Abstract
Background
While there is increasing interest in sharing genetic research results with participants, how best to communicate the risks, benefits and limitations of research results remains unclear.
Methods
Participants who received genetic research results answered open and closed‐ended questions about their experiences receiving results and interest in and advantages and disadvantages of a web‐based alternative to genetic counseling.
Results
107 BRCA1/2 negative women with a personal or family history of breast cancer consented to receive genetic research results and 82% completed survey items about their experience. Most participants reported there was nothing they disliked (74%) or would change (85%) about their predisclosure or disclosure session (78% and 89%). They most frequently reported liking the genetic counselor and learning new information. Only 24% and 26% would not be willing to complete predisclosure counseling or disclosure of results by a web‐based alternative, respectively. The most frequently reported advantages included convenience and reduced time. Disadvantages included not being able to ask questions, the risk of misunderstanding and the impersonal nature of the encounter.
Conclusion
Most participants receiving genetic research results report high satisfaction with telephone genetic counseling, but some may be willing to consider self‐directed web alternatives for both predisclosure genetic education and return of results.
Keywords: genetic counseling, genetic testing, preferences for return of genetic research results, return of genetic research results, web‐based alternatives to genetic counseling
1. INTRODUCTION
Advances in genetic sequencing have given researchers greater opportunities to understand genetic contributions to disease and the enhanced potential to improve clinical outcomes (Burke & Psaty, 2007; Khoury et al., 2009; Olopade, Grushko, Nanda, & Huo, 2008). The return of individual research results to research participants is a topic of vigorous debate (Burke, Evans, & Jarvik, 2014). While some regard return of research results as inappropriate due to their provisional and arguably confusing nature (Clayton & McGuire, 2012; Dressler, 2009; Miller, Christensen, Giacomini, & Robert, 2008), there is a growing perspective that an ethical duty exists for research teams to return genetic results obtained in the research setting that could impact a participant's clinical care (Dressler, 2009; Greely, 2007; Ramoni et al., 2013; Ravitsky & Wilfond, 2006). Researchers are now recommended to inform participants during the informed consent process about whether genetic research results will be returned, including how these results will be shared (Presidential Commission for the Study of Bioethical Issues, 2013), yet optimal methods for returning such remain unknown (Jarvik et al., 2014; Roberts, Wood, Gaieski, & Bradbury, 2017). Trained genetic counselors are the “gold standard” for educating patients about risks and benefits of genetic testing (Green & Fost, 1997; Roberts et al., 2017). However, the traditional two‐visit genetic counseling encounter is increasingly impractical owing to workforce limitations, state licensure barriers and professional costs (Biesecker et al., 2018; Roberts et al., 2017). To meet the growing demand for genetic services, alternative models need to be explored (Biesecker et al., 2018; Jarvik et al., 2014; Roberts et al., 2017). While others have investigated a variety of alternatives, including CD‐ROM and web‐based options to genetic education and results return with a counsellor (Biesecker et al., 2018; Jamal et al., 2014), none have reported outcomes with alternative delivery models in the context of returning individual genetic research results.
To address this gap, we sought to evaluate interest in alternative models for return of genetic research results among participants in the RESPECT study (2018), including participant experiences with return of results with a genetic counselor by telephone, willingness to complete predisclosure education and receive results by a self‐directed website, and perceived advantages and disadvantages of a web‐based alternative.
2. METHODS AND MATERIALS
2.1. Ethical compliance
This study was approved to recruit human subjects by the following Institutional Review Boards: University of Pennsylvania (lead site) Columbia University, University of Chicago.
2.2. Participants
As previously described (2018), potential participants in the RESPECT study had been consented to a research registry at the University of Pennsylvania and had available genetic research results, with one or more of the following: (a) diagnosis of breast cancer <40 years old, (b) multiple primary cancers (bilateral breast or breast and another nonmelanoma skin cancer primary), or (c) at least 3 first‐ or second‐degree relatives with breast cancer (2018). Participants had research sequencing of 24 breast cancer susceptibility genes (CDH1, CDKN2A, MLH2, MSH2, EPCAM, MSH6, MUTYH Homozygous, PMS2, PTEN, STK11, TP53, ATM, BAP1, BARD1, BMPR1A, BRIP1, CHEK2, MRE11A, MUTYH Heterozygous, NBN, PALB2, RAD50, RAD51C, and RAD51D). Institutional Review Board approval was obtained and potential participants were contacted between May 2014 and October 2015. Between May 2014 and October 2015, RESPECT study research staff mailed 402 invitation letters to potentially eligible participants from the registry explaining that research testing had been completed on their research sample and that they could enroll in a study evaluating the outcomes of returning research results.
2.3. Predisclosure counseling and result disclosure
Predisclosure counseling sessions were completed by phone or in‐person, based on patient preference. The five participating genetic counselors completed protocol training, at least one mock telephone counseling session and were blinded to results at predisclosure. Genetic counselors completed counseling checklists that were modeled after the tiered‐binned genetic counseling model (Bradbury, Patrick‐Miller, & Domchek, 2014; Bradbury et al., 2016, 2015). In this model, tier‐1 “indispensable” information is presented to all patients, and more specific tier‐2 information is provided to those patients who desire or require additional detail to make informed a decision (Berg, Khoury, & Evans, 2011; Bradbury et al., 2014). Key elements of predisclosure informed consent were provided to address return of research results and the unique limitations, risks and uncertainties of multigene panel testing. These included a discussion of the differences between clinical and research testing, the range of susceptibility genes tested and potential test results, the value of confirmatory testing in a CLIA‐approved clinical laboratory and the benefits and the limitations and risks of receiving research results.
