Abstract
Preeclampsia (PE), a multifactorial pregnancy-specific syndrome accounting for up to 8% of pregnancy complications, is a leading cause of maternal and fetal morbidity and mortality. PE is also associated with long-term risk of hypertension and stroke for both mother and fetus. Currently, the only “cure” is delivery of the baby and placenta, largely because the pathogenesis of PE is not yet fully understood. PE is associated with impaired vascular remodeling at the maternal-fetal interface and placental insufficiency; however, specific factors contributing to this impairment have not been identified. To identify molecular pathways involved in PE, we examined temporal transcriptomic changes occurring within the uterus, uterine implantation sites, and placentae from the Dahl salt-sensitive (Dahl S) rat model of superimposed PE compared with Sprague Dawley (SD) rats. We hypothesized that targeted gene analysis and whole transcriptome analysis would identify genetic factors that contribute to development of the preeclamptic phenotype in the Dahl S rat and unveil novel biomarkers, therapeutic targets, and mechanistic pathways in PE. Quantitative real-time PCR (qRT-PCR) and whole genome microarray analysis were performed on isolated total RNA from uterus (day 0), uterine implantation sites (days 7 and 10), and placenta (days 14 and 20). We found 624, 332, 185, and 366 genes to be differentially expressed between Dahl S (PE) and SD (normal pregnancy) on days 0, 7, 10, and 14, respectively. Our data revealed numerous pathways that may play a role in the pathophysiology of spontaneous superimposed PE and allow for further investigation of novel therapeutic targets and biomarker development.
Keywords: microarray, placenta, preeclampsia, pregnancy, uterus
INTRODUCTION
Preeclampsia (PE), a multifactorial pregnancy-specific syndrome, is a leading cause of maternal morbidity and mortality worldwide characterized by symptoms presenting after 20 wk of gestation namely: hypertension (>140/90 mmHg), proteinuria (>300 mg/day), thrombocytopenia (platelet count <100,000), impaired liver function, serum creatinine (>1.1 mg/dl), edema, and central nervous system disturbances (15). Worldwide, PE accounts for up to 8% of pregnancy complications and is considered a leading cause of maternal and fetal morbidity and mortality. The relative risk of superimposed PE in women with chronic hypertension is nearly eightfold higher than the risk of PE in the general population (9). Preeclamptic pregnancy is followed by long-term consequences for both mother and baby with increased risks of cardiovascular disease and stroke (7). Currently, preeclamptic women have limited treatment options with delivery of the baby and placenta being the only “cure.” There is little progress in developing treatments for PE, as well as a lack of robust biomarkers for early detection; therefore, further research is necessary to identify new therapeutic targets and potential biomarkers for early detection of the disease.
The exact mechanisms of the pathogenesis of PE are not yet fully understood, but given that the symptoms remit in the days to weeks following delivery, one can appreciate that the placenta plays a central role. In turn, PE is thought to be caused by placental insufficiency (20, 24, 78). However, little is known regarding the specific factors that contribute to this dysfunctional maternal-fetal interface. In normal placental development, cytotrophoblasts of fetal origin invade the maternal spiral arteries, transforming them from small-caliber resistance vessels to high-caliber capacitance vessels capable of providing placental perfusion adequate to sustain the growing fetus. This requires a complex development of maternal immune tolerance pathways to the developing fetus. In specific subsets of PE, spiral artery remodeling is insufficient, leaving them to remain as small-caliber resistance vessels (53).
Our laboratory has previously characterized the Dahl salt-sensitive rat (Dahl S), a known genetic model of hypertension and kidney disease, as a spontaneous model of superimposed PE that exhibits defects in placental vascular development and intrauterine growth restriction. These rats exhibit worsening hypertension and significant proteinuria during pregnancy, and this is associated with high uterine artery resistance, significantly smaller litter sizes, increased fetal resorptions, and intrauterine growth restriction (26). Therefore, in this present study we used the Dahl S rat model of superimposed PE to study temporal gene expression in the maternal uterus, uterine implantation sites, and placental tissues compared with normal control Sprague Dawley (SD) rat pregnancies. We hypothesized that targeted gene analyses and transcriptome analysis would identify genetic factors that contribute to development of the preeclamptic phenotype in the Dahl S rat and unveil novel biomarkers, therapeutic targets, and mechanistic pathways in PE. Separate microarray and quantitative (q)RT-PCR studies on uteri and placental samples at multiple time points (days 0, 7, 10, 14, 20), identified several hundred genes to be temporarily dysregulated in the Dahl S pregnancy. The data presented in this study set the stage for discovery of potential novel therapeutic targets and biomarkers for PE.
MATERIALS AND METHODS
Animals.
Dahl salt-sensitive S (SS/jr) rats were obtained from the colonies maintained by Dr. Michael Garrett at the University of Mississippi Medical Center (UMMC). SD rats were purchased from Harlan Laboratories (Indianapolis, IN). All rats were housed in a 12 h light-dark cycle and maintained on low-salt rodent chow (0.3% NaCl Teklad 7034) and water ad libitum. Timed breeding was performed for each strain at 16 wk of age, and gestational day 1 of pregnancy was defined by detection of sperm histologically from vaginal swab. Virgin rats from each group will be denoted as day 0. At days 0, 7, 10, 14, and 20, rats were anesthetized under isoflurane, perfused with 60 ml of saline via abdominal aortic puncture, and tissues isolated for RNA isolation were stored at −80°C until further use. These time points were chosen to represent virgin baseline (day 0; Dahl S = 5, SD = 5), early postimplantation/first trimester (day 7; Dahl S = 4, SD = 5), midgestation (day 10; Dahl S = 4, SD = 4), early chorioallantoic placental development/third trimester (day 14; Dahl S = 5, SD = 5), and near term (day 20; Dahl S = 6, SD = 6). Multiple implantation sites/placentas were isolated from each dam, and these samples were pooled together to reduce variability. All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved and monitored by the UMMC Institutional Animal Care and Use Committee.
RNA isolation and purification.
Total RNA was isolated from uterus (day 0), uterine implantation sites (days 7 and 10), and placenta (days 14 and 20) with TRIzol Plus RNA Purification Kit (Invitrogen) following manufacturer’s instructions from all groups of rats (n = 4–5 per group/time). RNA concentration was assessed with NanoDrop OneC Spectrophotometer (Thermo Scientific) and Qubit 2.0 Fluorometer (Invitrogen), and the 18S/28S ratio was assessed using Experion RNA StdSens Analysis Kit (Bio-Rad). Isolated RNA was stored at −80°C until further use.
qRT-PCR.
A total of 7.0 μg RNA was reverse-transcribed to cDNA with the iScript cDNA Synthesis Kit (Bio-Rad), according to manufacturer’s instructions. Real-time qPCR was performed with 2 μl of 350× diluted cDNA (50 ng/μl), 3 μl dH2O, 1 μl (10 μM) forward and reverse primer mix, and 5 μl SYBR-green PCR Master Mix (Bio-Rad) and run in duplicate on Bio-Rad CFX96/384 machine by the following protocol: 2 min 95°C, 40 cycles: 5 s 95°C and 30 s 60°C, followed by a 5 s 65°C melt curve to 5 s 95°C. Primers (Bio-Rad) were selected from predesigned and validated primers from Bio-Rad PrimePCR assays. PE-associated genes were chosen from literature searches of the PubMed database and included both human and animal studies (Table 1) (4, 10, 18, 27, 32, 37, 56a, 45, 59, 60, 76). Expression levels (∆∆Ct) were normalized to those of beta-actin (Actb) and glyceraldehyde 3-phosphate dehydrogenase (Gapdh). To evaluate differences between strains by day, Student’s t-tests were performed. Differences within strain across days for each gene were determined by performing ANOVA tests with Tukey honestly significant difference post hoc analysis. A P value < 0.05 was considered to be statistically significant with Bio-Rad Maestro software. The data are presented as means ± SE.
Table 1.
