Abstract
Actuarial senescence has been viewed for a long time as an inevitable and uniform process. However, the work on senescence has mainly focused on endotherms with deterministic growth and low regeneration capacity during the adult stage, leading to a strong taxonomic bias in the study of ageing. Recent studies have highlighted that senescence could indeed display highly variable trajectories that correlate with species life-history traits. Slow life histories and indeterminate growth seem to be associated with weak and late senescence. Furthermore, high regenerative abilities could lead to negligible senescence in ectotherms. However, demographic data for species that would allow testing of these hypotheses are scarce. Here, we investigated senescence patterns in ‘true salamanders’ from the western Palaearctic. Our results showed that salamanders have slow life histories and that they experience negligible senescence. This pattern was consistent at both intra- and interspecific levels, suggesting that the absence of senescence may be a phylogenetically conserved trait. The regenerative capacities of salamanders, in combination with other physiological and developmental features such as an indeterminate growth and a low metabolic rate, probably explain why these small ectotherms have lifespans similar to that of large endotherms and, in contrast with most amniotes, undergo negligible senescence. Our study seriously challenges the idea that senescence is a ubiquitous phenomenon in the tree of life.
Keywords: ageing, actuarial senescence, life history, urodeles, salamanders
1. Introduction
The great diversity of life histories continues to fascinate population biologists [1–3]. There have been many attempts to summarize the variety of life-history patterns that are observed (e.g. [4–6]). Among the spate of life-history traits, senescence is generally defined as a physiologically caused, irreversible increase in mortality (i.e. actuarial senescence; hereafter senescence) and/or a decline in fertility with age (i.e. reproductive senescence) [7,8]. Historically, senescence was expected to show a limited amount of variation across species. The senescence process was expected to be ubiquitous among age-structured populations [7], which led to the view that senescence was an unavoidable process in organisms with a clear distinction between somatic and germ lines [9–11]. In addition, regardless of the species considered, the age at the onset of senescence was expected to be immutably set at the age of first reproduction [7,12]. This long-held view was overturned when it was shown that the there is a bewildering diversity of senescence patterns across animal and plant species [13–16]. There is an obvious need to further describe and explain the diversity of senescence patterns which was recently reinforced by the observation that senescence patterns can strongly affect population growth rates and how species respond to environmental change [16].
In a recent analysis of senescence on a wide range of taxa, Jones et al. [13] demonstrated patterns of senescence are more diverse than previously thought. Although Jones et al. [13], and more recently Colchero et al. [16], present results from a broad variety of species, their studies highlighted the lack of data for many taxa and thus, there is still a great need to uncover and explain senescence patterns. In particular, it is necessary to evaluate the extent to which species can escape senescence or even show ‘negative senescence’ and to determinate the eco-evolutionary roots of such patterns [17–19]. As the life-history theory of ageing postulates that senescence is related to somatic maintenance [20,21], one may expect that species who invest more into this item of expenditure such as ‘slow’ species (i.e. low annual fecundity and high adult survival) should show slower rates of senescence (e.g. [22]). This expectation is supported by the findings of Jones et al. [23] who showed that in both mammals and birds, the onset and rate of senescence are predicted by generation time and age at maturity. Because the life histories of species can be arranged along a fast–slow life-history continuum [6,24–26], the speed of the life history can be used to predict senescence [23]. Later, Jones et al. [13] showed that the initial explanation of Jones et al. [23] did not fully account for the diversity of senescence patterns, suggesting that other mechanisms may be at work as well. For example, their study revealed the absence of senescence in Hydra (and few other organisms), potentially allowed by regenerative capacity [27].
Most of our understanding of senescence in wild animal populations is based on mammals and birds because there are many long-term individual-based datasets for these species [13]. Yet, patterns of senescence among mammals are rather uniform. For example, Colchero et al. [16] found that bathtub-shaped mortality trajectories were most commonly observed in ungulates and carnivores. The dataset of Colchero et al. [16] included only four amphibian species but four different models explained the data best (simple logistic, bathtub logistic, Gompertz, Weibull), suggesting great interspecific diversity within the amphibians. Interestingly, senescence was negligible in a salamander (Salamandra salamandra), an organism with an indeterminate (i.e. continuous) growth [28] that is well known for its regenerative capacity at the adult stage [29–31].
Here, we investigated how slow life histories with an implicit high level of investment in somatic maintenance are associated with a negligible actuarial senescence in a clade of salamanders from the western Palaearctic (known as the ‘true salamanders’). We used both unpublished and published capture–recapture data for our analyses. First, we analysed novel demographic data from a poorly known Mediterranean salamander (Lyciasalamandra fazilae) and examined if this species had a slow life history (i.e. high adult survival and low recruitment) consistent with the other species of true salamanders [32,33]. Then, we examined senescence patterns in L. fazilae and two other species, Salamandrina perspicillata and S. salamandra, from western Europe. We expected negligible senescence in the three taxa and hypothesized that this pattern was consistent among populations.
2. Material and methods
(a). Demography of Lyciasalamandra fazilae
(i). Study species and capture–recapture survey
Lyciasalamandra fazilae is terrestrial salamander occurring along the southern Anatolian coast in Turkey. This species is a member of the phylogenetic clade called ‘true’ salamanders that encompasses the genera Salamandra, Lyciasalamandra, Mertensiella, Salamandrina and Chioglossa [34]. Lyciasalamandra fazilae is viviparous salamander that gives birth to one or two fully metamorphosed young after 1 year of gestation. Sexual maturity is attained at an age of 3 years in both sexes [35]. The individual growth curve presents an asymptotic trend even if adult salamanders seem to continue to grow over their entire lifespan [35].