Participants who elected to receive their research result were scheduled for a disclosure session by phone or in‐person with a genetic counselor. Disclosure sessions included a review of the research results, the need for clinical confirmation when recommended, implications and potential medical management recommendations, if the results were confirmed, and the option for full clinical testing based on personal and family history consistent with current clinical practice standards. All participants with a pathogenic or likely pathogenic result or a variant of uncertain significance (VUS) in a high penetrance gene were recommended to have clinical confirmation testing of their research finding (2018).
2.4. Survey instrument
Participants completed surveys at baseline, after predisclosure counseling and results disclosure. In addition to quantitative scales assessing theoretically informed cognitive and affective outcomes (previously reported) (Burke & Psaty, 2007), surveys after predisclosure counseling and results disclosure included 14 items assessing experiences with genetic counseling and opinions regarding alternative delivery models for informed consent and disclosure of research results. These included 4 open‐ended items assessing what participants liked and disliked about their genetic counseling sessions (predisclosure or disclosure of results) and their recommendations for changes for participants undergoing genetic counseling in the future. Using a 5‐point Likert response from not at all willing to very willing, participants answered three survey questions that assessed interest in and the advantages and disadvantages of replacing phone counseling with a genetic counselor with web‐based education and disclosure of results by a private website (“Now that you have completed your counseling session, how willing would you have been to receive genetic counseling by a website where you can choose the information you want, rather than with a person?). Open‐ended assessment of advantages and disadvantages was intended to help in the development of future web‐based prototypes. In the final item (open‐ended), participants were asked to describe other ways they would be willing to complete predisclosure or disclosure of results counseling.
2.5. Data analyses
Descriptive statistics such as means, standard deviations (SDs) and proportions were used to characterize the baseline and response data. We used multiple linear regressions with forward stepwise selection to investigate patient level characteristics associated with the Likert measures assessing willingness to complete predisclosure education by web or disclosure of results by web as an alternative to telephone counseling with a genetic counselor. In the regressions, we accounted for clustering within family via the use of robust cluster corrected standard errors (Williams, 2000). We used a forward stepwise procedure to reduce the degrees of freedom used for model parameter estimation, and thereby enhance the power to detect associations. We used STATA (StataCorp, College Station, Texas) for the analyses. p‐values of less than .05 were used to assess statistical significance.
Framework analysis was utilized to examine open‐ended responses (Pope, Royen, & Baker, 2002; Ritchie & Spencer, 1994; Velikova et al., 2008). Investigators reviewed responses for a subsample (33%) of participants and developed a thematic framework of primary and secondary themes for each open‐ended item (Bradbury et al., 2008). Next, two investigators (JBG, SW) independently assigned thematic codes to the open‐ended responses. Inter‐coder agreement was high for all items (98%–100%) (Cohen, 1960). The thematic framework was then applied to the remaining samples’ open‐ended responses and themes were refined to include new ones as they emerged. Differences in code assignments were resolved by a third investigator (AB) and inter‐coder discussion, establishing agreement for all responses.
3. RESULTS
3.1. Participant characteristics
Of 107 consented participants, 88 (82%) completed genetic counseling experience items after their predisclosure counseling or result disclosure (Figure 1). The mean age of participants was 53.5 (SD = 12.7), 84 (95% of 88 completers) were white, and 76 (86%) had a college education or higher (Table 1).
Figure 1.
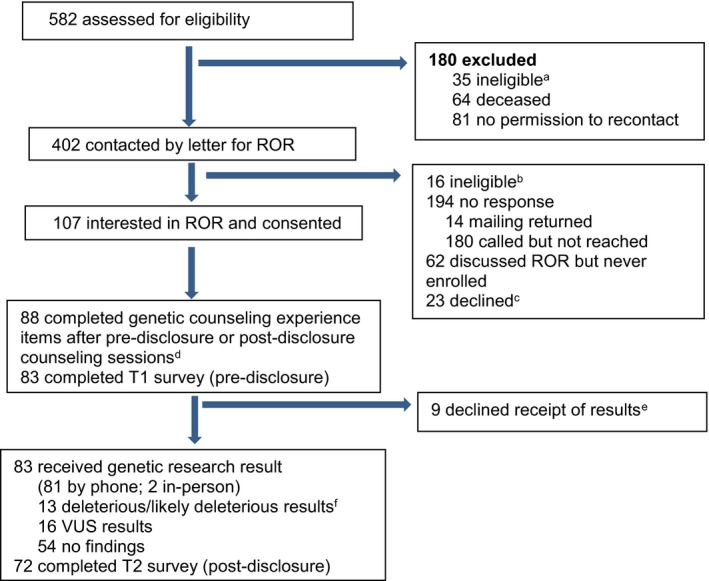
Study Flow Diagram
Table 1.
Characteristics of all research participants who answered predisclosure (T1) and/or postdisclosure (T2) genetic counseling experience items
| Participant characteristics |
Participants who consented to genetic counseling to consider return of research results (N = 88a) N (%) |
|---|---|
| Age (mean, SD) | 53.5 (12.7) |
| Race | |
| White | 84 (95%) |
| Non‐white | 4 (5%) |
| Missing | NA |
| Ethnicity | |
| Ashkenazi Jewish (AJ) | 18 (20%) |
| Non‐AJ | 67 (76%) |
| Missing | 3 (3%) |
| Education | |
| High school | 8 (9%) |
| Some college | 14 (16%) |
| College graduate or higher | 62 (70%) |
| Missing | 4 (5%) |
| Marital status | |
| Married | 69 (78%) |
| Not married | 19 (22%) |
| Missing | NA |
| Age at first cancer (mean, SD) | 39.4 (8.8)b |
| # FDR/SDR with breast cancer | 1.4 (1.4) |
| #FDR/SDR with cancer | 3.6 (2.5) |
| Result | |
| POS | 15 (17%) |
| VUS | 16 (18%) |
| NEGATIVE | 57 (65%) |
| Seen in cancer risk program | |
| Yes | 53 (60%) |
| Missing | 8 (9%) |
| Years since sample received (mean, SD) | 10.5 (12.5)c |
Includes 83 individuals who completed T1 and 5 additional participants who did not complete T1 but did complete T2 survey items.
