will be required for detecting PTB subtype-specific environmental effects.
Consideration of mixtures
Because many physical and social environmental exposures co-locate, considering the effects of mixtures is also extremely important given the small impacts of single exposures. For example, a single elevated phthalate may not alone cause spontaneous PTB, but combined with air pollution, lead, and violence, the phthalate could be the last necessary stressor to trigger premature myometrial contractility or cervical remodeling necessary for spontaneous PTB.
Getting closer to causation
Given the many variables that potentially confound an environmental exposure’s effect on PTB, demonstrating that a toxic chemical affects the risk of a specific PTB phenotype among black women within an educational or income stratum would help to identify true targets. Better causal inference modeling, including mediation may also facilitate a better understanding as to the relative contributions of multiple exposures.58 Multilevel modeling can also clarify the relative contribution of neighborhood factors versus individual-level factors.59,60 Additionally, collaborating with translational scientists and toxicologists to identify biologic pathways by which toxic chemicals could lead to a specific PTB phenotype in animal models or in-vitro can help to clarify whether as association is more likely to be truly causal.
Realizing policy change
It is important to focus on social and environmental determinants of health in order to reduce disparities, as opposed to simply improving medical care for all which can sometimes widen black-white gaps.61 Once the targets that are likely both causative agents for PTB and disproportionately concentrated in black communities are identified, policy changes could reduce disparities. The built environment can affect physical activity through walkability and recreational resources. Local food environments can affect diet quality. Together these factors can affect likelihood of diabetes and hypertension24 which can be associated with the risk of medically-indicated PTB.62 Additionally, clean-up efforts can improve birth outcomes. Recently, a study of over 57,000 births within 20 miles of coal power plants that were retired between 2001 and 2011 demonstrated significant reductions in PTB after the closures with the largest impact among black women.63 Ultimately, exposure reduction of multiple implicated exposures will be required to narrow the racial gap in PTB that has persisted for decades.
Conclusion
Longstanding racial disparities in PTB are likely largely due to social and physical exposures that vary by race due to enduring inequity in the United States. The next frontier in tackling perinatal health disparities will require studying and rectifying the injustices of unequal environments. Only then will black families have an equal chance of healthy, full term births.
Abbreviations:
- PTB
preterm birth
- BWT
birth weight
- CI
confidence interval
Footnotes
Competing Interest: None declared.
References
- 1.MacDorman MF, Matthews TJ, Mohangoo AD, Zeitlin J. International comparisons of infant mortality and related factors: United States and Europe, 2010. Natl Vital Stat Rep 2014;63(5):1–6. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Division of Prepoductive Health, National Center for Chronic Disease Prevention and Health Promotion 2018; https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm. Accessed January 2, 2019.
- 3.Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep 2015;64(9):1–30. [PubMed] [Google Scholar]
- 4.Rosenberg NA, Pritchard JK, Weber JL, et al. Genetic structure of human populations. Science 2002;298(5602):2381–2385. [DOI] [PubMed] [Google Scholar]
- 5.Zhang G, Feenstra B, Bacelis J, et al. Genetic Associations with Gestational Duration and Spontaneous Preterm Birth. N Engl J Med 2017;377(12):1156–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anum EA, Springel EH, Shriver MD, Strauss JF 3rd. Genetic contributions to disparities in preterm birth. Pediatr Res 2009;65(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David RJ, Collins JW Jr. Differing birth weight among infants of U.S.-born blacks, African-born blacks, and U.S.-born whites. N Engl J Med 1997;337(17):1209–1214. [DOI] [PubMed] [Google Scholar]
- 8.Elo IT, Vang Z, Culhane JF. Variation in birth outcomes by mother’s country of birth among non-Hispanic black women in the United States. Matern Child Health J 2014;18(10):2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.York TP, Eaves LJ, Neale MC, Strauss JF 3rd. The contribution of genetic and environmental factors to the duration of pregnancy. Am J Obstet Gynecol 2014;210(5):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.York TP, Strauss JF 3rd, Neale MC, Eaves LJ. Racial differences in genetic and environmental risk to preterm birth. PLoS One 2010;5(8):e12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ada ML, Hacker MR, Golen TH, Haviland MJ, Shainker SA, Burris HH. Trends in provider-initiated versus spontaneous preterm deliveries, 2004–2013. J Perinatol 2017;37(11):1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.In: Behrman RE, Butler AS, eds. Preterm Birth: Causes, Consequences, and Prevention Washington (DC)2007. [PubMed] [Google Scholar]
- 13.United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Division of Vital Statistics, Natality public-use data 2007–2017, on CDC WONDER Online Database, October 2018 http://wonder.cdc.gov/natality-current.html. Accessed January 3, 2019.