The study was conducted on a population of L. fazilae between 1999 and 2009 in western Turkey near Dalyan (36° 50′ N, 28° 41′ E). A detailed description of the study area can be found in Olgun et al. [35]. Several capture–recapture sessions (in February, March and April) were carried out each year. The salamanders were captured by hand, and were then released back in the place where they were initially caught after being marked using passive integrated transponder (PIT)-tags, identified and measured. The sex of the individuals was assessed using secondary sexual characters [35]. Juveniles were not included in the analysis because only 12 juveniles were encountered during the study but never recaptured. Furthermore, owing to small size of the dataset (133 individuals marked), we pooled the two sexes in analyses to avoid model overparametrization. We assumed that sex should have a little influence on adult survival as males and females have the same age structure in the population [35].
Body size of salamanders was determined by measuring snout–vent length (SVL): individuals were measured from the tip of the snout to the posterior margin of the vent. We used the size data in multievent capture–recapture models to estimate size-dependent survival. We also benefited from age data assessed using skeletochronological analyses (a robust approach to evaluate age in amphibians, [36]) for individuals marked over the period 1999–2003 [35]. Those age data were included in BaSTA models [37,38] to examine age-dependent survival and mortality rate.
(ii). Multievent model for size-dependent adult survival
We quantified size-dependent annual survival using multievent capture–recapture models [39]. Note that we did not perform goodness-of-fit tests before building the models because no test is currently available for multievent models; note that it was also possible to consider potential transience, trap-dependence and recapture heterogeneity in BaSTA models presented below. We considered a model based on three latent states that include information about individuals' size. The states s and l correspond to small (SVL, from 45 to 60 mm) and large (SVL, from 61 to 81 mm) adults, respectively; the classes were fixed to obtain a relatively similar number of observations in the two classes. The state d corresponds to the dead state. The models include three observations coded as following in the capture histories: individuals that are not captured are coded ‘0’; small and large individuals captured are coded ‘1’ and ‘2’, respectively.
At their first capture, individuals may occupy two distinct states of departure, s and l. At each time step, the information about individual state is progressively updated through two successive modelling steps: (i) survival and (ii) size transition. Each step is conditional on all previous steps. At the first modelling step, survival information is updated. A small individual may survive with a probability or die with a probability 1–. A large individual can survive with a probability or not with a probability 1–. These results are shown in the following matrix (the state of the individual at t − 1 is in column and state at t is in row):
In the second modelling step, information about individual size is updated. A small individual can become a large individual with a probability or remain in the same class with a probability 1–, leading to the following matrix:
The last component of the model links observation to states. Small and large individual can be captured with a probability or , which results in the following matrix:
This parametrization was implemented in the program E-SURGE [40]. We ranked models using Akaike information criteria adjusted for small sample size (AICc) and Akaike weights (w). If the Akaike weight of the best supported model was less than 0.9, we used model-averaging to obtain parameter estimates. We examined our hypotheses about survival and recapture probability from the following general model [(size), (.), p(t + size)]. The effects considered in the models were size and year (t). We hypothesized that survival probability differed between the two size classes (i.e. small and large). We also expected that recapture probability varies according to size and year. Age transition was set constant (.) in the model. We tested all the possible combinations of effects, resulting in the consideration of eight competing models (electronic supplementary material, table S2).
(iii). Multievent model for recruitment
We estimated recruitment rate of small-size adults using a modified Pradel [41] model in which recruitment is modelled by reversing capture histories and analysing them backwards. Recruitment probability was estimated as the probability that a small-sized individual present at t was not present at t − 1, i.e. the proportion of ‘new’ small individuals in the population at t. The model had a structure similar to that of the survival model. The survival matrix was replaced by the recruitment matrix. At each time step, small individuals may be recruited with a probability or not with a probability 1 – , leading to the following matrix:
The size transition matrix was also modified to allow reversed size transition:
This parametrization was implemented in the E-SURGE program. We considered the most general model [(.), (.), p(t + size)]. We examined all the possible combination of effects leading to the consideration of four candidate models.
(b). Age-dependent survival and senescence patterns in true salamanders
(i). Capture–recapture data
To estimate age-dependent survival and senescence, we re-analysed two datasets of true salamanders that were analysed previously (figure 1a). The capture–recapture data of S. perspicillata were collected over a 9-year period (1998–2006) in a population of central Italy (Monti Lepini, Latium; [42]). The capture–recapture data of S. salamandra were collected in two populations from southern France (Ardèche region, pop1) and northwestern Germany (Nordrhein-Westfalen, pop2). pop1 and pop2 were surveyed over 8 years (2008–2015) and 21 years (1965–1985) period, respectively [43,44]. A summary of the age-dependent capture–recapture data in the four populations of salamanders is provided in the electronic supplementary material, table S1. In all cases, the survey length was equal or longer than the mean lifespan of adults in the four populations (electronic supplementary material, table S1). Given the simulations of Colchero & Clark [37], we are therefore confident in our ability to detect senescence if it actually occurred. Note that the sex was not considered in the further analyses as only females were caught in S. perspicillata (males do not occur at breeding sites) and because sex cannot be easily ascertained by non-expert observers in S. salamandra.