1 missing.
2 missing.
3.2. Participant feedback on telephone predisclosure genetic counseling and disclosure of genetic research results with a genetic counselor
When asked what they liked about their predisclosure telephone genetic counseling session, most reported liking their genetic counselor (57%) and learning new information (53%) (Table 2). Some reported liking that they had the opportunity to ask questions and review information (9%), the visual aids were helpful (8%) and it was convenient (7%). The majority of respondents reported disliking nothing about their session (74%). The most frequently reported dislikes included the stressful nature of the session, including the possibility of getting a positive result (9%), that the information was repetitive (4%) and the large volume of information to process (3%). While most said there was nothing they would change about their session (85%), some respondents reported they wished they had had or would have liked changes to the visual aids (4%), a shorter/simpler session (3%) and learned their results during the predisclosure session (3%).
Table 2.
Patient feedback on telephone predisclosure genetic counseling and disclosure of genetic research results with a genetic counselor
| Pre‐disclosure telephone counseling with a genetic counselor | |
| What did you like about your genetic counseling session? ((n = 74)a | N (%) |
| Liked the genetic counselor (GC) | 42 (57) |
| GC was GC was kind/professional/caring | 27 |
| GC was knowledgeable/informative | 17 |
| GC answered my questions | 10 |
| GC was easy to talk to/interactive/one‐on‐one conversation | 9 |
| GC helped me manage my expectations | 1 |
| Session was informative/learned something new | 39 (53) |
| Was able to ask questions/review information | 7 (9) |
| The visual aids were helpful | 6 (8) |
| Session was convenient | 5 (7) |
| Session was the right pace/length | 3 (4) |
| Chance to help others | 1 (1) |
| Session was personal | 1 (1) |
| My family could listen and ask questions | 1 (1) |
| What did you dislike about your genetic counseling session? (n = 70)b | |
| Nothing | 52 (74) |
| Stressful: in general or specific to facing cancer, potentially getting a positive result or considering how to share with children | 6 (9) |
| Information was too repetitive | 3 (4) |
| A lot of information | 2 (3) |
| Length | 1 (1) |
| Made me realize getting my results would be cumbersome | 1 (1) |
| If they find I have mutation, info might not be helpful | 1 (1) |
| Might have preferred in‐person | 1 (1) |
| Confusing terminology | 1 (1) |
| Wished I’d had visual aids during session | 1 (1) |
| Is there anything you'd change about your genetic counseling session? (n = 68)c | |
| No/nothing | 58 (85) |
| Shorter/simpler | 2 (3) |
| Wish I could have gotten my results at the time/in first session | 2 (3) |
| Wish I’d had visual aids during the session | 2 (3) |
| Visual aids | 1 (1) |
| Space where I took call was not private enough | 1 (1) |
| Would have liked a written list of frequently asked questions to return to later | 1 (1) |
| Would have preferred in‐person session | 1 (1) |
| Telephone disclosure of genetic research results with a genetic counselor | |
| What did you like about genetic counseling session? (n = 67)d | N (%) |
| Liked the genetic counselor (GC) | 25 (37) |
| GC was GC was kind/professional/caring | 16 |
| GC was knowledgeable/informative | 8 |
| GC answered my questions | 4 |
| GC easy to talk to/interactive/one‐on‐one conversation | 4 |
| Session was informative/learned something new | 24 (36) |
| Session was the right pace/length | 8 (12) |
| Was able to ask questions/review information | 6 (9) |
| Session was convenient/easy | 5 (7) |
| GC told me my results early in session/discuss results right away | 5 (7) |
| Happy with my results | 5 (7) |
| Getting my results gave me peace of mind | 3 (4) |
| Results were explained in a way personalized to me and my family | 2 (3) |
| Session was reassuring | 1 (1) |
| Gave me another opportunity to discuss my results | 1 (1) |
| Important to be able to talk about next steps, especially if actionable | 1 (1) |
| What did you dislike about genetic counseling session? (n = 62)e | |
| Nothing | 48 (78) |
| Stressful in general or in facing cancer or getting a positive results | 3 (5) |
| Information was too repetitive | 3 (5) |
| Length | 2 (3) |
| Did not like my result | 2 (3) |
| Raised new questions for me that I don't know how to answer | 2 (3) |
| Wished I’d had visual aids during session | 1 (2) |
| A lot of information | 1 (2) |
| Inconvenient | 1 (2) |
| Would have preferred to get results during the first visit | 1 (2) |
| Is there anything you'd change about your genetic counseling session? (n = 65)f | |
| No/nothing | 58 (89) |
| Wait caused anxiety | 2 (3) |
| Shorter/simpler | 1 (2) |
| Wish I’d had visual aids with me | 1 (2) |
| Wish I could have gotten my results at the time/in first session | 1 (2) |
| Didn't like that I needed further testing | 1 (2) |
| Wish I had been better prepared to ask questions | 1 (2) |
10 did not answer this question.
14 did not answer this question.
16 did not answer this question.
6 did not answer this question.
11 did not answer this question.
8 did not answer this question.
When asked what they liked about their telephone results disclosure session, participants most commonly reporting liking their genetic counselor (37%), learning new information (36%), the pacing and length of the session (12%) and the ability to ask questions and review information (9%). While 78% reported there was nothing they disliked about their result disclosure session, the most frequently reported dislikes included the stressful nature of the session, including the possibility of getting a positive result (5%), repetitiveness of the information (5%), and that the session raised new questions they did not know how to answer (3%). While 89% of respondents stated they would not change anything about their result disclosure session, 2% reported that having to wait for results caused anxiety.