Known genes associated with preeclampsia
| Category | Symbol | Name | Reference |
|---|---|---|---|
| Vasoconstrictor | agt | angiotensinogen | 10, 58, 75 |
| avp | vasopressin | 59 | |
| edn1 | preproendothelin-1/endothelin | 10, 58 | |
| Angiogenesis | eng | soluble endoglin | 10, 26, 31, 58 |
| hif1a | hypoxia inducible factor 1a | 10, 31 | |
| hmox1 | heme oxygenase | 10, 58 | |
| il15 | interleukin 15 | 10 | |
| nos3 | nitric oxide synthase 3 (eNOS) | 4, 58, 75 | |
| vegfa | vascular endothelial growth factor-a | 4, 10, 18, 26, 31, 58, 75 | |
| Cytokine activity | edn1 | preproendothelin-1/endothelin | 10 |
| ifng | interferon gamma | 4, 10 | |
| il10 | interleukin 10 | 10, 75 | |
| il15 | interleukin 15 | 10 | |
| il17a | interleukin 17a | 58 | |
| il4 | interleukin 4 | 10 | |
| tgfb1 | transforming growth factor beta | 4, 10, 26, 31, 58 | |
| tnf | tumor necrosis factor alpha | 10, 26, 31, 37, 44, 58, 75 | |
| vegfa | vascular endothelial growth factor-a | 4, 10, 18, 26, 31, 58, 75 | |
| Inflammatory response | c3 | complement 3 | 10 |
| ephx2 | epoxide hydrolase 2 | 58, 75 | |
| il10 | interleukin 10 | 10, 75 | |
| il15 | interleukin 15 | 10 | |
| il17a | interleukin 17a | 58 | |
| tgfb1 | transforming growth factor beta | 4, 10, 26, 31, 58 | |
| tlr2 | toll-like receptor 2 | 37, 44, 58 | |
| tlr4 | toll-like receptor 4 | 26, 37, 44, 58 | |
| tnf | tumor necrosis factor alpha | 10, 26, 31, 37, 44, 58, 75 | |
| Misc. | |||
| Long-chain fatty acid transport and lipid storage | plin2 | perilipin 2 | 10 |
| G protein-coupled receptor binding | rln3 | relaxin-3 | 3, 18, 31 |
| Negative regulation of protein tyrosine kinase activity | sh3bp5 | SH3-domain binding protein 5 (BTK-associated) | 10 |
| Actin cytoskeleton organization | coro2a | coronin actin binding protein 2a | 10 |
| Negative regulation of immune response | fcrlb | Fc receptor-like B | 10 |
| Negative regulation of cell proliferation | agt | angiotensinogen | 10, 58, 75 |
| hmox1 | heme oxygenase | 10, 58 | |
| ifng | interferon gamma | 4, 10 | |
| il10 | interleukin 10 | 10, 75 | |
| nos1 | nitric oxide synthase 1 (nNOS) | 18 | |
| nos3 | nitric oxide synthase 3 (eNOS) | 4, 58, 75 | |
| notch2 | notch 2 | 36 | |
| pla2 g16 | phospholipase A2 group XVI | 10 | |
| tgfb1 | transforming growth factor beta | 4, 10, 26, 31, 58 | |
| tlr2 | toll like receptor 2 | 37, 44, 58 | |
| tnf | tumor necrosis factor alpha | 10, 26, 31, 37, 44, 58, 75 | |
| Nitric oxide-mediated signal transduction | apoe | apolipoprotein e | 75 |
| nos1 | nitric oxide synthase 1 (nNOS) | 18 | |
| Positive regulation of angiogenesis | nos3 | nitric oxide synthase 3 (eNOS) | 4, 58, 75 |
| c3 | complement 3 | 10 | |
| edn1 | preproendothelin-1/endothelin | 10 | |
| hif1a | hypoxia inducible factor 1a | 10, 31 | |
| hmox1 | heme oxygenase | 10, 58 | |
| nos3 | nitric oxide synthase 3 (eNOS) | 4, 58, 75 | |
| pgf | placental growth factor | 10, 18, 26, 31, 58 | |
| vegfa | vascular endothelial growth factor-a | 4, 10, 18, 26, 31, 58, 75 | |
| Positive regulation of apoptotic process | hif1a | hypoxia inducible factor 1a | 10, 31 |
| hmox1 | heme oxygenase | 10, 58 | |
| ifng | interferon gamma | 4, 10 | |
| nos3 | nitric oxide synthase 3 (eNOS) | 4, 58, 75 | |
| notch2 | notch 2 | 36 | |
| rln1 | relaxin-1 | 4, 18, 31 | |
| tgfb1 | transforming growth factor beta | 10, 26, 31, 32, 58 | |
| tlr4 | toll like receptor 4 | 26, 37, 44, 58 | |
| tnf | tumor necrosis factor alpha | 10, 26, 31, 37, 44, 58, 75 | |
| Positive regulation of cell proliferation | agt | angiotensinogen | 10, 58, 75 |
| avp | vasopressin | 59 | |
| edn1 | preproendothelin-1/endothelin | 10 | |
| hif1a | hypoxia inducible factor 1a | 10, 31 | |
| ifng | interferon gamma | 4, 10 | |
| il15 | interleukin 15 | 10 | |
| il4 | interleukin 4 | 10 | |
| notch2 | notch 2 | 36 | |
| pgf | placental growth factor | 10, 18, 26, 31, 58 | |
| rln1 | relaxin-1 | 4, 18, 31 | |
| tgfb1 | transforming growth factor beta | 4, 10, 26, 31, 58 | |
| vegfa | vascular endothelial growth factor-a | 4, 10, 18, 26, 31, 58, 75 | |
| eng | soluble endoglin | 10, 26, 31, 58 | |
| Response to hypoxia | edn1 | preproendothelin-1/endothelin | 10 |
| hif1a | hypoxia inducible factor 1a | 10, 31 | |
| hmox1 | heme oxygenase | 10, 58 | |
| nos1 | nitric oxide synthase 1 (nNOS) | 18 | |
| nos3 | nitric oxide synthase 3 (eNOS) | 4, 58, 75 | |
| pgf | placental growth factor | 10, 18, 26, 31, 58 | |
| tgfb1 | transforming growth factor beta | 4, 10, 26, 31, 58 | |
| tlr2 | toll like receptor 2 | 37, 44, 58 | |
| tlr4 | toll like receptor 4 | 26, 37, 44, 58 | |
| tnf | tumor necrosis factor alpha | 10, 26, 31, 37, 44, 58, 75 | |
| vegfa | vascular endothelial growth factor-a | 4, 10, 18, 26, 31, 58, 75 |
Microarray.
In a subset of the samples obtained from SD and Dahl S rats used in the PrimePCR assays, uterus day 0, uterine implantation sites from days 7 and 10, and placenta from day 14 were used for microarray experiments. RNA was processed using manufacturer directions for rat GeneChip 2.0 ST using Affymetrix equipment (Scanner 3000 7G System). Hybridized chips were automatically washed, stained, and scanned at the UMMC Institutional Molecular and Genomics Core using Affymetrix equipment as previously described by Spann et al. (2018) (66).
Microarray data analysis.
Statistical analysis of microarray data was performed using Bioconductor, Limma, and other R Packages (2, 6, 12–14, 21, 22, 28, 36, 40, 41, 46, 48, 51, 57, 68–70, 72–75, 79, 80). Quality control was performed and all arrays met predefined criteria as described in the workflow package “maEndtoEnd” (43). In brief, row-wise medians from expression data were filtered to exclude transcripts that did not have intensities larger than a 2.5 median expression intensity (539 for days 0–10 and 909 for day 14 of 36,685). Additionally, probes that did not match to a specific gene/gene symbol as well as probes that had multiple matches to a single gene were filtered out (1,790 for days 0–10 and 1,783 for day 14), resulting in a final total of 34,356 genes to be evaluated. Expression values were calculated by the robust multichip average method, which included quantile normalization. All microarray data are MIAME compliant and have been submitted to the Gene Expression Omnibus (GSE130102). A linear model consisting of six different contrasts was used: “SD day 7 vs. SD day 0” for gene expression pregnancy induced changes in normal uterus; “Dahl S day 7 vs. Dahl S day 0” for gene expression pregnancy induced changes in preeclamptic uterus; and “Dahl S day 0 vs. SD day 0,” “Dahl S day 7 vs. SD day 7,” “Dahl S day 10 vs. SD day 10,” and “Dahl S day 14 vs. SD day 14” for significant differences in transcriptome between normal pregnant and preeclamptic rats. Empirical Bayes variance moderation was used for correction. Gene expression differences between groups were considered to be statistically significant with a P value < 0.001. Gene Set Enrichment Analysis (GSEA) was performed to compare the groups with the ReactomePA R Package (79).
qRT-PCR was performed as described above to validate the microarray data for 20 genes identified as differentially regulated in the GeneChip experiment. All primers were predesigned, validated primers from Bio-Rad PrimePCR assays. A significant linear correlation for all 20 genes was found between Real-time PCR data and microarray data.
RESULTS
qRT-PCR analysis of known PE-associated genes.