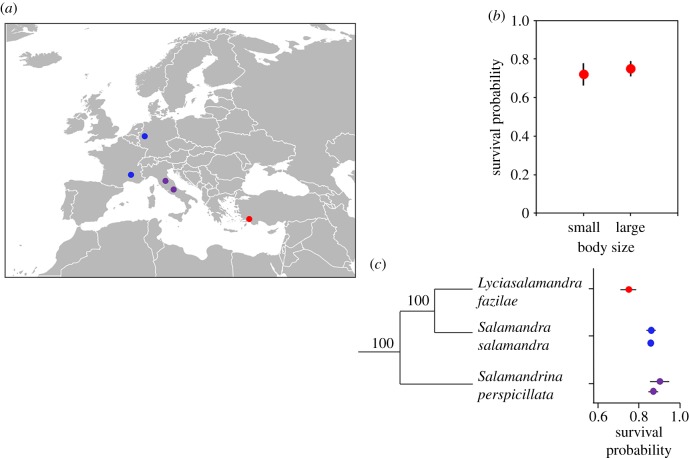
Figure 1.
Annual adult survival (and standard errors) in three species of salamanders from the western Palaearctic. (a) Map showing the populations of L. fazilae (in red), S. salamandra (in blue) and Salamandra perspicillata (in violet) considered in this study. Note that data of only one population of S. perspicillata were available for the present study (the southernmost population on the map). (b) Survival of small and large adults of L. fazilae and their standard errors (model-averaged) estimated using our survival and recruitment models. (c) Survival estimates of L. fazilae (from this study) and S. salamandra and S. perspicillata, which are extracted from two previous capture–recapture studies ([42,43], respectively). The phylogenetic tree is the one presented in [34].
(ii). Age-dependent survival and mortality rate
We investigated actuarial senescence patterns in the three salamander species using Bayesian survival trajectory analyses implemented in the R package BaSTA [37,38]. BaSTA allowed us to account for imperfect detection, left-truncated (i.e. unknown birth date) and right-censored (i.e. unknown death date) capture–recapture data in our analysis. It allows estimating two demographic functions: cumulative probability survival until a given age and mortality rate (i.e. hazard rate) at a given age. Given the results of previous analyses [42–44], we allowed recapture probabilities to vary among years. As the study period and number of survey years differ among populations (electronic supplementary material, table S1), the four populations and species were analysed separately. We used deviance information criterion (DIC) to select models that fit the data best and we compared the outputs of the best supported model of the four populations by inspecting mean estimates and 95% confidence intervals (CI). This allowed us to investigate population/species-specific variation in the shape of the age-specific mortality patterns. We considered the four mortality functions implemented in BaSTA: exponential, Gompertz, Weibull and logistic. For the three last functions, we considered three potential shapes: simple that only uses the basic functions described above; Makeham [45]; and bathtub [46]. As individuals cannot be individually surveyed before their sexual maturity (3 years old in the three species), we conditioned the analyses at a minimum age of 3. Four Markov chain Monte Carlo chains were run with 50 000 iterations and a burn-in of 5000. Chains were thinned by a factor of 50. Model convergence was evaluated using the diagnostic analyses implemented in BaSTA, which calculate the potential scale reduction for each parameter to assess convergence. For all populations, we used DIC to compare the predictive power of each mortality function and its refinements [38,47].
3. Results
(a). Demography of Lyciasalamandra fazilae
Over the 8-year study period, we made 179 captures of salamanders. We identified 121 adults (51 males and 70 females) and 12 juveniles. The mean age was 5.5 years and the maximum was 10 years.
The best supported survival model was [(.), (.), p(t + size)] (electronic supplementary material, table S2); its Akaike weight was 0.54 and we therefore model-averaged the estimates. The recapture probability of small individuals was higher than that of large individuals and varied over time. In 2001, the recapture probability of small individuals was 0.05 ± 0.05, while it was 0.01 ± 0.01 in large individuals; in 2007, the recapture probability of small and large individuals was 0.78 ± 0.18 and 0.41 ± 0.17, respectively. The probability that a small individual changed size class and became a large individual was 0.28 ± 0.09. Annual survival did not differ between small (0.72 ± 0.06) and large (0.75 ± 0.04) individuals (figure 1b).
The best supported recruitment model was [(.), (.), p(t + size)] (electronic supplementary material, table S2); its AICc weight was 0.54 and we therefore model-averaged the estimates. The recapture probabilities were relatively similar to that provided by survival model; we did not report them for this reason. The rate of small individual recruitment was 0.15 ± 0.15.
(b). Age-dependent survival and senescence in true salamanders
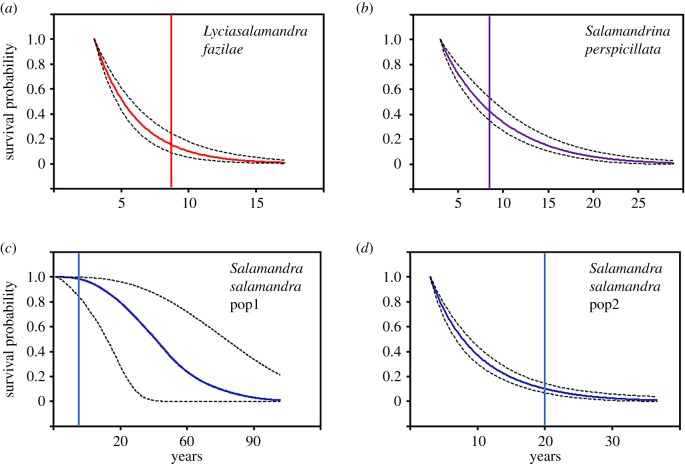
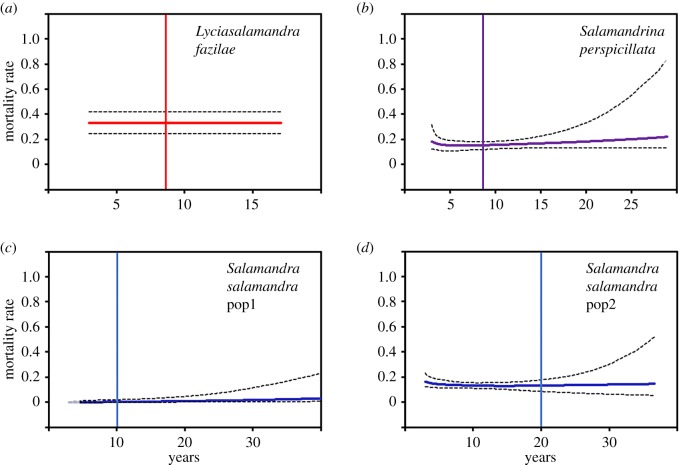
In L. fazilae, the age-specific capture–recapture data were best described by an exponential function (electronic supplementary material, table S3). The probability of surviving was 0.75 until age 4, 0.50 until age 5 and 0.25 until age 7 (figure 2a). Furthermore, the model indicates the absence of age-dependent mortality rate: mortality rate remained stable (around 0.34) regardless of age (figure 3a).