3.3. Willingness to complete predisclosure education and disclosure of research results by a website
In response to hypothetical queries, approximately 24% of respondents reported being “not at all willing” to consider using a website as an alternative to predisclosure education with a genetic counselor by telephone. Others reported variable levels of wiliness (Table 3). Similarly, 26% said they would be “not at all willing” to consider receiving result disclosure by a website, with the remainder reporting variable levels of willingness (Table 3). In secondary exploratory analyses with model selection, those with a VUS were less willing to consider predisclosure by a website as compared to those who received a negative result (p < .01). Those with a positive result did not differ significantly in their willingness compared to other test subgroups. In additional exploratory analyses, older age was also associated with less willingness to complete results disclosure by a website (p = .001). Race/ethnicity, education, marital status and family history of cancer were not associated with willingness to consider website alternatives after adjustment for result or age.
Table 3.
Willingness to complete predisclosure education by web or disclosure of results by web as an alternative to telephone counseling with a genetic counselor
| Not at all willing | Somewhat unwilling | Neither willing or unwilling | Somewhat willing | Very willing | |
|---|---|---|---|---|---|
| Pretest education and informed consent (N = 83) | 20 (24%) | 34 (41%) | 16 (19%) | 5 (6%) | 8 (10%) |
| Disclosure of genetic research results (N = 72) | 19 (26%) | 25 (35%) | 6 (8%) | 7 (10%) | 15 (21%) |
| Willingness to complete disclosure of genetic research results by test result received | |||||
| POSITIVE (N = 11) | 3 (27%) | 2 (18%) | 2 (18%) | 1 (9%) | 3 (27%) |
| VUS (N = 14) | 7 (50%) | 4 (29%) | 1 (7%) | 0 | 2 (14%) |
| NEGATIVE (N = 47) | 9 (19%) | 19 (10%) | 3 (6%) | 6 (13%) | 10 (21%) |
Abbreviation: VUS, variant of uncertain significance.
3.4. Advantages and disadvantages of predisclosure education or disclosure of results by a website
When asked to report advantages of a website as an alternative to predisclosure education and counseling by phone, many patients (37%) reported no advantages to a website alternative (Table 4). Among those reporting advantages, the most frequently reported advantages were convenience (24%) and reduced time commitment (14%). Examples include “could do it whenever I had time”, “wouldn't need an appointment” (convenience), “takes less time”, “can do any time of day or night” (reduction in time commitment). Others reported the benefit of being able to self‐select information (10%), having access to more information (8%) and privacy reviewing and responding to the information (7%). Examples include, “one could look further into items that interest you and less at ones that do not”, “I can choose the amount of information I wanted” (ability to self‐select information), “could review the information in private in the event it is upsetting, no one could see/hear your reaction” (privacy). A minority (8%) indicated specifically that they preferred speaking with a person for predisclosure education and therefore did not report any advantages.
Table 4.
Advantages and disadvantages of predisclosure education by web or disclosure of results by web
| Predisclosure education and informed consent by web | |
| What do you feel would be the advantages to having genetic counseling by a website where you can choose the information you want, rather than with a person? | N = 59a |
| N (%) | |
| No advantages | 22 (37) |
| Convenience | 14 (24) |
| Reduce time commitment | 8 (14) |
| Can self‐select information | 6 (10) |
| Can provide more information | 5 (8) |
| Permits privacy and/or anonymity | 4 (7) |
| One can control the pace of the session | 1 (2) |
| Would be useful after speaking with a person (but not as an alternative) | 1 (2) |
| What do you feel would be the disadvantages to having genetic counseling by a web‐site where you can choose the information you want, rather than with a person? | N = 62b |
| N (%) | |
| No disadvantages | 1 (2) |
| No one to ask/answer my questions | 22 (35) |
| Risk of misunderstanding | 20 (32) |
| Impersonal, lack of personal touch, face‐to‐face communication | 17 (27) |
| No one to make sure I understand or to explain things | 5 (8) |
| May be too generic and not individualized | 2 (3) |
| May not be proficient with technology | 2 (3) |
| Might result in GC follow‐up session anyway | 1 (2) |
| No one to help me if I get upset/give me support | 1 (2) |
| Time | 1 (2) |
| Having a session with GC will help me prioritize this process | 1 (2) |
| Disclosure of genetic research results by web | |
| What do you feel would be the advantages to having genetic counseling disclosure session by a website where you can choose the information you want, rather than with a person? | N = 62c |
| N (%) | |
| No advantages | 22 (36) |
| Convenience | 14 (23) |
| Reduce time commitment | 6 (10) |
| Can self‐select information | 6 (10) |
| One can control the pace of the session | 5 (8) |
| Permits privacy and/or anonymity | 3 (5) |
| Available as a resource to revisit | 3 (5) |
| Better than no counseling | 1 (2) |
| Can provide more information | 1 (2) |
| What do you feel would be the disadvantages to having genetic counseling disclosure session by a website where you can choose the information you want, rather than with a person? | N = 59d |
| N (%) | |
| No disadvantage | 9 (15) |
| Risk of misunderstanding | 15 (25) |
| Impersonal, lack of personal touch, fact‐to‐face communication | 15 (25) |
| No one to ask/answer my questions | 9 (15) |
| No one to make sure I understand or explain things | 7 (12) |
| Not acceptable/must be in‐person | 3 (5) |
| No one to help me if I get upset/give me support | 2 (3) |
| Too much information | 2 (3) |
| Generally may not work for some people | 1 (2) |
| May not be proficient with technology | 1 (2) |
14 did not answer the question and 6 reported they were unsure, and 5 indicated that they would prefer a person (removed for not reporting an advantage).
excluded 15 who did not answer this item and 2 reported they were unsure.