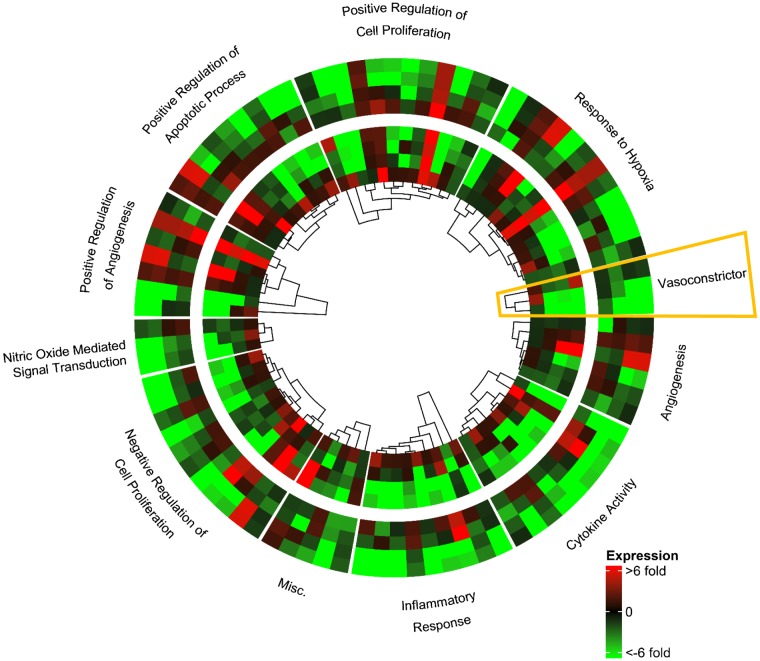
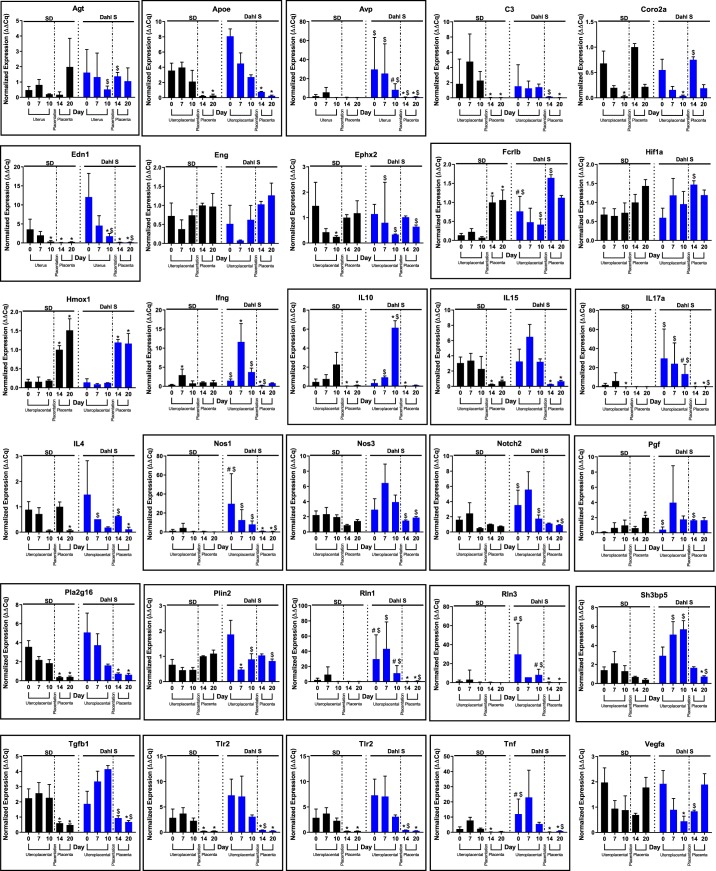
Table 1 provides a list of known genes associated with PE, along with their categorization based on DAVID functional annotation terms (34, 35). The expression pattern for each of these genes was evaluated in both the Dahl S and SD rats across the time-course of pregnancy (days 0, 7, 10, 14, and 20) by qRT-PCR. Figure 1 shows expression levels of these known genes associated with PE on days 7, 10, 14, and 20 relative to day 0 using circlize and ComplexHeatmap R Packages (29, 30), with the Dahl S strain in the outer track and the SD strain in the inner track. Strikingly, there are obvious parallels in the expression patterns that occur during the time-course of both Dahl S and SD pregnancies, underscoring the common theme of pregnancy. However, out of the 30 known associated PE genes assessed, 21 showed quantitatively higher expression levels for four or more of the five time points in the Dahl S rat compared with the SD rat (Fig. 2, genes in boxes). For example, the vasoconstrictor category genes (Angiotensinogen, Vasopressin, and Endothelin) demonstrates similar patterns of expression changes occurring during pregnancy but had significantly higher expression levels of these three genes in the uterus/uterine implantation sites of the Dahl S rat.
Fig. 1.
Gene expression of known preeclampsia associated genes. Heat map of Dahl S (outer track) and SD (inner track) expression for day 7 (innermost) to day 20 (outermost) relative to day 0. Yellow box denotes vasoconstrictor genes selected for Fig. 2; n = 4–6 per group/time.
Fig. 2.
Individual gene expression levels of known preeclampsia associated genes. Boxed genes denote genes showing quantitatively higher expression levels for 4 or more of the 5 time points in the Dahl salt-sensitive (Dahl S) rat compared with the SD rat. $P < 0.05 t-test fold change same day Dahl S vs. SD; #P < 0.05 same day ANOVA, *P < 0.05 vs. day 0 same strain; n = 4–6 per group/time.
Gene expression changes in uterine tissue during early pregnancy.
The preeclamptic phenotype previously described from Gillis et al. (2015 and 2016) (25, 26) was confirmed by verification of a reduction in litter size. The total number of fetuses present in Dahl S dams on both day 10 (10 ± 1 vs. 13 ± 1, P < 0.05, n = 7–12) and day 14 (11 ± 1 vs. 14 ± 1, P < 0.05, n = 7–11) was significantly lower compared with SD by unpaired t-test with Welch’s correction. Two out of seven of the Dahl S dams examined on day 14 had evidence of fetal resorptions (3, 5). A subset of these samples were used in an unbiased microarray approach, using Affymetrix GeneChip Microarrays, to identify genes that changed in the uterine tissue during healthy and preeclamptic pregnancy.
Microarray results from SD early pregnant (day 7) vs. virgin (day 0).
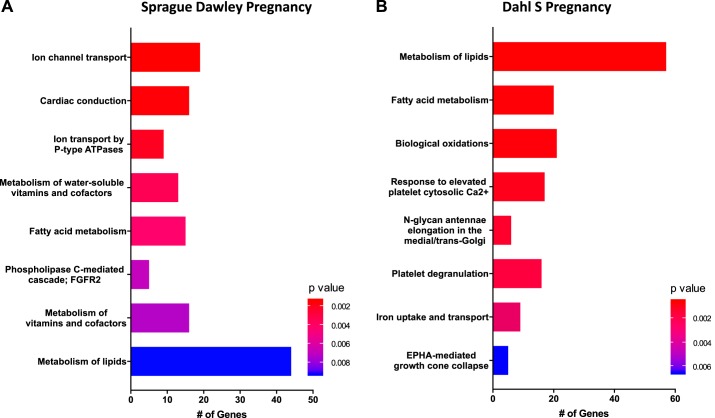
Microarray experiments were performed on uterus tissue isolated from virgin SD (day 0) and pregnant SD (implantation sites on day 7) to establish early pregnancy-induced changes under conditions of normal pregnancy. We observed 734 genes to be differentially expressed in SD from day 0 to 7, with 364 upregulated and 370 downregulated. The top 10 significantly up- and downregulated genes are listed in Table 2. GSEA using the Reactome Pathway was performed to determine which biological pathways change during the first week of pregnancy. The top eight most significantly expressed gene sets in normal pregnancy at day 7 were related to ion channel transport (n = 19), cardiac conduction (n = 16), ion transport by P-type ATPases (n = 9), metabolism of water-soluble vitamins and cofactors (n = 13), fatty acid metabolism (n = 15), phospholipase c-mediated cascade; FGFR2 (n = 5), metabolism of vitamins and cofactors (n = 16), and metabolism of lipids (n = 44) (Fig. 3A, Table 3).
Table 2.
Top 10 significantly up- and downregulated genes at day 7
| Normal Pregnancy | Preeclamptic Pregnancy | ||||
|---|---|---|---|---|---|
| Symbol | Log Fold Change | P Value | Symbol | Log Fold Change | P Value |
| Igfbp1 | 4.43 | <0.001 | Igfbp1 | 5.47 | <0.001 |
| Cyp26a1 | 3.84 | <0.001 | Cyp26a1 | 5.07 | <0.001 |
| LOC691970 | 3.64 | <0.001 | LOC259244 | 4.69 | <0.001 |
| LOC102556288 | 3.47 | <0.001 | Acod1 | 4.61 | <0.001 |
| Unc93a | 3.39 | <0.001 | Igfbp3 | 4.36 | <0.001 |
| Erbb4 | 2.97 | <0.001 | Obp3 | 4.28 | <0.001 |
| Atp6v0a4 | 2.86 | <0.001 | Pgf | 3.97 | <0.001 |
| Depp1 | 2.63 | <0.001 | Slc5a8 | 3.96 | <0.001 |
| Spp1 | 2.63 | <0.001 | F5 | 3.72 | <0.001 |
| Pla2 g2a | 2.49 | <0.001 | Depp1 | 3.70 | <0.001 |
| Mmp7 | −5.17 | <0.001 | A2ml1 | −6.14 | <0.001 |
| A2ml1 | −3.80 | <0.001 | Gabrp | −5.57 | <0.001 |
| Trappc31 | −3.25 | <0.001 | Mmp7 | −5.15 | <0.001 |
| Cuzd1 | −3.12 | 0.04 | Trappc31 | −3.98 | <0.001 |
| Plekhs1 | −3.12 | <0.001 | Nxpe5 | −3.61 | <0.001 |
| Fam3b | −3.01 | <0.001 | Cd8a | −3.55 | <0.001 |
| Far2 | −2.99 | <0.001 | Alox12 | −3.55 | <0.001 |
| B4 galnt2 | −2.83 | <0.001 | Rnf128 | −3.38 | <0.001 |
| Tmem229a | −2.74 | <0.001 | Cuzd1 | −3.36 | <0.001 |
| Col6a4 | −2.68 | <0.001 | Far2 | −3.33 | <0.001 |
Fig. 3.
Early pregnancy reactome pathway gene set enrichment analysis (GSEA) in Sprague Dawley (SD, A) and Dahl S (B) uterine tissue; n = 4–5 per group/time.
Table 3.
