Abstract
The active uptake of exogenous nucleic acids by spermatozoa of virtually all animal species is a well-established phenomenon whose significance has long been underappreciated. A growing body of published data demonstrates that extracellular vesicles released from mammalian somatic tissues pass an RNA-based flow of information to epididymal spermatozoa, thereby crossing the Weismann barrier. That information is delivered to oocytes at fertilization and affects the fate of the developing progeny. We propose that this essential process of epigenetic transmission depends upon the documented ability of epididymal spermatozoa to bind and internalize foreign nucleic acids in their nuclei. In other words, spermatozoa are not passive vectors of exogenous molecules but rather active participants in essential somatic communication across generations.
Keywords: spermatozoa, embryogenesis, microvesicles, retrotransposons, transgenerational inheritance, Weismann barrier
1. Introduction
The development of epigenetic studies in recent years has profoundly enriched our view of genetic inheritance. Most significantly, the nuclear genome has gradually lost its function as the unique and exclusive determinant of inherited characteristics due to mounting waves of data revealing the complex epigenetic networks that heritably control genome expression. A comprehensive picture of epigenetic patterns is now emerging where DNA methylation, histone modification, chromatin patterning and RNA-mediated functions play key regulatory roles on a variety of cellular processes [1,2] and on the programming of early embryonic development [3].
Growing evidence indicates that epigenetic states can be transmitted to the germline—most significantly via spermatozoa—and then delivered to the offspring at fertilization and inherited by the progeny (extensively reviewed by Lane et al. [4] and Rando [5]). A fundamental role in this process is played by extracellular vesicles, heterogeneous membrane-coated particles that can transfer RNA, DNA, proteins and lipids between a broad range of cell types and across species [6]. The cargo of extracellular vesicles is predominantly constituted by a wide range of RNAs including regulatory miRNA, tRNA, lncRNA, piRNA and snRNA, which collectively can modulate the expression of an ample spectrum of genes [7]. In the past decade, extracellular vesicles, particularly exosomes, have emerged as crucial vehicles mediating intercellular communication in a variety of physiological [8] and pathological processes [9,10]. Extracellular vesicle-mediated intercellular trafficking is not restricted to somatic cells, but is also a phenomenon involving germline cells—most significantly, mature spermatozoa. During sperm maturation, the regulatory RNA content is selectively modified by the interaction with epididymosomes, a class of extracellular vesicles released from somatic epididymis that deliver their cargoes to epididymal spermatozoa [11–16]. Some of these epididymal RNAs are essential for proper embryonic development [17].
In summary, a growing body of published data now supports the idea that spermatozoa provide an active system of soma-to-germline communication that crosses the Weismann barrier and contributes to epigenetic formatting of progeny development (for an exhaustive review, see [18]).
2. Programmed transport of RNA to spermatozoa
A unifying concept in the field is emerging, according to which an RNA-based flow of information connects somatic tissues to the germline—particularly mature spermatozoa—and can be delivered to the next generation of embryos at fertilization. Soma-to-sperm RNA delivery is an unprecedented dynamic, well-regulated process, mediated by extracellular vesicles occurring during the epididymal maturation of sperm cells in mice [14,18,19]. These RNAs comprise mostly regulatory miRNAs and tRNA fragments [11,12,14,20] that are delivered to oocytes at fertilization, are essential to early embryonic development [17] and have been demonstrated to affect the fate of the progeny [21–26] up to the fourth generation [27,28]. The effect on embryonic development is a consequence of spermatozoa delivering their RNA cargos to oocytes at fertilization [29]. Together, these results highlight a central role of epididymal spermatozoa in the rising tide of evidence for radically new modes of transgenerational epigenetic inheritance that can cross the Weismann barrier. As remarked, such a model would be consistent with a Lamarckian model of inheritance and the related idea of somatic-derived transmission known as Darwinian Pangenesis [30–33].
3. Unprogrammed extracellular vesicle transport from somatic tissues to germ cells and next-generation embryos
Extracellular vesicles are recognized as effective mediators of intercellular communication. They are released from diverse cellular sources and can pass many different RNA molecules, which may vary in response to stressing stimuli [34] and with the health of the donors [35].