11 did not answer the question, (removed for not reporting an advantage).
14 did not answer the question, 2 removed for not reporting a disadvantage.
The most frequently reported disadvantages to using a website as an alternative to predisclosure phone education and counseling included not having access to a person to whom they could ask or who could answer questions (35%), a risk of misunderstanding (32%) and the impersonal nature of the encounter (27%). Examples include “I think it's important to have someone to answer questions and explain the results whether or not they are good or bad, “I might have questions and no one to ask them to” (no person to ask/answer questions), “possibility of confusing information”, “may miss important information if you don't look for it” (risk of misunderstanding) and “too cold and impersonal”, “I prefer the personal approach” (impersonal). Other reported disadvantages include: “better to have someone walking you through it” (no one to make sure I understand or explain things) and “I may not be as focused choosing the information that seems most appealing and may miss pertinent information that may otherwise be communicated to me by a counselor” (not individualized enough).
When asked to report advantages of receiving results by a website as an alternative to receiving results and postdisclosure counseling with a genetic counselor by phone, many patients (36%) reported no advantages to a website alternative (Table 4). Among those reporting advantages, the most frequently reported advantages included convenience (23%), reduced time commitment (10%) and the ability to self‐select information (10%). Examples include: “would be easier to schedule, and possibly this could be done after work hours.”, “the session would not have to be scheduled in advance” (convenience), “faster, easier”, “not as time consuming (reduced time‐commitment), “would allow you to see just the information you wish”, “would eliminate risk factors and screening recommendations that don't apply to you” (self‐selection of information). Other reported benefits include: “might list information that I wouldn't have thought of asking about” (the opportunity to have additional information) and “not having to show your response or feelings to the counselor” (privacy). The most frequently reported disadvantages included risk of misunderstanding (25%), the impersonal nature of the encounter (25%) and no person to ask/answer questions (15%). Examples include: “if a person is anxious or depressed or unintelligent, they might come to the wrong conclusions”, “some of the context surrounding the results would be lost” (risk of misunderstanding), “very cold”, “I like to have personal contacts when getting information of this importance” (impersonal) and “wouldn't be able to ask about things not listed on the website”, “results may be misinterpreted and questions may not be answered” (no person to ask/answer questions). Other reported disadvantages include: “can be a scary and overwhelming process and it is very helpful to have someone guide you through it” (no one to help me if I get upset/give me support) and “too much information for some people to sort through” (too much information).
4. DISCUSSION
To our knowledge, this is the first published study to report patient qualitative experiences with return of high and moderate penetrance genetic research results by telephone with a genetic counselor and opinions regarding using web‐based alternatives to genetic counseling. In this study, research participants reported many benefits to telephone predisclosure education and counseling and most would not have changed anything about their experience. Nonetheless, providing individual counseling for all participants who receive research results could create considerable resource and financial burdens for research teams and if established as a standard could reduce available funds for scientific discovery (Bledsoe et al., 2013; Bledsoe, Grizzle, Clark, & Zeps, 2012). Thus, there has been great interest in identifying alternative models for return of research results including web‐based delivery. In this study, approximately 25% of participants indicated that they would not want to replace either visit (predisclosure or disclosure) with a genetic counselor with a website alternative, but the majority reported some level of willingness to consider this approach, suggesting web‐based models may be a reasonable and acceptable alternative to some research participants. Further, research participants identified several advantages to web‐based alternatives and potential disadvantages, which could be helpful for designing patient‐focused web‐based alternatives for return of genetic research results.
Overall, respondents were satisfied with tiered‐binned counseling, most frequently reporting there was nothing they would change about their pre‐ or postdisclosure genetic counseling sessions (85% and 89% respectively). The small number who offered suggestions for changes to telephone counseling stated they would have preferred shorter or simpler sessions, changes to visual aids, a more private space to receive the information, a printed list of FAQs, and in‐person counseling instead of telephone. Likewise, respondents reported valuing personal interactions they had with their counselor, the ability to ask questions, and that they learned something new. Consistent with our quantitative data revealing increases in genetic knowledge after predisclosure and results disclosure (Burke & Psaty, 2007), participants reported that both genetic counseling sessions were informative. Given the risks, benefits and limitations of receiving genetic information, the two‐visit model including predisclosure counseling and result disclosure with a trained genetic counselor may be the most comprehensive model for returning genetic research results (Roberts et al., 2017). Our qualitative and quantitative data support favorable outcomes with return of genetic research results with trained genetic professionals employing the established two‐visit telephone model utilizing a tiered‐binned counseling model.
However, while genetic counselors may be the ideal brokers of informed consent and delivering important clinically relevant information to research participants, there are limitations to this approach. Potential barriers to the two‐visit model including genetic counselor workforce shortages, varying state licensure requirements limiting the geographic scope of genetic counseling practices and the professional costs associated with utilizing genetic providers have led to a growing concern that relying exclusively on genetic counselors for return of genetic research results may be infeasible (Forrest & Young, 2016; Jarvik et al., 2014; Roberts et al., 2017). The costs of implementing the two‐visit genetic counseling model means that many research programs will lack the necessary resources to afford this standard, which could ultimately limit the return of actionable results (Bledsoe et al., 2013, 2012). Thus, alternative delivery models for returning clinically significant genetic research findings must be considered (Buchanan, Rahm, & William, 2016; Jarvik et al., 2014; Roberts et al., 2017; Trepanier et al., 2004; Yu, Harrell, Jamal, Tabor, & Bamshad, 2014).