Figure 2.
(a–d) Survival until age x (full lines) and their 95% CI (broken lines) in three salamanders of the western Palaearctic. The vertical line indicates the length of the capture–recapture survey.
Figure 3.
(a–d) Mortality rate at age x (full lines) and their 95% CI (broken lines) in three salamanders of the western Palaearctic. The vertical line indicates the length of the capture–recapture survey.
In S. perspicillata, the best supported model included a Gompertz function (electronic supplementary material, table S3). The probability of surviving was 0.75 until age 5, 0.50 until age 8 and 0.25 until age 12 (figure 2b). Moreover, our results indicate that age had no influence on mortality rate (figure 3b). Mortality rate remained stable (around 0.20) regardless of age.
In S. salamandra, the best supported model included a Weibull function in pop1 and a Gompertz function in pop2 (electronic supplementary material, table S3). The probability of surviving was 0.75 until age 5, 0.50 until age 8 and 0.25 until age 13 in pop2 (figure 2c); in pop1, the survival estimates were very imprecise (figure 2d). Moreover, our results indicate that mortality rate was not affected by age (figure 3c,d). Mortality rate remained stable (around 0.05 and 0.15 in pop1 and pop2, respectively) regardless of age.
4. Discussion
The three species of true salamanders analysed in this study are characterized by a slow life-history strategy (i.e. low recruitment and high adult survival). Survival of all three species of salamanders decreased slowly with age and mortality rate remained constant regardless of salamander age, suggesting negligible actuarial senescence in this clade.
(a). Demography of Lyciasalamandra fazilae
Using capture–recapture data, we have provided, to our knowledge, the first detailed demographic characterization of L. fazilae and demonstrated that it is a species with a relatively ‘slow’ life history. The survival estimate of our multievent model (mean annual adult survival = 0.74 ± 0.05) was very close to the survival estimate (0.79) provided by the skeletochronological analysis in the same population [35]. We are therefore confident that the survival estimate of L. fazilae is not biased by a methodological artefact; for example, permanent emigration leads to underestimated survival rates (i.e. apparent survival) in capture–recapture studies [48]. Our study revealed that the probability of surviving was 0.50 until age 5, and less than 0.05 until age 12. These results are also congruent with skeletochronological data that reported a maximum longevity of 10 years in the population. Furthermore, we did not find evidence of an effect of body size on adult survival. It is possible that a small body size negatively affects survival at the juvenile stage (as commonly reported in amphibians; e.g. [49,50]) and that the effect of size on survival vanishes at either some point before or rapidly after sexual maturation. Furthermore, our study also revealed that recruitment was relatively low in L. fazilae (0.15). This is probably owing to the low fecundity of females that produce one or two young after a gestation of 1 year [35]. All together, these results indicate that L. fazilae has a relatively slow life history.
(b). Slow life histories in true salamanders
Our study and previous ones [42–44] indicate that true salamanders have slow life-history strategies. First, adult survival is relatively high in this clade (figure 1c). In S. salamandra, survival probability was 0.85 in both populations, while it ranges from 0.86 to 0.90 among S. perspicillata populations, indicating little variation at the intraspecific level (figure 1c). At the interspecific scale, survival is relatively high in these three genera, which suggests that a long lifespan is a highly conserved trait in salamanders of the western Palaearctic. Yet, L. fazilae has a lower survival (0.74 and 0.79 from capture–recapture and skeletochronological analyses, respectively) than S. salamandra and S. perspicillata (figure 1c). This pattern was also perceptible in the survival estimates provided by our study: a survival probability of 0.50 was reached at 5 years in L. fazilae, 7.5 years in S. perspicillata and 8 years in S. Salamandra (in pop2, the estimate of pop1 was very imprecise). These adult survival rates for true salamanders are higher than for most anurans [51] and many newts and plethodontid salamanders [52–54] supporting the idea that these species exhibit slow life histories.
Through a trade-off, the relatively long lifespan of salamanders is also associated with a low annual fecundity (compared to other amphibians; [55,56]) that is modulated by their reproductive modes. Oviparous species have the highest annual fecundity (e.g. Salamandra infraimmaculata, 218 eggs per female per year, [57]; S. perspicillata, 40–65 eggs, [58,59]). Salamanders with lecithotrophic viviparity (i.e. giving birth to larvae) have an intermediate annual fecundity (e.g. S. salamandra, 23 larvae per female per year, [60]; Salamandra algira, 13 larvae, [61]), whereas species with matrotrophic viviparity (i.e. giving birth to fully developed young) have the lowest annual fecundity (e.g. L. fazilae and Salamandra atra, 1 young per female per year; [62,63]). Fecundity adjustments of true salamander result from potentially rapid (less than 1000 generations, [64]) changes in reproductive modes that may occur at the intraspecific level [64]. These characteristics make ‘true’ salamanders an exceptional biological system to extend our understanding of the evolution reproductive modes and the modularity of life-history strategies in amniotes.