Day 7 Reactome Pathway Gene Set Enrichment Analysis
| Pathway | Genes | P Value |
|---|---|---|
| Normal Pregnancy | ||
| Ion channel transport | Atp1b1/Atp6v0a4/Atp1b3/Atp7b/Fxyd4/Atp2b2/Trpc5/Atp2a2/Tpcn2/Asic1/Camk2 g/Atp8b1/Trpc6/Slc17a3/ Trpm8/Clic2/Ttyh3/Atp1a2/Tctn2 | 0.001438 |
| Cardiac conduction | Atp1b1/Atp1b3/Kcnj12/Fxyd4/Npr1/Kcne1/Atp2b2/Atp2a2/Camk2g/Kcnk18/Cacnb2/Cacng3/Clic2/Kcnk2/ Kcne3/Atp1a2 | 0.001565 |
| Ion transport by P-type ATPases | Atp1b1/Atp1b3/Atp7b/Fxyd4/Atp2b2/Atp2a2/Camk2 g/Atp8b1/Atp1a2 | 0.002054 |
| Metabolism of water-soluble vitamins and cofactors | Btd/Parp14/Parp9/Cyb5r3/Cyb5a/Nt5e/Vnn1/Slc19a3/Nadk2/Ctrc/Mmachc/Nnmt/Slc2a1 | 0.003505 |
| Fatty acid metabolism | Fads1/Phyh/Slc25a17/Ptgds/Elovl5/Acadm/Cpt1a/Dpep1/Nudt19/Ppt1/Acot9/Acsl3/Acads/Gpx4/Hacl1 | 0.004295 |
| Phospholipase C-mediated cascade; FGFR2 | Fgf9/Fgf10/Fgf7/Fgf23/Fgf8 | 0.006907 |
| Metabolism of vitamins and cofactors | Lpl/Btd/Parp14/Parp9/Sdc3/Cyb5r3/Cyb5a/Nt5e/Vnn1/Slc19a3/Nadk2/Ctrc/Lrat/Mmachc/Nnmt/Slc2a1 | 0.00728 |
| Metabolism of lipids | Slc10a1/Fabp5/Far2/Fads1/Pla2 g2a/Pla2 g5/Phyh/Pik3c2a/Osbpl3/Slc25a17/Sumf2/Pemt/Hsd17b14/Ptgds/ Osbpl8/Ormdl1/Elovl5/Pik3r3/Acadm/Dgat2/Prkd3/Cpt1a/Degs2/Glb1l/Pik3r5/Dpep1/Nudt19/Ppt1/Acot9/ Agpat4/Agpat3/Akr1b1/Asah1/Acsl3/S ts/Acads/Arsb/B4 galnt1/Gpx4/Inpp5j/ Pik3c2b/Mboat2/Hacl1/Ptdss2 | 0.009268 |
| Preeclamptic Pregnancy | ||
| Metabolism of lipids | Slc10a1/Fabp5/Osbpl8/Pemt/Far2/Osbpl3/Cyp4b1/Acot5/Ddhd1/Hacd4/Ormdl1/Ptgds/Mboat1/Cds1/Hacd3/ Agpat5/Ddhd2/Acsl3/Pik3r3/Acot9/Acadm/Hadh/Gpam/Alb/Fads1/Ugcg/Bdh1/Chka/Lpin3/Slc25a17/ Oxct1/Dpep2/Abcc3/Cpt1a/Gpat3/Osbpl2/Dgat2/Mid1ip1/Tecr/Acly/Dpep1/Sacm1l/Hacl1/Sts/Stard3/ Sphk1/Acot7/Arsk/Pik3r1/Elovl5/Gk/Acss3/Pla2r1/Hrasls5/Idi1/Tnfaip8/Plpp1 | 0.000557 |
| Fatty acid metabolism | Cyp4b1/Acot5/Hacd4/Ptgds/Hacd3/Acsl3/Acot9/Acadm/Hadh/Fads1/Slc25a17/Dpep2/Cpt1a/ Mid1ip1/Tecr/Acly/Dpep1/Hacl1/Acot7/Elovl5 | 0.000637 |
| Biological oxidations | Aldh2/Fmo3/Cyp4b1/Cyp2f4/Slc35b3/Gstm5/Gstm7/Cmbl/Fmo2/Comt/Dpep2/Gstp1/Cyb5b/Bpnt1/ Tpmt/Dpep1/Papss2/Por/Arnt2/Adh6/Svs1 | 0.000778 |
| Response to elevated platelet cytosolic Ca2+ | Mmrn1/Sod1/Alb/Clu/Clec3b/Tgfb2/Fam49b/Lgals3bp/Anxa5/Tagln2/Fermt3/Pcyox1l/A1bg/Tor4a/ Srgn/Pdgfa/Prkca | 0.001129 |
| N-glycan antennae elongation in the medial/trans-Golgi | B4 galt4/Man2a1/St6 gal1/Chst8/B4 galt3/B4 galt5 | 0.001615 |
| Platelet degranulation | Mmrn1/Sod1/Alb/Clu/Clec3b/Tgfb2/Fam49b/Lgals3bp/Anxa5/Tagln2/Fermt3/Pcyox1l/A1bg/Tor4a/ Srgn/Pdgfa | 0.002093 |
| Iron uptake and transport | Atp6v0a4/Tcirg1/Atp6v0a1/Mcoln1/Cybrd1/Abcg3l3/Abcg2/Fbxl5/Skp1 | 0.003067 |
| EPHA-mediated growth cone collapse | Epha2/Epha7/Efna1/Ngef/Efna4 | 0.006601 |
Microarray results from Dahl S early pregnant (day 7) vs. virgin (day 0).
To determine if there was dysregulation of normal gene expression changes during preeclamptic pregnancy, gene expression levels were compared between uterine tissue from virgin Dahl S (day 0) and pregnant Dahl S (implantation sites on day 7). In total, 884 genes were observed to be differentially expressed in Dahl S from day 0 to 7, with 428 upregulated and 456 downregulated. The top 10 significantly up- and downregulated genes in the Dahl S are listed in Table 2, all of which exhibited a log2 fold change >3.7 (upregulated) or <−3.33 (downregulated). While few of these genes were observed to change in a similar degree to that of the SD pregnancy, 14 of the 20 genes were not in the top 20 for the SD comparison (day 7 vs. 0). The top eight most significantly expressed gene sets from Reactome Pathway GSEA for preeclamptic pregnancy at day 7 include metabolism of lipids (n = 57), fatty acid metabolism (n = 20), biological oxidations (n = 21), response to elevated platelet cytosolic Ca2+ (n = 17), N-glycan antennae elongation in the medial/trans-Golgi (n = 6), platelet degranulation (n = 16), iron uptake and transport (n = 9), and EPHA-mediated growth cone collapse (n = 5) (Fig. 3B, Table 3). Interestingly, while these pathways share similarities to that of normal pregnancy (SD), the genes that contributed to this Reactome Pathway Enrichment were different. For example, the fatty acid metabolism pathway was enriched in both Dahl S and SD analysis; however, different isozymes of Acyl-CoA thioesterase, enzymes that catalyze the hydrolysis activated fatty acids to their respective free fatty acid and coenzyme A (CoASH) (11), were expressed in the Dahl S and SD (Acot5 vs. Acot9, respectively).
Microarray analysis of transcriptome differences between uterine tissue from Dahl S and SD rats.
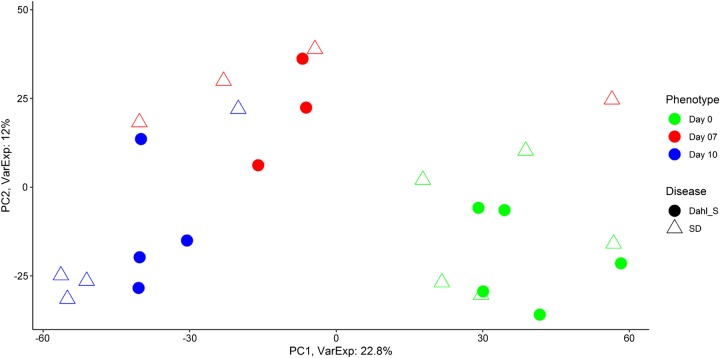
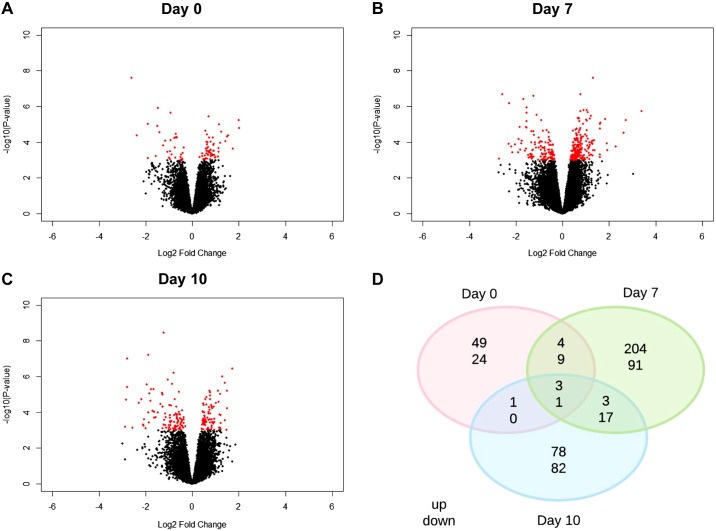
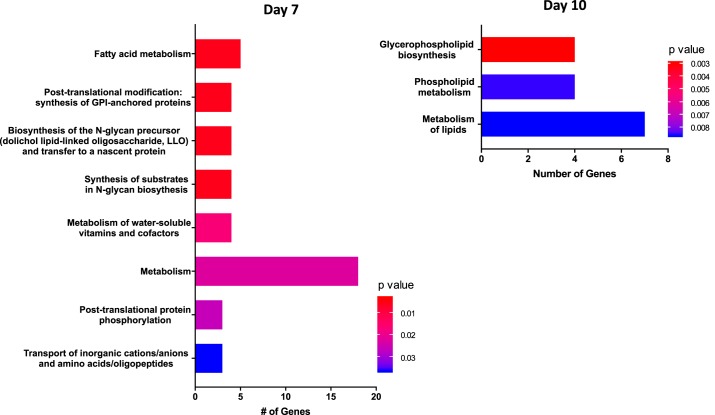
Microarray analysis was performed between SD (normal) and Dahl S (PE) rats through midpregnancy (day 10) to investigate transcriptome differences in the uterus between normal and preeclamptic pregnancy. A Principal Component Analysis (Fig. 4) for days 0, 7, and 10, demonstrates that that for the most part, each stage of pregnancy has distinct transcriptome differences with only minor differences between normal and preeclamptic pregnancy. Of the total number of genes evaluated (n = 34,356), 566 genes were significantly different across all time points in Dahl S rats compared with SD 73 genes were uniquely differentially expressed at day 0, 295 to day 7, and 160 to day 10 (Fig. 5, A–C; P < 0.001). Importantly, while some of these genes have been previously defined as having known associations with PE, many have yet to be studied in terms of their functional role in regards to PE. For example, three genes (Atat1, LOC100362031, RT1-CE5) were upregulated, and one (Tatdn3) was downregulated in the Dahl S across all experimental days (Fig. 5D); however, there is no known role for any of these genes in PE to date. Reactome Pathway Analysis on genes that were significantly differentially expressed on gestational days 7 and 10 was enriched for several pathways, most notably Fatty Acid Metabolism (Acot5, Acot6, Cyp4b1, Acads, and Acot7) and Metabolism of Lipids (Cyp2e1, Hpgd, Osbpl8, Pla2g16, Ppt1, Cept1, and Pld1), respectively (Fig. 6, Table 4). No significant enrichment in Reactome pathway analysis was observed for day 0.