Because epididymal spermatozoa are permeable to both naked nucleic acid molecules and extracellular vesicles (as discussed in more depth in the next section), it is not unreasonable to speculate that extracellular vesicles of somatic cell origin can deliver their ‘altered’ RNA cargo to epididymal spermatozoa and, eventually, to the germline. Indeed, experimental data are available in support of this view. When human melanoma cells were engineered to express EGFP, and subsequently inoculated subcutaneously in nude mice, they released traceable EGFP RNA-containing extracellular vesicles into the bloodstream, which reached the epididymis and transferred EGFP mRNA to spermatozoa [36]. These results suggest that the RNA-based information can actually reach the epididymal spermatozoa even when released from a distant tissue, not necessarily from closely related epididymal tissue [14,19,37]. Another possible interpretation is that tumour cell-released extracellular vesicles first transfer their RNA cargo to epididymosomes, which then deliver it to epididymal spermatozoa. In either pathway, epididymosomes would consistently mediate the flow of somatic RNA-based information to spermatozoa.
We can now retrospectively appreciate earlier results indicating a role of epididymal spermatozoa as collectors of somatic RNA-based epigenetic information and vectors delivering that information to fertilized oocytes and embryos [2]. That is of particular interest in the light of the notion that sperm RNA acts as a transgenerational modifier, transmitting paternal responses to environmental stressors [21,22,24,25,38] (for comprehensive reviews, see [26,39]).
4. Spermatozoa actively internalize, process and transmit exogenous DNA
In 1971, it was first demonstrated that rabbit spermatozoa, depleted of seminal fluid, were able to incorporate Simian Virus 40 DNA and deliver it to oocytes at fertilization [40]. In the pre-Internet era, that work remained long ignored by the scientific community. Eighteen years later, we rediscovered this phenomenon demonstrating that surgically withdrawn mouse epididymal spermatozoa could spontaneously bind and internalize plasmid DNA and deliver it to oocytes during in vitro fertilization (IVF). The introduced DNA sequences became heritable to the extent that they were identified in tail tissue samples of F0 and F1 offspring [41]. The process was called sperm-mediated gene transfer (SMGT) and is mediated by specific surface proteins acting as DNA-binding substrates [42] that trigger the internalization process [43] (reviewed in [44]).
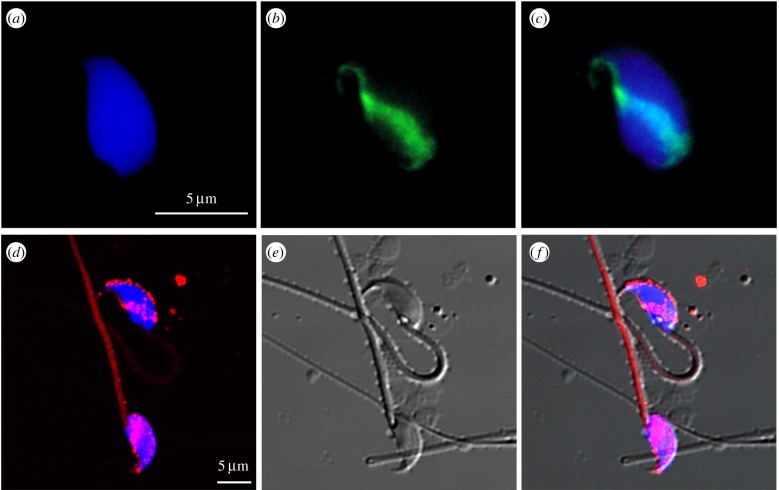
Microscopic inspection shows that epididymal spermatozoa, or ejaculated spermatozoa depleted of seminal fluid, incorporate large amounts of exogenous DNA in a spatially organized manner preferentially in the subacrosomal segment of spermatozoa (figure 1a–c). That interaction is strongly antagonized by the mammalian seminal fluid: thus, only epididymal spermatozoa, or ejaculated spermatozoa subjected to thorough washes, are ‘permeable’ to exogenous molecules [44]. It is interesting to remark that the epididymis-to-spermatozoa communication, mediated by epididymosomes, and the ability of spermatozoa to take up exogenous DNA and RNA molecules, both reflect the constitutive high permeability of mature sperm cells to molecules and extracellular vesicles. The finding that the ejaculated seminal fluid contains factors able to inhibit the permeability of spermatozoa likely reflects the need to preserve the paternal genome from undesired intrusions of foreign molecules in their travel towards fertilization. Prior to ejaculation, epididimal spermatozoa are permeable and a specific network is in place to regulate their permeablility: the internalization of exogenous nucleic acid molecules requires CD4 molecules present on the spermatozoa surface and is inhibited in sperm cells from CD4 knock-out mice [44,45].