(c). Negligible actuarial senescence in true salamanders
We observed negligible actuarial senescence in the three species of true salamanders considered in our study. The mortality rate was relatively stable and weakly affected by age in the three taxa and the two populations of S. salamandra. This mortality pattern markedly differs from age-dependent mortality rates found in various species of anurans and newts (using capture–recapture data with relatively similar sample size, and a similar modelling approach), which may experience a sharp, early senescence [16,65]; but also see [13]. The detection of actuarial senescence in other species using similar modelling tools [16,65] indicates that the negligible senescence in true salamanders is not a methodological artefact. It has previously been suggested that detecting senescence would be difficult in wild animals because high levels of mortality would remove individuals from the population before they start to senesce [66]. However, by revealing a great diversity of senescence patterns, studies using capture–recapture data collected across a broad range of taxa showed that this assumption was untrue ([13,16,23]; see also [19] for a review). Furthermore, Colchero's model has proven to be particularly efficient for detecting senescence when it is actually present [37,38]. Simulations showed that the model is able to detect senescence when the study period is equal or longer than the mean lifespan in the population [37], which was the case in the four datasets considered in our study.
A long lifespan, a high level of iteroparity and a low reproductive effort appear to be closely associated with negligible actuarial senescence in the three species of true salamanders considered in our study. This pattern is congruent with the results of Jones et al. [23] showing that senescence rate is negatively correlated with generation time and age at primiparity, and is positively associated with maximum fecundity in endotherm vertebrates; the opposite relationships were detected with the age at senescence onset. Interestingly, our results suggest an absence of trade-off between senescence and offspring production in true salamanders. Although they display large variation in annual offspring numbers, the three species experience negligible senescence. This seems to indicate a partial decoupling of senescence and reproductive effort whose variation is rather associated with species-specific reproductive mode (oviparity, lecithotrophic viviparity and matrotrophic viviparity). However, our study focused on a limited number of salamander species and further investigations are required to examine in detail covariation between senescence (speed and age at onset of senescence) and life-history traits at the clade level.
The negligible senescence of salamanders probably relies on their high regenerative capacities. Contrary to other amniotes, salamanders are able to retain near perfect regeneration of most organs and appendages (e.g. spinal cord, heart, brain, skin, digit and lens) well into adulthood [31]. Although almost no studies have tested these abilities in old animals [31], their great potential for tissue repair and regeneration probably allow true salamanders to escape actuarial senescence. In parallel, although we did not detect size-dependent survival in L. fazilae, an indeterminate growth [19] could also contribute to the negligible senescence reported in our study. Furthermore, a low body temperature and a low metabolic rate [67,68] might also limit actuarial senescence.
Our results also showed that negligible senescence is a consistent pattern at both intraspecific and interspecific levels in true salamanders (at least in the species considered in this study). This indicates that negligible senescence may be a phylogenetically conserved trait within a clade containing species that have diverged a long time ago (several million years; [34]). These results suggest a strong genetic determinism in ageing mechanisms and the existence of orthologous genes involved in the repression of actuarial senescence in urodeles. However, the genomic architecture of life-history components as senescence rate and onset remains poorly understood, except in few model species (e.g. Drosophila: [69,70]; human: [71]). The recent development of powerful genomic tools should allow identification of candidate genes and gene networks involved in senescence regulation.
5. Conclusion
Negligible actuarial senescence is highlighted in a growing number of taxa, mainly ectotherms (e.g. corals, hydras and amphibians; [13]). These cases have been considered for a long time as exceptions or the product of methodological artefacts, in the light of senescence having been presented as a nearly ubiquitous phenomenon in the living world. We argue that this representation was partly owing to a taxonomic bias where the study of senescence has for many years been focused on endotherm vertebrates (mainly mammals) with reduced regenerative capacities at adult stages [31]. The regenerative capacities of true salamanders, and urodeles in general, probably explains why these small ectotherm amniotes (the body mass of the largest true salamanders is approx. 50 g) have lifespans similar to that of large endotherm amniotes (e.g. ungulates, large birds) and undergo a negligible actuarial senescence contrary to most vertebrates including humans [13].
Supplementary Material
Data accessibility
Capture–recapture datasets necessary to reproduce all analyses in this paper are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.6f407n9 [72].