Fig. 4.
Principle Component Analysis of days 0, 7, 10; n = 4–5 per group/time.
Fig. 5.
Midpregnancy significantly differentially expressed genes in Dahl S vs. SD uterine tissue. P < 0.001; n = 4–5 per group/time. A–C: volcano plots showing total differentially expressed genes. D: Venn diagram showing overlap of differentially expressed genes across all time points.
Fig. 6.
Midpregnancy Reactome pathway Gene Set Enrichment Analysis (GSEA) in Dahl S vs. SD uterine tissue; n = 4–5 per group/time.
Table 4.
Preplacentation Reactome Pathway Gene Set Enrichment Analysis
| Pathway | Genes | P value |
|---|---|---|
| Day 7 | ||
| Fatty acid metabolism | Acot5/Acot6/Cyp4b1/Acads/Acot7 | 0.00331 |
| Posttranslational modification: synthesis of GPI-anchored proteins | Msln/Lypd1/Pgap1/Vnn1 | 0.003386 |
| Biosynthesis of the N-glycan precursor (dolichol lipid-linked oligosaccharide, LLO) and transfer to a nascent protein | St6 galnac2/Pmm1/Nans/St3 gal6 | 0.00552 |
| Synthesis of substrates in N-glycan biosynthesis | St6 galnac2/Pmm1/Nans/St3 gal6 | 0.00552 |
| Metabolism of water-soluble vitamins and cofactors | Slc19a3/Slc5a8/Slc52a3/Vnn1 | 0.016794 |
| Metabolism | Slc19a3/Acot5/Dcxr/Acot6/Slc5a8/Slc22a1/Pla2 g2a/Cyp4b1/Slc52a3/Bdh1/Acads/Acot7/Plcd3/Alad/Vnn1/Etfa/Idi1/St3 gal6 | 0.022344 |
| Posttranslational protein phosphorylation | Msln/Fgg/Spp1 | 0.026206 |
| Transport of inorganic cations/anions and amino acids/oligopeptides | Slc26a3/Slc5a8/Slc15a2 | 0.036759 |
| Day 10 | ||
| Glycerophospholipid biosynthesis | Osbpl8/Pla2 g16/Cept1/Pld1 | 0.002969 |
| Phospholipid metabolism | Osbpl8/Pla2 g16/Cept1/Pld1 | 0.008546 |
| Metabolism of lipids | Cyp2e1/Hpgd/Osbpl8/Pla2 g16/Ppt1/Cept1/Pld1 | 0.008658 |
Microarray analysis of transcriptome differences between day 14 placenta samples from Dahl S and SD rats.
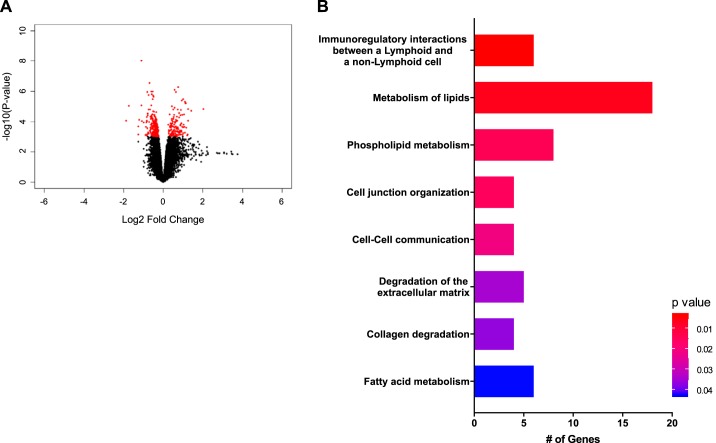
Because the placenta plays a crucial role in the pathogenesis of PE, transcriptomes differences between placentae of normal (SD) and preeclamptic (Dahl S) rats were also investigated. Day 14 placentae were selected for this analysis to determine potential changes in gene expression that correspond to early placentation and the onset of the symptoms of PE. Figure 7A shows 366 differentially expressed genes in the Dahl S rat compared with SD on day 14 (P < 0.001). Reactome Pathway Analysis for day 14 again highlights the role of fatty acid metabolism (Prkag2, Hpgds, Pon2, Ptgs1, Nudt19, Mid1ip1) and metabolism of lipids (Pik3cg, Prkag2, Pla2g4d, Hpgds, Mtmr7, Pon2, Prkd3, Ddhd1, Tnfaip8, Ptgs1, Phospho1, Nudt19, Mid1ip1, Plekha1, Cyp2d2, Fabp5, Pla2r1, and Mogat2) (Fig. 7B). Notably, while the pathways of metabolism of lipids and fatty acid metabolism are shared both pre- and postplacentation, the specific genes that make up these pathways are different (Table 5). Interestingly, the top Reactome Pathway identified in this analysis was immunoregulatory interactions between lymphoid and non-lymphoid cells. This pathway included the genes Cd19, Cd200, and C3.
Fig. 7.
Differentially expressed genes (A) and Reactome Pathway Gene Set Enrichment Analysis (GSEA) (B) in Dahl S vs. SD day 14 placenta samples; n = 4–5 per group/time.
Table 5.
Postplacentation (day 14) Reactome Pathway Gene Set Enrichment Analysis
| Pathway | Genes | P Value |
|---|---|---|
| Immunoregulatory interactions between a lymphoid and a nonlymphoid cell | Nectin2/Cd19/RT1-CE5/Cd200/ C3/PVR | 0.003355 |
| Metabolism of lipids | Pik3cg/Prkag2/Pla2 g4d/Hpgds/Mtmr7/Pon2/Prkd3/Ddhd1/Tnfaip8/Ptgs1/ Phospho1/Nudt19/Mid1ip1/Plekha1/Cyp2d2/Fabp5/Pla2r1/Mogat2 | 0.0059 |
| Phospholipid metabolism | Pik3cg/Pla2 g4d/Mtmr7/Ddhd1/Tnfaip8/Phospho1/Plekha1/Pla2r1 | 0.013763 |
| Cell junction organization | Nectin2/Parvb/PVR/Lamc2 | 0.015111 |
| Cell-cell communication | Nectin2/Parvb/PVR/Lamc2 | 0.021028 |
| Degradation of the extracellular matrix | Mmp1/Mmp15/Lamc2/Col4a1/Ctsq | 0.034465 |
| Collagen degradation | Mmp1/Mmp15/Col4a1/Ctsq | 0.036627 |
| Fatty acid metabolism | Prkag2/Hpgds/Pon2/Ptgs1/Nudt19/Mid1ip1 | 0.042755 |
Microarray validation.
We selected 20 genes for qRT-PCR validation of the microarray results based on the largest fold changes in expression, most significant differential expression, and relevant Reactome pathways (Table 6). Fold changes in expression of the Dahl S compared with SD for all genes were calculated separately for both microarray and qRT-PCR data. Figure 8 shows a significant linear correlation in fold changes between data sets with a Pearson correlation coefficient of 0.9096 (P < 0.0001). Most relevant to the Reactome lipid pathway analysis, six of the genes were related to the apolipoprotein family (Apoa2, Apoa4, Apob, Apoc2, Apoh, and Apom), of which five of the six genes show a significant difference in expression fold change between Dahl S and SD rats at day 14, again highlighting the significance of placentation. Interestingly, alpha-fetoprotein (Afp), the most significantly differentially expressed gene with the highest fold change (13.62, P = 0.01) in the microarray analysis for day 14, was also significantly elevated in the Dahl S rat compared with SD on day 14 in qRT-PCR analysis.
Table 6.
Validation of microarray with quantitative RT-PCR
| Symbol | Microarray Fold Change (P value) | Quantitative RT-PCR Fold Change (P value) |
|---|---|---|
| Afp | 13.61652 (0.014) | 96.64089 (0.019) |
| Apoa2 | 10.86055 (0.009) | 89.46349 (0.003) |
| Apoa4 | 3.556006 (0.003) | 27.0976 (0.002) |
| Apob | 11.0689 (0.014) | 30.17789 (0.011) |
| Apoc2 | 7.142272 (0.012) | 52.48363 (0.004) |
| Apoh | 4.80271 (0.012) | 40.58866 (0.147) |
| Apom | 2.920876 (0.004) | 21.96439 (< 0.001) |
| Cd68 | 1.898543 (0.017) | 2.58181 (0.005) |
| Flt3 | 0.736441 (< 0.001) | 0.87781 (0.337) |
| Lox | 3.368831 (0.002) | 3.21418 (0.022) |
| Ly6i | 2.674769 (< 0.001) | 5.46058 (0.011) |
| Pla2 g2a | 0.832463 (0.013) | 0.88423 (0.304) |
| Prl8a2 | 1.707548 (< 0.001) | 3.46549 (< 0.001) |
| Psg29 | 0.300977 (< 0.001) | 0.32297 (0.009) |
| Rgs5 | 0.572762 (< 0.001) | 0.5518 (0.031) |
| Rps16 | 0.465481 (< 0.001) | 0.34474 (0.004) |
| Rt1-ce5 | 0.657241718 (< 0.001) | 0.00134 (0.025) |
| Rt1-db1 | 0.833484 (0.020) | 1.41131 (0.322) |
| Sned1 | 2.416386 (< 0.001) | 3.78484 (< 0.001) |
| Upk1b | 1.14187 (0.050) | 2.43309 (0.136) |
Fig. 8.