- 14.Hiza HA, Casavale KO, Guenther PM, Davis CA. Diet quality of Americans differs by age, sex, race/ethnicity, income, and education level. J Acad Nutr Diet 2013;113(2):297–306. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 16.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2013;26(9):889–899. [DOI] [PubMed] [Google Scholar]
- 17.Wagner CL, McNeil R, Hamilton SA, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol 2013;208(2):137 e131–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth DE, Morris SK, Zlotkin S, et al. Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth. N Engl J Med 2018;379(6):535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeiffer CM, Sternberg MR, Caldwell KL, Pan Y. Race-ethnicity is related to biomarkers of iron and iodine status after adjusting for sociodemographic and lifestyle variables in NHANES 2003–2006. J Nutr 2013;143(6):977S–985S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le CH. The Prevalence of Anemia and Moderate-Severe Anemia in the US Population (NHANES 2003–2012). PLoS One 2016;11(11):e0166635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong X, Buekens P, Alexander S, Demianczuk N, Wollast E. Anemia during pregnancy and birth outcome: a meta-analysis. Am J Perinatol 2000;17(3):137–146. [DOI] [PubMed] [Google Scholar]
- 22.Haider BA, Olofin I, Wang M, et al. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 2013;346:f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Mei Z, Zhang L, et al. Effects of Prenatal Micronutrient Supplementation on Spontaneous Preterm Birth: A Double-Blind Randomized Controlled Trial in China. Am J Epidemiol 2017;186(3):318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 25.White K, Borrell LN. Racial/ethnic residential segregation: framing the context of health risk and health disparities. Health Place 2011;17(2):438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morello-Frosch R, Lopez R. The riskscape and the color line: examining the role of segregation in environmental health disparities. Environ Res 2006;102(2):181–196. [DOI] [PubMed] [Google Scholar]
- 27.Mehra R, Boyd LM, Ickovics JR. Racial residential segregation and adverse birth outcomes: A systematic review and meta-analysis. Soc Sci Med 2017;191:237–250. [DOI] [PubMed] [Google Scholar]
- 28.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 2012;117:100–111. [DOI] [PubMed] [Google Scholar]
- 29.Li XY, Huang SQ, Jiao AQ, et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environ Pollut 2017;227:596–605. [DOI] [PubMed] [Google Scholar]
- 30.Ash M, Boyce JK. Racial disparities in pollution exposure and employment at US industrial facilities. P Natl Acad Sci USA 2018;115(42):10636–10641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian N, Xue JP, Barzyk TM. Evaluating socioeconomic and racial differences in traffic-related metrics in the United States using a GIS approach. J Expo Sci Env Epid 2013;23(2):215–222. [DOI] [PubMed] [Google Scholar]
- 32.Liu WY, Yu ZB, Qiu HY, Wang JB, Chen XY, Chen K. Association between ambient air pollutants and preterm birth in Ningbo, China: a time-series study. BMC Pediatr 2018;18(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller C, Sampson RJ, Winter AS. Environmental Inequality: The Social Causes and Consequences of Lead Exposure. Annu Rev Sociol 2018;44:263–282. [Google Scholar]
- 34.Environmental Protection Agency. America’s Children and the Environment 2017; https://www.epa.gov/sites/production/files/2017-07/documents/biomonitoring_lead_data_7-26-17.pdf. Accessed January 6, 2019.