Authors' contributions
The manuscript was written by H.C. who also conducted the statistical analyses. B.R.S. and J.-F.L. contributed to the writing of the manuscript. Data collection was mainly carried out by K.O., C.A., N.U., O.P., C.M. and A.A.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Stearns SC. 1976. Life-history tactics: a review of the ideas. Q. Rev. Biol. 51, 3–47. ( 10.1086/409052) [DOI] [PubMed] [Google Scholar]
- 2.Dammhahn M, Dingemanse NJ, Niemelä PT, Réale D. 2018. Pace-of-life syndromes: a framework for the adaptive integration of behaviour, physiology and life history. Behav. Ecol. Sociobiol. 72, 62 ( 10.1007/s00265-018-2473-y) [DOI] [Google Scholar]
- 3.Wright J, Bolstad GH, Araya-Ajoy YG, Dingemanse NJ. 2019. Life-history evolution under fluctuating density-dependent selection and the adaptive alignment of pace-of-life syndromes. Biol. Rev. 94, 230–247. ( 10.1111/brv.12451) [DOI] [PubMed] [Google Scholar]
- 4.Pianka ER. 1970. On r- and K-selection. Am. Nat. 104, 592–597. ( 10.1086/282697) [DOI] [Google Scholar]
- 5.Sæther BE. 1988. Pattern of covariation between life-history traits of European birds. Nature 331, 616 ( 10.1038/331616a0) [DOI] [PubMed] [Google Scholar]
- 6.Bielby J, Mace GM, Bininda-Emonds OR, Cardillo M, Gittleman JL, Jones KE, Orme CDL, Purvis A. 2007. The fast–slow continuum in mammalian life history: an empirical reevaluation. Am. Nat. 169, 748–757. ( 10.1086/516847) [DOI] [PubMed] [Google Scholar]
- 7.Hamilton WD. 1966. Moulding of senescence by natural selection. J. Theor. Biol. 12, 12–45. ( 10.1016/0022-5193(66)90184-6) [DOI] [PubMed] [Google Scholar]
- 8.Rose MR. 1994. Evolutionary biology of aging. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Kirkwood TB. 1977. Evolution of ageing. Nature 270, 301 ( 10.1038/270301a0) [DOI] [PubMed] [Google Scholar]
- 10.Ackermann M, Stearns SC, Jenal U. 2003. Senescence in a bacterium with asymmetric division. Science 300, 1920 ( 10.1126/science.1083532) [DOI] [PubMed] [Google Scholar]
- 11.Nussey DH, Froy H, Lemaitre JF, Gaillard JM, Austad SN. 2013. Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res. Rev. 12, 214–225. ( 10.1016/j.arr.2012.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams GC. 1957. Pleiotrophy, natural selection, and the evolution of senescence. Evolution 11, 398–411. [Google Scholar]
- 13.Jones OR, et al. 2014. Diversity of ageing across the tree of life. Nature 505, 169–173. ( 10.1038/nature12789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baudisch A, Salguero-Gómez R, Jones OR, Wrycza T, Mbeau-Ache C, Franco M, Colchero F. 2013. The pace and shape of senescence in angiosperms. J. Ecol. 101, 596–606. ( 10.1111/1365-2745.12084) [DOI] [Google Scholar]
- 15.Gaillard JM, Lemaître JF. 2017. The Williams’ legacy: a critical reappraisal of his nine predictions about the evolution of senescence. Evolution 71, 2768–2785. ( 10.1111/evo.13379) [DOI] [PubMed] [Google Scholar]
- 16.Colchero F, et al. 2019. The diversity of population responses to environmental change. Ecol. Lett. 22, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finch CE. 1998. Variations in senescence and longevity include the possibility of negligible senescence. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 53, B235–B239. ( 10.1093/gerona/53A.4.B235) [DOI] [PubMed] [Google Scholar]
- 18.Vaupel JW, Baudisch A, Dölling M, Roach DA, Gampe J. 2004. The case for negative senescence. Theor. Popul. Biol. 65, 339–351. ( 10.1016/j.tpb.2003.12.003) [DOI] [PubMed] [Google Scholar]
- 19.Jones OR, Vaupel JW. 2017. Senescence is not inevitable. Biogerontology 18, 965–971. ( 10.1007/s10522-017-9727-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemaître JF, Berger V, Bonenfant C, Douhard M, Gamelon M, Plard F, Gaillard JM. 2015. Early–late life trade-offs and the evolution of ageing in the wild. Proc. R. Soc. B 282, 20150209 ( 10.1098/rspb.2015.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkwood TB. 2017. The disposable soma theory. In Evolution of senescence in the tree of life (eds Shefferson RP, Jones OR, Salguero-Gomez R), pp. 23–39. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.Ma S, Gladyshev VN. 2017. Molecular signatures of longevity: insights from cross-species comparative studies. Semin. Cell Dev. Biol. 70, 90–203. ( 10.1016/j.semcdb.2017.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones OR, et al. 2008. Senescence rates are determined by ranking on the fast–slow life-history continuum. Ecol. Lett. 11, 664–673. ( 10.1111/j.1461-0248.2008.01187.x) [DOI] [PubMed] [Google Scholar]
- 24.Stearns SC. 1983. The influence of size and phylogeny on patterns of covariation among life-history traits in the mammals. Oikos 41, 173–187. ( 10.2307/3544261) [DOI] [Google Scholar]
- 25.Harvey PH, Zammuto RM. 1985. Patterns of mortality and age at first reproduction in natural populations of mammals. Nature 315, 319 ( 10.1038/315319a0) [DOI] [PubMed] [Google Scholar]
- 26.Promislow DE, Harvey PH. 1990. Living fast and dying young: a comparative analysis of life-history variation among mammals. J. Zool. 220, 417–437. ( 10.1111/j.1469-7998.1990.tb04316.x) [DOI] [Google Scholar]
- 27.Martinez DE. 1998. Mortality patterns suggest lack of senescence in Hydra. Exp. Gerontol. 33, 217–225. ( 10.1016/S0531-5565(97)00113-7) [DOI] [PubMed] [Google Scholar]