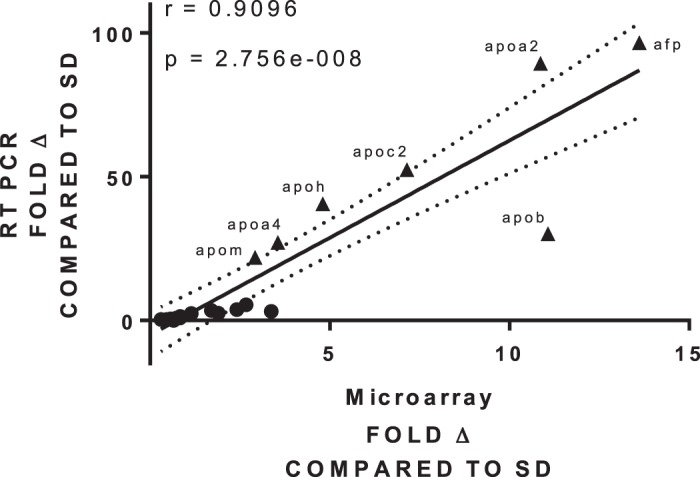
Microarray validation of 20 genes with quantitative RT-PCR; n = 4–5 per group/time.
DISCUSSION
Previous work found that the Dahl S demonstrate a robust and consistent PE phenotype involving increased blood pressure and proteinuria during late pregnancy. Final pregnancy outcomes involve decreases in pup number, weight, and length as well as an increase in fetus resorptions (25, 26). The aim of this study was to assess early differences in temporal transcriptomic profiles in the maternal-fetal interface in a rat model of superimposed PE. The Dahl S rat model was compared with normal healthy pregnancy (SD) to investigate expression patterns in both strains at days 0, 7, 10, 14, and 20 of pregnancy with qRT-PCR and Affymetrix GeneChip technology. While similarities were observed in expression changes across the time-course of pregnancy irrespective of strain, indicating a common theme of pregnancy itself, there were significant differences in the expression patterns between normal and preeclamptic pregnancy. Our data confirmed that 21 of the 30 genes previously associated with PE (either in human or animal studies) change across the course of pregnancy in the Dahl S compared with SD. In particular, quantitatively higher expression levels for four or more of the five time points in the Dahl S rat model of PE compared with the healthy SD were observed. Notably, 80% of these genes illustrate large increases in expression in the Dahl S compared with SD, demonstrating that this preclinical animal model of PE exhibits similar expression to that which would be expected. For example, arginine vasopressin (Avp), a peptide hormone with vasoconstrictive activity, causes a preeclamptic phenotype when chronically infused in C57BL6/J mice (61). Consistent with Avp playing a role in PE, our data show increased expression levels of Avp in the Dahl S (PE) compared with SD (normal pregnancy). Similarly, Endothelin-1 (Edn-1), a potent vasoconstrictor that has been shown to be increased in human PE, was upregulated in the Dahl S compared with SD, further underscoring the pregnant Dahl S rat as a model of PE (23). Surprisingly there were increases in transcript levels of Nitric Oxide Synthase (Nos) isoforms 1 and 3 as well as Relaxin (Rln) isoforms 1 and 3. These changes in transcript levels would not be expected in PE; however, these changes in RNA expression may not correlate with protein expression or activity. The qRT-PCR analysis of these known associated PE genes coupled with the pregnancy phenotype we and others have previously described further supports the use of the Dahl S rat as a genetic model of superimposed PE (26, 67).
A second aim of the study was to identify novel pathways involved in the early pathogenesis of PE in an unbiased way. Whole transcriptome analysis was performed on uterine tissue during the first half of pregnancy and samples obtained during early development of the placenta by microarray technology. These analyses identified several pathways that may provide a deeper understanding of the pathophysiology of PE and future targets for intervention and/or biomarkers of PE, including fatty acid metabolism and metabolism of lipids as well as additional pathways involved in inflammatory responses.
Fatty acid metabolism and metabolism of lipids.
The placenta’s primary role in pregnancy is to supply key nutrients to the fetus, namely oxygen, glucose, amino acids, and fatty acids. Of these, lipids and fatty acids are important factors for proper maintenance and development of fetal cellular membranes and can be used as a substrate for energy (64). An increased flux of these nutrients through their metabolic pathways could result in increases of oxidative stress and inflammatory cascades, both of which are key constituents in the development of PE (19, 31, 39). Additionally, circulating inflammatory factors such as TNF-α and proinflammatory cytokines are upregulated in PE and are known to mediate lipid dysregulation by inhibiting lipogenesis, stimulating lipolysis and release of free fatty acids, which result in increased circulating triglycerides and nonesterified free fatty acids (19, 31, 39, 58, 64). As our analysis has shown, Reactome pathways highlight significant differential expression in genes involved in both the metabolism of lipids as well as fatty acid metabolism across all experimental time-points (days 0–14) between Dahl S (PE) and SD (normal) pregnant rats.
For example, paraoxonase 2 (Pon2), a gene highlighted in the Reactome pathway for both metabolism of lipids and fatty acid metabolism on day 14, is an enzyme that plays a protective role against oxidative stress. While clinical studies examining the expression of this gene in PE are limited, Pon2 has been shown to be decreased in women with PE, and we observed a similar decrease in Pon2 in the Dahl S (PE) compared with SD (normal pregnancy) (1, 62).
Various apolipoproteins have been implicated in playing a role in PE. However, several studies have assessed the roles of these lipid metabolizers and transporters with unclear results. In our study, we identified a large number of apolipoproteins, including Apoa2, Apoa4, Apob, Apoc2, Apoe, Apoh, and Apom that demonstrated significant differences between Dahl S (PE) compared with SD (normal pregnant). Higher levels of Apoe, for example, have been associated with increased risk of PE and increases in frequency of Apoe alleles have been detected in women with severe PE (50, 63). However, other studies suggest Apoe is decreased in PE, while Apob is significantly elevated in preeclamptic human pregnancy (5, 44, 49). Adding to the complexity of the apolipoprotein story, an additional study looking at Apoa1 showed increased levels in preeclamptic pregnancies (81). While the exact role that these apolipoproteins play in PE in humans remains uncertain, it is evident from our analysis that they also appear to play a role in the Dahl S (PE) compared with SD (normal pregnant).
Inflammatory placental environment.
In this study, Cd19, a biomarker for B cells, was found to be significantly upregulated in the placenta of our Dahl S rat compared with SD. Several studies have attempted to link the relationship of CD19+CD5+ B cells and their production of autoantibodies to an increased risk of PE (8, 52). In the Dahl S model, this increased B cell population could result in an increase in circulating inflammatory factors, such as TNF-α, which has been shown to be upregulated in this model (26). However, in this setting, it can only be said that there is an upregulation of expression of Cd19 suggesting an increase in B cell population within the placental unit.
Pregnancy necessitates a delicate balance in the immune system, and deviations in maternal T cell polarization have been implicated in the pathogenesis of PE. Normal pregnancy is understood to be a T helper (Th) 2/T regulatory (Treg)-immunotolerant state; whereas preeclamptic pregnancies have been characterized as a proinflammatory environment, with Th1 and Th17 cells predominating. Cd200, an anti-inflammatory signaling molecule found on the surface of trophoblast cells, is an important immune tolerance and inflammatory response regulator and has been closely linked to pregnancy and complications therein, via its ability to alter the immune response from a Th1 to Th2 cytokine shift via binding to the CD200R, suppressing NK cell activity and promotion of Th2 cytokines (16). Reduced expression of Cd200 has been previously implicated in the trophoblasts of preeclamptic placentas and was associated with increased levels of TNF-α, IL-6 and other inflammatory factors (77). Cd200 was found to be downregulated in the Dahl S placenta (day 14) compared with SD, which could contribute to an increased inflammatory response phenotype characteristically found in women with PE, potentially due to the lack of Th1 to Th2 shift typically seen in normal pregnancy.
Similarly, a delicate balance in the activation of the complement system is necessary for normal placental development (54, 55). Excessive activation of this system leads to chemotaxis of inflammatory cells and enhances phagocytosis by neutrophils and macrophages. C3 stands at the crux of the complement cascade as it is the final common pathway in complement activation (47). This gene has been previously indicated to have increased expression in the BPH/5 mouse placenta, an animal model that develops spontaneous PE (56, 65). Similar to this model, our Dahl S rat exhibited increases in C3 expression of placental tissues on day 14.
Validation of notable differentially expressed genes.
In addition to the apolipoprotein genes discussed above, additional genes were included in the qRT-PCR validation analysis. Regulator of G protein signaling 5 (Rgs5) was included due to having a significant decrease in fold change in the Dahl S compared with SD on day 14. Rgs5 has been shown to play an important role in blood pressure control during pregnancy. Rgs5-deficient mice have increased blood pressure during gestation due to increased sensitivity to angiotensin II (ANG II) and when challenged with ANG II results in a preeclamptic phenotype (hypertension, proteinuria, placental pathology, and reduced fetal weight) (33). Alpha-fetoprotein (Afp), on the other hand, showed significant increased fold change in the Dahl S compared with SD on day 14 for both the microarray and qRT-PCR validation analysis. Interestingly, women with PE, uteroplacental insufficiency, preterm birth, or placental abnormalities have elevated levels of Afp in their second-trimester as compared with gestational age-matched controls (3, 17, 69a, 71).