Our data suggest that while some participants may be unwilling to consider a self‐directed web‐based approach to either predisclosure education and consent or disclosure of research results, the majority would not rule it out. Interestingly, while a small subset (16%) reported being very or somewhat willing to complete predisclosure education online, almost double that number (31%) were willing to consider web‐based return of results. This may be an indication that a positive experience with predisclosure education from a genetic counselor resulted in less apprehension over considering receiving results through web‐disclosure, or that web‐disclosure of genetic research results would have been an acceptable alternative following actual receipt of results with a genetic counselor.
Other studies similarly suggest that given cost constraints, workforce shortages and the complexity of genomic information, innovative approaches, like web‐based education and results disclosure, could be an acceptable alternative to the two‐visit genetic counseling model (Biesecker et al., 2018; Green, Biesecker, McInerney, Mauger, & Fost, 2001; Green et al., 2005, 2004; Roberts et al., 2017; Sweet et al., 2017). Two studies that used an on‐line educational tool as an adjunct in advance of pretest counseling, reported the intervention was an effective way to learn about the issues, participants valued it for being self‐paced, private and an efficient use of their time (Green et al., 2001), and was associated with increased knowledge about heredity breast cancer and genetic testing, without increasing anxiety (Green et al., 2004). A recent randomized study that compared education and disclosure of carrier results by a web‐platform versus a genetic counselor, reported that the web‐based arm was noninferior for knowledge, distress and decisional regret (Biesecker et al., 2018). Of note, and as acknowledged by the authors, this was a highly select population who were undergoing relatively low risk testing (e.g., carrier testing with less direct implication for health care). Thus, these findings are not sufficient to conclude that web‐based pretesting or predisclosure counseling or disclosure of results for more complex testing is an acceptable alternative. However, they do support the premise that further evaluation of web‐based alternatives in these and other settings is warranted, particularly given the cost and other barriers to provision of traditional genetic counseling for return of genetic results.
Similarly, our data suggest several potential advantages to a web‐based alternative for delivery of genetic education and informed consent or disclosure of research results. Participants reported advantages might include the ability to independently receive, peruse and pursue information, which could result in an increase in comprehension. In provider–patient interactions, patients can quickly get lost in complex information. The ability to revisit information provides the opportunity to increase understanding, leading to better decision‐making and greater overall satisfaction. These advantages, coupled with others respondents cited, including a reduction in time commitment, the ability to control the pace of the session, choose the information that interests them and the freedom to return to the website as an information resource all indicate that a web‐based alternative may provide advantages that telephone counseling cannot (Jamal et al., 2014; Roberts et al., 2017; Tabor, Berkman, Hull, & Bamshad, 2011).
Participants also highlighted some of the potential challenges and perceived risks of web‐based alternatives. The most frequently reported concerns, which will be important to evaluate in future web‐based return of research results studies, include the inability to ask questions, misunderstandings about results, uncertainty regarding next steps, including implications for their medical care, the impersonal nature of a web‐intervention and lack of psychosocial support. Other studies have similarly found that patients prefer computer‐based interventions for their privacy and efficiency (Green et al., 2001, 2005), but favor a genetic counselor for their emotional support. Future studies could evaluate what aspects patients value most (e.g., convenience or emotional support, etc.) and who benefits less or more from talking with a provider. Biesecker et al. (2018) found that while those randomized to a web‐based platform were less satisfied with the session than those randomized to a genetic counselor, the costs of in‐person results delivery with a counselor may be difficult to justify in light of the noninferiority assessment and overall high satisfaction scores. Future studies assessing web‐based models could address these concerns by offering participants the option to speak with a genetic counselor by telephone, provide local referrals or even to offer emotional support, should the need arise. Short‐term follow‐up for patients demonstrating any of these risks could be instituted to mitigate any negative impact. In addition, while web‐based platforms may be sufficient for certain individuals or test results (Biesecker et al., 2018), reserving genetic counselors for return of results that pose a greater health threat may not only help to alleviate these concerns, but could also ease genetic counselor workforce shortages.
We acknowledge several limitations. These were hypothetical questions in patients who had already experienced genetic counseling with a provider, both pre‐ and postdisclosure and participants did not have actual screen shots to review. Thus, uptake of a web alternative (e.g., real willingness) in those who had not had genetic counseling could be higher or lower. Our cohort consisted of mostly educated white women with a history of breast cancer, therefore, these findings may not apply to more diverse populations. Additionally, this was a single‐site study at a major academic center, our findings may not reflect other research cohorts and setting.
In summary, participants receiving genetic research results report high satisfaction with predisclosure education and results disclosure with a genetic counselor. However, given the costs of the two‐visit model and genetic counseling workforce shortages, alternative models must be considered. Our data suggest that some patients may be willing to consider self‐directed web alternatives for either predisclosure genetic education and consent or return of results. Patients report several potential advantages and disadvantages to web‐based alternatives, which can inform future studies evaluating the outcomes of novel delivery models aimed at improving access to and optimizing the outcomes of returning genetic research results to research participants.
CONFLICT OF INTERESTS
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
The first and last authors each contributed significantly to the writing of the manuscript. Other authors provided research strategy and manuscript feedback.
Gaieski JB, Patrick‐Miller L, Egleston BL, et al. Research participants’ experiences with return of genetic research results and preferences for web‐based alternatives. Mol Genet Genomic Med. 2019;7:e898 10.1002/mgg3.898
Funding information
Primary financial support for this work was provided by a Breast Cancer Research Foundation/ASCO Conquer Cancer Award (Bradbury). Additional support from: Susan G. Komen SAC100003 (Domchek); Department of Defense W81XWH‐13‐1‐0338 (Maxwell); NIH 5T32GM008638‐15 (Maxwell); American Association for Cancer Research (Maxwell); BCRF (Nathanson, Domchek); CURE (Commonwealth Universal Research Enhancement) Program (Nathanson), NIH R01 CA176785 (Nathanson). The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Views and opinions of, and endorsements by the authors do not reflect those of the US Army of the Department of Defense.