- 28.Bouzid S, Konecny L, Grolet O, Douady CJ, Joly P, Bouslama Z. 2017. Phylogeny, age structure, growth dynamics and colour pattern of the Salamandra algira algira population in the Edough Massif, northeastern Algeria. Amphibia-Reptilia 38, 461–471. ( 10.1163/15685381-00003127) [DOI] [Google Scholar]
- 29.Yokoyama H. 2008. Initiation of limb regeneration: the critical steps for regenerative capacity. Dev. Growth Differ. 50, 13–22. ( 10.1111/j.1440-169X.2007.00973.x) [DOI] [PubMed] [Google Scholar]
- 30.Poss KD. 2010. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 11, 710 ( 10.1038/nrg2879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seifert AW, Voss SR. 2013. Revisiting the relationship between regenerative ability and aging. BMC Biol. 11, 2 ( 10.1186/1741-7007-11-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warburg MR. 2007. Longevity in Salamandra infraimmaculata from Israel with a partial review of life expectancy in urodeles. Salamandra 43, 21–34. [Google Scholar]
- 33.Sinsch U, Böcking H, Leskovar C, Öz M, Veith M. 2017. Demography and lifetime growth patterns in viviparous salamanders (genus Lyciasalamandra): living underground attenuates interspecific variation. Zoologischer Anzeiger 269, 48–56. ( 10.1016/j.jcz.2017.07.005) [DOI] [Google Scholar]
- 34.Weisrock DW, Papenfuss TJ, Macey JR, Litvinchuk SN, Polymeni R, Ugurtas IH, Zhao E, Jowkar H, Larson A. 2006. A molecular assessment of phylogenetic relationships and lineage accumulation rates within the family Salamandridae (Amphibia, Caudata). Mol. Phylogenet. Evol. 41, 368–383. ( 10.1016/j.ympev.2006.05.008) [DOI] [PubMed] [Google Scholar]
- 35.Olgun K, Miaud C, Gautier P. 2001. Age, growth, and survivorship in the viviparous salamander Mertensiella luschani from southwestern Turkey. Can. J. Zool. 79, 1559–1567. ( 10.1139/z01-111) [DOI] [Google Scholar]
- 36.Sinsch U. 2015. Skeletochronological assessment of demographic life-history traits in amphibians. Herpetol. J. 25, 5–13. [Google Scholar]
- 37.Colchero F, Clark JS. 2012. Bayesian inference on age-specific survival for censored and truncated data. J. Anim. Ecol. 81, 139–149. ( 10.1111/j.1365-2656.2011.01898.x) [DOI] [PubMed] [Google Scholar]
- 38.Colchero F, Jones OR, Rebke M. 2012. BaSTA: an R package for Bayesian estimation of age-specific survival from incomplete mark–recapture/recovery data with covariates. Methods Ecol. Evol. 3, 466–470. ( 10.1111/j.2041-210X.2012.00186.x) [DOI] [Google Scholar]
- 39.Pradel R. 2005. Multievent: an extension of multistate capture–recapture models to uncertain states. Biometrics 61, 442–447. ( 10.1111/j.1541-0420.2005.00318.x) [DOI] [PubMed] [Google Scholar]
- 40.Choquet R, Rouan L, Pradel R. 2009. Program E-SURGE: a software application for fitting multievent models. In Modeling demographic processes in marked populations (eds Thomson DL, Cooch EG, Conroy MJ), pp. 845–865. Boston, MA: Springer. [Google Scholar]
- 41.Pradel R. 1996. Utilization of capture-mark-recapture for the study of recruitment and population growth rate. Biometrics 52, 703–709. ( 10.2307/2532908) [DOI] [Google Scholar]
- 42.Angelini C, Antonelli D, Utzeri C. 2010. Capture-mark-recapture analysis reveals survival correlates in Salamandrina perspicillata (Savi, 1821). Amphibia-Reptilia 31, 21–26. ( 10.1163/156853810791069047) [DOI] [Google Scholar]
- 43.Cayuela H, Joly P, Schmidt BR, Pichenot J, Bonnaire E, Priol P, Peyronel O, Laville M, Besnard A. 2017. Life history tactics shape amphibians' demographic responses to the North Atlantic Oscillation. Glob. Change Biol. 23, 4620–4638. ( 10.1111/gcb.13672) [DOI] [PubMed] [Google Scholar]
- 44.Schmidt BR, Feldmann R, Schaub M. 2005. Demographic processes underlying population growth and decline in Salamandra salamandra. Conserv. Biol. 19, 1149–1156. ( 10.1111/j.1523-1739.2005.00164.x) [DOI] [Google Scholar]
- 45.Pletcher SD. 1999. Model fitting and hypothesis testing for age-specific mortality data. J. Evol. Biol. 12, 430–439. ( 10.1046/j.1420-9101.1999.00058.x) [DOI] [Google Scholar]
- 46.Siler W. 1979. A competing-risk model for animal mortality. Ecology 60, 750–757. ( 10.2307/1936612) [DOI] [Google Scholar]
- 47.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. 2002. Bayesian measures of model complexity and fit. J. R. Stat. Soc. B 64, 583–639. ( 10.1111/1467-9868.00353) [DOI] [Google Scholar]
- 48.Schmidt BR, Schaub M, Steinfartz S. 2007. Apparent survival of the salamander Salamandra salamandra is low because of high migratory activity. Front. Zool. 4, 19 ( 10.1186/1742-9994-4-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt BR, Hödl W, Schaub M. 2012. From metamorphosis to maturity in complex life cycles: equal performance of different juvenile life history pathways. Ecology 93, 657–667. ( 10.1890/11-0892.1) [DOI] [PubMed] [Google Scholar]
- 50.Cayuela H, et al. 2016. Contrasting patterns of environmental fluctuation contribute to divergent life histories among amphibian populations. Ecology 97, 980–991. ( 10.1002/ecy.1489) [DOI] [PubMed] [Google Scholar]