Conclusion
Since the cause of PE may be rooted in the placenta, its development and the events leading up to proper placentation warrant investigation. While genomic and proteomic studies in human PE placental tissue have been beneficial, most have consisted of placentas obtained at delivery, leaving a broad knowledge gap in the understanding of preceding events. This study identified differences in this maternal-fetal interface along the time-course of pregnancy (days 0, 7, 10, 14, and 20) in a rat model of spontaneous superimposed PE (Dahl S) compared with a normal pregnant control rats (SD). The prevalence of fatty acid metabolism and metabolism of lipids throughout this study highlights future points of potential intervention as well as possible mechanisms underlying the pathology of PE. Future studies to examine this pathway, as well as others identified through this analysis, are needed to identify potential therapeutic targets for the treatment of PE or determine the potential for use of markers in this pathway to aid in the diagnosis of superimposed PE.
GRANTS
This work was supported by National Institutes of Health Grants T32HL-105324 (K. J. Maeda), R01HL-134711, K01DK-095018, and P20GM-103476 (J. M. Sasser), and R01HL-137673 (M. R. Garrett). The work performed through the UMMC Molecular and Genomics Facility is supported, in part, by funds from the National Institute of General Medical Sciences, including Mississippi INBRE (P20GM-103476), Obesity, Cardiorenal and Metabolic Diseases- COBRE (P20GM-104357), and Mississippi Center of Excellence in Perinatal Research (MS-CEPR)-COBRE (P20GM-121334).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.J.M. and A.C.J. performed experiments; K.J.M. and K.C.S. analyzed data; K.J.M. interpreted results of experiments; K.J.M. prepared figures; K.J.M. drafted manuscript; K.J.M., K.C.S., A.C.J., M.R.G., and J.M.S. edited and revised manuscript; K.J.M., K.C.S., A.C.J., M.R.G., and J.M.S. approved final version of manuscript; M.R.G. and J.M.S. conceived and designed research.
ACKNOWLEDGMENTS
The authors thank Jennifer Mooney and Divya Patel for technical assistance.
REFERENCES
- 1.Aksoy AN, Ozturk N, Aksoy H, Akcay F. Paraoxonase and arylesterase activities in patients with preeclampsia. Eurasian J Med 40: 10–13, 2008. [PMC free article] [PubMed] [Google Scholar]
- 2.Alexa A, Rahnenfuhrer J. topGO: Enrichment Analysis for Gene Ontology. Bioconductor, 2018. [Google Scholar]
- 3.Allen R, Marleen S, Velauthar L, Harrington K, Aquilina J. The relationship between second trimester alpha fetoprotein levels and adverse pregnancy outcome. OJOG 3: 262–266, 2013. doi: 10.4236/ojog.2013.32049. [DOI] [Google Scholar]
- 4.Anand-Ivell R, Ivell R. Regulation of the reproductive cycle and early pregnancy by relaxin family peptides. Mol Cell Endocrinol 382: 472–479, 2014. doi: 10.1016/j.mce.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Belo L, Gaffney D, Caslake M, Santos-Silva A, Pereira-Leite L, Quintanilha A, Rebelo I. Apolipoprotein E and cholesteryl ester transfer protein polymorphisms in normal and preeclamptic pregnancies. Eur J Obstet Gynecol Reprod Biol 112: 9–15, 2004. doi: 10.1016/S0301-2115(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson H, Corrada Bravo H, Gentleman R, Hossjer O, Jaffee H, Jiang D, Langfelder P, Hickey P. matrixStats: Functions that Apply to Rows and Columns of Matrices and to Vectors. 2018.
- 7.Bokslag A, van Weissenbruch M, Mol BW, de Groot CJM. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev 102: 47–50, 2016. doi: 10.1016/j.earlhumdev.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Borchers AT, Naguwa SM, Keen CL, Gershwin ME. The implications of autoimmunity and pregnancy. J Autoimmun 34: J287–J299, 2010. doi: 10.1016/j.jaut.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 348, apr15 7: g2301, 2014. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brew O, Sullivan MHF, Woodman A. Comparison of Normal and Pre-Eclamptic Placental Gene Expression: A Systematic Review with Meta-Analysis. PLoS One 11: e0161504, 2016. doi: 10.1371/journal.pone.0161504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brocker C, Carpenter C, Nebert DW, Vasiliou V. Evolutionary divergence and functions of the human acyl-CoA thioesterase gene (ACOT) family. Hum Genomics 4: 411–420, 2010. doi: 10.1186/1479-7364-4-6-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho B. pd.ragene.2.0.st: Platform Design Info for Affymetrix RaGene-2_0-st. R package version 3.14.1. Bioconductor, 2015. doi: 10.18129/B9.bioc.pd.ragene.2.0.st. [DOI] [Google Scholar]
- 13.Carvalho B, Scharpf R. oligoClasses: Classes for high-throughput arrays supported by oligo and crlmm. R package version 1.40.0. Bioconductor, 2018. [Google Scholar]
- 14.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics 26: 2363–2367, 2010. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 10: 466–480, 2014. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark DA, Arredondo JL, Dhesy-Thind S. The CD200 tolerance-signaling molecule and its receptor, CD200R1, are expressed in human placental villus trophoblast and in peri-implant decidua by 5 weeks’ gestation. J Reprod Immunol 112: 20–23, 2015. doi: 10.1016/j.jri.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Clayton-Hopkins JA, Olsen PN, Blake AP. Maternal serum alfa-fetoprotein levels in the pregnancy complicated by hypertension. Prenat Diagn 2: 47–54, 1982. doi: 10.1002/pd.1970020108. [DOI] [Google Scholar]
- 18.Conrad KP, Davison JM. The renal circulation in normal pregnancy and preeclampsia: is there a place for relaxin? Am J Physiol Renal Physiol 306: F1121–F1135, 2014. doi: 10.1152/ajprenal.00042.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding X, Yang Z, Han Y, Yu H. Fatty acid oxidation changes and the correlation with oxidative stress in different preeclampsia-like mouse models. PLoS One 9: e109554, 2014. doi: 10.1371/journal.pone.0109554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fushima T, Sekimoto A, Minato T, Ito T, Oe Y, Kisu K, Sato E, Funamoto K, Hayase T, Kimura Y, Ito S, Sato H, Takahashi N. Reduced Uterine Perfusion Pressure (RUPP) Model of Preeclampsia in Mice. PLoS One 11: e0155426, 2016. doi: 10.1371/journal.pone.0155426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gentleman R, Biocore geneplotter: Graphics related functions for Bioconductor. Bioconductor, 2018. [Google Scholar]
- 22.Gentleman R, Carey V, Huber W, Hahne F. genefilter: genefilter: methods for filtering genes from high-throughput experiments. Bioconductor, 2018. doi: 10.18129/B9.bioc.genefilter. [DOI] [Google Scholar]
- 23.George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens 24: 964–969, 2011. doi: 10.1038/ajh.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 294: H541–H550, 2008. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 25.Gillis EE, Mooney JN, Garrett MR, Granger JP, Sasser JM. Sildenafil Treatment Ameliorates the Maternal Syndrome of Preeclampsia and Rescues Fetal Growth in the Dahl Salt-Sensitive Rat. Hypertension 67: 647–653, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol 309: R62–R70, 2015. doi: 10.1152/ajpregu.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goulopoulou S, Davidge ST. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol Med 21: 88–97, 2015. doi: 10.1016/j.molmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Graffelman J. calibrate: Calibration of Scatterplot and Biplot Axes. rdrr.io, 2013. [Google Scholar]
- 29.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32: 2847–2849, 2016. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 30.Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize Implements and enhances circular visualization in R. Bioinformatics 30: 2811–2812, 2014. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 31.Herrera E, Ortega-Senovilla H. Maternal lipid metabolism during normal pregnancy and its implications to fetal development. Clin Lipidol 5: 899–911, 2010. doi: 10.2217/clp.10.64. [DOI] [Google Scholar]
- 32.Hod T, Cerdeira AS, Karumanchi SA. Molecular Mechanisms of Preeclampsia. Cold Spring Harb Perspect Med 5: a023473, 2015. doi: 10.1101/cshperspect.a023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holobotovskyy V, Chong YS, Burchell J, He B, Phillips M, Leader L, Murphy TV, Sandow SL, McKitrick DJ, Charles AK, Tare M, Arnolda LF, Ganss R. Regulator of G protein signaling 5 is a determinant of gestational hypertension and preeclampsia. Sci Transl Med 7: 290ra88, 2015. doi: 10.1126/scitranslmed.aaa5038. [DOI] [PubMed] [Google Scholar]
- 34.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13, 2009. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 36.Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, Gottardo R, Hahne F, Hansen KD, Irizarry RA, Lawrence M, Love MI, MacDonald J, Obenchain V, Oleś AK, Pagès H, Reyes A, Shannon P, Smyth GK, Tenenbaum D, Waldron L, Morgan M. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods 12: 115–121, 2015. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunkapiller NM, Gasperowicz M, Kapidzic M, Plaks V, Maltepe E, Kitajewski J, Cross JC, Fisher SJ. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development 138: 2987–2998, 2011. doi: 10.1242/dev.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaaja R. Lipid abnormalities in pre-eclampsia: implications for vascular health. Clin Lipidol 6: 71–78, 2011. doi: 10.2217/clp.10.82. [DOI] [Google Scholar]
- 40.Kauffmann A, Gentleman R, Huber W. arrayQualityMetrics–a bioconductor package for quality assessment of microarray data. Bioinformatics 25: 415–416, 2009. doi: 10.1093/bioinformatics/btn647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kauffmann A, Rayner TF, Parkinson H, Kapushesky M, Lukk M, Brazma A, Huber W. Importing ArrayExpress datasets into R/Bioconductor. Bioinformatics 25: 2092–2094, 2009. doi: 10.1093/bioinformatics/btp354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klaus B, Reisenauer S. An end to end workflow for differential gene expression using Affymetrix microarrays. F1000 Res 5: 1384, 2016. doi: 10.12688/f1000research.8967.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi S, Tanaka M, Masaki K, Hirakawa S, Momose K. Apolipoprotein levels in preeclamptic pregnancies. Nippon Sanka Fujinka Gakkai Zasshi 44: 223–228, 1992. [PubMed] [Google Scholar]
- 45.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol 63: 587–600, 2010. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolde R. pheatmap: Pretty Heatmaps. 2018.