Figure 1.
Exogenous plasmid DNA and human exosomes are taken up by mouse epididymal spermatozoa: (a) DAPI staining of sperm DNA; (b) subacrosomal localization of foreign plasmid DNA revealed by FISH analysis; (c) merge of the DAPI and FISH images. Confocal microscopic images of: (d) murine spermatozoa incubated for 2 h with rhodamine-stained human exosomes (red hue) and nuclei counterstained with Hoechst (blue hue); (e) spermatozoa morphology visualized by differential interference contrast (DIC); (f) merged Rhodamine/Hoechst/DIC signals. Exosomes were extracted from A-375 human melanoma cell line as in [36]. The exosome samples shown in (d–f) were provided by courtesy of Drs S. Fais and A. Logozzi (Italian National Institute of Health). (Online version in colour.)
Similar to DNA, foreign exosomes can also interact and deliver their cargo to sperm heads (figure 1d–f). Recent reports have shown that the subacrosomal domain of sperm cells is the predominant docking and cargo delivery site of epididymosome–spermatozoa interaction [15,16].
The binding and internalization of foreign nucleic acids trigger metabolic functions such as endonucleases [46] and reverse transcriptase (RT) [47] that, under normal conditions, remains otherwise silent in spermatozoa.
Initially, SMGT had been wrongly interpreted as the result of an ‘anomalous’ behaviour of spermatozoa which, nevertheless, could potentially provide an exploitable tool for the generation of transgenic animals. However, attempts to establish it as a biotechnological application declined in subsequent years as the real nature of the phenomenon became clear. Indeed, it has been clarified that free exogenous nucleic acid molecules delivered to oocytes at fertilization are further propagated as low-copy episomes throughout embryogenesis and inherited in a mosaic pattern within progeny tissues [48,49]. Both DNA and RNA sequences introduced by SMGT were expressed in the F0 and F1 offspring, yet were propagated mainly as non-integrated extrachromosomal structures [48,49]. The SMGT-delivered DNA sequences are (i) maintained unintegrated as low-copy episomes (less than one copy per genome), (ii) sexually transmitted in a non-Mendelian fashion from founders to the next generation (reviewed in [50]) and (iii) transcriptionally competent by means of RNA polymerase activity present in sperm cells [51] (and, remarkably, they are highly expressed in progeny tissues [48,49]).
5. Reverse transcription in spermatozoa
A similar unstable transmission of exogenous nucleic acid occurs using spermatozoa incubated with RNA, in a process called sperm-mediated reverse gene transfer (SMRGT) [48]. SMRGT requires the LINE-1-encoded RT activity present in both mature spermatozoa [47] and early preimplantation embryos [49]. The RT activity can reverse-transcribe RNA molecules either internalized in sperm heads after direct incubation or transcribed from internalized DNA molecules. In both the direct (DNA-mediated) and the reverse (RNA-mediated) process, biologically active ‘retrogenes’ are generated via reverse transcription in spermatozoa and early embryos, where the LINE-1-encoded RT is active [47,49] (reviewed in [52]). The resulting cDNA copies then become the actual substrates of the SMGT process. Moreover, since RT activity can interact with the products of sperm RNA polymerase [51], a kind of ‘natural’ PCR/RT–PCR cycle arises capable of replicating both RNA and DNA molecules incorporated by spermatozoa.
6. Spermatozoa as agents for acquisition, processing, replication and delivery of somatic nucleic acids across generations
Together, the data summarized above show that sperm are not mere passive containers, permeable to foreign nucleic acid sequences and extracellular vesicles, but are functional cells with an active biochemical machinery that generates, processes and amplifies additional information via transcription and reverse transcription [52]. In other words, mature spermatozoa act as active collectors of somatic information, carrying it across the Weismann barrier and delivering it in episomal fashion to the next generation.
In an evolutionary context, the ability of spermatozoa to take up exogenous nucleic acids is conserved in virtually all animal species, from sea urchins to mammals [53]. Sperm-mediated transgenerational inheritance can be regarded as an active process that plays more than one key role in early development. Spermatozoa transmit programmed epigenetic signals necessary for embryonic development [17]. But they are also capable of transmitting information contained in extracellular vesicles and free nucleic acids generated in response to external stimuli. Thus, sperm-mediated transgenerational inheritance has the potential to remodel the embryonic epigenetic landscape favouring the adaptation of newborns when their fathers encounter stressful conditions [54].