- 51.Muths E, et al. 2017. Heterogeneous responses of temperate-zone amphibian populations to climate change complicates conservation planning. Sci. Rep. 7, 17102 ( 10.1038/s41598-017-17105-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jehle R, Hödl W, Thonke A. 1995. Structure and dynamics of central European amphibian populations: a comparison between Triturus dobrogicus (Amphibia, Urodela) and Pelobates fuscus (Amphibia. Anura). Aust. J. Ecol. 20, 362–366. ( 10.1111/j.1442-9993.1995.tb00551.x) [DOI] [Google Scholar]
- 53.Bendik NF. 2017. Demographics, reproduction, growth, and abundance of Jollyville Plateau salamanders (Eurycea tonkawae). Ecol. Evol. 7, 5002–5015. ( 10.1002/ece3.3056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cayuela H, Schmidt BR, Weinbach A, Besnard A, Joly P. 2019. Multiple density-dependent processes shape the dynamics of a spatially structured amphibian population. J. Anim. Ecol. 88, 164–177. ( 10.1111/1365-2656.12906) [DOI] [PubMed] [Google Scholar]
- 55.Wells KD. 2010. The ecology and behavior of amphibians. Chicago, IL: University of Chicago Press. [Google Scholar]
- 56.Oliveira BF, São-Pedro VA, Santos-Barrera G, Penone C, Costa GC. 2017. AmphiBIO, a global database for amphibian ecological traits. Sci. Data 4, 1 ( 10.1038/sdata.2017.123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharon R, Degani G, Warburg MR. 1997. Oogenesis and the ovarian cycle in Salamandra salamandra infraimmaculata Mertens (Amphibia; Urodela; Salamandridae) in fringe areas of the taxon's distribution. J. Morphol. 231, 149–160. () [DOI] [PubMed] [Google Scholar]
- 58.Angelini C. 2006. Ecologia di popolazione di Salamandrina perspicillata (Savi, 1821) (Amphibia, Salamandridae). PhD thesis, Università ‘la Sapienza’ di Roma, Rome, Italy. [Google Scholar]
- 59.Rovelli V, Randi E, Davoli F, Macale D, Bologna MA, Vignoli L. 2015. She gets many and she chooses the best: polygynandry in Salamandrina perspicillata (Amphibia: Salamandridae). Biol. J. Linn. Soc. 116, 671–683. ( 10.1111/bij.12613) [DOI] [Google Scholar]
- 60.Kopp M, Baur B. 2000. Intra- and inter-litter variation in life-history traits in a population of fire salamanders (Salamandra salamandra terrestris). J. Zool. 250, 231–236. ( 10.1111/j.1469-7998.2000.tb01073.x) [DOI] [Google Scholar]
- 61.Reinhard S, Renner S, Kupfer A. 2015. Age and fecundity in Salamandra algira (Caudata: Salamandridae). Salamandra 51, 19–24. [Google Scholar]
- 62.Greven H, Thiesmeier B. 1994. Biology of Salamandra and Mertensiella. Bonn, Germany: DGHT. [Google Scholar]
- 63.Luiselli L, Andreone F, Capizzi D, Anibaldi C. 2001. Body size, population structure and fecundity traits of a Salamandra atra atra (Amphibia, Urodela, Salamandridae) population from the northeastern Italian Alps. Ital. J. Zool. 68, 125–130. ( 10.1080/11250000109356396) [DOI] [Google Scholar]
- 64.Velo-Antón G, Zamudio KR, Cordero-Rivera A. 2012. Genetic drift and rapid evolution of viviparity in insular fire salamanders (Salamandra salamandra). Heredity 108, 410 ( 10.1038/hdy.2011.91) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller DA, Janzen FJ, Fellers GM, Kleeman PM, Bronikowski AM. 2014. Biodemography of ectothermic tetrapods provides insights into the evolution and plasticity of mortality patterns. In Sociality, hierarchy, health: comparative biodemography (eds Weinstein M, Lane MA), pp. 1–30. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 66.Kirkwood TB, Austad SN. 2000. Why do we age? Nature 408, 233 ( 10.1038/35041682) [DOI] [PubMed] [Google Scholar]
- 67.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. 2007. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 87, 1175–1213. ( 10.1152/physrev.00047.2006) [DOI] [PubMed] [Google Scholar]
- 68.Flouris AD, Piantoni C. 2015. Links between thermoregulation and aging in endotherms and ectotherms. Temperature 2, 73–85. ( 10.4161/23328940.2014.989793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Remolina SC, Chang PL, Leips J, Nuzhdin SV, Hughes KA. 2012. Genomic basis of aging and life-history evolution in Drosophila melanogaster. Evolution 66, 3390–3403. ( 10.1111/j.1558-5646.2012.01710.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ivanov DK, Escott-Price V, Ziehm M, Magwire MM, Mackay TF, Partridge L, Thornton JM. 2015. Longevity GWAS using the Drosophila genetic reference panel. J. Gerontol. A: Biomed. Sci. Med. Sci. 70, 1470–1478. ( 10.1093/gerona/glv047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deelen J, Beekman M, Capri M, Franceschi C, Slagboom PE. 2013. Identifying the genomic determinants of aging and longevity in human population studies: progress and challenges. Bioessays 35, 386–396. ( 10.1002/bies.201200148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cayuela H, Olgun K, Angelini C, Üzüm N, Peyronel O, Miaud C, Avcı A, Lemaitre J-F, Schmidt BR.2019. Data from: Slow life-history strategies are associated with negligible actuarial senescence in western Palaearctic salamanders. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cayuela H, Olgun K, Angelini C, Üzüm N, Peyronel O, Miaud C, Avcı A, Lemaitre J-F, Schmidt BR.2019. Data from: Slow life-history strategies are associated with negligible actuarial senescence in western Palaearctic salamanders. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Capture–recapture datasets necessary to reproduce all analyses in this paper are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.6f407n9 [72].