- 47.Lokki AI, Kaartokallio T, Holmberg V, Onkamo P, Koskinen LLE, Saavalainen P, Heinonen S, Kajantie E, Kere J, Kivinen K, Pouta A, Villa PM, Hiltunen L, Laivuori H, Meri S. Analysis of Complement C3 Gene Reveals Susceptibility to Severe Preeclampsia. Front Immunol 8: 589, 2017. doi: 10.3389/fimmu.2017.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacDonald JW. ragene20sttranscriptcluster.db: Affymetrix ragene20 annotation data (chipragene20sttranscriptcluster). Bioconductor, 2017. [Google Scholar]
- 49.Makkonen N, Heinonen S, Hiltunen M, Helisalmi S, Mannermaa A, Kirkinen P. Apolipoprotein E alleles in women with pre-eclampsia. J Clin Pathol 54: 652–654, 2001. doi: 10.1136/jcp.54.8.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagy B, Rigó J Jr, Fintor L, Karádi I, Tóth T. Apolipoprotein E alleles in women with severe pre-eclampsia. J Clin Pathol 51: 324–325, 1998. doi: 10.1136/jcp.51.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuwirth E. RColorBrewer: ColorBrewer Palettes. 2014.
- 52.Nguyen TG, Ward CM, Morris JM. To B or not to B cells-mediate a healthy start to life. Clin Exp Immunol 171: 124–134, 2013. doi: 10.1111/cei.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parikh SM, Karumanchi SA. Putting pressure on pre-eclampsia. Nat Med 14: 810–812, 2008. doi: 10.1038/nm0808-810. [DOI] [PubMed] [Google Scholar]
- 54.Qing X, Redecha PB, Burmeister MA, Tomlinson S, D’Agati VD, Davisson RL, Salmon JE. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int 79: 331–339, 2011. doi: 10.1038/ki.2010.393. [DOI] [PubMed] [Google Scholar]
- 55.Regal JF, Burwick RM, Fleming SD. The Complement System and Preeclampsia. Curr Hypertens Rep 19: 87, 2017. doi: 10.1007/s11906-017-0784-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reijnders D, Liu CC, Xu X, Zhao AM, Olson KN, Butler SD, Douglas NC, Sones JL. Celecoxib restores angiogenic factor expression at the maternal-fetal interface in the BPH/5 mouse model of preeclampsia. Physiol Genomics 50: 385–392, 2018. doi: 10.1152/physiolgenomics.00115.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56a.Riley JK, Nelson DM. Toll-like receptors in pregnancy disorders and placental dysfunction. Clin Rev Allergy Immunol 39: 185–193, 2010. doi: 10.1007/s12016-009-8178-2. [DOI] [PubMed] [Google Scholar]
- 57.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson NJ, Minchell LJ, Myers JE, Hubel CA, Crocker IP. A potential role for free fatty acids in the pathogenesis of preeclampsia. J Hypertens 27: 1293–1302, 2009. doi: 10.1097/HJH.0b013e328329fbfe. [DOI] [PubMed] [Google Scholar]
- 59.Sado T, Naruse K, Noguchi T, Haruta S, Yoshida S, Tanase Y, Kitanaka T, Oi H, Kobayashi H. Inflammatory pattern recognition receptors and their ligands: factors contributing to the pathogenesis of preeclampsia. Inflamm Res 60: 509–520, 2011. doi: 10.1007/s00011-011-0319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandgren JA, Scroggins SM, Santillan DA, Devor EJ, Gibson-Corley KN, Pierce GL, Sigmund CD, Santillan MK, Grobe JL. Vasopressin: the missing link for preeclampsia? Am J Physiol Regul Integr Comp Physiol 309: R1062–R1064, 2015. doi: 10.1152/ajpregu.00073.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GK, Gibson-Corley KN, Grobe JL. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension 64: 852–859, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawant SD, Mogarekar MR. Human Monocytic Paraoxonse2 (PON2) Association with Birth weight In Preeclamptic Patients. Int J Biotechnol Biochem 12: 43–54, 2016. [Google Scholar]
- 63.Serrano NC, Guio-Mahecha E, Quintero-Lesmes DC, Becerra-Bayona S, Paez MC, Beltran M, Herrera VM, Leon LJ, Williams D, Casas JP. Lipid profile, plasma apolipoproteins, and pre-eclampsia risk in the GenPE case-control study. Atherosclerosis 276: 189–194, 2018. doi: 10.1016/j.atherosclerosis.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 64.Shin EK, Kang HY, Yang H, Jung EM, Jeung EB. The Regulation of Fatty Acid Oxidation in Human Preeclampsia. Reprod Sci 23: 1422–1433, 2016. doi: 10.1177/1933719116641759. [DOI] [PubMed] [Google Scholar]
- 65.Sones JL, Merriam AA, Seffens A, Brown-Grant DA, Butler SD, Zhao AM, Xu X, Shawber CJ, Grenier JK, Douglas NC. Angiogenic factor imbalance precedes complement deposition in placentae of the BPH/5 model of preeclampsia. FASEB J 32: 2574–2586, 2018. doi: 10.1096/fj.201701008R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spann RA, Lawson WJ, Bidwell GL III, Zamarripa CA, Maranon RO, Bandyopadhyay S, Taylor ER, Reckelhoff JF, Garrett MR, Grayson BE. Rodent vertical sleeve gastrectomy alters maternal immune health and fetoplacental development. Clin Sci (Lond) 132: 295–312, 2018. doi: 10.1042/CS20171416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takushima S, Nishi Y, Nonoshita A, Mifune H, Hirata R, Tanaka E, Doi R, Hori D, Kamura T, Ushijima K. Changes in the nitric oxide-soluble guanylate cyclase system and natriuretic peptide receptor system in placentas of pregnant Dahl salt-sensitive rats. J Obstet Gynaecol Res 41: 540–550, 2015. doi: 10.1111/jog.12602. [DOI] [PubMed] [Google Scholar]
- 68.Turner S. Tmisc: Turner Miscellaneous. rdrr.io, 2018. [Google Scholar]
- 69.Walker A. openxlsx: Read, Write and Edit XLSX Files. rdrr.io, 2018. [Google Scholar]
- 69a.Waller DK, Lustig LS, Cunningham GC, Feuchtbaum LB, Hook EB. The association between maternal serum alpha-fetoprotein and preterm birth, small for gestational age infants, preeclampsia, and placental complications. Obstet Gynecol 88: 816–822, 1996. doi: 10.1016/0029-7844(96)00310-9. [DOI] [PubMed] [Google Scholar]
- 70.Warnes GR, Bolker B, Bonebakker L, Gentleman R, Liaw WHA, Lumley T, Maechler M, Magnusson A, Moeller S, Schwartz M, Venables B. gplots: Various R Programming Tools for Plotting Data. rdrr.io, 2016. [Google Scholar]
- 71.Weitzner O, Yagur Y, Weissbach T, Man El G, Biron-Shental T. Preeclampsia: risk factors and neonatal outcomes associated with early- versus late-onset diseases. J Matern Fetal Neonatal Med Sep 6: 1–5, 2018. doi: 10.1080/14767058.2018.1500551. [DOI] [PubMed] [Google Scholar]
- 72.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag, 2016. [Google Scholar]
- 73.Wickham H. stringr: Simple, Consistent Wrappers for Common String Operations. rdrr.io, 2018. [Google Scholar]
- 74.Wickham H, François R, Henry L, Müller K. dplyr: A Grammar of Data Manipulation. rdrr.io, 2018. [Google Scholar]
- 75.Wickham H, Henry L. tidyr: Easily Tidy Data with ‘spread()’ and ‘gather()’ Functions. rdrr.io, 2018. [Google Scholar]
- 76.Williams PJ, Broughton Pipkin F. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 25: 405–417, 2011. doi: 10.1016/j.bpobgyn.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu J, Gu Y, Sun J, Zhu H, Lewis DF, Wang Y. Reduced CD200 expression is associated with altered Th1/Th2 cytokine production in placental trophoblasts from preeclampsia. Am J Reprod Immunol 79: e12763, 2018. doi: 10.1111/aji.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol 5: 173–192, 2010. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 79.Yu G, He Q-Y. ReactomePA: an R/Bioconductor package for reactome pathway analysis and visualization. Mol Biosyst 12: 477–479, 2016. doi: 10.1039/C5MB00663E. [DOI] [PubMed] [Google Scholar]
- 80.Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16: 284–287, 2012. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang H, Zhang Y, Yang F, Li L, Liu S, Xu Z, Wang J, Sun S. Complement component C4A and apolipoprotein A-I in plasmas as biomarkers of the severe, early-onset preeclampsia. Mol Biosyst 7: 2470–2479, 2011. doi: 10.1039/c1mb05142c. [DOI] [PubMed] [Google Scholar]