Clearly, spermatozoa are more complex cells than previously believed and are endowed with many more functions than just the delivery of the male genome.
Supplementary Material
Acknowledgement
We are grateful to Drs S. Fais and A. Logozzi (Italian National Institute of Health, Rome, Italy) for providing exosome samples used in interaction assays with mouse sperm cell preparations.
Data accessibility
This article has no additional data.
Authors' contributions
I.S.: investigation, data organization. A.S.: investigation. J.A.S.: contribution to the final version of the manuscript. C.S.: wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Chen Z, Li S, Subramaniam S, Shyy JY, Chien S. 2017. Epigenetic regulation: a new frontier for biomedical engineers. Annu. Rev. Biomed. Eng. 19, 195–219. ( 10.1146/annurev-bioeng-071516-044720) [DOI] [PubMed] [Google Scholar]
- 2.Zhang G, Pradhan S. 2014. Mammalian epigenetic mechanisms. IUBMB Life 66, 240–256. ( 10.1002/iub.1264) [DOI] [PubMed] [Google Scholar]
- 3.Eckersley-Maslin MA, Alda-Catalinas C, Reik W. 2018. Dynamics of the epigenetic landscape during the maternal-to-zygotic transition . Nat. Rev. Mol. Cell Biol. 19, 436–450. ( 10.1038/s41580-018-0008-z) [DOI] [PubMed] [Google Scholar]
- 4.Lane M, Robker RL, Robertson SA. 2014. Parenting from before conception. Science 345, 756–760. ( 10.1126/science.1254400) [DOI] [PubMed] [Google Scholar]
- 5.Rando OJ. 2016. Intergenerational transfer of epigenetic information in sperm. Cold Spring Harb. Perspect. Med. 6, a022988 ( 10.1101/cshperspect.a022988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. ( 10.1038/ncb1596) [DOI] [PubMed] [Google Scholar]
- 7.Schwarzenbach H, Gahan PB. 2019. MicroRNA shuttle from cell-to-cell by exosomes and its impact in cancer. Non-coding RNA 5, 28 ( 10.3390/ncrna5010028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanez-Mo M, et al. 2015. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 ( 10.3402/jev.v4.27066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang-Doran I, Zhang CY, Vidal-Puig A. 2017. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol. Metab. 28, 3–18. ( 10.1016/j.tem.2016.10.003) [DOI] [PubMed] [Google Scholar]
- 10.Wan Z, Gao X, Dong Y, Zhao Y, Chen X, Yang G, Liu L. 2018. Exosome-mediated cell–cell communication in tumor progression. Am. J. Cancer Res. 8, 1661–1673. [PMC free article] [PubMed] [Google Scholar]
- 11.Belleannee C, Calvo E, Caballero J, Sullivan R. 2013. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol. Reprod. 89, 1e11 ( 10.1095/biolreprod.113.110486) [DOI] [PubMed] [Google Scholar]
- 12.Sharma U, et al. 2016. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351, 391–396. ( 10.1126/science.aad6780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rompala GR, Mounier A, Wolfe CM, Lin Q, Lefterov I, Homanics GE. 2018. Heavy chronic intermittent ethanol exposure alters small noncoding RNAs in mouse sperm and epididymosomes. Front. Genet. 9, 32 ( 10.3389/fgene.2018.00032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma U, Sun F, Conine CC, Reichholf B, Kukreja S, Herzog VA, Ameres SL, Rando OJ. 2018. Small RNAs are trafficked from the epididymis to developing mammalian sperm. Dev. Cell 46, 481–494. ( 10.1016/j.devcel.2018.06.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Stanger SJ, Anderson AL, Bernstein IR, De Iuliis GN, Mccluskey A, Mclaughlin EA, Dun MD, Nixon B. 2019. Mechanisms of tethering and cargo transfer during epididymosome–sperm interactions. BMC Biol. 17, 35 ( 10.1186/s12915-019-0653-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nixon B, et al. 2019. Proteomic profiling of mouse reveals their contributions to post-testicular sperm maturation. Mol. Cell. Proteomics 18, S91–S108. ( 10.1074/mcp.RA118.000946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conine CC, Sun F, Song L, Rivera-Pérez JA, Rando OJ. 2018. Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Dev. Cell 46, 470–480. ( 10.1016/j.devcel.2018.06.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bošković A, Rando OJ. 2018. Transgenerational epigenetic inheritance. Annu. Rev. Genet. 52, 21–41. ( 10.1146/annurev-genet-120417-031404) [DOI] [PubMed] [Google Scholar]