REFERENCES
- Berg, J. S. , Khoury, M. J. , & Evans, J. P. (2011). Deploying whole genome sequencing in clinical practice and public health: Meeting the challenge one bin at a time. Genetics in Medicine, 13, 499–504. 10.1097/GIM.0b013e318220aaba [DOI] [PubMed] [Google Scholar]
- Biesecker, B. B. , Lewis, K. L. , Umstead, K. L. , Johnston, J. J. , Turbitt, E. , Fishler, K. P. , … Biesecker, L. G. (2018). Web platform vs in‐person genetic counselor for return of carrier results from exome sequencing. JAMA Internal Medicine, 178(3), 338– 10.1001/jamainternmed.2017.8049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe, M. J. , Clayton, E. W. , McGuire, A. L. , Grizzle, W. E. , O'Rourke, P. P. , & Zeps, N. (2013). Return of research results from genomic biobanks: A call for data. Genetics in Medicine, 15, 159–160. 10.1038/gim.2012.163 [DOI] [PubMed] [Google Scholar]
- Bledsoe, M. J. , Clayton, E. W. , McGuire, A. L. , Grizzle, W. E. , O'Rourke, P. P. , & Zeps, N. (2013). Return of research results from genomic biobanks: Cost matters. Genetics in Medicine, 15, 103–105. 10.1038/gim.2012.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe, M. J. , Grizzle, W. E. , Clark, B. J. , & Zeps, N. (2012). Practical implementation issues and challenges for biobanks in the return of individual research results. Genetics in Medicine, 14, 478–483. 10.1038/gim.2011.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury, A. R. , Patrick‐Miller, L. , & Domchek, S. (2014). Multiplex genetic testing: Reconsidering utility and informed consent in the era of next‐generation sequencing. Genetics in Medicine, 17, 97 10.1038/gim.2014.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury, A. R. , Patrick‐Miller, L. , Egeleston, B. L. , Maxwell, K. N. , DiGiovanni, L. , Brower, J. , … Domchek, M. (2018). Returning individual genetic research results to research participants: Uptake and outcomes among breast cancer patients. JCO Precision Oncology, 2, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury, A. R. , Patrick‐Miller, L. J. , Egleston, B. L. , DiGiovanni, L. , Brower, J. , Harris, D. , … Domchek, S. M. (2016). Patient feedback and early outcome data with a novel tiered‐binned model for multiplex breast cancer susceptibility testing. Genetics in Medicine, 18, 25–33. 10.1038/gim.2015.19 [DOI] [PubMed] [Google Scholar]
- Bradbury, A. R. , Patrick‐Miller, L. , Long, J. , Powers, J. , Stopfer, J. , Forman, A. , … Domchek, S. M. (2015). Development of a tiered and binned genetic counseling model for informed consent in the era of multiplex testing for cancer susceptibility. Genetics in Medicine, 17, 485–492. 10.1038/gim.2014.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury, A. R. , Patrick‐Miller, L. , Pawlowski, K. , Ibe, C. N. , Cummings, S. A. , Olopade, O. I. , & Daugherty, C. K. (2008). Should genetic testing for BRCA1/2 be permitted for minors? Opinions of BRCA mutation carriers and their adult offspring. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 148C, 70–77. 10.1002/ajmg.c.30163 [DOI] [PubMed] [Google Scholar]
- Buchanan, A. H. , Rahm, A. K. , & William, J. L. (2016). Alternative delivery service models in cancer genetic counseling: A mini‐review. Frontiers in Genetics, 6, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, W. , Evans, B. J. , & Jarvik, G. P. (2014). Return of results: Ethical and legal distinctions between research and clinical care. American Journal of Medical Genetics Part C, Seminars in Medical Genetics, 166C, 105–111. 10.1002/ajmg.c.31393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, W. , & Psaty, B. M. (2007). Personalized medicine in the era of genomics. JAMA, 298, 1682–1684. 10.1001/jama.298.14.1682 [DOI] [PubMed] [Google Scholar]
- Clayton, E. W. , & McGuire, A. L. (2012). The legal risks of returning results of genomics research. Genetics in Medicine, 14, 473–477. 10.1038/gim.2012.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. (1960). A coefficient of agreement for nominal scales. Educational and Psychological Measurement, 20, 37–46. 10.1177/001316446002000104 [DOI] [Google Scholar]
- Dressler, L. G. (2009). Disclosure of research results from cancer genomic studies: State of the science. Clinical Cancer Research, 15, 4270–4276. [DOI] [PubMed] [Google Scholar]
- Forrest, L. , & Young, M. A. (2016). Clinically significant germline mutations in cancer causing genes identified through research studies should be offered to research participants by genetic counselors. Journal of Clinical Oncology, 898–901. 10.1200/JCO.2015.60.9388 [DOI] [PubMed] [Google Scholar]
- Greely, H. T. (2007). The uneasy ethical and legal underpinnings of large‐scale genomic biobanks. Annual Review of Genomics and Human Genetics, 8, 343–364. 10.1146/annurev.genom.7.080505.115721 [DOI] [PubMed] [Google Scholar]
- Green, M. J. , Biesecker, B. B. , McInerney, A. M. , Mauger, D. , & Fost, N. (2001). An interactive computer program can effectively educate patients about genetic testing for breast cancer susceptibility. American Journal of Medical Genetics, 103, 16–23. 10.1002/ajmg.1500 [DOI] [PubMed] [Google Scholar]
- Green, M. J. , & Fost, N. (1997). Who should provide genetic education prior to gene testing? Computers and other methods for improving patient understanding. Genetic Testing, 1, 131–136. 10.1089/gte.1997.1.131 [DOI] [PubMed] [Google Scholar]
- Green, M. J. , Peterson, S. K. , Baker, M. W. , Friedman, L. C. , Harper, G. R. , Rubinstein, W. S. , … Mauger, D. T. (2005). Use of an educational computer program before genetic counseling for breast cancer susceptibility: Effects on duration and content of counseling sessions. Genetics in Medicine, 7, 221–229. 10.1097/01.GIM.0000159905.13125.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. J. , Peterson, S. K. , Baker, M. W. , Harper, G. R. , Friedman, L. C. , Rubinstein, W. S. , & Mauger, D. T. (2004). Effect of a computer‐based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: A randomized controlled trial. JAMA, 292, 442–452. 10.1001/jama.292.4.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal, S. M. , Shankar, A. G. , Yu, J. H. , Crouch, J. , Harrell, T. M. , Bamshad, M. J. … Tabor, H. K. (2014). Use of My46 to return individual research results to families of children with Joubert syndrome. ASHG 2014 Meeting; San Diego, CA.