- 35.Tsoi MF, Cheung CL, Cheung TT, Cheung BM. Continual Decrease in Blood Lead Level in Americans: United States National Health Nutrition and Examination Survey 1999–2014. Am J Med 2016;129(11):1213–1218. [DOI] [PubMed] [Google Scholar]
- 36.Andrews KW, Savitz DA, Hertz-Picciotto I. Prenatal lead exposure in relation to gestational age and birth weight: a review of epidemiologic studies. Am J Ind Med 1994;26(1):13–32. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B, Xia W, Li Y, et al. Prenatal exposure to lead in relation to risk of preterm low birth weight: A matched case-control study in China. Reprod Toxicol 2015;57:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr 2014;168(1):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Branch F, Woodruff TJ, Mitro SD, Zota AR. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001–2004. Environ Health 2015;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 2004;112(3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins JW, David RJ, Handler A, Wall S, Andes S. Very low birthweight in African American infants: The role of maternal exposure to interpersonal racial discrimination. Am J Public Health 2004;94(12):2132–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, Kiefe CI. Self-reported experiences of racial discrimination and black-white differences in preterm and low-birthweight deliveries: The CARDIA study. Am J Public Health 2004;94(12):2125–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krivo LJ, Peterson RD, Kuhl DC. Segregation, Racial Structure, and Neighborhood Violent Crime. Am J Sociol 2009;114(6):1765–1802. [DOI] [PubMed] [Google Scholar]
- 44.Messer LC, Kaufman JS, Dole N, Herring A, Laraia BA. Violent crime exposure classification and adverse birth outcomes: a geographically-defined cohort study. Int J Health Geogr 2006;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown R The Mexican Drug War and Early-Life Health: The Impact of Violent Crime on Birth Outcomes. Demography 2018;55(1):319–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis 1992;2(3):207–221. [PubMed] [Google Scholar]
- 47.McEwen BS. Stress, adaptation, and disease - Allostasis and allostatic load. Ann Ny Acad Sci 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 48.Shmool JL, Bobb JF, Ito K, et al. Area-level socioeconomic deprivation, nitrogen dioxide exposure, and term birth weight in New York City. Environ Res 2015;142:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponce NA, Hoggatt KJ, Wilhelm M, Ritz B. Preterm birth: the interaction of traffic-related air pollution with economic hardship in Los Angeles neighborhoods. Am J Epidemiol 2005;162(2):140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padula AM, Huang H, Baer RJ, et al. Environmental pollution and social factors as contributors to preterm birth in Fresno County. Environ Health 2018;17(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frumkin H, Walker ED, Friedman-Jimenez G. Minority workers and communities. Occup Med 1999;14(3):495–517. [PubMed] [Google Scholar]
- 52.Karasek RA. Job Demands, Job Decision Latitude, and Mental Strain - Implications for Job Redesign. Admin Sci Quart 1979;24(2):285–308. [Google Scholar]
- 53.Meyer JD. Race-based job discrimination, disparities in job control, and their joint effects on health. American Journal of Industrial Medicine 2014;57(5):587–595. [DOI] [PubMed] [Google Scholar]
- 54.Croteau A, Marcoux S, Brisson C. Work activity in pregnancy, preventive measures, and the risk of preterm delivery. Am J Epidemiol 2007;166(8):951–965. [DOI] [PubMed] [Google Scholar]
- 55.Brett KM, Strogatz DS, Savitz DA. Employment, job strain, and preterm delivery among women in North Carolina. Am J Public Health 1997;87(2):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schoendorf KC, Hogue CJR, Kleinman JC, Rowley D. Mortality among Infants of Black as Compared with White College-Educated Parents. New Engl J Med 1992;326(23):1522–1526. [DOI] [PubMed] [Google Scholar]
- 57.Shachar BZ, Mayo JA, Lyell DJ, Stevenson DK, Shaw GM, Blumenfeld YJ. Risk for spontaneous preterm birth among inter-racial/ethnic couples(). J Matern Fetal Neonatal Med 2018;31(5):633–639. [DOI] [PubMed] [Google Scholar]
- 58.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 59.Diez-Roux AV. Bringing context back into epidemiology: Variables and fallacies in multilevel analysis. Am J Public Health 1998;88(2):216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evans CR, Williams DR, Onnela JP, Subramanian SV. A multilevel approach to modeling health inequalities at the intersection of multiple social identities. Soc Sci Med 2018;203:64–73. [DOI] [PubMed] [Google Scholar]
- 61.Thornton RL, Glover CM, Cene CW, Glik DC, Henderson JA, Williams DR. Evaluating Strategies For Reducing Health Disparities By Addressing The Social Determinants Of Health. Health Aff (Millwood) 2016;35(8):1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meis PJ, Goldenberg RL, Mercer BM, et al. The preterm prediction study: risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol 1998;178(3):562–567. [DOI] [PubMed] [Google Scholar]
- 63.Casey JA, Karasek D, Ogburn EL, et al. Retirements of Coal and Oil Power Plants in California: Association With Reduced Preterm Birth Among Populations Nearby. American Journal of Epidemiology 2018;187(8):1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital Stat Rep 2017;66(1):1. [PubMed] [Google Scholar]
- 65.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: Final Data for 2016. Natl Vital Stat Rep 2018;67(1):1–55. [PubMed] [Google Scholar]