- 19.Reilly JN, et al. 2016. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 6, 31794 ( 10.1038/srep31794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vojtech L, et al. 2014. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 42, 7290–7304. ( 10.1093/nar/gku347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fullston T, Teague EMCO, Palmer NO, Deblasio MJ, Mitchell M, Corbett M, Print CG, Owens JA, Lane M. 2013. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 27, 4226–4243. ( 10.1096/fj.12-224048) [DOI] [PubMed] [Google Scholar]
- 22.Dias BG, Ressler KJ. 2014. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 17, 89–96. ( 10.1038/nn.3594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM. 2014. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 17, 667–669. ( 10.1038/nn.3695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodgers AB, Morgan CP, Leu NA, Bale TL. 2015. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl Acad. Sci. USA 112, 13 699–13 704. ( 10.1073/pnas.1508347112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huypens P, Sass S, Wu M, Dyckhoff D, Tschop M. 2016. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat. Genet. 48, 497–499. ( 10.1038/ng.3527) [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Yan W, Duan E. 2016b. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 17, 733–743. ( 10.1038/nrg.2016.106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Steenwyk G, Roszkowski M, Manuella F, Franklin TB, Mansuy IM.. 2018. Transgenerational inheritance of behavioral and metabolic effects of paternal exposure to traumatic stress in early postnatal life: evidence in the 4th generation. Environ. Epigenet. 4, dvy023 ( 10.1093/eep/dvy023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrero-Bosagna C, Covert TR, Haque MM, Settles M, Nilsson EE, Anway MD, Skinner MK. 2012. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod. Toxicol. 34, 694–707. ( 10.1016/j.reprotox.2012.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. 2004. Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature 429, 154 ( 10.1038/429154a) [DOI] [PubMed] [Google Scholar]
- 30.Barry G. 2013. Lamarckian evolution explains human brain evolution and psychiatric disorders. Front. Neurosci. 7, 224 ( 10.3389/fnins.2013.00224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma A. 2014. Bioinformatic analysis revealing association of exosomal mRNAs and proteins in epigenetic inheritance. J. Theor. Biol. 357, 143–149. ( 10.1016/j.jtbi.2014.05.019) [DOI] [PubMed] [Google Scholar]
- 32.Smythies J, Edelstein L, Ramachandran V. 2014. Molecular mechanisms for the inheritance of acquired characteristics—exosomes, microRNA shuttling, fear and stress: Lamarck resurrected? Front. Genet. 5, 133 ( 10.3389/fgene.2014.00133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eaton SA, Jayasooriah N, Buckland ME, Martin DIK, Cropley JE, Suter CM. 2015. Roll over Weismann: extracellular vesicles in the transgenerational transmission of environmental effects. Epigenomics 7, 1165–1171. ( 10.2217/epi.15.58) [DOI] [PubMed] [Google Scholar]
- 34.de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, Van Balkom BWM. 2012. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exo somes. J. Extracell. Vesicles 1, 18396 ( 10.3402/jev.v1i0.18396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476. ( 10.1038/ncb1800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cossetti C, Lugini L, Astrologo L, Saggio I, Fais S, Spadafora C. 2014. Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: possible transport by exosomes. PLoS ONE 9, e101629 ( 10.1371/journal.pone.0101629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nixon B, Stanger SJ, Mihalas BP, Reilly JN, Anderson AL. 2015. The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol. Reprod. 93, 1–20. ( 10.1095/biolreprod.115.132209) [DOI] [PubMed] [Google Scholar]