- Jarvik, G. P. , Amendola, L. M. , Berg, J. S. , Brothers, K. , Clayton, E. W. , Chung, W. , … Sun, K. (2014). Return of genomic results to research participants: The floor, the ceiling, and the choices in between. American Journal of Human Genetics, 94, 818–826. 10.1016/j.ajhg.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury, M. J. , McBride, C. M. , Schully, S. D. , Ioannidis, J. P. A. , Feero, W. G. , Janssens, A. C. J. W. , … Xu, J. (2009). The Scientific Foundation for personal genomics: Recommendations from a National Institutes of Health‐Centers for Disease Control and Prevention multidisciplinary workshop. Genetics in Medicine, 11, 559–567. 10.1097/GIM.0b013e3181b13a6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, F. A. , Christensen, R. , Giacomini, M. , & Robert, J. S. (2008). Duty to disclose what? Querying the putative obligation to return research results to participants. Journal of Medical Ethics, 34, 210–213. 10.1136/jme.2006.020289 [DOI] [PubMed] [Google Scholar]
- Olopade, O. I. , Grushko, T. A. , Nanda, R. , & Huo, D. (2008). Advances in breast cancer: Pathways to personalized medicine. Clinical Cancer Research, 14, 7988–7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope, C. , van Royen, P. , & Baker, R. (2002). Qualitative methods in research on healthcare quality. Quality & Safety in Health Care, 11, 148–152. 10.1136/qhc.11.2.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presidential Commission for the Study of Bioethical Issues . (2013). Anticipate and Communicate: Ethical Management of Incidental and Secondary Findings in the Clinical, Research, and Direct‐to‐Consumer Contexts. Washington, DC. [DOI] [PubMed]
- Ramoni, R. B. , McGuire, A. L. , Robinson, J. O. , Morley, D. S. , Plon, S. E. , & Joffe, S. (2013). Experiences and attitudes of genome investigators regarding return of individual genetic test results. Genetics in Medicine, 15, 882–887. 10.1038/gim.2013.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravitsky, V. , & Wilfond, B. S. (2006). Disclosing individual genetic results to research participants. The American Journal of Bioethics, 6, 8–17. 10.1080/15265160600934772 [DOI] [PubMed] [Google Scholar]
- Ritchie, J. , & Spencer, L. (1994). Qualitative data analysis for applied policy research In Bryman A., & Burgess R. (Eds.), Analysing qualitative data (pp. 173–194). London, UK: Routledge. [Google Scholar]
- Roberts, M. C. , Wood, E. M. , Gaieski, J. B. , & Bradbury, A. R. (2017). Possible barriers for genetic counselors returning actionable genetic research results across state lines. Genetics in Medicine, 19, 1202–1204. 10.1038/gim.2017.34 [DOI] [PubMed] [Google Scholar]
- Sweet, K. , Hovick, S. , Strum, A. , Schmidlen, T. , Gordon, E. , Bernhardt, B. , … Christman, M. (2017). Counselees' perspectives of genomic counseling following online receipt of multiple actionable complex diseases and pharmacogenomic results: A qualitative research study. Journal of Genetic Counseling, 26, 738–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor, H. K. , Berkman, B. E. , Hull, S. C. , & Bamshad, M. J. (2011). Genomics really gets personal: How exome and whole genome sequencing challenge the ethical framework of human genetics research. American Journal of Medical Genetics Part A, 155A, 2916–2924. 10.1002/ajmg.a.34357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanier, A. , Ahrens, M. , McKinnon, W. , Peters, J. , Stopfer, J. , Grumet, S. C. , … Vockley, C. W. (2004). Genetic cancer risk assessment and counseling: Recommendations of the national society of genetic counselors. Journal of Genetic Counseling, 13, 83–114. 10.1023/B:JOGC.0000018821.48330.77 [DOI] [PubMed] [Google Scholar]
- Velikova, G. , Awad, N. , Coles‐Gale, R. , Wright, E. P. , Brown, J. M. , & Selby, P. J. (2008). The clinical value of quality of life assessment in oncology practice‐a qualitative study of patient and physician views. Psycho‐oncology, 17, 690–698. 10.1002/pon.1295 [DOI] [PubMed] [Google Scholar]
- Williams, R. L. (2000). A note on robust variance estimation for cluster‐correlated data. Biometrics, 56, 645–646. 10.1111/j.0006-341X.2000.00645.x [DOI] [PubMed] [Google Scholar]
- Yu, J. H. , Harrell, T. M. , Jamal, S. M. , Tabor, H. K. , & Bamshad, M. J. (2014). Attitudes of genetics professionals toward the return of incidental results from exome and whole‐genome sequencing. The American Journal of Human Genetics, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