- 38.Chen Q, et al. 2016. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400. ( 10.1126/science.aad7977) [DOI] [PubMed] [Google Scholar]
- 39.Sharma U, Rando OJ. 2017. Metabolic inputs into the epigenome. Cell Metab. 25, 544–558. ( 10.1016/j.cmet.2017.02.003) [DOI] [PubMed] [Google Scholar]
- 40.Bracket BG, Boranska W, Swicki W, Koprowski H. 1971. Uptake of heterologous genome by mammalian spermatozoa and its transfer to ova through fertilization. Proc. Natl Acad. Sci. USA 68, 353–357. ( 10.1073/pnas.68.2.353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavitrano M, Camaioni A, Fazio VM, Dolci S, Farace MG, Spadafora C. 1989. Sperm cells as vectors for introducing foreign DNA into eggs: genetic transformation of mice. Cell 57, 717–723. ( 10.1016/0092-8674(89)90787-3) [DOI] [PubMed] [Google Scholar]
- 42.Zani M, Lavitrano M, French D, Lulli V, Maione B, Sperandio S, Spadafora C. 1995. Mechanism of binding of exogenous DNA to sperm cells: factors controlling DNA uptake. Exp. Cell Res. 217, 57–64. ( 10.1006/excr.1995.1063) [DOI] [PubMed] [Google Scholar]
- 43.Francolini M, Lavitrano M, Lora Lamia C, French D, Frati L, Cotelli F, Spadafora C. 1993. Evidence for nuclear internalization of exogenous DNA into mammalian sperm cells. Mol. Reprod. Dev. 34, 133–139. ( 10.1002/mrd.1080340204) [DOI] [PubMed] [Google Scholar]
- 44.Spadafora C. 1998. Sperm cells and foreign DNA: a controversial relation. Bioessays 20, 955–964. () [DOI] [PubMed] [Google Scholar]
- 45.Lavitrano M, Maione B, Forte E, Francolini M, Sperandio S, Testi R, Spadafora C. 1997. The interaction of sperm cells with exogenous DNA: a role of CD4 and major histocompatibility complex class II molecules. Exp. Cell Res. 233, 56–62. ( 10.1006/excr.1997.3534) [DOI] [PubMed] [Google Scholar]
- 46.Maione B, Pittoggi C, Achene L, Lorenzini R, Spadafora C. 1997. Activation of endogenous nucleases in mature sperm cells upon interaction with exogenous DNA. DNA Cell Biol. 16, 1087–1097. ( 10.1089/dna.1997.16.1087) [DOI] [PubMed] [Google Scholar]
- 47.Giordano R, Magnano AR, Zaccagnini G, Pittoggi C, Moscufo N, Lorenzini R, Spadafora C. 2000. Reverse transcriptase activity in mature spermatozoa of mouse. J. Cell Biol. 148, 1107–1113. ( 10.1083/jcb.148.6.1107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sciamanna I, Barberi L, Martire A, Pittoggi C, Beraldi R, Giordano R, Rosa Magnano A, Hogdson C, Spadafora C. 2003. Sperm endogenous reverse transcriptase as mediator of new genetic information. Biochem. Biophys. Res. Commun. 312, 1039–1046. ( 10.1016/j.bbrc.2003.11.024) [DOI] [PubMed] [Google Scholar]
- 49.Pittoggi C, Beraldi R, Sciamanna I, Barberi L, Giordano R, Magnano AR, Torosantucci L, Pescarmona E, Spadafora C. 2006. Generation of biologically active retro-genes upon interaction of mouse spermatozoa with exogenous DNA. Mol. Reprod. Dev. 73, 1239–1246. ( 10.1002/mrd.20550) [DOI] [PubMed] [Google Scholar]
- 50.Spadafora C. 2008. Sperm-mediated ‘reverse’ gene transfer: a role of reverse transcriptase in the generation of new genetic information. Hum. Reprod. 23, 735–740. ( 10.1093/humrep/dem425) [DOI] [PubMed] [Google Scholar]
- 51.Fuster CD, Farrell D, Stern FA, Hecht NB. 1977. RNA polymerase activity in bovine spermatozoa. J. Cell Biol. 74, 698–706. ( 10.1083/jcb.74.3.698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sciamanna I, Vitullo P, Curatolo A, Spadafora C. 2009. Retrotransposons, reverse transcriptase and the genesis of new genetic information. Gene 448, 180–186. ( 10.1016/j.gene.2009.07.011) [DOI] [PubMed] [Google Scholar]
- 53.Smith K, Spadafora C. 2005. Sperm-mediated gene transfer: applications and implications. Bioessays 27, 551–562. ( 10.1002/bies.20211) [DOI] [PubMed] [Google Scholar]
- 54.Spadafora C. 2018. The ‘evolutionary field’ hypothesis: non-Mendelian transgenerational inheritance mediates diversification and evolution. Prog. Biophys. Mol. Biol. 134, 27–37 ( 10.1016/j.pbiomolbio.2017.12.001) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.